Key Points
Question
For patients with blunt trauma at high risk of spleen rupture, does prophylactic splenic artery embolization improve the rate of spleen rescue compared with surveillance and embolization only if necessary?
Findings
In this randomized clinical trial, the number of patients with an at least 50% viable spleen detected on a computed tomography scan at 1 month was not significantly different between patients receiving immediate prophylactic splenic artery embolization and those receiving surveillance only, with embolization only if necessary. Many patients in the surveillance group received embolization within a few days and were hospitalized for significantly longer.
Meaning
For patients with severe splenic trauma, both strategies resulted in a spleen rescue rate greater than 93%.
Abstract
Importance
Splenic arterial embolization (SAE) improves the rate of spleen rescue, yet the advantage of prophylactic SAE (pSAE) compared with surveillance and then embolization only if necessary (SURV) for patients at high risk of spleen rupture remains controversial.
Objective
To determine whether the 1-month spleen salvage rate is better after pSAE or SURV.
Design, Setting, and Participants
In this randomized clinical trial conducted between February 6, 2014, and September 1, 2017, at 16 institutions in France, 133 patients with splenic trauma at high risk of rupture were randomized to undergo pSAE or SURV. All analyses were performed on a per-protocol basis, as well as an intention-to-treat analysis for specific events.
Interventions
Prophylactic SAE, preferably using an arterial approach via the femoral artery, or SURV.
Main Outcomes and Measures
The primary end point was an intact spleen or a spleen with at least 50% vascularized parenchyma detected on an arterial computed tomography scan at 1 month after trauma, assessed by senior radiologists masked to the treatment group. Secondary end points included splenectomy and pseudoaneurysm, secondary SAE after inclusion, complications, length of hospital stay, quality-of-life score, and length of time off work or studies during the 6-month follow-up.
Results
A total of 140 patients were randomized, and 133 (105 men [78.9%]; median age, 30 years [interquartile range, 23-47 years]) were retained in the study. For the primary end point, data from 117 patients (57 who underwent pSAE and 60 who underwent SURV) could be analyzed. The number of patients with at least a 50% viable spleen detected on a computed tomography scan at month 1 was not significantly different between the pSAE and SURV groups (56 of 57 [98.2%] vs 56 of 60 [93.3%]; difference, 4.9%; 95% CI, −2.4% to 12.1%; P = .37). By the day 5 visit, there were significantly fewer splenic pseudoaneurysms among patients in the pSAE group than in the SURV group (1 of 65 [1.5%] vs 8 of 65 [12.3%]; difference, −10.8%; 95% CI, −19.3% to −2.1%; P = .03), significantly fewer secondary embolizations among patients in the pSAE group than in the SURV group (1 of 65 [1.5%] vs 19 of 65 [29.2%]; difference, −27.7%; 95% CI, −41.0% to −15.9%; P < .001), and no difference in the overall complication rate between the pSAE and SURV groups (19 of 65 [29.2%] vs 27 of 65 [41.5%]; difference, −12.3%; 95% CI, −28.3% to 4.4%; P = .14). Between the day 5 and month 1 visits, the overall complication rate was not significantly different between the pSAE and SURV groups (11 of 59 [18.6%] vs 12 of 63 [19.0%]; difference, −0.4%; 95% CI, −14.4% to 13.6%; P = .96). The median length of hospitalization was significantly shorter for patients in the pSAE group than for those in the SURV group (9 days [interquartile range, 6-14 days] vs 13 days [interquartile range, 9-17 days]; P = .002).
Conclusions and Relevance
Among patients with splenic trauma at high risk of rupture, the 1-month spleen salvage rate was not statistically different between patients undergoing pSAE compared with those receiving SURV. In view of the high proportion of patients in the SURV group needing SAE, both strategies appear defendable.
Trial Registration
ClinicalTrials.gov Identifier: NCT02021396
This randomized clinical trial assesses whether the 1-month spleen salvage rate among patients with splenic trauma at high risk of rupture is higher after prophylactic splenic arterial embolization or surveillance and then embolization only if necessary.
Introduction
The spleen is the organ most frequently affected in the event of blunt abdominal trauma, with an incidence of approximatively 40 000 splenic traumas per year in the United States.1 In 10% to 20% of splenic trauma cases,2,3 the patient is admitted in a state of hemorrhagic shock and requires immediate surgical management with, in most cases, hemostatic splenectomy. Complications of splenectomy are either immediately postoperative4,5,6 or lifelong, principally fulminant infections for which the occurrence is 100 times higher than that in individuals who did not undergo splenectomy.7,8 In the approximately 85% of patients who are hemodynamically stable on arrival, the aim is to obtain the best splenic rescue rate. Nonoperative management of splenic trauma has been recommended for 20 years,9 but, in practice, secondary splenectomy owing to hemorrhage is often needed.1 The final rate of spleen rescue was only 60% in a major retrospective review of the trauma experience in east coast US centers prior to the era of embolization.10
The development of splenic arterial embolization (SAE) in expert trauma centers has increased the rate of spleen rescue to more than 80%,11,12,13,14 and it has been shown that trauma centers with high rates of angiography have a lesser incidence of splenectomy in the management of blunt splenic injury than elsewhere.15,16 However, for the most part, these results come from retrospective series, and the question of operative vs nonoperative management of splenic trauma has never been rigorously evaluated in a randomized clinical trial, to our knowledge. Splenic arterial embolization is not free of complications and has a failure rate of up to 30%.2,17 The risk factors for complications are not yet well established.18,19 As for any organ, the current internationally accepted indication for SAE is the presence of an active leak of contrast medium detected on a computed tomography (CT) scan,20 but in view of its efficacy, expert centers have extended the indication for embolization to patients who have predisposing factors for secondary splenic hemorrhage that have been well identified in retrospective series: splenic pseudoaneurysms (SPAs) and splenic arteriovenous fistulas (SAVFs), a large hemoperitoneum, and severe damage (American Association for the Surgery of Trauma Organ Injury Scale [OIS] grade 3-5).2,13,21,22,23,24 In these situations, the failure rate of nonoperative treatment is greater than 50%.1,2,13 Owing to the greater than 80% risk of secondary rupture,25 preventive embolization of SPAs and SAVFs is currently performed as routine practice, as recommended by US and international guidelines1,21,26,27 and, ethically, cannot be questioned in a randomized clinical trial; nevertheless, for patients at high risk of secondary splenic hemorrhage, such as a large hemoperitoneum and severe damage, practices are still very heterogeneous.
Owing to the relatively high incidence of splenic trauma, it is important that the potential benefits and risks of SAE are clearly defined. In the present multi-institutional randomized clinical trial (NCT02021396), our hypothesis was that prophylactic SAE (pSAE) would improve the rate of spleen rescue at 1 month compared with surveillance alone followed by embolization only if necessary (SURV) among hemodynamically stable adult patients with severe spleen trauma at high risk of splenectomy. Secondary goals were to evaluate the adverse effects of pSAE compared with SURV at day 5, month 1, and month 6.
Methods
Design, Setting, and Participants
This was a prospective randomized multicenter clinical trial (trial protocol in Supplement 1). Patients admitted via the emergency department, shock treatment unit, or intensive care unit or for surgery in 1 of the 16 participating level 1 trauma centers throughout France between February 6, 2014, and September 1, 2017, were screened. Each participating institution provided institutional review board approval of the study protocol, and each patient, or their legal representative, provided written informed consent before participation. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Adult (≥18 years) hemodynamically stabilized patients (according to the French Society of Anesthesia & Intensive Care Medicine criteria28) with blunt splenic trauma that had occurred within the previous 48 hours and spleen damage with high risk of splenectomy assessed by injected abdominal CT were enrolled and randomized to receive pSAE or SURV. Inclusion criteria were either OIS grade 3 splenic trauma22 with a large pelvic hemoperitoneum (defined as large if there was perisplenic effusion associated with pelvic effusion) and/or serious damage with a New Injury Severity Score29 of 15 or more30; OIS grade 4 splenic trauma22; or OIS grade 5 splenic trauma with persisting vascularization of the spleen.22,31 Unstable patients, patients with OIS grade 5 shattered spleen, and patients who were stable but immediately needed embolization of the spleen or another organ (ie, an active leak and/or SPA or SAVF detected on the initial CT scan) were excluded.
To avoid classification bias, a preliminary consensus meeting of radiologists from participating centers was organized before the launch of the study to agree on definitions and provide specific training in grading splenic trauma OIS detected on CT scans22 (see consensus definitions in the eAppendix in Supplement 2). Moreover, assessment of all CT scans was performed by 2 expert radiologists (J.F. and V.M.-B.) who were blinded to the randomization group. If they both disagreed with the OIS classification proposed by the enrolling center, the patient was excluded. If 1 radiologist disagreed, a third assessment was made by a senior expert, and the patient was included or excluded. Records were kept in each center of the characteristics of eligible, included, and excluded patients (eTable 1 in Supplement 2).
Randomization and Masking
Included patients were randomly assigned in a 1:1 ratio to undergo either pSAE or SURV. Randomization was performed with stratification according to center. Centralized randomized assignment was performed electronically. Neither the patients nor their clinicians were blinded to treatment assignment. Both the criteria for inclusion (day 0) and the primary end point (month 1) were validated by 2 senior radiologists (J.F. and V.M.-B.) blinded to the treatment assignment.
Study Treatment
For pSAE, an arterial approach via the femoral artery was preferred, or, for patients with unfavorable anatomy, a humeral approach via the celiac trunk, using a maximum 6F catheter, was preferred. The choice of catheterization equipment was at the discretion of the operator. Rigid coils of 0.089 cm (0.035 in) were preferred to reduce the risk of emboli migration. The use of microcoils was discouraged, and the use of glue, gelatin fragments, or microparticles was prohibited, as described in the eFigure in Supplement 2. Proximal or combined proximal and distal splenic artery embolization was required (eFigure in Supplement 2).
Assessments
Before enrollment and randomization, each patient underwent a baseline evaluation consisting of medical history taking and a physical examination (including age, sex, OIS splenic injury severity grade, and New Injury Severity Score trauma severity).29,30 In routine practice, all hemodynamically stable patients with abdominal trauma underwent a whole-body multibarrel CT scan with contrast injection at admission. Patients with a history of allergy or demonstrated intolerance to iodine were treated using the antiallergic protocol in use in the hospital. The admission CT scan included abdominopelvic sections without injection, and then, after opacification, at arterial and parenchymal times (60-90 seconds). The quantity of iodine injected was at least 1 mL/kg at a concentration of 300 to 350 mg/mL. The imaging data collected during the study (CT scans and embolization) on CD or DVD were archived in a centralized and secure archiving system. For included patients, the medical evaluation, an assessment of complications, and whole-body multibarrel CT scans with contrast injection were repeated at day 5 (−1 day/+3 days) while the patient was still hospitalized and at 1 month and 6 months after enrollment. Management of patients followed the French28 and international guidelines, including the prevention of thromboembolic complications.32 Unplanned SAE was performed in the event of clinical deterioration and/or an urgent indication according to consensus recommendations.1,21,26,28 The amount of time a patient was off work or studies were interrupted (many patients were students) was noted. The duration of hospitalization and the Western Ontario and McMaster Universities Osteoarthritic Index (WOMAC) score33 (a patient-reported functional activity score, where 0 corresponds to no impairment and 96 [the maximum score] corresponds to severe handicap) were recorded at each follow-up visit.
End Points
The primary end point was the composite criterion of a spleen with at least 50% vascularized parenchyma detected on the arterial CT scan and no splenectomy at the 1-month postinclusion visit, assessed by senior radiologists (J.F. and V.M.-B.) masked to the study group. Secondary end points included death; splenectomy; vascular spleen abnormalities; need for urgent embolization or reembolization; hemorrhagic, infectious, and thromboembolic complications; length of hospital stay; spleen rescue rate at 6 months; total time off work or studies; and physical activity. Criteria for complications are listed in eTable 2 in Supplement 2.
Sample Size Calculation
Assuming an expected rate of splenectomy (or rate of spleen necrosis) of 60% in the SURV group and 10% in the SAE group1,2,13 and a 5% α risk and 20% β risk, 120 evaluable patients (60 in each group) were required. Thus, 140 patients were to be included with a maximal number misclassified and with 15% of images lost at 1 month.
Statistical Analysis
All analyses were performed on a per-protocol basis, as well as an intention-to-treat analysis for specific events. For all descriptive analyses, median values and interquartile ranges (IQRs) are given for continuous variables, and numbers and percentages are given for categorical variables. For categorical variables, the χ2 test was used to compare the 2 randomization groups, considering the Cochran34 criteria. If these were not validated, a nonparametric Fisher exact test was used. Continuous parameters were analyzed using the t test, or the Mann-Whitney test if normal distribution was not (graphically) validated. For the primary end point, a sensitivity analysis was performed using multiple imputation (10 imputations) of missing data in a simple logistic regression to validate the result. Confidence intervals for the difference of proportions were calculated using the Fisher z approximation. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Statistical analysis was performed using Stata, version 15 software (StataCorp).
Results
Study Patients
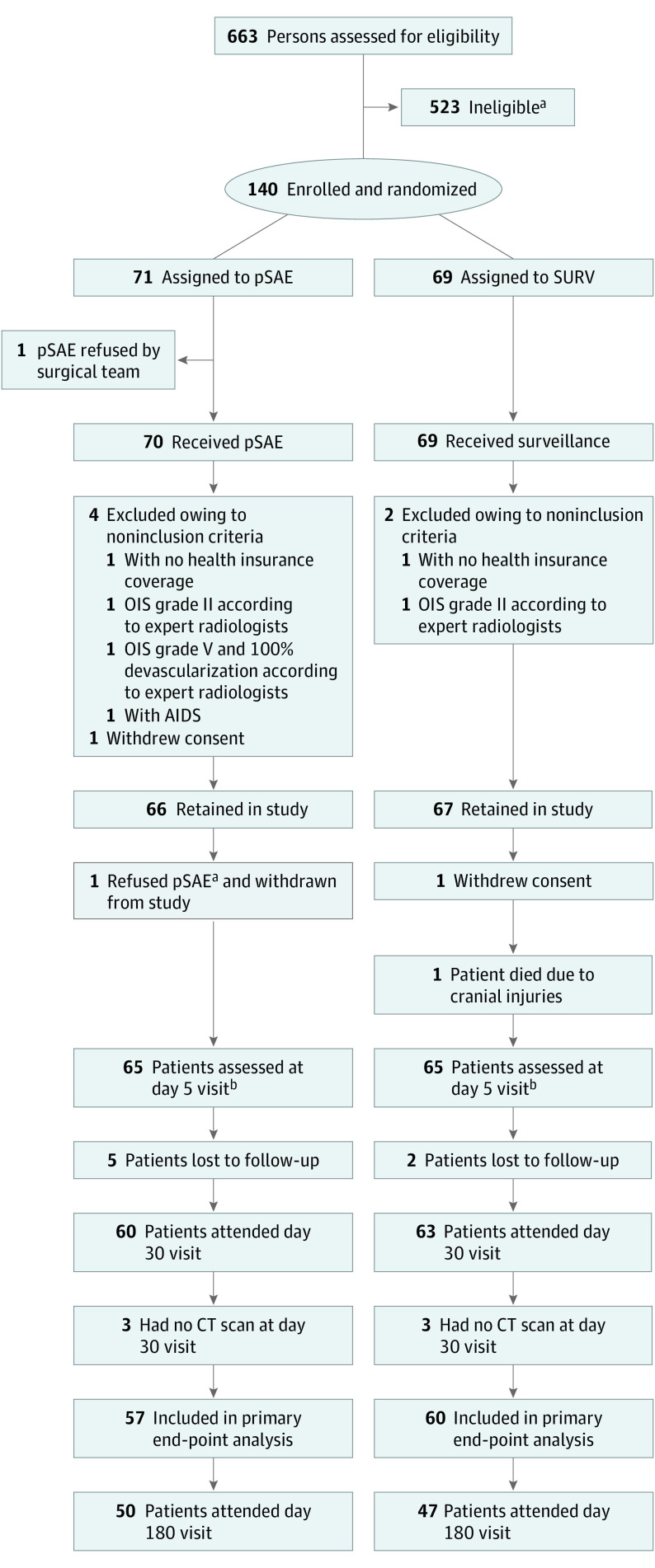
A total of 663 patients presented with splenic trauma in the 16 participating French level 1 trauma centers, of whom 140 eligible patients were enrolled in an emergency context and randomized to undergo immediate pSAE (n = 71) or SURV alone (n = 69). Seven patients were subsequently found to meet the exclusion criteria and were excluded from the study (Figure 1). The baseline characteristics of the remaining 133 patients were well balanced between the groups (Table 1).22,29,30,33 For the primary end point, 117 patients (83.6%) were evaluable, with a median interval since enrollment of 38 days (IQR, 32-47 days) and a completion rate of 57 of 71 (80.3%) in the SAE group and 60 of 69 (87.0%) in the SURV group (P = .29, determined by use of the Fisher exact test). There was no statistical difference between evaluable and nonevaluable patients for all criteria except for mean hospital-to-home distance and circumstances of the unintentional injury (Table 2).22,29,30,33
Figure 1. Study Flowchart.
CT indicates computed tomography; OIS, Organ Injury Scale; pSAE, prophylactic splenic arterial embolization; and SURV, surveillance with embolization only if necessary.
aSee eTable 1 in Supplement 2 for reasons for ineligibility.
bDay 5 visit occurred between day 4 and day 8 after inclusion.
Table 1. Baseline Characteristics of the Patients at Inclusion.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| pSAE (n = 66) | SURV (n = 67) | Total (N = 133) | |
| Sex | |||
| Female | 11 (16.7) | 17 (25.4) | 28 (21.1) |
| Male | 55 (83.3) | 50 (74.6) | 105 (78.9) |
| Employed or student | |||
| No | 18 (27.3) | 16 (23.9) | 34 (25.6) |
| Yes | 48 (72.7) | 50 (74.6) | 98 (73.7) |
| Missing | 0 | 1 (1.5) | 1 (0.8) |
| Age, median (IQR), y | 30 (22-42) | 30 (23-48) | 30 (23-47) |
| Distance between hospital and residence, median (IQR), km | 45 (15-95) | 30 (19-100) | 30 (15-100) |
| Time from unintentional injury to enrollment in trial, median (IQR), h | |||
| 0-23 | 29 (43.9) | 42 (62.7) | 71 (53.4) |
| 24-48 | 37 (56.1) | 25 (37.3) | 62 (46.6) |
| Circumstances of unintentional injury | |||
| Traffic | 39 (59.1) | 39 (58.2) | 78 (58.6) |
| Domestic | 3 (4.5) | 5 (7.5) | 8 (6.0) |
| Sport | 16 (24.2) | 16 (23.9) | 32 (24.1) |
| Work | 5 (7.6) | 6 (9.0) | 11 (8.3) |
| Other | 3 (4.5) | 1 (1.5) | 4 (3.0) |
| OIS grade (after expert rereading of CT scan images)a | |||
| 3 | 37 (56.1) | 43 (64.2) | 80 (60.2) |
| 4 | 28 (42.4) | 22 (32.8) | 50 (37.6) |
| 5 | 1 (1.5) | 2 (3.0) | 3 (2.3) |
| NISS, median (IQR)b | 19 (12-25) | 20 (13-29) | 19 (13-27) |
| WOMAC score available before unintentional injuryc | 60 (90.9) | 56 (83.6) | 116 (87.2) |
| Missing | 6 (9.1) | 11 (16.4) | 17 (12.8) |
| WOMAC score = 0 before unintentional injury, No./total No. (%)c | 46/60 (76.7) | 46/56 (82.1) | 92/116 (79.3) |
Abbreviations: CT, computed tomography; IQR, interquartile range; NISS, New Injury Severity Score; OIS, Organ Injury Scale; pSAE, prophylactic splenic arterial embolization; SURV, surveillance, with embolization only if necessary; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The OIS from the American Association for the Surgery of Trauma22 is a widely used scanographic score. It takes into account the type of damage (eg, hematoma or tearing), the localization in the splenic gland (eg, intraparenchymal or subcapsular), and the percentage of devascularized tissue. Grade 3 includes subcapsular hematoma of more than 50% of the spleen surface, rupture, spreading, or bleeding and/or intraparenchymal hematoma evolutive or diameter greater than 5 cm and/or capsular tear with a depth greater than 3 cm or that involves trabecular vessels. Grade 4 includes ruptured hematoma, lesion reaching segmental or hilar vessels, or more than 25% devascularized spleen. Grade 5 corresponds to complete splenic fragmentation.
The NISS29 is a widely used anatomical score giving an overall score for the anatomical lesions of a person with multiple traumas. Each organ involved is scored according to the OIS from 1 (mild) to 5 (total destruction or devascularization of the organ) according to the criteria of the American Association for the Surgery of Trauma. The NISS is calculated from the OIS of the 3 most serious lesions as follows: NISS = a2 + b2 + c2 (eg, a patient with a cerebral contusion rated OIS = 3, a spleen fracture rated OIS = 4, and minor hepatic injury rated OIS = 2 will have an NISS of 9 + 16 + 4 = 29). A trauma is considered severe when the NISS is 15 or more.30
The WOMAC33 self-administered questionnaire uses a Likert scale with 5 possible answers (none = 0, minimum = 1, moderate = 2, severe = 3, and extreme = 4) to several questions about physical functional impairment, pain, and stiffness. The minimum score (0) corresponds to no affliction and the maximum score (96) corresponds to severe distress and disability.
Table 2. Comparison of Baseline Characteristics of Nonevaluable and Evaluable Patients for the Primary End Point at Month 1.
| Characteristic | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| Nonevaluable (n = 16) | Evaluable (n = 117) | Total (N = 133) | ||
| Randomization arm | ||||
| pSAE | 9 (56.3) | 57 (48.7) | 66 (49.6) | .61 |
| SURV | 7 (43.8) | 60 (51.3) | 67 (50.4) | |
| Sex | ||||
| Female | 1 (6.3) | 27 (23.1) | 28 (21.1) | .19 |
| Male | 15 (93.8) | 90 (76.9) | 105 (78.9) | |
| Age, median (IQR), y | 27 (22-35) | 31 (23-48) | 30 (23-47) | .56 |
| Distance between hospital and residence, median (IQR), km | 104 (35-950) | 30 (15-92) | 30 (15-100) | .002 |
| Time from unintentional injury to enrollment in trial, median (IQR), h | ||||
| 0-23 | 11 (68.8) | 60 (51.3) | 71 (53.4) | .22 |
| 24-48 | 5 (31.3) | 57 (48.7) | 62 (46.6) | |
| Circumstances of unintentional injury | ||||
| Traffic | 5 (31.3) | 73 (62.4) | 78 (58.6) | .04 |
| Domestic | 2 (12.5) | 6 (5.1) | 8 (6.0) | |
| Sport | 8 (50.0) | 24 (20.5) | 32 (24.1) | |
| Work | 1 (6.3) | 10 (8.5) | 11 (8.3) | |
| Other | 0 | 4 (3.4) | 4 (3.0) | |
| OIS grade (after expert rereading of CT scan images)a | ||||
| 3 | 12 (75.0) | 68 (58.1) | 80 (60.2) | .51 |
| 4 | 4 (25.0) | 46 (39.3) | 50 (37.6) | |
| 5 | 0 | 3 (2.6) | 3 (2.3) | |
| NISS, median (IQR)b | 19.5 (10-31.5) | 19 (13-26) | 19 (13-27) | .71 |
| WOMAC score available before unintentional injuryc | 12 (75.0) | 104 (88.9) | 116 (87.2) | .13 |
| Missing | 4 (25.0) | 13 (11.1) | 17 (12.8) | |
| WOMAC score = 0 before unintentional injury, No./total No. (%)c | 10/12 (83.3) | 82/104 (78.8) | 92/116 (79.3) | >.99 |
Abbreviations: CT, computed tomography; IQR, interquartile range; NISS, New Injury Severity Score; OIS, Organ Injury Scale; pSAE, prophylactic splenic arterial embolization; SURV, surveillance, with embolization only if necessary; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The OIS from the American Association for the Surgery of Trauma22 is a widely used scanographic score. It takes into account the type of damage (eg, hematoma or tearing), the localization in the splenic gland (eg, intraparenchymal or subcapsular), and the percentage of devascularized tissue. Grade 3 includes subcapsular hematoma of more than 50% of the spleen surface, rupture, spreading, or bleeding and/or intraparenchymal hematoma evolutive or diameter greater than 5 cm and/or capsular tear with a depth greater than 3 cm or that involves trabecular vessels. Grade 4 includes ruptured hematoma, lesion reaching segmental or hilar vessels, or more than 25% devascularized spleen. Grade 5 corresponds to complete splenic fragmentation.
The NISS29 is a widely used anatomical score giving an overall score for the anatomical lesions of a person with multiple traumas. Each organ involved is scored according to the OIS from 1 (mild) to 5 (total destruction or devascularization of the organ) according to the criteria of the American Association for the Surgery of Trauma. The NISS is calculated from the OIS of the 3 most serious lesions as follows: NISS = a2 + b2 + c2 (eg, a patient with a cerebral contusion rated AIS = 3, a spleen fracture rated AIS = 4, and minor hepatic injury rated AIS = 2 will have an NISS of 9 + 16 + 4 = 29). A trauma is considered severe when the NISS is 15 or more.30
The WOMAC33 self-administered questionnaire uses a Likert scale with 5 possible answers (none = 0, minimum = 1, moderate = 2, severe = 3, and extreme = 4) to several questions about physical functional impairment, pain, and stiffness. The minimum score (0) corresponds to no affliction and the maximum score (96) corresponds to severe distress and disability.
Primary End Point
For the primary end point, the number of patients with at least 50% viable spleen detected on the CT scan at month 1 was not significantly different between the pSAE and SURV groups (56 of 57 [98.2%] vs 56 of 60 [93.3%]; difference, 4.9%; 95% CI, −2.4% to 12.1%; P = .37). The results of the sensitivity analysis are consistent with this conclusion (P = .16). The 5 cases of failed spleen rescue occurred in 5 different centers (eTable 3 in Supplement 2).
Overall Mortality and Morbidity
One patient from the SURV group died before the day 5 visit of cranial trauma (1 of 133 [0.8%]). There was no significant difference in the overall complication rate between the pSAE and SURV groups detected at day 5 (19 of 65 [29.2%] vs 27 of 65 [41.5%]; difference, −12.3%; 95% CI, −28.3% to 4.4%; P = .14), between day 5 and month 1 (11 of 59 [18.6%] vs 12 of 63 [19.0%]; difference, −0.4%; 95% CI, −14.4% to 13.6%; P = .96) (Table 3), or at the month 1 visit (eTable 4 in Supplement 2).
Table 3. Complications Reported at Day 5 Visit and at Month 1 Visit.
| Type of complication | Patients at day 5 visit, No. (%)a | P value | Patients at month 1 visit, No. (%)b | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 130) | pSAE (n = 65) | SURV (n = 65) | Total (N = 122) | pSAE (n = 59) | SURV (n = 63) | |||
| Need for splenic embolization | 20 (15.4) | 1 (1.5) | 19 (29.2) | <.001 | 4 (3.3) | 1 (1.7) | 3 (4.8) | .62 |
| Due to SAE procedure | ||||||||
| Hematoma on femoral access | 1 (0.8) | 1 (1.5) | 0 | >.99 | 1 (1.3)c | 1 (1.7) | 0d | >.99 |
| Thrombosis on femoral access | 1 (0.8) | 1 (1.5) | 0 | >.99 | 0c | 0 | 0d | NA |
| Aneurysm on femoral access | 1 (0.8) | 1 (1.5) | 0 | >.99 | 1 (1.3)c | 1 (1.7) | 0d | >.99 |
| Allergy to contrast agent | 0 | 0 | 0 | NA | 0 | 0 | 0 | NA |
| Kidney insufficiency | 0 | 0 | 0 | NA | 0 | 0 | 0 | NA |
| Splenic | ||||||||
| Abscess | 0 | 0 | 0 | NA | 0 | 0 | 0 | NA |
| Splenectomy | 3 (2.3) | 0 | 3 (4.6) | .12 | 1 (0.8) | 0 | 1 (1.6) | .52 |
| Arteriovenous fistula | 2 (1.5) | 0 | 2 (3.1) | .50 | 2 (1.6) | 1 (1.7) | 1 (1.6) | >.99 |
| Pseudoaneurysm | 9 (6.9) | 1 (1.5) | 8 (12.3) | .03 | 3 (2.5) | 0 | 3 (4.8) | .25 |
| Pseudocyst | 1 (0.8) | 1 (1.5) | 0 | >.99 | 1 (0.8) | 0 | 1 (1.6) | >.99 |
| Hemorrhagic | ||||||||
| Decrease in hemoglobin >3 g/dL with an identified bleeding site or a decrease in hemoglobin >4 g/dL without an identified bleeding site | 18 (13.8) | 7 (10.8) | 11 (16.9) | .31 | 3 (2.5) | 0 | 3 (4.8) | .25 |
| Transfusion | 15 (11.5) | 7 (10.8) | 8 (12.3) | >.99 | 2 (1.6) | 0 | 2 (3.2) | .50 |
| No. of packed RBC units transfused, median (IQR) | 2 (2-4) | 2 (1-3) | 2 (2-5) | .13 | 5 (4-6) | 0 | 5 (4-6) | NA |
| Infectious | ||||||||
| ≥1 Infectious complications | 4 (3.1) | 3 (4.6) | 1 (1.5) | .62 | 4 (3.3) | 1 (1.7) | 3 (4.8) | .62 |
| Septicemia | 1 (0.8) | 1 (1.5) | 0 | >.99 | 2 (1.6) | 0 | 2 (3.2) | .50 |
| Pancreatic | ||||||||
| Pancreatitis | 0 | 0 | 0 | NA | 2 (1.6) | 0 | 2 (3.2) | .50 |
| Thrombotic | ||||||||
| Thrombosis of splenoportal trunk | 1 (0.8) | 0 | 1 (1.5) | >.99 | 0 | 0 | 0 | NA |
| Phlebitis | 0 | 0 | 0 | NA | 1 (0.8) | 0 | 1 (1.6) | NA |
| Pulmonary embolism | 2 (1.5) | 1 (1.5) | 1 (1.5) | >.99 | 1 (0.8) | 0 | 1 (1.6) | NA |
| Pulmonary | ||||||||
| Pleural effusion | 11 (8.5) | 5 (7.7) | 6 (9.2) | .75 | 4 (3.3) | 2 (3.4) | 2 (3.2) | >.99 |
| Thoracic drain if pleural effusion | 5 (3.8) | 2 (3.1) | 3 (4.6) | >.99 | 1 (0.8) | 0 | 1 (1.6) | >.99 |
| Pulmonary infection | 5 (3.8) | 4 (6.2) | 1 (1.5) | .37 | 2 (1.6) | 1 (1.7) | 1 (1.6) | >.99 |
| ≥1 Complications (all) | 46 (35.4) | 19 (29.2) | 27 (41.5) | .14 | 23 (18.9) | 11 (18.6) | 12 (19.0) | .96 |
Abbreviations: IQR, interquartile range; NA, not applicable; pSAE, prophylactic splenic arterial embolization; RBC, red blood cell; SAE, splenic arterial embolization; SURV, surveillance, with embolization only if necessary.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.0.
Events occurring between day 0 and day 5 visits (where the day 5 visit occurred between day 4 and day 8 after inclusion).
Events occurring between day 5 visit and month 1 visit.
For 79 patients.
For 20 patients.
Specific Complications of SAE
In the pSAE group, there were 5 minor complications: 3 reported at day 5 and 2 more reported at month 1. In the SURV group, there was 1 minor complication after urgent embolization (Table 3). There was no failure of embolization.
Splenic Complications
At the day 5 visit, there were significantly fewer SPAs among patients in the pSAE group compared with the SURV group (1 of 65 [1.5%] vs 8 of 65 [12.3%]; difference, −10.8%; 95% CI, −19.3% to −2.1%; P = .03) (Table 3) as well as at the month 1 visit (eTable 4 in Supplement 2). Rates of SAVF and pseudocyst were not significantly different between the 2 groups (Table 3; eTable 4 and eTable 5 in Supplement 2). At the day 5 visit, there were significantly fewer secondary embolizations among patients in the pSAE group than in the SURV group (1 of 65 [1.5%] vs 19 of 65 [29.2%]; difference, −27.7%; 95% CI, −41.0% to −15.9%; P < .001) (Table 3). During the whole follow-up period, there were 4 splenectomies (days 0, 2, 6, and 44), for an overall rate of splenectomy of 3.3% (4 of 122). Rates of splenectomy were not significantly different between the pSAE and SURV groups (0 of 59 [0%] vs 4 of 63 [6.3%]; difference, −6.3%; 95% CI, −12.5% to −0.2%; P = .12).
Other Complications
Rates of other complications were not significantly different between the 2 groups (eTable 5 in Supplement 2). The occurrence of hemorrhagic syndrome (see definition in eTable 2 in Supplement 2) did not differ significantly between the pSAE and SURV groups at day 5, nor did the necessity for transfusion. In addition, there was no significant difference between the pSAE and SURV groups in hemorrhagic complications occurring after day 5 (Table 3; eTable 4 and eTable 5 in Supplement 2). There was no significant difference between the pSAE and SURV groups in the rates of thrombotic complications, pleural effusion, and pulmonary infection detected at day 5 (Table 3), between day 5 and month 1 (Table 3), in the whole first month (eTable 4 in Supplement 2), or between the month 1 and month 6 visits (eTable 5 in Supplement 2).
Failures in Each Group
Between day 0 and month 1, 2 of 65 patients (3.1%) in the pSAE group had undergone urgent reembolization, and 21 of 65 patients (32.3%) in the SURV group had undergone splenic embolization (eTable 4 in Supplement 2). The characteristics of the 21 patients in the SURV group requiring a delayed intervention (19 emergency embolizations and 3 splenectomies [including 1 after emergency embolization]) up to the day 5 visit showed that splenic trauma of OIS grade 4 or higher was a risk factor compared with OIS grade 3 (15 of 21 [71.4%] vs 9 of 44 [20.4%]; difference, 51.0% [95% CI, 24.8%-87.6%]; P < .001) (eTable 6 in Supplement 2).
Length of Hospitalization
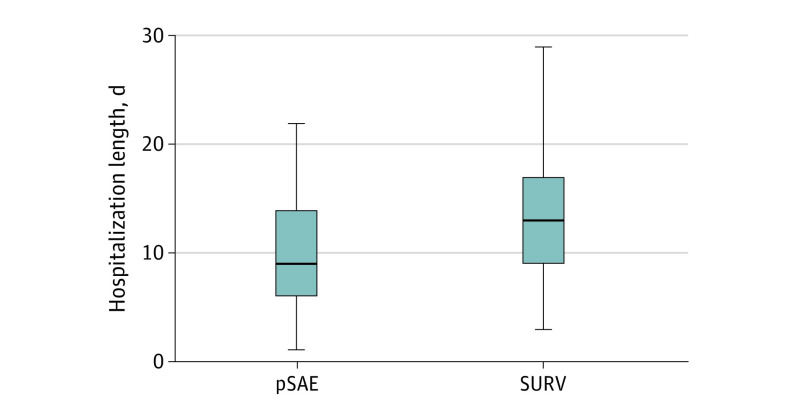
The median length of hospitalization was 11 days (IQR, 7-15 days). As shown in Figure 2, patients in the pSAE group had a shorter median length of hospitalization compared with the SURV group (9 days [IQR, 6-14 days] vs 13 days [IQR, 9-17 days]; P = .002).
Figure 2. Comparison of the Total Length of Hospitalization.
For prophylactic splenic arterial embolization (pSAE), the median length of hospitalization was 9 days (interquartile range, 6-14 days). For surveillance and then embolization only if necessary (SURV), the median length of hospitalization was 13 days (interquartile range, 9-17 days) (P = .002). The horizontal lines indicate the median. The vertical lines indicate the 2 groups.
Activity Score
There was no statistical difference between the pSAE and the SURV groups in the median WOMAC score before the unintentional injury (0 [IQR, 0-0] vs 0 [IQR, 0-0]; P = .38), at month 1 (4 [IQR, 0-13] vs 4 [IQR, 0-26]; P = .51), and at month 6 (0 [IQR, 0-7] vs 0 [IQR, 0-6.5]; P = .63) (eTable 7 in Supplement 2).
Length of Time Off Work or Studies
At month 1, there was no significant difference between the pSAE and SURV groups in return to work or studies (6 of 43 [14.0%] vs 5 of 45 [11.1%]; difference, 2.9%; 95% CI, –11.2% to 16.8%; P = .69). There was also no significant difference between the 2 groups in the total time off work or studies at 6 months (27 of 36 [75.0%] vs 22 of 36 [61.1%]; difference, 13.9%; 95% CI, –8.2% to 36.2%; P = .21) (eTable 8 in Supplement 2).
Discussion
In this randomized clinical trial that enrolled selected hemodynamically stable patients presenting with spleen trauma at high risk of rupture, immediate pSAE did not provide any significant difference in the spleen rescue rate compared with SURV (98.2% vs 93.3%), with a rate of spleen rescue in the SURV group of 93.3% (56 of 60 patients) at 1 month that was much higher than expected from the data in the literature when we designed the trial.2,22 The predominant feature of our study was that 32.3% of patients in the SURV group required embolization or splenectomy, with the only risk factor being the severity of the splenic trauma, because 71.4% of patients with splenic trauma of OIS grade 4 or higher required embolization or splenectomy. Patients in the pSAE group had statistically fewer occurrences of SPA (in line with a recent retrospective study24), seen both at day 5 and at 1 month, as well as significantly shorter lengths of hospitalization.
The precise assessment of the risk of secondary spleen rupture in a patient with spleen trauma who is stable with no active leakage of contrast medium or SPA or SAVF is crucial to reducing the rate of splenectomies for trauma. In this study, we assessed these criteria using the initial CT scan.23,35,36,37 Several publications have highlighted the difficulty of comparing studies with imprecise CT criteria, particularly regarding the severity of spleen lesion(s) and the volume of the hemoperitoneum.37,38,39 To avoid any bias of inclusion and interpretation, we organized a consensus conference and specific training for radiologists from the participating centers prior to the inclusion of patients. Furthermore, we imposed a double-blind reading of both the inclusion CT scan and the CT scan at 1 month, which was used to validate the main outcome. We used the current Advisory Committee on Immunization Practices recommendations (ie, if ≥50% of the splenic mass is lost, patients should be treated as though they are asplenic).40
In this trial, the rate of splenic rescue was greater than 93% in both groups, confirming the efficacy of SAE for splenic trauma already reported in the literature.24,26 The embolization complication rate in our trial was low, less than 10%. Only 1 case of splenic necrosis involving more than half the volume of the gland occurred in the pSAE group. In the literature, there are significant variations in the rate of specific complications of embolization,11,19 which can be attributed to the degree of expertise of the radiologists and the embolization techniques used. Proximal splenic artery embolization decreases the perfusion pressure in the spleen and allows for the viability of the spleen to be maintained via collateral pathways. Distal embolization can be used in cases of focal injury.41 In terms of efficacy, the results seem comparable.42 A meta-analysis by Schnüriger et al17 showed that a recurrence of bleeding was the most common reason for failure and did not differ statistically between the techniques used. In our trial, we used a maximum 6F catheter and rigid coils of 0.089 cm (0.035 inches) for pSAE, which reduced the risk of complications in terms of vascular access. The recommended technique of proximal or combined embolization, the avoidance of microcoils, and the prohibition of the use of glue, gelatin fragments, or microparticles made it possible to avoid extensive splenic necrosis.
Limitations
There were several limitations to our trial. We cannot exclude the possibility of imbalances in unknown confounders between the evaluable groups. Other limitations were the absence of an individual calculation of the irradiated volume to which patients in the 2 groups were exposed and no medicoeconomic comparison of the 2 strategies. We acknowledge that the data available in the literature when we designed the study in 2012 led us to underestimate the sample size. Another limitation was the possibility that, outside the context of a clinical trial, the surveillance of posttrauma patients was not as rigorous as it should have been.
Conclusions
For hemodynamically stable patients with splenic trauma at high risk of rupture, there was no significant difference in the rates of splenic rescue and complications or in their effects on activities between immediate pSAE and SURV with SAE performed only if necessary. A significant proportion of patients in the SURV group needed SAE (in particular, those with higher OIS grade splenic injuries). Performing control CT scans on about day 5 and day 30, with SAE if necessary, seems to provide a good rate of spleen salvage for trauma patients at high risk of splenic rupture, but the practice needs to be validated in further studies.
Trial Protocol
eAppendix. Consensus Definitions
eFigure. Technique of Splenic Artery Embolization Used in the Study
eTable 1. Motives of Noninclusion Before Randomization
eTable 2. Criteria for Complications
eTable 3. Spleen Salvage Rate for Each Center
eTable 4. Events Occurring Between Day 0 and Month 1 Visit (ie, Over the Whole 30 Days)
eTable 5. Complications at Month 6 Visit
eTable 6. Characteristics of Patients in SURV Arm Requiring Delayed Intervention (SAE and/or Splenectomy) at Day 5 Visit
eTable 7. Comparison of WOMAC Activity Scores Between pSAE and SURV
eTable 8. Ability of Patients to Return to Work or Studies (No Missing Data for Patients Declared as Employed or Students on D0) N = 96
Data Sharing Statement
References
- 1.Zarzaur BL, Rozycki GS. An update on nonoperative management of the spleen in adults. Trauma Surg Acute Care Open. 2017;2(1):e000075. doi: 10.1136/tsaco-2017-000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Requarth JA, D’Agostino RB Jr, Miller PR. Nonoperative management of adult blunt splenic injury with and without splenic artery embolotherapy: a meta-analysis. J Trauma. 2011;71(4):898-903. doi: 10.1097/TA.0b013e318227ea50 [DOI] [PubMed] [Google Scholar]
- 3.Zarzaur BL, Croce MA, Fabian TC. Variation in the use of urgent splenectomy after blunt splenic injury in adults. J Trauma. 2011;71(5):1333-1339. doi: 10.1097/TA.0b013e318224d0e4 [DOI] [PubMed] [Google Scholar]
- 4.Arnoletti JP, Karam J, Brodsky J. Early postoperative complications of splenectomy for hematologic disease. Am J Clin Oncol. 1999;22(2):114-118. doi: 10.1097/00000421-199904000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Aiolfi A, Inaba K, Strumwasser A, et al. Splenic artery embolization versus splenectomy: analysis for early in-hospital infectious complications and outcomes. J Trauma Acute Care Surg. 2017;83(3):356-360. doi: 10.1097/TA.0000000000001550 [DOI] [PubMed] [Google Scholar]
- 6.Demetriades D, Scalea TM, Degiannis E, et al. Blunt splenic trauma: splenectomy increases early infectious complications: a prospective multicenter study. J Trauma Acute Care Surg. 2012;72(1):229-234. doi: 10.1097/TA.0b013e31823fe0b6 [DOI] [PubMed] [Google Scholar]
- 7.Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23(2):153-161. doi: 10.1081/IPH-100103856 [DOI] [PubMed] [Google Scholar]
- 8.Kotsanas D, Al-Souffi MH, Waxman BP, King RW, Polkinghorne KR, Woolley IJ. Adherence to guidelines for prevention of postsplenectomy sepsis: age and sex are risk factors: a five-year retrospective review. ANZ J Surg. 2006;76(7):542-547. doi: 10.1111/j.1445-2197.2006.03775.x [DOI] [PubMed] [Google Scholar]
- 9.Bain IM, Kirby RM. 10 Year experience of splenic injury: an increasing place for conservative management after blunt trauma. Injury. 1998;29(3):177-182. doi: 10.1016/S0020-1383(97)00170-8 [DOI] [PubMed] [Google Scholar]
- 10.Harbrecht BG, Zenati MS, Ochoa JB, et al. Management of adult blunt splenic injuries: comparison between level I and level II trauma centers. J Am Coll Surg. 2004;198(2):232-239. doi: 10.1016/j.jamcollsurg.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 11.Haan JM, Biffl W, Knudson MM, et al. ; Western Trauma Association Multi-Institutional Trials Committee . Splenic embolization revisited: a multicenter review. J Trauma. 2004;56(3):542-547. doi: 10.1097/01.TA.0000114069.73054.45 [DOI] [PubMed] [Google Scholar]
- 12.Liu PP, Lee WC, Cheng YF, et al. Use of splenic artery embolization as an adjunct to nonsurgical management of blunt splenic injury. J Trauma. 2004;56(4):768-772. doi: 10.1097/01.TA.0000129646.14777.ff [DOI] [PubMed] [Google Scholar]
- 13.Sabe AA, Claridge JA, Rosenblum DI, Lie K, Malangoni MA. The effects of splenic artery embolization on nonoperative management of blunt splenic injury: a 16-year experience. J Trauma. 2009;67(3):565-572. doi: 10.1097/TA.0b013e3181b17010 [DOI] [PubMed] [Google Scholar]
- 14.Gaarder C, Dormagen JB, Eken T, et al. Nonoperative management of splenic injuries: improved results with angioembolization. J Trauma. 2006;61(1):192-198. doi: 10.1097/01.ta.0000223466.62589.d9 [DOI] [PubMed] [Google Scholar]
- 15.Capecci LM, Jeremitsky E, Smith RS, Philp F. Trauma centers with higher rates of angiography have a lesser incidence of splenectomy in the management of blunt splenic injury. Surgery. 2015;158(4):1020-1024. doi: 10.1016/j.surg.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 16.Banerjee A, Duane TM, Wilson SP, et al. Trauma center variation in splenic artery embolization and spleen salvage: a multicenter analysis. J Trauma Acute Care Surg. 2013;75(1):69-74. doi: 10.1097/TA.0b013e3182988b3b [DOI] [PubMed] [Google Scholar]
- 17.Schnüriger B, Inaba K, Konstantinidis A, Lustenberger T, Chan LS, Demetriades D. Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma. 2011;70(1):252-260. doi: 10.1097/TA.0b013e3181f2a92e [DOI] [PubMed] [Google Scholar]
- 18.Haan J, Ilahi ON, Kramer M, Scalea TM, Myers J. Protocol-driven nonoperative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma. 2003;55(2):317-321. doi: 10.1097/01.ta.0000083336.93868.f7 [DOI] [PubMed] [Google Scholar]
- 19.Ekeh AP, McCarthy MC, Woods RJ, Haley E. Complications arising from splenic embolization after blunt splenic trauma. Am J Surg. 2005;189(3):335-339. doi: 10.1016/j.amjsurg.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 20.Zarzaur BL, Dunn JA, Leininger B, et al. Natural history of splenic vascular abnormalities after blunt injury: a Western Trauma Association multicenter trial. J Trauma Acute Care Surg. 2017;83(6):999-1005. doi: 10.1097/TA.0000000000001597 [DOI] [PubMed] [Google Scholar]
- 21.Stassen NA, Bhullar I, Cheng JD, et al. ; Eastern Association for the Surgery of Trauma . Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5)(suppl 4):S294-S300. doi: 10.1097/TA.0b013e3182702afc [DOI] [PubMed] [Google Scholar]
- 22.Kozar RA, Crandall M, Shanmuganathan K, et al. ; AAST Patient Assessment Committee . Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85(6):1119-1122. doi: 10.1097/TA.0000000000002058 [DOI] [PubMed] [Google Scholar]
- 23.Smith SR, Morris L, Spreadborough S, et al. Management of blunt splenic injury in a UK major trauma centre and predicting the failure of non-operative management: a retrospective, cross-sectional study. Eur J Trauma Emerg Surg. 2018;44(3):397-406. doi: 10.1007/s00068-017-0807-5 [DOI] [PubMed] [Google Scholar]
- 24.Lauerman MH, Brenner M, Simpson N, Shanmuganathan K, Stein DM, Scalea T. Angioembolization significantly improves vascular injuries in blunt splenic trauma. Eur J Trauma Emerg Surg. 2019. doi: 10.1007/s00068-019-01151-z [DOI] [PubMed] [Google Scholar]
- 25.Sugg SL, Gerndt SJ, Hamilton BJ, Francis IR, Taheri PA, Rodriguez JL. Pseudoaneurysms of the intraparenchymal splenic artery after blunt abdominal trauma: a complication of nonoperative therapy and its management. J Trauma. 1995;39(3):593-595. doi: 10.1097/00005373-199509000-00034 [DOI] [PubMed] [Google Scholar]
- 26.Coccolini F, Montori G, Catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12:40. doi: 10.1186/s13017-017-0151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coccolini F, Fugazzola P, Morganti L, et al. The World Society of Emergency Surgery (WSES) spleen trauma classification: a useful tool in the management of splenic trauma. World J Emerg Surg. 2019;14:30. doi: 10.1186/s13017-019-0246-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David JS, Lambert A, Bouzat P, et al. Fibrinolytic shutdown diagnosed with rotational thromboelastometry represents a moderate form of coagulopathy associated with transfusion requirement and mortality: a retrospective analysis. Eur J Anaesthesiol. 2020;37(3):170-179. doi: 10.1097/EJA.0000000000001096 [DOI] [PubMed] [Google Scholar]
- 29.Stevenson M, Segui-Gomez M, Lescohier I, Di Scala C, McDonald-Smith G. An overview of the injury severity score and the new injury severity score. Inj Prev. 2001;7(1):10-13. doi: 10.1136/ip.7.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie A, Moore L, LeSage N, Liberman M, Sampalis JS. The New Injury Severity Score: a more accurate predictor of in-hospital mortality than the Injury Severity Score. J Trauma. 2004;56(6):1312-1320. doi: 10.1097/01.TA.0000075342.36072.EF [DOI] [PubMed] [Google Scholar]
- 31.Olthof DC, Joosse P, van der Vlies CH, de Haan RJ, Goslings JC. Prognostic factors for failure of nonoperative management in adults with blunt splenic injury: a systematic review. J Trauma Acute Care Surg. 2013;74(2):546-557. doi: 10.1097/TA.0b013e31827d5e3a [DOI] [PubMed] [Google Scholar]
- 32.Khatsilouskaya T, Haltmeier T, Cathomas M, Eberle B, Candinas D, Schnüriger B. Thromboembolic prophylaxis with heparin in patients with blunt solid organ injuries undergoing non-operative treatment. World J Surg. 2017;41(5):1193-1200. doi: 10.1007/s00268-016-3820-7 [DOI] [PubMed] [Google Scholar]
- 33.Marot V, Murgier J, Carrozzo A, et al. Determination of normal KOOS and WOMAC values in a healthy population. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):541-548. doi: 10.1007/s00167-018-5153-6 [DOI] [PubMed] [Google Scholar]
- 34.Cochran WG. The χ2 test of goodness of fit. Ann Math Statistics. 1952;23(3):315–345. doi: 10.1214/aoms/1177729380 [DOI] [Google Scholar]
- 35.Gamblin TC, Wall CE Jr, Royer GM, Dalton ML, Ashley DW. Delayed splenic rupture: case reports and review of the literature. J Trauma. 2005;59(5):1231-1234. doi: 10.1097/01.ta.0000197270.25280.7c [DOI] [PubMed] [Google Scholar]
- 36.Bessoud B, Duchosal MA, Siegrist CA, et al. Proximal splenic artery embolization for blunt splenic injury: clinical, immunologic, and ultrasound-Doppler follow-up. J Trauma. 2007;62(6):1481-1486. doi: 10.1097/TA.0b013e318047dfb8 [DOI] [PubMed] [Google Scholar]
- 37.Margari S, Garozzo Velloni F, Tonolini M, et al. Emergency CT for assessment and management of blunt traumatic splenic injuries at a level 1 trauma center: 13-year study. Emerg Radiol. 2018;25(5):489-497. doi: 10.1007/s10140-018-1607-x [DOI] [PubMed] [Google Scholar]
- 38.Harmon L, Bilow R, Shanmuganathan K, et al. Delayed splenic hemorrhage: myth or mystery? a Western Trauma Association multicenter study. Am J Surg. 2019;218(3):579-583. doi: 10.1016/j.amjsurg.2019.06.025 [DOI] [PubMed] [Google Scholar]
- 39.Adibi A, Ferasat F, Baradaran Mahdavi MM, Kazemi K, Sadeghian S. Assessment of blunt splenic trauma: which imaging scoring system is superior? J Res Med Sci. 2018;23:29. doi: 10.4103/jrms.JRMS_875_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crooker KG, Howard JM, Alvarado AR, et al. Splenic embolization after trauma: an opportunity to improve best immunization practices. J Surg Res. 2018;232:293-297. doi: 10.1016/j.jss.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 41.Quencer KB, Smith TA. Review of proximal splenic artery embolization in blunt abdominal trauma. CVIR Endovasc. 2019;2(1):11. doi: 10.1186/s42155-019-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frandon J, Rodière M, Arvieux C, et al. Blunt splenic injury: outcomes of proximal versus distal and combined splenic artery embolization. Diagn Interv Imaging. 2014;95(9):825-831. doi: 10.1016/j.diii.2014.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Consensus Definitions
eFigure. Technique of Splenic Artery Embolization Used in the Study
eTable 1. Motives of Noninclusion Before Randomization
eTable 2. Criteria for Complications
eTable 3. Spleen Salvage Rate for Each Center
eTable 4. Events Occurring Between Day 0 and Month 1 Visit (ie, Over the Whole 30 Days)
eTable 5. Complications at Month 6 Visit
eTable 6. Characteristics of Patients in SURV Arm Requiring Delayed Intervention (SAE and/or Splenectomy) at Day 5 Visit
eTable 7. Comparison of WOMAC Activity Scores Between pSAE and SURV
eTable 8. Ability of Patients to Return to Work or Studies (No Missing Data for Patients Declared as Employed or Students on D0) N = 96
Data Sharing Statement