Key Points
Question
What is the long-term stability of bicuspid aortic valve repair for aortic regurgitation and/or aneurysm, and what are the risk factors associated with valve failure?
Findings
In this case series of 1024 patients who underwent bicuspid aortic valve repair with a differentiated anatomic repair approach, 15-year survival was 82%. Cusp calcification, asymmetric commissural orientation, and the need for partial cusp replacement were risk factors associated with valve failure.
Meaning
Bicuspid aortic valve repair with or without concomitant aortic replacement is associated with long-term stability if all pathologic components of the aortic valve and root, including commissural orientation, are addressed.
Abstract
Importance
Bicuspid aortic valve (BAV) repair has been used in limited cohorts, but its long-term results in a large population are unknown.
Objectives
To analyze the long-term stability of BAV repair for survival and the factors associated with repair failure and to evaluate whether a differentiated anatomic repair approach may improve repair stability.
Design, Setting, and Participants
In this case series, 1024 patients underwent BAV repair for aortic regurgitation or aneurysm between October 1995 and June 2018, with a mean (SD) follow-up time of 56 (49) months and maximum follow-up of 271 months. Systematic modifications in technique based on anatomic principles were introduced in 2009 and applied for the last 727 patients. Data were acquired prospectively and analyzed retrospectively.
Exposures
Repair of BAV with or without concomitant aortic replacement, as well as postoperative clinical and echocardiographic follow-up.
Main Outcomes and Measures
Survival and incidence of reoperation or recurrent aortic regurgitation, as well as factors associated with valve repair failure.
Results
Among the 1024 patients in the study (920 male [89.8%]; mean [SD] age, 47 [13] years [range, 15-86 years]), the survival rate at 15 years was 82.1%. The cumulative incidence of reoperation was 30.7% (95% CI, 22.7%-38.7%) at 15 years. Cusp calcification (subdistribution hazard ratio, 1.78; 95% CI, 1.14-2.77; P = .01), asymmetric commissural orientation (subdistribution hazard ratio, 1.95; 95% CI, 1.02-3.72; P = .04), and use of a pericardial patch for cusp repair (subdistribution hazard ratio, 5.25; 95% CI, 3.52-7.82; P < .001) were associated with time to reoperation. At 10 years, the incidence of reoperation was significantly reduced among patients who received the anatomic repair concept compared with those who had undergone surgery in the earlier period (8.8% vs 24.6%; P < .001).
Conclusions and Relevance
This study suggests that survival after BAV repair is excellent and that a large proportion of BAV repairs will remain stable. Repair stability can be markedly improved by an anatomic repair concept. Cusp calcification and the need for cusp repair using a patch remain the factors most strongly associated with valve failure. In those instances, valve replacement should be preferred.
This case series analyzes the long-term stability of bicuspid aortic valve repair for survival and the factors associated with repair failure and evaluates whether a differentiated anatomic repair approach may improve repair stability.
Introduction
The bicuspid aortic valve (BAV) is the most frequent congenital cardiac anomaly.1 It is characterized by a variable degree of fusion between 2 cusps and the presence of a nonfused cusp that is almost always larger than the 2 components of the fused cusp (Figure 1A).2,3 There is further anatomic variability that is less frequently appreciated; there are different fusion patterns, and the degree of fusion varies (Figure 1A).2,3 In addition, there is variability of commissural orientation ranging from a symmetric configuration to one that is close to a tricuspid design (Figure 1A).2,3 A relevant proportion of individuals with BAV will need treatment for either aortic regurgitation (AR) or ascending aortic aneurysm.1,4
Figure 1. Different Bicuspid Aortic Valve (BAV) Phenotypes and Landmarks of the Aortic Root.
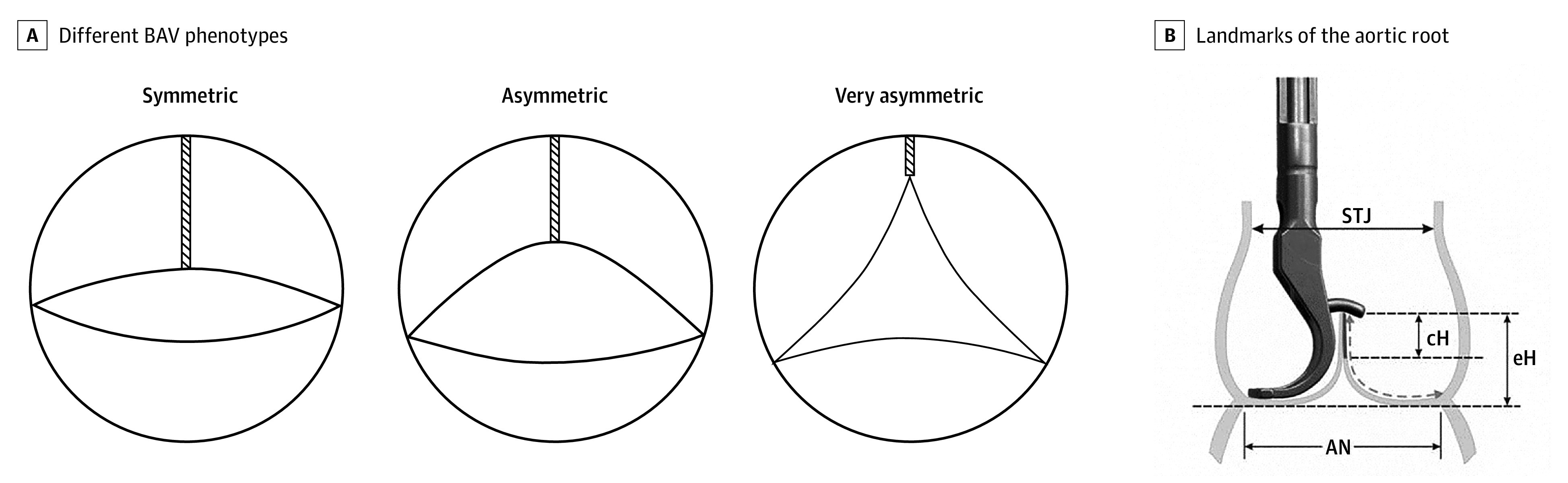
A, Different BAV phenotypes: symmetric (commissural orientation, 160°-180°), asymmetric (commissural orientation, 140°-155°), and very asymmetric (commissural orientation, <140°). The length of fusion varies between the phenotypes. It is longest in symmetric and shortest in asymmetric BAVs (modified according to de Kerchove et al2). B, Landmarks of the aortic root: effective height (eH), aortic annulus (AN), sinotubular junction (STJ), and coaptation height (cH). Effective height is measured using a special caliper.
Reconstruction of the regurgitant BAV was already proposed more than 25 years ago with good early results.5 Regurgitation was assumed to be solely due to prolapse of the fused cusp, which was corrected as the key intervention. With longer follow-up, results were sobering because a relevant proportion of repaired valves failed within the first 5 postoperative years.6 Initially, a specific repair type (ie, triangular resection) was assumed to predispose the repaired valve to failure.6
Based on the analysis of failed BAVs, we realized that symmetric prolapse was an important mode of failure.7 This could be avoided using the effective height (eH) concept, measured as the height difference between the basal plane and free margins in diastole8 (Figure 1B). Normal data on tissue height allowed for improved selection of adequate valves for repair.9 The analysis of a larger patient cohort then identified important factors associated with valve failure (namely, annular size and circumferential orientation of the commissures of the nonfused cusp).10 Moreover, the use of autologous pericardium as partial cusp replacement was also associated with unacceptable rates of failure.10
These results led us to modify our repair approach by selecting patients more carefully. More important, we developed a suture annuloplasty11 to address annular dilatation, with good midterm results.12 We also modified the circumferential orientation, attempting to come as close as possible to a symmetric configuration.13,14
During the past 10 years, we have consistently applied this differentiated anatomic concept in repairing BAVs. With follow-up reaching 20 years, we now intend to analyze the long-term durability of BAV repair and to evaluate current results of the anatomic repair concept.
Methods
Between October 1995 and June 2018, 1024 patients with a BAV underwent valve-preserving surgery for AR (n = 745) and/or ascending aortic aneurysm (n = 249) in our institution. Patients’ ages ranged from 15 to 86 years (mean [SD] age, 47 [13] years; 920 male [89.8%]) (Table 1). Preoperative AR was relevant (III° or IV°) in most patients (784 [76.6%]). The left ventricular end-systolic diameter ranged from 22 to 71 mm (mean [SD], 43 [8] mm), the left ventricular end-diastolic diameter ranged from 38 to 86 mm (mean [SD], 61 [9] mm), the maximum sinus diameter was 71 mm (mean [SD], 42 [8] mm), and a basal diameter of 28 mm or more was present in 795 patients (77.6%). The investigation was approved by the Saarland University Regional Ethics Committee for the analysis and publication of patient data in anonymized fashion. All patients provided written and oral consent.
Table 1. Perioperative Patient Data.
| Characteristic | Patients, No. (%) (N = 1024) |
|---|---|
| Male sex | 920 (89.8) |
| Age, mean (SD), y | 47 (13) |
| AR preoperatively | |
| <Grade III | 240 (23.4) |
| ≥Grade III | 784 (76.6) |
| Fusion | |
| Right-left | 873 (85.3) |
| Right-nonfused | 144 (14.1) |
| Left-nonfused | 7 (0.7) |
| Primary indication | |
| AR | 745 (72.8) |
| Aneurysm | 249 (24.3) |
| Other | 30 (2.9) |
| Preoperative LVESD, mean (SD), mm | 43 (8) |
| Preoperative LVEDD, mean (SD), mm | 61 (9) |
| Postoperative LVESD, mean (SD), mm | 41 (7) |
| Postoperative LVEDD, mean (SD), mm | 54 (7) |
| Preoperative sinus, mean (SD), mm | 42 (8) |
| Preoperative basal ring, mean (SD), mm | 30 (4) |
| Standard repair | 917 (89.6) |
| Fused cusp | |
| Central plication | 593/917 (64.7) |
| Triangular resection | 210/917 (22.9) |
| Nonfused cusp intervention | 546/917 (59.5) |
| Tricuspid-like repair | 107 (10.4) |
| Pericardial patch | 138 (13.5) |
| Suture annuloplasty | 591 (57.7) |
| Subcommissural plication | 107 (10.4) |
| Concomitant procedures | 244 (23.8) |
| Partial arch replacement | 122 (11.9) |
| Total arch replacement | 4 (0.4) |
| Elephant trunk | 2 (0.2) |
| MVR | 32 (3.1) |
| TVR | 12 (1.2) |
| Ablation | 44 (4.3) |
| CABG | 50 (4.9) |
| Closure | |
| PFO | 13 (1.3) |
| ASD | 3 (0.3) |
| VSD | 2 (0.2) |
| Septal myectomy | 6 (0.6) |
| PTE | 1 (0.1) |
| PDA | 1 (0.1) |
Abbreviations: AR, aortic regurgitation; ASD, atrial septal defect; CABG, coronary artery bypass grafting; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MVR, mitral valve repair; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PTE, pulmonary thromboendarterectomy; TVR, tricuspid valve repair; VSD, ventricular septal defect.
Cusp fusion was mostly seen between the right and the left coronary cusp (873 [85.3%]) (Table 1), with varying extent of fusion from minimal (3-4 mm) to complete (771 [75.3%]). The commissural orientation of the nonfused cusp varied (Figure 1A), with predominant orientation of 160° or more (712 [69.5%]). An asymmetric orientation (140°-155°) was present in 205 patients (20.0%) and a very asymmetric orientation (<140°) was observed in 107 individuals (10.4%). Cusp calcification was present in 182 individuals (17.8%). Cardiac comorbidities requiring additional surgical treatment were present in 244 individuals (23.8%).
Technique
Aortic valve repair (AVr) was performed in the presence of preserved aortic root dimensions (sinus diameter, <42-45 mm; 618 individuals [60.4%]). Of those individuals, 180 (29.1%) required concomitant tubular aortic replacement. In the presence of root dilatation (sinus diameter, >42-45 mm), root remodeling (RR) was performed (406 [39.6%]).
Aortic valve function and root dimensions were assessed intraoperatively by transesophageal echocardiography (HDI 3000; Advanced Technology Laboratories or Vivid e9 or S70; General Electric). The chest was opened via a median sternotomy followed by aortic and right atrial cannulation. The aorta was transected transversely above the sinotubular junction, and blood cardioplegia was given directly into the coronary ostia. Standard BAV repair consisted of plication of the 2 components of the fused cusp, which was performed for all symmetric and asymmetric BAVs (917 [89.6%]). In the presence of limited fusion and very asymmetric commissural orientation (<140°), the repair was performed according to the principles of tricuspid AVr (107 [10.4%]).15
Initially, cusp prolapse was visually assessed by comparison of the cusp margins. Starting in 2004, valve assessment included measurement of both geometric height9 and eH8 (Figure 1B). With a geometric height of 20 mm or more, valve repair was pursued. Prolapse requiring correction was defined as an eH of less than 9 mm on the nonfused cusp. Fused cusp prolapse was evaluated by direct comparison of the position of the free margin relative to the nonfused cusp. In very asymmetric BAVs, eH was measured in all 3 cusps and corrected accordingly. The diameter of the basal ring was assessed by direct intubation with graded Hegar dilators.
The different surgical techniques remained principally constant over time and have been described previously.16 If concomitant tubular aortic replacement was necessary in addition to AVr, the graft was sutured to the root at the level of the sinotubular junction; graft size was chosen according to the patient’s body surface area (<2 m2, 22 or 24 mm; 2-2.2 m2, 26 mm; and >2.2 m2, 28 mm). If aortic root dilatation required RR, a standard technique was applied.16 A Dacron graft was tailored to accommodate the circumferential orientation of the commissures (n = 118); since 2009, 2 symmetric tongues were created (n = 288).
Correction of prolapse of the fused cusp was performed mainly by central plication (593 of 917 [64.7%]). Extensive tissue redundancy, dense fibrosis, or limited calcification required triangular resection (210 of 917 [22.9%]). Prolapse of both cusps required correction in 546 of 917 individuals (59.5%).
In 107 individuals, a very asymmetric commissural orientation combined with only minimal fusion was found. Thus, near-tricuspid morphologic characteristics were present, and the aortic valve was repaired according to the principles of tricuspid AVr.15
Pericardial patches (n = 138) were used for central cusp replacement, closure of defects, or fenestrations. An autologous pericardium was fixed in glutaraldehyde (1.5% for 3 minutes) followed by rinsing in normal saline for 3 minutes. The patch was sutured into the tissue defect using polypropylene sutures (Prolene 5-0; Ehicon).
Annular dilatation (basal ring <26 mm) was initially corrected by subcommissural plication (107 [10.4%]).17 Since 2009, a suture annuloplasty (591 [57.7%])11 was used to address annular dilatation. It was tied around a graded Hegar dilator according to body surface area (<1.8 m2, 21 mm; 1.8-2.0 m2, 23 mm; and >2.0 m2, 25 mm). Also since 2009, in the absence of RR, an asymmetric commissural orientation was modified by sinus plication (138 [13.5%]) to approximate a symmetric configuration. During the past 10 years, we have consistently applied this differentiated anatomic concept in BAV repair.
All patients were followed up clinically and by echocardiography (mean [SD] follow-up, 56 [49] months; maximum follow-up, 271 months). Twenty-four patients (2.3%) were lost to follow-up. The degree of AR was evaluated according to current guidelines,18 and systolic gradients were measured by continuous wave Doppler.18
Statistical Analysis
Sample characteristics are expressed as absolute and relative frequencies for categorical variables, mean (SD) values for approximately normally distributed continuous variables, and median values with interquartile ranges for nonnormally distributed continuous variables. Statistical significance testing was conducted using the χ2 test for categorical variables and the t test for approximately normally distributed continuous variables.
Cumulative incidences for reoperation or recurrent AR of grade II or higher and associations of preoperative and intraoperative characteristics with respect to time to the first event were assessed in the framework of competing risks to take death into account.19 Statistically significant univariable Fine-Gray models (P < .01) were adjusted for covariates that altered crude effect estimates by more than 10% and were considered clinically relevant. For fitted (multivariable) Fine-Gray models, the proportional subdistribution hazards assumption was assessed using (scaled) Schoenfeld residuals.20 Overall survival was analyzed in a similar fashion using the proportional hazards model. Expected survival for an age- and sex-matched general population was estimated using a suggested approach21 and life tables from the Federal Statistical Office of Germany.
The significance level was set at P < .05, and reported 2-sided P values were not adjusted for multiple comparisons owing to the explorative nature of this investigation. All analyses were conducted using the cmprsk, stats, and survival packages in R, version 3.5.1.22,23
Results
Among the 1024 patients in the study, the mean (SD) cardiopulmonary bypass time was 70 (30) minutes, and the cross clamp times was 49 (22) minutes. Twenty-two patients (2.1%) required reexploration for hemorrhage. Thromboembolic strokes were observed in 1 patient (0.1%) during the hospital stay and in another 7 patients (0.7%) during follow-up. All late strokes occurred in the context of paroxysmal atrial fibrillation.
Postoperatively, transvalvular gradients were significantly higher after repair of asymmetric BAVs without commissural modification (mean [SD], 12 [10] mm Hg; mean [SD] peak, 23 [17] mm Hg) compared with symmetric or modified asymmetric BAVs (mean [SD], 7 [4] mm Hg; mean [SD] peak, 12 [7] mm Hg; mean gradient, P = .02; maximum gradient, P = .01). At hospital discharge, significantly decreased left ventricular dimensions were observed compared with the preoperative size (mean [SD] left ventricular end-systolic diameter, 41 [7] mm; mean [SD] left ventricular end-diastolic diameter, 54 [7] mm; P < .001) (Table 1).
Survival
Four patients (0.4%) died during the hospital stay, while another 40 individuals died during follow-up, for a 15-year-survival of 82.1% (Figure 2A). The following results summarize estimates for 10-year and 15-year survival. Reported P values refer to comparisons of hazard functions between groups and were derived from the log-rank test.
Figure 2. Survival of the Study Population.
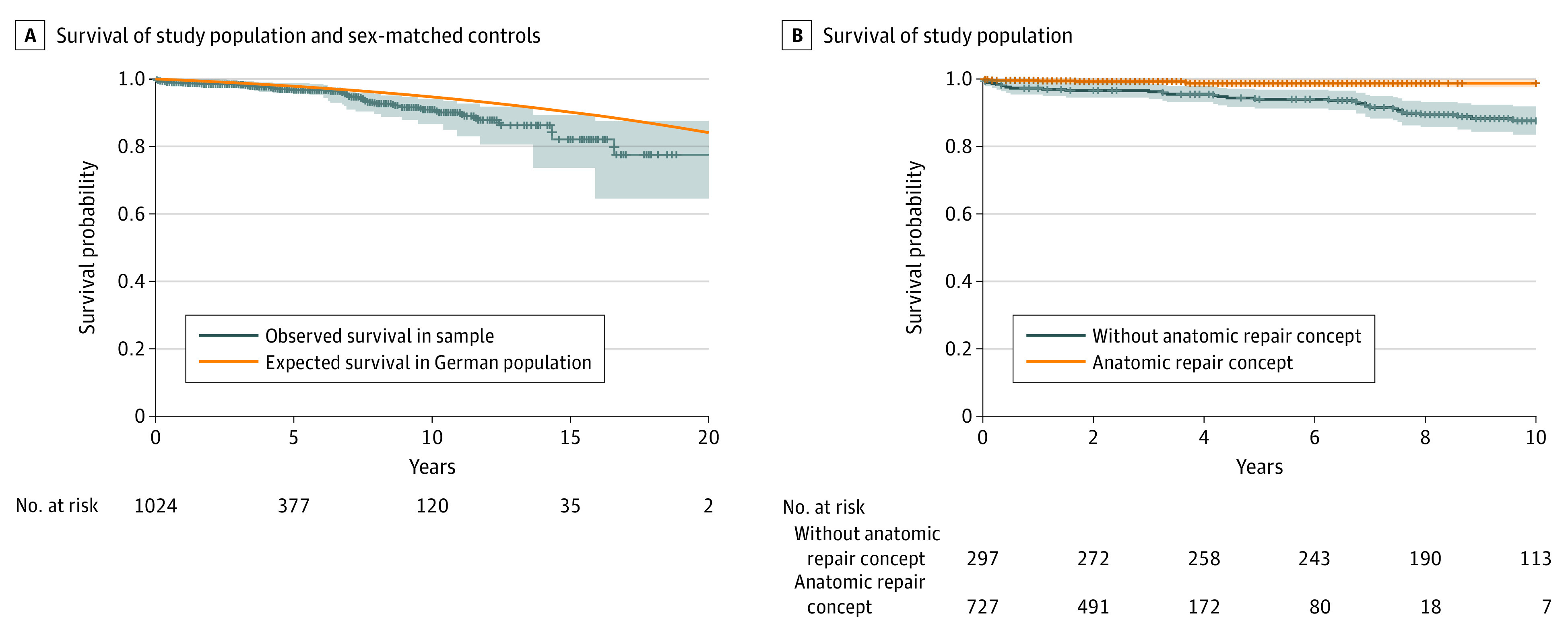
A, Survival of the study population and expected survival for age- and sex-matched German population. B, Survival of the study population was superior for patients who underwent surgery with the anatomic repair concept compared with patients whose surgery was prior to 2009 (P < .001).
Patients whose operation was performed with the anatomic repair concept showed a significantly superior 10-year survival compared with patients whose operation was performed prior to 2009 (98.7% vs 87.6%; P < .001) (Figure 2B). Ten-year survival was also better among patients without cardiac comorbidities (92.9% vs 84.3%; P = .02) and after the addition of annuloplasty (98.7% vs 88.7%; P = .001).
At 10 years, the survival rate was lower after the use of a pericardial patch (80.6% vs 93.3%; P < .001) among patients with limited cusp calcification (86.9% vs 92.2%; P = .03), and after tricuspid-like repair compared with all other orientations (10 years, 55.6%; P < .001). After adjustment for potential confounders, a strong association was found for the use of a pericardial patch (hazard ratio, 2.95; 95% CI, 1.43-6.11; P = .003). No association was found for preoperative left ventricular end-systolic diameter (hazard ratio, 0.99; 95% CI, 0.95-1.04; P = .74) or left ventricular end-diastolic diameter (hazard ratio, 0.97; 95% CI, 0.93-1.00; P = .08).
Reoperation
A total of 101 patients required reoperation on the aortic valve, of whom 32 underwent re-repair. Of the 101 patients who required a reoperation, 72 had undergone surgery without the differentiated anatomic repair concept. The cumulative incidence of reoperation was 19.8% (95% CI, 15.0%-24.7%) at 10 years and 30.7% (95% CI, 22.7%-38.7%) at 15 years.
The following results summarize the estimates of cumulative incidence for reoperation at 10 and 15 years. Reported P values refer to comparisons of cumulative incidence functions between groups and were derived from the Fine-Gray test.
The cumulative incidence of reoperation at 10 years was significantly lower among patients who underwent surgery since 2009 with the current repair concept compared with those who had undergone surgery in the earlier period (8.8% vs 24.6%; P < .001) (Figure 3A). For patients who underwent surgery prior to 2009, the 10-year incidence of reoperation was lower after RR compared with AVr (10.2% vs 35.0%; P < .001), while there was no difference after 2009 in reoperation rates between RR and AVr among patients who underwent surgery with the anatomic repair concept. The incidence of reoperation at 10 years after RR was not altered by the addition of annuloplasty (5.8% vs 9.6%; P = .50), but a decreased incidence of reoperation at 10 years was observed for AVr after the addition of annuloplasty (5.9% vs 22.4%; P = .01).
Figure 3. Cumulative Incidence of Reoperation After Bicuspid Aortic Valve (BAV) Repair.
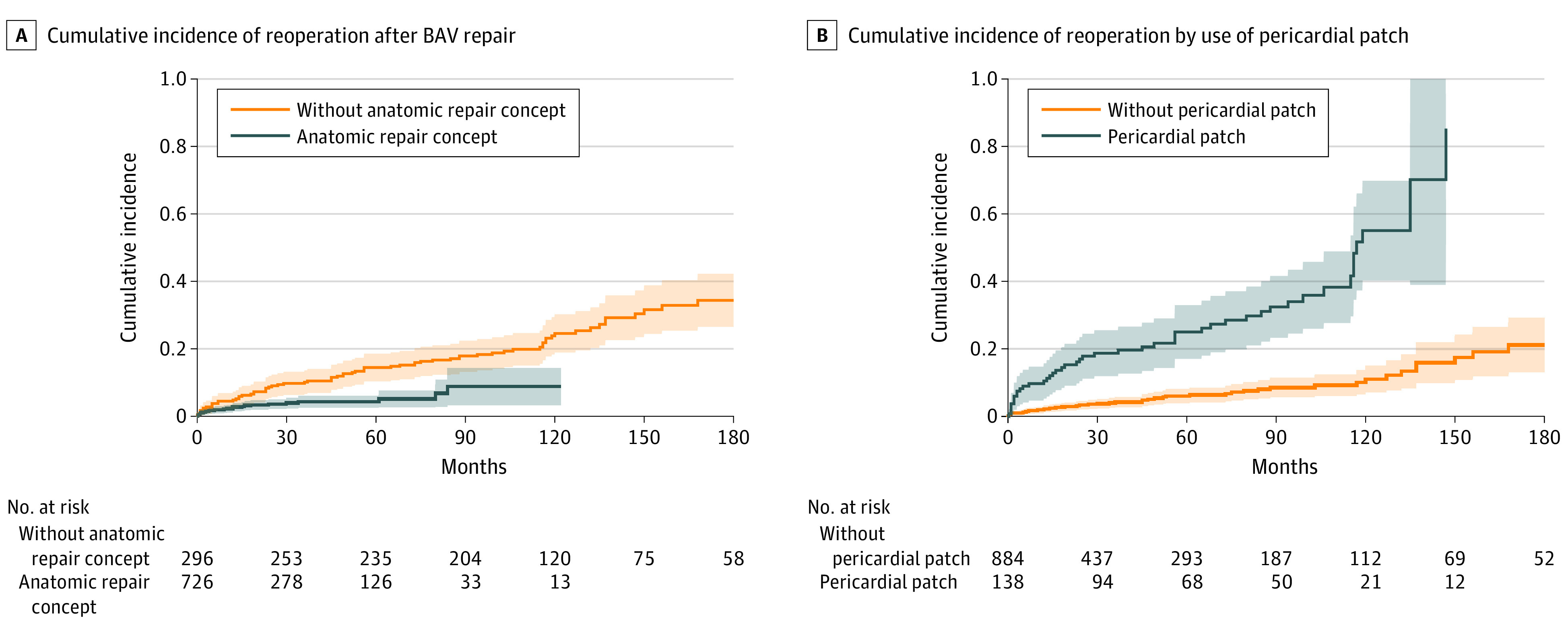
A, Cumulative incidence of reoperation after BAV repair was significantly lower among patients who underwent surgery with the differentiated anatomic repair concept (P < .001). B, Cumulative incidence of reoperation according to the use of a pericardial patch. A higher cumulative incidence of reoperation was found after use of a pericardial patch (P < .001).
The factors associated with an increased incidence of reoperation included use vs no use of a pericardial patch (55.0% vs 11.0% at 10 years; P < .001) (Figure 3B), presence or absence of cusp calcification (43.7% vs 26.2% at 15 years; P < .001), and use vs no use of a subcommissural plication (38.4% vs 27.7% at 15 years; P = .01). Asymmetric orientation without surgical alteration was also associated with a significantly higher incidence of reoperation compared with symmetric orientation (47.1% vs 18.6% at 10 years; P < .001).
The cumulative postoperative incidence of severe aortic stenosis was 4.2% at 10 years and 10.6% at 15 years. It was higher among patients showing cusp calcification at the time of surgery (11.6% at 10 years and 15.2% at 15 years) compared with individuals without cusp calcification at the time of surgery (1.5% at 10 years and 8.8% at 15 years; P = .02). After adjustment for potential confounders, strong associations remained for cusp calcification (subdistribution hazard ratio [SHR], 1.78; 95% CI, 1.14-2.77; P = .01), pericardial patch (SHR, 5.25; 95% CI, 3.52-7.82; P < .001), and asymmetric commissural orientation (SHR, 1.95; 95% CI, 1.02-3.72; P = .04) (Table 2).
Table 2. Associations With Time to Reoperation From Fine-Gray Models.
| Characteristic | Crude model | Adjusted model | ||
|---|---|---|---|---|
| SHR (95% CI) | P value | SHR (95% CI) | P value | |
| Annuloplastya | 0.52 (0.32-0.86) | .01 | 0.67 (0.37-1.19) | .17 |
| Commissural orientationb | ||||
| Tricuspid-like vs symmetricb | 0.93 (0.43-2.03) | .86 | 0.74 (0.34-1.63) | .45 |
| Asymmetric without modification vs symmetricb | 3.87 (2.09-7.17) | <.001 | 1.95 (1.02-3.72) | .04 |
| Modified asymmetric vs symmetric | 0.79 (0.37-1.66) | .53 | 0.99 (0.46-2.12) | .97 |
| Cusp calcificationc | 2.44 (1.63-3.64) | <.001 | 1.78 (1.14-2.77) | .01 |
| Pericardial patch | 5.25 (3.52-7.82) | <.001 | 5.25 (3.52-7.82) | <.001 |
| Root replacementd | 0.47 (0.31-0.72) | .001 | 0.71 (0.45-1.15) | .16 |
| 1995-2008 | 0.30 (0.17-0.52) | <.001 | ||
| 2009-2016 | 1.22 (0.59-2.53) | .59 | ||
| Annuloplasty | 1.32 (0.60-2.90) | .50 | ||
| Subcommissural plicatione | 1.84 (1.18-2.88) | .007 | 1.11 (0.67-1.84) | .70 |
Abbreviation: SHR, subdistribution hazard ratio.
Adjusted for chronological age, cusp calcification, commissural orientation, effective height, pericardial patch, primary indication, and subcommissural plication.
Adjusted for pericardial patch, primary indication, and root replacement.
Adjusted for chronological age, pericardial patch, and primary indication.
Adjusted for commissural orientation, pericardial patch, primary indication, and subcommissural plication.
Adjusted for annuloplasty, commissural orientation, effective height, pericardial patch, primary indication, and root replacement.
Reoperations associated with annuloplasty were necessary for 9 of 591 patients (1.5%) for lateral wall ischemia owing to occlusion of the circumflex artery requiring subsequent removal (n = 4) or closure of a ventricular septal defect caused by erosion of the membranous septum (n = 5). Those complications occurred in the early phase of the technique and were not seen in the last 350 patients. Two patients required a postoperative pacemaker implant owing to complete atrioventricular block.
Recurrent Aortic Regurgitation
The cumulative incidence of recurrent AR of grade II or higher was 24.6% (95% CI, 19.8%-29.4%) at 10 years and 30.8% (95% CI, 23.8%-37.8%) at 15 years. It was lower for patients who underwent RR primarily for aneurysm and without severe preoperative AR (8.9% at 10 years and 13.0% at 15 years) compared with patients with severe preoperative AR undergoing either AVr (29.7% at 10 years and 34.3% at 15 years) or RR (24.6% at 10 years and 32.7% at 15 years; P < .001).
Preoperative severe AR was associated with time to recurrent AR of grade II or higher (SHR, 1.96; 95% CI, 1.25-3.06; P = .003). Recurrent AR was also associated with the operative covariate use of a pericardial patch (SHR, 3.10; 95% CI, 2.16-4.47; P < .001) and the use of the eH measurement (SHR, 2.16; 95% CI, 1.19-3.91; P = .01), along with the presence of cusp calcification (SHR, 1.79; 95% CI, 1.23-2.59; P = .002).
Prior to 2009, RR was inversely associated with time to recurrent AR (SHR, 0.46; 95% CI, 0.28-0.76; P = .003), while there was no association after 2009 with the current repair concept. After adjustment for potential confounders, associations remained for use of a pericardial patch (SHR, 3.10; 95% CI, 2.16-4.47; P < .001), preoperative severe AR (SHR, 1.88; 95% CI, 1.17-3.02; P = .009), primary indication (aneurysm compared with AR: SHR, 0.37; 95% CI, 0.23-0.62; P < .001), and cusp calcification (SHR, 1.68; 95% CI, 1.10-2.57; P = .02).
Discussion
With the development of heart valve prostheses in the 1960s, aortic valve replacement became the standard treatment for AR. It became apparent that, at 10 years, a proportion of the patients had developed valve-related complications.24 Valve-related mortality was observed,24 which was associated with reduced life expectancy after aortic valve replacement.25,26 In addition, there exists the need for reoperation, even after mechanical prosthetic replacement.24,25,26,27 Moreover, many young patients do not wish to undergo anticoagulation. For these reasons, AVr appears as a promising alternative to replacement. A possible positive association between valve repair and survival has been proposed in a very limited cohort of patients.28 The current results indicate that survival after AVr is similar to the estimated survival of a matched population that experiences very little, if any, excess mortality.
Repair of regurgitant BAVs has been performed for more than 2 decades. Initially, surgeons relied on a visual assessment of their procedures. Although the early results were good,5 there was attrition of valve function during later follow-up in several series.6,10,29 Based on the analysis of valve failures, we proposed eH of the valve as an objective and quantitative indicator of valve configuration.8 This observation has been confirmed by others.30 Nevertheless, failures of repaired BAVs continued to occur with frequency.10 In a cohort of more than 300 patients with repaired BAVs, additional anatomic factors associated with valve failure were identified (in particular, annular dilatation),10 which were later confirmed by others.31 Based on these findings, the repair strategy was adapted to the anatomic characteristics of the valve. Probably the most important change was the introduction of the annuloplasty concept for treating annular dilatation. Use of annuloplasty has improved early valve competence,11 and it has also improved the durability of isolated BAV repairs.12 This observation could be confirmed by the current analysis. In addition, we have now been able to report a positive association between annuloplasty and survival.
Root remodeling has consistently been associated with the best durability of BAV repair.10 At 15 years, the incidence of reoperation is only 20.0%; the durability is even better if solely recurrent AR is taken as the reason for reoperation (15 years, 10.7%). So far, the addition of annuloplasty to RR has not been associated with any further improvement. This observation indicates that either the annular stabilization is not as important as generally assumed or that RR, in fact, addresses the anatomic annulus32 and may also have an association with the basal plane.33
Local complications associated with the suture annuloplasty were almost exclusively seen early in the experience and with the use of braided polyester sutures. The complications have been largely eliminated using expanded polytetrafluorethylene as the suture material. Thus, our current results for both RR and AVr show a stability that is comparable to that described by others applying an external annuloplasty ring34 or using a valve reimplant more liberally.35
More important, we found that an asymmetric configuration of the valve is associated with decreased stability10 and increased turbulence in the ascending aorta.36 We showed that modifying commissural orientation close to a symmetric design was associated with improved systolic valve function and repair durability.13,14 In the current analysis, patients with an unchanged asymmetric commissural orientation were associated with a higher incidence of reoperation as well as higher postoperative transvalvular gradients, thus confirming earlier findings.13,14
There is a low 15-year postoperative incidence of calcific aortic stenosis of 10.6% despite calcific plaques being present at the time of initial surgery in more than 50% of patients. With these calcium deposits, the 15-year incidence of valve stenosis was only 15.2%, still comparing favorably with bioprostheses in a similar age group. In the absence of calcium deposits, the incidence of stenosis at 15 years was 8.8%, which implies that noncalcific BAVs may have a high probability of acceptable function at 20 years and beyond.
Despite the initial enthusiasm about excision of calcium with partial cusp replacement using the pericardium, the stability of this modification was disappointing, with a high incidence of early reoperation. Although resection of limited plaques with direct approximation of cusp tissue seems to be an acceptable approach, the use of the pericardium for cusp replacement should be avoided, and such valves should be replaced.
The current data seem to confirm the clinical relevance of the factors associated with valve failure identified previously.10 More important, they confirm the applicability and stability of the anatomy-based repair strategy, and we have described the stability of the repair up to 10 years, which is significantly superior to the stability described in early experience. Using this concept, we are now able to achieve a stable repair result for most patients.
Limitations
The present study has certain limitations owing to its retrospective and monocentric design because it does include modifications introduced over time. Nevertheless, surgical bias is probably limited by the fact that 1 surgeon (H.-J.S.) substantially participated in all procedures.
Moreover, our findings need to be interpreted with caution owing to multiple testing and the size of different subgroups. Despite the adjustment for possible confounders, residual confounding may have been present.
Nonetheless, to our knowledge, this is the first study on BAV repair incorporating a population of more than 1000 participants with a follow-up now reaching 24 years. Nonetheless, all long-term observations must be carefully interpreted owing to the fact that only a limited proportion of patients had a follow-up longer than 10 years. Longer follow-up will be necessary to evaluate the true long-term durability of BAV repair.
Conclusions
This study suggests that BAV repair has excellent durability if all pathologic components of the aortic valve and root are addressed by an anatomy-based repair concept. Survival after BAV repair is greater than 80% at 15 years and similar to the expected survival of the general population. If partial cusp replacement is required or cusp calcification is present, valve replacement should be preferred.
References
- 1.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83(1):81-85. doi: 10.1136/heart.83.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg. 2019;ezz033. doi: 10.1093/ejcts/ezz033 [DOI] [PubMed] [Google Scholar]
- 3.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74(1):14-26. doi: 10.4065/74.1.14 [DOI] [PubMed] [Google Scholar]
- 4.Michelena HI, Della Corte A, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: incidence, etiology, and clinical significance. Int J Cardiol. 2015;201:400-407. doi: 10.1016/j.ijcard.2015.08.106 [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove DM, Rosenkranz ER, Hendren WG, Bartlett JC, Stewart WJ. Valvuloplasty for aortic insufficiency. J Thorac Cardiovasc Surg. 1991;102(4):571-576. doi: 10.1016/S0022-5223(20)31429-X [DOI] [PubMed] [Google Scholar]
- 6.Casselman FP, Gillinov AM, Akhrass R, Kasirajan V, Blackstone EH, Cosgrove DM. Intermediate-term durability of bicuspid aortic valve repair for prolapsing leaflet. Eur J Cardiothorac Surg. 1999;15(3):302-308. doi: 10.1016/S1010-7940(99)00003-2 [DOI] [PubMed] [Google Scholar]
- 7.Schäfers HJ, Aicher D, Langer F, Lausberg HF. Preservation of the bicuspid aortic valve. Ann Thorac Surg. 2007;83(2):S740-S745. doi: 10.1016/j.athoracsur.2006.11.017 [DOI] [PubMed] [Google Scholar]
- 8.Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg. 2006;132(2):436-438. doi: 10.1016/j.jtcvs.2006.04.032 [DOI] [PubMed] [Google Scholar]
- 9.Schäfers HJ, Schmied W, Marom G, Aicher D. Cusp height in aortic valves. J Thorac Cardiovasc Surg. 2013;146(2):269-274. doi: 10.1016/j.jtcvs.2012.06.053 [DOI] [PubMed] [Google Scholar]
- 10.Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123(2):178-185. doi: 10.1161/CIRCULATIONAHA.109.934679 [DOI] [PubMed] [Google Scholar]
- 11.Schneider U, Aicher D, Miura Y, Schäfers HJ. Suture annuloplasty in aortic valve repair. Ann Thorac Surg. 2016;101(2):783-785. doi: 10.1016/j.athoracsur.2015.07.068 [DOI] [PubMed] [Google Scholar]
- 12.Schneider U, Hofmann C, Aicher D, Takahashi H, Miura Y, Schäfers HJ. Suture annuloplasty significantly improves the durability of bicuspid aortic valve repair. Ann Thorac Surg. 2017;103(2):504-510. doi: 10.1016/j.athoracsur.2016.06.072 [DOI] [PubMed] [Google Scholar]
- 13.Schneider U, Schmied W, Aicher D, Giebels C, Winter L, Schäfers HJ. Sinus plication to improve valve configuration in bicuspid aortic valve repair—early results. Ann Thorac Surg. 2017;103(2):580-585. doi: 10.1016/j.athoracsur.2016.06.064 [DOI] [PubMed] [Google Scholar]
- 14.Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg. 2017;153(4):S65-S71. doi: 10.1016/j.jtcvs.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 15.Lausberg HF, Aicher D, Kissinger A, Langer F, Fries R, Schäfers HJ. Valve repair in aortic regurgitation without root dilatation—aortic valve repair. Thorac Cardiovasc Surg. 2006;54(1):15-20. doi: 10.1055/s-2005-872961 [DOI] [PubMed] [Google Scholar]
- 16.Schneider U, Schäfers H-J. Repair of the bicuspid aortic valve. Oper Tech Thorac Cardiovasc Surg. 2017;22(2):91-109. doi: 10.1053/j.optechstcvs.2018.02.003 [DOI] [Google Scholar]
- 17.Cabrol C, Cabrol A, Guiraudon G, Bertrand M. Treatment of aortic insufficiency by means of aortic annuloplasty. Article in French. Arch Mal Coeur Vaiss. 1966;59(9):1305-1312. [PubMed] [Google Scholar]
- 18.Lancellotti P, Tribouilloy C, Hagendorff A, et al. ; European Association of Echocardiography . European Association of Echocardiography recommendations for the assessment of valvular regurgitation, part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. 2010;11(3):223-244. doi: 10.1093/ejechocard/jeq030 [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 20.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994; 81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 21.Finkelstein DM, Muzikansky A, Schoenfeld DA. Comparing survival of a sample to that of a standard population. J Natl Cancer Inst. 2003;95(19):1434-1439. doi: 10.1093/jnci/djg052 [DOI] [PubMed] [Google Scholar]
- 22.Gray B. cmprsk: Subdistribution analysis of competing risks. R package version 2.2-7. Published 2014. Updated June 9, 2020. Accessed August 7, 2020. https://cran.r-project.org/web/packages/cmprsk/index.html
- 23.R Core Team R: a language and environment for statistical computing. R version 3.5.1. R Foundation for Statistical Computing. Published 2018. Accessed August 7, 2020. http://www.r-project.org/
- 24.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36(4):1152-1158. doi: 10.1016/S0735-1097(00)00834-2 [DOI] [PubMed] [Google Scholar]
- 25.Korteland NM, Etnel JRG, Arabkhani B, et al. Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation. Eur Heart J. 2017;38(45):3370-3377. doi: 10.1093/eurheartj/ehx199 [DOI] [PubMed] [Google Scholar]
- 26.Bouhout I, Stevens LM, Mazine A, et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148(4):1341-1346.e1. doi: 10.1016/j.jtcvs.2013.10.064 [DOI] [PubMed] [Google Scholar]
- 27.Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35(3):747-756. doi: 10.1016/S0735-1097(99)00584-7 [DOI] [PubMed] [Google Scholar]
- 28.de Meester C, Pasquet A, Gerber BL, et al. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: a propensity score analysis. J Thorac Cardiovasc Surg. 2014;148(5):1913-1920. doi: 10.1016/j.jtcvs.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 29.Moidl R, Moritz A, Simon P, Kupilik N, Wolner E, Mohl W. Echocardiographic results after repair of incompetent bicuspid aortic valves. Ann Thorac Surg. 1995;60(3):669-672. doi: 10.1016/0003-4975(95)00508-I [DOI] [PubMed] [Google Scholar]
- 30.le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging. 2009;2(8):931-939. doi: 10.1016/j.jcmg.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 31.Navarra E, El Khoury G, Glineur D, et al. Effect of annulus dimension and annuloplasty on bicuspid aortic valve repair. Eur J Cardiothorac Surg. 2013;44(2):316-322. doi: 10.1093/ejcts/ezt045 [DOI] [PubMed] [Google Scholar]
- 32.Anderson RH. Clinical anatomy of the aortic root. Heart. 2000;84(6):670-673. doi: 10.1136/heart.84.6.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunihara T, Arimura S, Sata F, Giebels C, Schneider U, Schäfers HJ. Aortic annulus does not dilate over time after aortic root remodeling with or without annuloplasty. J Thorac Cardiovasc Surg. 2018;155(3):885-894.e3. doi: 10.1016/j.jtcvs.2017.10.074 [DOI] [PubMed] [Google Scholar]
- 34.Lansac E, Di Centa I, Sleilaty G, et al. Remodeling root repair with an external aortic ring annuloplasty. J Thorac Cardiovasc Surg. 2017;153(5):1033-1042. doi: 10.1016/j.jtcvs.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 35.de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142(6):1430-1438. doi: 10.1016/j.jtcvs.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 36.Stephens EH, Hope TA, Kari FA, et al. Greater asymmetric wall shear stress in Sievers’ type 1/LR compared with 0/LAT bicuspid aortic valves after valve-sparing aortic root replacement. J Thorac Cardiovasc Surg. 2015;150(1):59-68. doi: 10.1016/j.jtcvs.2015.04.020 [DOI] [PubMed] [Google Scholar]


