This pooled analysis investigates the association between the length of reproductive life span and risk of incident cardiovascular disease events, while also considering the timing of menarche and menopause.
Key Points
Question
Is the length of reproductive life span associated with future risk of cardiovascular disease (CVD) events?
Findings
In a pooled analysis of 307 855 women from 12 studies participating in the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events consortium, short reproductive life span (<33 years) was associated with an increased risk of CVD events in midlife. Women who had both a short reproductive life span and early menarche (age ≤11 years) had the most pronounced risk of CVD events.
Meaning
These findings highlight reproductive life span as a potential marker of women’s risk of CVD events in midlife.
Abstract
Importance
Early menarche and early menopause are associated with increased risk of cardiovascular disease (CVD) in midlife, but little is known about the association between reproductive life span and the risk of CVD.
Objective
To investigate the association between the length of reproductive life span and risk of incident CVD events, while also considering the timing of menarche and menopause.
Design, Setting, and Participants
Individual-level data were pooled from 12 studies participating in the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events consortium. Women provided complete information on the timing of menarche and menopause, nonfatal CVD events, and covariates. Cox proportional hazards models were used to estimate hazard ratios and 95% CIs, adjusted for covariates. The association between reproductive life span and CVD was adjusted for age at menarche and age at menopause separately. Analysis began March 2018 and ended December 2019.
Exposures
Reproductive life span was calculated by subtracting age at menarche from age at menopause and categorized as younger than 30, 30 to 32, 33 to 35, 36 to 38 (reference group), 39 to 41, 42 to 44, and 45 years or older.
Main Outcomes and Measures
First nonfatal CVD event, including coronary heart disease and stroke events.
Results
A total of 307 855 women were included. Overall, the mean (SD) ages at menarche, menopause, and reproductive life span were 13.0 (1.5) years, 50.2 (4.4) years, and 37.2 (4.6) years, respectively. Pooled analyses showed that women with a very short reproductive life span (<30 years) were at 1.71 (95% CI, 1.58-1.84) times higher risk of incident CVD events than women with a reproductive life span of 36 to 38 years after adjustment for covariates. This association remained unchanged when adjusted for age at menarche but was attenuated to 1.26 (95% CI, 1.09-1.46) when adjusted for age at menopause. There was a significant interaction between reproductive life span and age at menarche associated with CVD risk (P < .001). Women who had both short reproductive life span (<33 years) and early menarche (age ≤11 years) had the highest risk of CVD (hazard ratio, 2.06; 95% CI, 1.76-2.41) compared with those with a reproductive life span of 36 to 38 years and menarche at age 13 years.
Conclusions and Relevance
Short reproductive life span was associated with an increased risk of nonfatal CVD events in midlife, and the risk was significantly higher for women with early age at menarche.
Introduction
Globally, cardiovascular disease (CVD) contributes to a high burden of mortality and morbidity for women.1,2 Women who experience early menarche3,4 and early menopause3,5 are at increased risk of CVD, which suggests a potential role for the female reproductive life span in the risk of CVD in later life.2,6,7
Two systematic review and meta-analysis studies have shown that early menarche and early menopause are associated with higher risk of all-cause mortality, while the associations with cardiovascular mortality and nonfatal cardiovascular outcomes were less conclusive.8,9 Subsequently, 3 large studies have shown fairly consistent evidence for links of early menarche and early menopause with CVD events.3,4,5 Besides the timing of menarche and menopause, recent interest has emerged in the association between CVD risk and reproductive life span (defined by age at menopause − age at menarche). The Nurses’ Health Study3 reported that a shorter reproductive life span (<30 years) was associated with a 1.32 (95% CI, 1.16-1.49) times higher risk of CVD events compared with a reproductive life span of 42 years or longer. Further, the association with long reproductive life span was either null10,11 or mixed.3,12,13 These findings suggest that the association between reproductive life span and CVD is not entirely clear and requires further investigation. Additionally, our previous study showed that women with early menarche were associated with increased risk of experiencing premature and early menopause,14 which may consequently lead to a shorter reproductive life span compared with those with normal ages at menarche and menopause. To date and to our knowledge, no study has examined whether the timing of menarche or menopause affects the association between reproductive life span and risk of CVD events.
For the present study, individual-level data pooled from 12 studies15,16,17,18,19,20,21,22,23,24,25,26 participating in the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events (InterLACE) consortium27 were used to examine the association between reproductive life span and risk of incident CVD events, while considering the timing of menarche and menopause.
Methods
Ethics
Each study participating in InterLACE received ethics approval from the institutional review board or human research ethics committee at each participating institution. All the participants provided informed consent.
Study Participants
The InterLACE consortium consists of more than 20 observational studies across 10 countries. Briefly, each study collected prospective or retrospective survey data on women’s reproductive health across the life span, sociodemographic and lifestyle factors, and chronic disease events. Key variables were harmonized into the simplest level of detail that would incorporate information from as many studies as possible. The eligibility criteria for participating studies and data harmonization procedures have been published elsewhere.27,28
In this study, we pooled data from 12 studies15,16,17,18,19,20,21,22,23,24,25,26 that had collected information on ages at menarche and menopause and CVD events (Table 1). A total of 484 870 women were included at analytic baseline. In our analyses, we first excluded women who had missing data on CVD events (n = 4544) and age at onset of CVD events (n = 8794). Women who did not report their age at menarche and whose menopause status was unknown (n = 56 232) and those who were using menopausal hormone therapy before menopause and who had undergone a hysterectomy or oophorectomy were excluded from this study (n = 70 597). Further, women who had missing data on covariates (n = 36 848) were also excluded (eFigure 1 in the Supplement).
Table 1. Characteristics of 12 Individual Studies of a Subset of Women With Information on Reproductive Life Span and Cardiovascular Disease Events in the InterLACE Consortium.
| Study | Country | No. of individuals | Age, median (IQR), y | |
|---|---|---|---|---|
| At baseline | At last follow-up | |||
| Australian Longitudinal Study on Women’s Health, 201520 | Australia | 6516 | 47.6 (46.3-48.9) | 63.8 (62.4-65.4) |
| Melbourne Collaborative Cohort Study, 200221 | Australia | 14 394 | 56.7 (48.0-63.4) | 65.6 (57.4-72.1) |
| Danish Nurse Cohort Study, 201222 | Denmark | 16 098 | 50.0 (47.0-59.0) | 61.0 (47.0-71.0) |
| Women’s Lifestyle and Health Study, 201718 | Sweden | 26 216 | 38.0 (34.0-42.0) | 47.0 (42.0-53.0) |
| Japan Nurses’ Health Study, 200719 | Japan | 39 889 | 41.0 (35.0-47.0) | 41.0 (35.0-47.0) |
| Hilo Women's Health Study, 200723 | United States | 614 | 50.4 (45.6-55.1) | 50.4 (45.6-55.1) |
| Study of Women's Health Across the Nation, 200024 | United States | 2717 | 46.0 (44.0-48.0) | 54.0 (52.0-57.0) |
| MRC National Survey of Health and Development, 200615a | United Kingdom | 573 | 47.0 (47.0-47.0) | 54.0 (54.0-54.0) |
| National Child Development Study, 200616a | United Kingdom | 2454 | 50.0 (50.0-50.0) | 55.0 (55.0-55.0) |
| English Longitudinal Study of Aging, 201325 | United Kingdom | 2621 | 57.0 (51.0-66.0) | 67.0 (61.0-76.0) |
| UK Women’s Cohort Study, 201726 | United Kingdom | 15 768 | 48.6 (43.1-56.5) | 50.9 (45.2-59.1) |
| UK Biobank, 201517 | United Kingdom | 179 995 | 57.0 (49.0-62.0) | 57.0 (50.0-63.0) |
| Total | NA | 307 855 | 51.0 (44.0-60.0) | 54.0 (46.0-62.0) |
Abbreviations: InterLACE, International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events; IQR, interquartile range; MRC, Medical Research Council; NA, not applicable.
National Survey of Health and Development (1946 British Birth Cohort) and National Child Development Study (1958 British Birth Cohort) first collected information on women’s health in 1993 (age 47 years) and 2008 (age 50 years), respectively.
Assessment of Reproductive Markers
The main exposure variables were reproductive life span, age at menarche, and age at menopause. Age at menarche was collected retrospectively by most studies except for the 2 British birth cohort studies15,16 and was categorized as age 10 or younger, 11, 12, 13 (reference group), 14, 15, and 16 years or older.4 Women who experienced CVD events before menopause or who were still premenopausal (menstrual cycle in past 3 months with no change in regularity over past 12 months) or perimenopausal (who had a menstrual cycle in past 12 months with change in regularity) were categorized as premenopausal or perimenopausal and were included in the analyses as a separate group. Age at natural menopause (amenorrhea for at least 12 months without an intervention such as hysterectomy or bilateral oophorectomy) was categorized as younger than 40, 40 to 44, 45 to 49, 50 to 51 (reference group), 52 to 53, 54 to 55, and 56 years or older.14 Reproductive life span was calculated by subtracting age at menarche from age at natural menopause (using actual ages) and was categorized into 7 categories with 3-year increments: younger than 30, 30 to 32, 33 to 35, 36 to 38 (reference group), 39 to 41, 42 to 44, and 45 years or older.
Case Ascertainment
A CVD event was defined as the first event of nonfatal CVD reported in survey questionnaires, including coronary heart disease (CHD; myocardial infarction and angina) and stroke (ischemic stroke and hemorrhagic stroke). Three studies (UK Biobank,17 Women’s Lifestyle and Health Study,18 and Danish Nurse Cohort Study19) also provided hospital admission data.27 Thus, both the self-reported physician diagnoses and hospital admissions were used to define nonfatal CVD events in these studies. Incident CHD events were classified by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes I21-I25, or classified by ICD-9 codes 410-413. Stroke was classified by ICD-10 codes I60, I61, I63, and I64, or ICD-9 codes 430-434.29
Covariates
We included the following factors as covariates: women’s year of birth (<1940, 1940-1949, and ≥1950), age at last follow-up (<60, 60-64, and ≥65 years), education (≤10, 11-12, and >12 years), smoking status at baseline (never, past, and current), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) at baseline (<18.5, 18.5-22.9, 23.0-24.9, 25.0-29.9, and ≥30.0), age at first birth (no children, ≤20, 21-25, 26-30, and >30 years), number of children (no children, 1, 2, 3, and ≥4 children), and use of menopausal hormone therapy (never, past, and current). Participants self-reported race/ethnicity. In the studies where this information was not available, race/ethnicity was defined based on country of birth, language spoken at home, or the country of residence. This pooled study included data from 12 studies across 6 countries. Thus, we categorized race/ethnicity (region) as follows: White–Australian/New Zealand, White-European, White–North American, Asian (including Japanese, Chinese, South Asian, and Southeast Asian), and other (including African American/Black, Hispanic/Latino, Middle Eastern, Aboriginal, and mixed).
Statistical Analyses
First, tests for associations between demographic variables and CVD events by reproductive life span were examined using χ2 tests for categorical variables. Cox proportional hazards models were used to examine the association of reproductive life span, age at menarche, and age at menopause with the incidence of CVD events (ie, 3 separate regression models), providing estimates of hazard ratios (HRs) and 95% CIs. The proportional hazards assumption was checked using log-cumulative hazard plots and found to be reasonable. We adjusted for within-study correlation by treating the study-level as a random effect in all models. For women with CVD, the total follow-up time was calculated as age at first CVD event. For women without CVD, the total follow-up time was calculated as age at last follow-up. For postmenopausal women, the time leading up to menopause was classified as the unexposed period and was included in the survival analyses to account for immortal time bias. First, we built 2 models: (1) the partially adjusted model was adjusted for women’s years of birth, race/ethnicity, education, smoking status at baseline, and baseline BMI and (2) the fully adjusted model was additionally adjusted for other reproductive factors: age at first birth, number of children, and menopausal hormone therapy according to evidence from prior studies.3,13,14
Excluding 127 648 women who were premenopausal or perimenopausal, we examined which combination of exposures provided the model with the best fit. Separate models for reproductive life span (model 1), age at menarche (model 2), and age at menopause (model 3) were adjusted for women’s year of birth, race/ethnicity, education, smoking status at baseline, BMI at baseline, age at first birth, number of children, and menopausal hormone therapy use. Model 4 included both reproductive life span and age at menarche in addition to the covariates. Model 5 included both reproductive life span and age at menopause in addition to the covariates. Model 6 included both age at menarche and age at menopause in addition to the covariates. The −log likelihood (−logL) value was used in 2 ways. For nested models (eg, models 1 or 2 compared with model 4), it was used to calculate the deviance (−2 × difference in logL values) and test the statistical significance of inclusion of the additional factor. Also, because the factors all had the same number of categories (7), it was used to compare goodness of fit between models 4, 5, and 6 (as the Akaike Information Criterion = constant – 2logL, in this case). Separate analyses were undertaken for CHD and stroke events. For the model including reproductive life span and age at menarche, we further examined the combined association of reproductive life span (<33, 33-35, 36-38, 39-41, ≥42 years) with different ages at menarche (≤11, 12, 13, 14, ≥15 years) using a 5 × 5 heat map.
In addition, a sensitivity analysis was conducted by restricting the sample to women who experienced CVD events at least 5 years (n = 136 267), 7 years (n = 116 305), and 10 years (n = 87 832) after menopause to minimize the possible influence of subclinical CVD causing earlier age at menopause. A further sensitivity analysis was undertaken using the 3 studies (UK Biobank,17 Women’s Lifestyle and Health Study,18 Danish Nurse Cohort Study19; n = 142 763) that provided hospital admission data on nonfatal CVD events to check the robustness of findings.
The primary analyses were conducted by using PHREG procedure (SAS Enterprise Guide, version 9.4 [SAS Institute Inc]) following a sequential Cox proportional hazards models in SAS version 9.4 (SAS Institute Inc), also taking into account study level as random effects. All statistical analyses were based on 2-sided 5% level of significance. Analysis began March 2018 and ended December 2019.
Results
Study Characteristics
Altogether, 307 855 women were included in this pooled analysis (eFigure 1 in the Supplement). The data were predominantly from White populations from the UK, US, Australia, Denmark, and Sweden (Table 1). Overall, the mean (SD) ages at menarche, menopause, and reproductive life span were 13.0 (1.5) years, 50.2 (4.4) years, and 37.2 (4.6) years, respectively. The incidence of nonfatal CVD, CHD, and stroke events after menopause were 3.3% (n = 10 235), 2.5% (n = 7610), and 1.0% (n = 3161), respectively. Baseline characteristics according to reproductive life span and occurrence of CVD events were shown in eTable 1 in the Supplement.
Association of Reproductive Life Span, Age at Menarche, and Age at Menopause With Nonfatal CVD Events
Women who had reached menopause showed an inverse association between increasing reproductive life span and nonfatal CVD events (P < .001 for trend) (Table 2). After adjusting for covariates (fully adjusted model), women with less than 30 years of reproductive life span had 71% higher risk of CVD (HR, 1.71; 95% CI, 1.58-1.84) compared with those with a reproductive life span of 36 to 38 years. Similar results were also observed for nonfatal CHD (HR, 1.66; 95% CI, 1.52-1.82) and stroke (HR, 1.75; 95% CI, 1.52-2.01) (eTable 2 in the Supplement). Having a reproductive life span of 30 to 32 years and 33 to 35 years was also associated with higher risk of CVD, CHD, and stroke but to a lesser extent.
Table 2. Association Between Reproductive Life Span, Age at Menarche, Age at Menopause, and Risk of Nonfatal CVD Events.
| Characteristic | No. of women | No. of CVD events | Person-years at risk | No. of events per 10 000 person-years | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Partially adjusted modela | Fully adjusted modelb | |||||
| Reproductive life span, y | ||||||
| Premenopause or perimenopause | 127 648 | 2019 | 5 685 573 | 3.6 | 0.28 (0.25-0.30) | 0.25 (0.23-0.27) |
| <30 | 11 442 | 906 | 6 77 722 | 13.4 | 1.73 (1.60-1.87) | 1.71 (1.58-1.84) |
| 30-32 | 14 075 | 875 | 844 714 | 10.4 | 1.32 (1.22-1.43) | 1.31 (1.21-1.42) |
| 33-35 | 29 046 | 1475 | 1 737 737 | 8.5 | 1.16 (1.08-1.24) | 1.16 (1.08-1.24) |
| 36-38 | 51 552 | 2221 | 3 114 752 | 7.1 | 1 [Reference] | 1 [Reference] |
| 39-41 | 45 647 | 1752 | 2 781 965 | 6.3 | 0.90 (0.85-0.96) | 0.91 (0.85-0.96) |
| 42-44 | 21 901 | 776 | 1 359 193 | 5.7 | 0.77 (0.71-0.84) | 0.78 (0.72-0.85) |
| ≥45 | 6544 | 211 | 416 146 | 5.1 | 0.61 (0.53-0.70) | 0.62 (0.54-0.72) |
| P value for trend | NA | NA | NA | NA | <.001 | <.001 |
| Age at menarche, y | ||||||
| ≤10 | 12 498 | 410 | 658 174 | 6.2 | 1.16 (1.05-1.29) | 1.16 (1.04-1.29) |
| 11 | 43 192 | 1438 | 2 302 984 | 6.2 | 1.16 (1.08-1.24) | 1.15 (1.07-1.22) |
| 12 | 64 616 | 1815 | 3 401 406 | 5.3 | 0.99 (0.93-1.05) | 0.98 (0.92-1.04) |
| 13 | 79 760 | 2460 | 4 288 161 | 5.7 | 1 [Reference] | 1 [Reference] |
| 14 | 61 268 | 2119 | 3 356 786 | 6.3 | 1.00 (0.94-1.06) | 0.99 (0.93-1.05) |
| 15 | 30 418 | 1193 | 1 698 551 | 7.0 | 1.05 (0.98-1.13) | 1.04 (0.97-1.11) |
| ≥16 | 16 103 | 800 | 911 741 | 8.8 | 1.16 (1.07-1.26) | 1.15 (1.06-1.24) |
| Age at menopause, y | ||||||
| Premenopause or perimenopause | 127 648 | 2019 | 5 685 573 | 3.6 | 0.27 (0.21-0.29) | 0.25 (0.23-0.27) |
| <40 | 3366 | 272 | 193 608 | 14.0 | 1.97 (1.73-2.23) | 1.92 (1.69-2.18) |
| 40-44 | 13 184 | 921 | 783 746 | 11.8 | 1.58 (1.46-1.71) | 1.57 (1.45-1.70) |
| 45-49 | 45 112 | 2298 | 2 681 408 | 8.6 | 1.21 (1.13-1.28) | 1.21 (1.14-1.29) |
| 50-51 | 44 072 | 1904 | 2 671 383 | 7.1 | 1 [Reference] | 1 [Reference] |
| 52-53 | 35 737 | 1407 | 2 179 906 | 6.5 | 0.91 (0.85-0.97) | 0.92 (0.86-0.98) |
| 54-55 | 24 196 | 892 | 1 500 466 | 5.9 | 0.79 (0.73-0.85) | 0.80 (0.73-0.86) |
| ≥56 | 14 540 | 522 | 921 714 | 5.7 | 0.71 (0.64-0.78) | 0.72 (0.65-0.79) |
| P value for trend | NA | NA | NA | NA | <.001 | <.001 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HR, hazard ratio; NA, not applicable.
Adjusted for women’s year at birth, race/ethnicity, education, smoking status at baseline, and baseline BMI.
Additionally adjusted for number of children, age at first birth, and menopausal hormone therapy use.
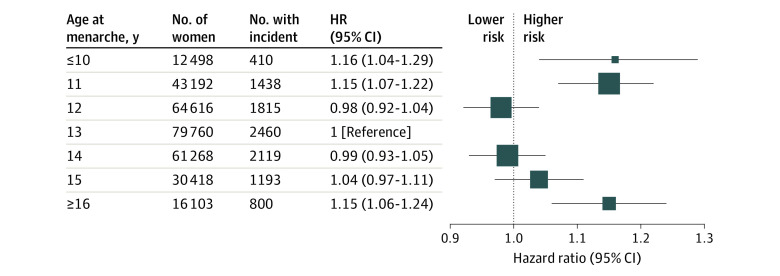
A U-shaped association was evident between age at menarche and CVD events (Figure 1). Compared with women with menarche at age 13 years, those with early menarche (age ≤10 years) had 16% (HR, 1.16; 95% CI, 1.04-1.29) increased risk of CVD, and those with late menarche (age ≥16 years) had 15% (HR, 1.15; 95% CI, 1.06-1.24) increased risk of CVD (fully adjusted model). A similar U-shaped association was observed for CHD and stroke events (eFigure 2 in the Supplement).
Figure 1. Hazard Ratio (HR) and 95% CI of Incident Cardiovascular Disease by Age at Menarche.
The estimates used in the figure are shown in Table 2. The HRs were fully adjusted for women’s year at birth, race/ethnicity, education, smoking status at baseline, body mass index at baseline, number of children, age at first birth, and menopausal hormone therapy use. The reference category was menarche at age 13 years. The area of the square was inversely proportional to the variance of log HR.
Premature (age <40 years) and early menopause (age 40-44 years) were strongly associated with the incidence of nonfatal CVD, CHD, and stroke events. Compared with women aged 50 to 51 years at menopause, those with premature menopause had 92% (HR, 1.92; 95% CI, 1.69-2.18) higher risk of incident CVD events, and those with early menopause had 57% (HR, 1.57; 95% CI, 1.45-1.70) higher risk of CVD. In contrast, those with late menopause at 56 years or older had a 28% (HR, 0.72; 95% CI, 0.65-0.79) lower risk of CVD. Similar findings were found for CHD and stroke events.
Association of Reproductive Life Span Adjusted for Age at Menarche or Age at Menopause With CVD Risk
For the combined associations, the model that included reproductive life span and age at menarche (model 4) had the best fit (Table 3). The association of very short reproductive life span (<30 years) with CVD risk remained unchanged after adjusting for age at menarche (HR, 1.70; 95% CI, 1.57-1.84), while the association of early menarche was strengthened and the association of late menarche disappeared. In contrast, after adjusting for age at menopause, the association of short reproductive life span with CVD was attenuated, from an HR of 1.69 (95% CI, 1.57-1.83) to 1.25 (95% CI, 1.08-1.45) for duration of less than 30 years, but it still remained statistically significant (model 5). In the model of age at menarche and age at menopause (model 6), the association of premature menopause (<40 years) remained unaffected after adjusting for age at menarche. Similar patterns of results were evident for CHD and stroke events (eTable 3 in the Supplement).
Table 3. Nested Models Between Reproductive Life Span, Age at Menarche, Age at Menopause, and Risk of Nonfatal Cardiovascular Disease Events.
| Characteristic | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2a | Model 3a | Model 4b | Model 5c | Model 6d | |
| –log Likelihood | 87 117 | 87 317 | 87 102 | 87 078 | 87 092 | 87 086 |
| Reproductive life span, y | ||||||
| <30 | 1.69 (1.57-1.83) | NA | NA | 1.70 (1.57-1.84) | 1.25 (1.08-1.45) | NA |
| 30-32 | 1.31 (1.21-1.42) | NA | NA | 1.31 (1.21-1.42) | 1.09 (0.98-1.20) | NA |
| 33-35 | 1.16 (1.09-1.24) | NA | NA | 1.16 (1.09-1.24) | 1.04 (0.96-1.12) | NA |
| 36-38 | 1 [Reference] | NA | NA | 1 [Reference] | 1 [Reference] | NA |
| 39-41 | 0.90 (0.84-0.96) | NA | NA | 0.86 (0.81-0.92) | 1.01 (0.94-1.09) | NA |
| 42-44 | 0.76 (0.70-0.83) | NA | NA | 0.71 (0.66-0.78) | 0.95 (0.84-1.07) | NA |
| ≥45 | 0.58 (0.50-0.67) | NA | NA | 0.52 (0.45-0.60) | 0.75 (0.61-0.91) | NA |
| Age at menarche, y | ||||||
| ≤10 | NA | 1.13 (1.00-1.27) | NA | 1.34 (1.19-1.52) | NA | 1.12 (1.00-1.27) |
| 11 | NA | 1.16 (1.07-1.24) | NA | 1.27 (1.18-1.37) | NA | 1.14 (1.06-1.23) |
| 12 | NA | 0.98 (0.91-1.05) | NA | 1.03 (0.96-1.10) | NA | 0.98 (0.91-1.05) |
| 13 | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] |
| 14 | NA | 1.00 (0.93-1.06) | NA | 0.95 (0.89-1.01) | NA | 1.00 (0.94-1.07) |
| 15 | NA | 1.07 (0.99-1.15) | NA | 0.97 (0.89-1.04) | NA | 1.07 (0.99-1.16) |
| ≥16 | NA | 1.17 (1.07-1.28) | NA | 0.98 (0.90-1.08) | NA | 1.16 (1.07-1.27) |
| Age at menopause, y | ||||||
| <40 | NA | NA | 1.92 (1.69-2.18) | NA | 1.54 (1.27-1.87) | 1.90 (1.67-2.15) |
| 40-44 | NA | NA | 1.55 (1.44-1.68) | NA | 1.33 (1.16-1.52) | 1.55 (1.43-1.68) |
| 45-49 | NA | NA | 1.22 (1.15-1.29) | NA | 1.18 (1.09-1.27) | 1.22 (1.14-1.29) |
| 50-51 | NA | NA | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] |
| 52-53 | NA | NA | 0.92 (0.85-0.98) | NA | 0.92 (0.85-0.99) | 0.92 (0.86-0.98) |
| 54-55 | NA | NA | 0.78 (0.72-0.85) | NA | 0.81 (0.73-0.90) | 0.78 (0.72-0.85) |
| ≥56 | NA | NA | 0.68 (0.62-0.75) | NA | 0.79 (0.68-0.92) | 0.68 (0.62-0.75) |
Abbreviation: NA, not applicable.
Models 1 to 3 were adjusted for women’s year at birth, race/ethnicity, education, smoking status at baseline, baseline body mass index, number of children, age at first birth, and menopausal hormone therapy use.
Model 4 included both reproductive life span and age at menarche in addition to the covariates.
Model 5 included both reproductive life span and age at menopause in addition to the covariates.
Model 6 included both age at menarche and age at menopause in addition to the covariates.
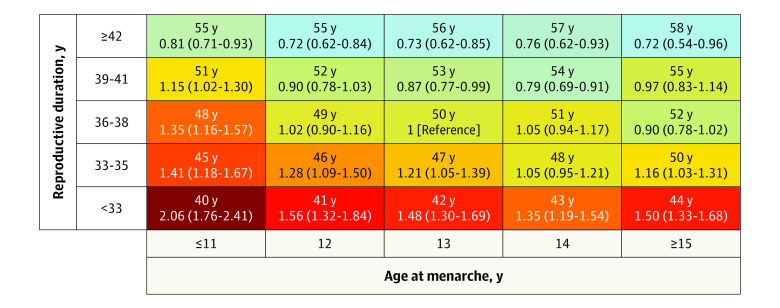
An interaction was detected between reproductive life span and age at menarche (P for interaction < .001). Compared with women with menarche at age 13 years and a reproductive life span of 36 to 38 years, those with menarche at 13 years and short reproductive life span less than 33 years had almost 50% higher risk (HR, 1.48; 95% CI, 1.30-1.69) of CVD events. Early menarche (age ≤11 years) strengthened the association, with the risk of CVD even higher for women with early age at menarche and a short reproductive life span (HR, 2.06; 95% CI, 1.76-2.41), while late age at menarche (age ≥15 years) did not influence the association (Figure 2). The estimates used in the Figure 2 are shown in eTable 4 in the Supplement.
Figure 2. Heat Map for the Association Between Reproductive Life Span and Age at Menarche.
The association (hazard ratios and 95% CI) between the combination of reproductive life span (<33, 33-35, 36-38, 39-41, ≥42 years) and age at menarche (≤11, 12, 13, 14, ≥15 years) with nonfatal cardiovascular disease events is displayed. The hazard ratios were fully adjusted for women’s year at birth, race/ethnicity, education, smoking status at baseline, body mass index at baseline, number of children, age at first birth, and menopausal hormone therapy use. The number above each hazard ratio shows the mean age at menopause for that combination. A darker color (in a gradient from green to red) shows increasing risk of nonfatal cardiovascular disease. The estimates are shown in eTable 4 in the Supplement.
Sensitivity Analysis
A sensitivity analysis was performed to examine the estimates restricted to the onset of CVD at least 5 years after menopause. The findings for the association between reproductive life span, age at menarche, age at menopause, and risk of CVD events were increased with longer follow-up after menopause (eTables 5, 6, and 7 in the Supplement). Furthermore, when the analysis was restricted to the 3 studies with hospital data on CVD events (n = 142 763), the results remained unchanged (eTable 8 in the Supplement).
Discussion
This large pooled analysis of data from 307 855 women provides robust evidence for the association between a shorter reproductive life span and the first nonfatal CVD event. Having a short reproductive life span (<30 years) was associated with a 71% higher risk of CVD after adjusting for covariates. A U-shaped association was found between age at menarche and CVD, with a higher risk of CVD for both early menarche (age ≤11 years) and late menarche (age ≥15 years). Premature (age <40 years) and early menopause (age 40-44 years) were strongly associated with higher risk of nonfatal CVD events. The association of short reproductive life span with incident CVD remained unchanged after adjusting for age at menarche but was attenuated substantially after adjusting for age at menopause, showing the strong association between premature and early menopause and short reproductive life span. Additionally, there was an interaction between reproductive life span and age at menarche. We found the risk of nonfatal CVD was highest for women with early menarche and a short reproductive life span (more than 2-fold increased risk). Women in this group had a mean age at menopause of 40 years.
Our findings are consistent with earlier studies that suggested a shorter reproductive life span is associated with a higher risk of CVD,3,11 CHD,3,12 and stroke.3,12 Our study further expanded these findings by showing whether the association between reproductive life span and risk of CVD is affected by the timing of menarche or menopause. Previous studies have suggested that menarche at age 12 to 14 years was optimal in terms of future CVD risk,3,4 but our study has demonstrated that this does not capture the increased risk for women with a short reproductive life span who have an average age at menarche. A previous study that looked at the combined association of age at menarche with different lengths of reproductive life span included a small sample of individuals with stroke (189 individuals with stroke and 192 controls).10 Women with later age at menarche (age >15 years) and short reproductive life span (≤36 years) had a higher risk of stroke compared with those with menarche at 15 years or younger and reproductive life span more than 36 years. No evidence of an association for early age at menarche (age ≤15 years) and short reproductive life span (≤36 years) was reported.10 Our study showed that both combinations, early menarche (age ≤11 years) and short reproductive life span (<33 years) as well as late menarche (age ≥15 years) and short reproductive life span (<33 years) were associated with increased risk of CVD events compared with menarche at age 13 years and reproductive life span at 36 to 38 years.
Our finding of a lower risk of nonfatal CVD events for women who had a long reproductive life span (≥42 years) is consistent with the cardioprotective effect of endogenous estrogen in blood-vascular systems by regulating the levels of metabolic markers such as lipids, inflammatory markers, and coagulants.2,7 On the other hand, a short reproductive life span may indicate accelerated aging in midlife. The end of the reproductive life span serves as a marker for a range of biologic mechanisms linked with progressive damage to tissues and organs (aging) increasing the risk not only for CVD, but also for Parkinson disease, dementia, depression, and osteoporosis.9,30 Some shared genetic and environmental factors also contribute to risk, along with the timing of menarche and menopause that together explain the risk of CVD for women in the middle age.31 Further, some evidence suggests a possible link between socioeconomic factors (eg, higher education, being employed, and having better self-reported health)32 and risk of later menopause as well as exposure to persistent organic pollutants (eg, polyfluoroalkyl chemicals) and risk of early menopause.33 Given the interrelationship between timing of menarche and menopause,14 we suggest that for women in midlife, CVD risk assessment should take into account the timing of both menarche and menopause.
Strengths and Limitations
The strengths of our study include its large sample size from 6 countries and the availability of information on other reproductive variables. By adjusting for age at menarche or menopause, we were able to compare women at same timing of either menarche or menopause relative to their reproductive life span. Further, this pooled study had sufficient power to detect the association at extreme age ranges of menarche, menopause, and reproductive life span to allow replication of findings from earlier studies.3,4,5,13
Our study also had some limitations. First, information on reproductive markers was mostly collected based on retrospective recall (except for data collected from the longitudinal cohorts), which could have introduced measurement errors due to recall bias. Second, the nonfatal CVD events were based on self-report of physician diagnosis in most studies, which may have resulted in misclassification of CVD outcomes. However, 3 large studies, UK Biobank,17 Women’s Lifestyle and Health Study (UK),18 and Danish Nurse Cohort Study (Denmark),19 also provided hospital admissions data and a sensitivity analysis using only these data confirmed the main results. Third, we could not adjust for genetic factors, early life factors, diet, physical activity, and comorbidities (eg, diabetes, cancer, chronic obstructive pulmonary disease), which might be related to both the exposure and outcomes variables, but earlier estimates from the Nurses’ Health Study were unchanged after adjusting for history of diabetes, hypertension, and hypercholesterolemia.3
Conclusions
Short reproductive life span was associated with higher risk of nonfatal CVD events in midlife. Early age at menarche and menopause were also associated with a higher risk of nonfatal CVD events. Women who had both short reproductive life span and early menarche had the most pronounced risk of nonfatal CVD events. These findings highlight reproductive life span as a potential marker of women’s risk of CVD events in midlife; however, further research is needed on the underlying mechanisms linking fertility, reproductive aging, with CVD risk across the life span.
eFigure 1. Flow diagram of sample for analysis of the associations between reproductive lifespan and non-fatal CVD events in the InterLACE consortium
eFigure 2. Hazard ratio and 95% confidence interval (CI) of incident (A) coronary heart disease (CHD) and (B) stroke by age at menarche
eTable 1. Baseline characteristics according to reproductive lifespan and occurrence of first non-fatal CVD events (N=307,855)
eTable 2. Association between reproductive lifespan, age at menarche, age at menopause, and risk of non-fatal CHD and stroke events
eTable 3. Nested models between reproductive lifespan, age at menarche, age at menopause, and risk of non-fatal CHD and stroke events
eTable 4. Reproductive lifespan in combination with age at menarche and the risk of non-fatal CVD events (N=180,207)
eTable 5. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 5-years after menopause (n=136,267)
eTable 6. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 7-years after menopause (N=116,305)
eTable 7. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 10-years after menopause (n=87,832)
eTable 8. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and diagnosed CVD events using hospital registry data (N=142,763*)
eAppendix. Acknowledgments
References
- 1.Naghavi M, Abajobir AA, Abbafati C, et al. ; GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151-1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18(12):598-602. doi: 10.1007/s12471-010-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. doi: 10.1161/JAHA.117.006713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canoy D, Beral V, Balkwill A, et al. ; Million Women Study Collaborators* . Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237-244. doi: 10.1161/CIRCULATIONAHA.114.010070 [DOI] [PubMed] [Google Scholar]
- 5.Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069-1075. doi: 10.1136/heartjnl-2017-312289 [DOI] [PubMed] [Google Scholar]
- 6.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47(6):1019-1026. doi: 10.1161/01.HYP.0000223064.62762.0b [DOI] [PubMed] [Google Scholar]
- 7.Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause. Nat Rev Dis Primers. 2015;1:15004. doi: 10.1038/nrdp.2015.4 [DOI] [PubMed] [Google Scholar]
- 8.Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):29-40. doi: 10.1093/aje/kwu113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767-776. doi: 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 10.Hsieh Y-C, Hwang L-C, Hsieh F-I, et al. Early menarche and ischemic stroke risk among postmenopausal women. Int J Gerontol. 2010;4(1):16-22. doi: 10.1016/S1873-9598(10)70017-X [DOI] [Google Scholar]
- 11.Mansoor H, Elgendy IY, Segal R, Hartzema A. Duration of reproductive years and the risk of cardiovascular and cerebrovascular events in older women: insights from the National Health and Nutrition Examination Survey. J Womens Health (Larchmt). 2017;26(10):1047-1052. doi: 10.1089/jwh.2016.6013 [DOI] [PubMed] [Google Scholar]
- 12.Jung KJ, Kim M-R, Yun YD, Kim HC, Jee SH. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: the Korean Heart Study. Menopause. 2016;23(1):60-66. doi: 10.1097/GME.0000000000000489 [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Lin L, Kartsonaki C, et al. ; China Kadoorie Biobank Study Collaborative Group . Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese Women. Circ Cardiovasc Qual Outcomes. 2017;10(11):e004235. doi: 10.1161/CIRCOUTCOMES.117.004235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra GD, Pandeya N, Dobson AJ, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017;32(3):679-686. doi: 10.1093/humrep/dew350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 national birth cohort (MRC National Survey of Health and Development). Int J Epidemiol. 2006;35(1):49-54. doi: 10.1093/ije/dyi201 [DOI] [PubMed] [Google Scholar]
- 16.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol. 2006;35(1):34-41. doi: 10.1093/ije/dyi183 [DOI] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort profile: the Swedish Women’s Lifestyle and Health cohort. Int J Epidemiol. 2017;46(2):e8. doi: 10.1093/ije/dyv089 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Mizunuma H, Fujita T, et al. Design of the Japan Nurses’ Health Study: a prospective occupational cohort study of women’s health in Japan. Ind Health. 2007;45(5):679-686. doi: 10.2486/indhealth.45.679 [DOI] [PubMed] [Google Scholar]
- 20.Dobson AJ, Hockey R, Brown WJ, et al. Cohort profile update: Australian Longitudinal Study on Women’s Health. Int J Epidemiol. 2015;44(5):1547–, 1547a-1547f.. doi: 10.1093/ije/dyv110 [DOI] [PubMed] [Google Scholar]
- 21.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69-70. [PubMed] [Google Scholar]
- 22.Hundrup YA, Simonsen MK, Jørgensen T, Obel EB. Cohort profile: the Danish nurse cohort. Int J Epidemiol. 2012;41(5):1241-1247. doi: 10.1093/ije/dyr042 [DOI] [PubMed] [Google Scholar]
- 23.Sievert LL, Morrison L, Brown DE, Reza AM. Vasomotor symptoms among Japanese-American and European-American women living in Hilo, Hawaii. Menopause. 2007;14(2):261-269. doi: 10.1097/01.gme.0000233496.13088.24 [DOI] [PubMed] [Google Scholar]
- 24.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, R Marcus, eds. Menopause: Biology and Pathobiology. Academic Press, 2000:175-188. [Google Scholar]
- 25.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42(6):1640-1648. doi: 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cade JE, Burley VJ, Alwan NA, et al. Cohort profile: the UK Women’s Cohort Study (UKWCS). Int J Epidemiol. 2017;46(2):e11. doi: 10.1093/ije/dyv173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra GD, Chung H-F, Pandeya N, et al. The InterLACE study: design, data harmonization and characteristics across 20 studies on women’s health. Maturitas. 2016;92:176-185. doi: 10.1016/j.maturitas.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra GD, Anderson D, Schoenaker DA, et al. InterLACE: a new international collaboration for a life course approach to women’s reproductive health and chronic disease events. Maturitas. 2013;74(3):235-240. doi: 10.1016/j.maturitas.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Littlejohns TJ, Sudlow C, Allen NE, Collins R. UK Biobank: opportunities for cardiovascular research. Eur Heart J. 2019;40(14):1158-1166. doi: 10.1093/eurheartj/ehx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocca WA, Shuster LT, Grossardt BR, et al. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond). 2009;5(1):39-48. doi: 10.2217/17455057.5.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolk L, Perry JR, Chasman DI, et al. ; LifeLines Cohort Study . Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44(3):260-268. doi: 10.1038/ng.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70-83. doi: 10.1093/aje/kws421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ Health Perspect. 2014;122(2):145-150. doi: 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow diagram of sample for analysis of the associations between reproductive lifespan and non-fatal CVD events in the InterLACE consortium
eFigure 2. Hazard ratio and 95% confidence interval (CI) of incident (A) coronary heart disease (CHD) and (B) stroke by age at menarche
eTable 1. Baseline characteristics according to reproductive lifespan and occurrence of first non-fatal CVD events (N=307,855)
eTable 2. Association between reproductive lifespan, age at menarche, age at menopause, and risk of non-fatal CHD and stroke events
eTable 3. Nested models between reproductive lifespan, age at menarche, age at menopause, and risk of non-fatal CHD and stroke events
eTable 4. Reproductive lifespan in combination with age at menarche and the risk of non-fatal CVD events (N=180,207)
eTable 5. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 5-years after menopause (n=136,267)
eTable 6. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 7-years after menopause (N=116,305)
eTable 7. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and incident CVD events disaggregated by onset of CVD at 10-years after menopause (n=87,832)
eTable 8. Sensitivity analysis for the association between reproductive lifespan, age at menarche, age at menopause, and diagnosed CVD events using hospital registry data (N=142,763*)
eAppendix. Acknowledgments