Recent outbreaks of chikungunya fever have increased its clinical importance. Neither a specific antiviral drug nor a commercial vaccine for CHIKV infection are available. Here, we show a detailed model of the docking between the envelope glycoprotein of CHIKV and our unique anti-CHIKV-neutralizing monoclonal antibody (CHE19), which inhibits CHIKV membrane fusion and virion release from CHIKV-infected cells. Homology modeling of the neutralizing antibody CHE19 and protein-protein docking analysis of the CHIKV envelope glycoprotein and CHE19 suggested that CHE19 inhibits the viral membrane fusion by stabilizing the E2-E1 heterodimer and inhibits virion release by facilitating the formation of virus aggregation due to the connecting virions, and these predictions were confirmed by experiments. Sequence information of CHE19 and the CHIKV envelope glycoprotein and their docking model will contribute to future development of an effective prophylactic and therapeutic agent.
KEYWORDS: aggregation, chikungunya, monoclonal antibody, protein-protein docking analysis, viral membrane fusion
ABSTRACT
Chikungunya fever, a mosquito-borne disease manifested by fever, rash, myalgia, and arthralgia, is caused by chikungunya virus (CHIKV), which belongs to the genus Alphavirus of the family Togaviridae. Anti-CHIKV IgG from convalescent patients is known to directly neutralize CHIKV, and the state of immunity lasts throughout life. Here, we examined the epitope of a neutralizing mouse monoclonal antibody against CHIKV, CHE19, which inhibits viral fusion and release. In silico docking analysis showed that the epitope of CHE19 was localized in the viral E2 envelope and consisted of two separate segments, an N-linker and a β-ribbon connector, and that its bound Fab fragment on E2 overlapped the position that the E3 glycoprotein originally occupied. We showed that CHIKV-E2 is lost during the viral internalization and that CHE19 inhibits the elimination of CHIKV-E2. These findings suggested that CHE19 stabilizes the E2-E1 heterodimer instead of E3 and inhibits the protrusion of the E1 fusion loop and subsequent membrane fusion. In addition, the antigen-bound Fab fragment configuration showed that CHE19 connects to the CHIKV spikes existing on the two individual virions, leading us to conclude that the CHE19-CHIKV complex was responsible for the large virus aggregations. In our subsequent filtration experiments, large viral aggregations by CHE19 were trapped by a 0.45-μm filter. This virion-connecting characteristic of CHE19 could explain the inhibition of viral release from infected cells by the tethering effect of the virion itself. These findings provide clues toward the development of effective prophylactic and therapeutic monoclonal antibodies against the Alphavirus infection.
IMPORTANCE Recent outbreaks of chikungunya fever have increased its clinical importance. Neither a specific antiviral drug nor a commercial vaccine for CHIKV infection are available. Here, we show a detailed model of the docking between the envelope glycoprotein of CHIKV and our unique anti-CHIKV-neutralizing monoclonal antibody (CHE19), which inhibits CHIKV membrane fusion and virion release from CHIKV-infected cells. Homology modeling of the neutralizing antibody CHE19 and protein-protein docking analysis of the CHIKV envelope glycoprotein and CHE19 suggested that CHE19 inhibits the viral membrane fusion by stabilizing the E2-E1 heterodimer and inhibits virion release by facilitating the formation of virus aggregation due to the connecting virions, and these predictions were confirmed by experiments. Sequence information of CHE19 and the CHIKV envelope glycoprotein and their docking model will contribute to future development of an effective prophylactic and therapeutic agent.
INTRODUCTION
Chikungunya fever is a mosquito-borne disease that occurs mainly in tropical regions such as those in Africa, South Asia, and Southeast Asia (1). An epidemic of chikungunya fever over the years 2004 to 2011 resulted in 1.4 to 6.5 million cases, with imported cases reported in nearly 40 countries (2, 3), and the disease has recently spread to Europe and the Americas (4–6). This infectious disease is transmitted by the bite of Aedes mosquitoes infected with the chikungunya virus (CHIKV), which is a member of the genus Alphavirus in the family Togaviridae. Chikungunya fever usually begins 2 to 12 days after the mosquito bite and is characterized by the sudden onset of a high fever that is frequently accompanied by severe joint pain, muscle pain, headache, nausea, fatigue, and rash (1, 7). Viral loads of up to 109 viral RNA copies per ml of blood plasma occur during early infection, and viremia may last for 5 to 7 days (5, 8). Humoral immunity plays a pivotal role in the control of infection and rapid virus clearance and thus in the fight against CHIKV infection. IgMs against CHIKV are produced during the acute and early convalescent phases of infection (days 3 to 8 after the onset of clinical symptoms), and IgGs against CHIKV are produced during the convalescent phases (day 4 until postvirus clearance) (3, 9) and may persist in immune individuals for life (10, 11). Neither a commercial vaccine nor a specific antiviral drug for the treatment of chikungunya fever are available. The treatment of joint pain due to chikungunya fever is focused on relieving the symptoms with antipyretics, optimal analgesics, and fluids.
The structural proteins making up the CHIKV virions are translated from a subgenomic 26S mRNA as a single polyprotein, which is processed cotranslationally into five structural proteins: capsid, E3, E2, 6K, and E1 (12). The CHIKV virions have a diameter of 65 to 70 nm and are enveloped with a lipid bilayer membrane containing 80 viral envelope spikes trimerized with the E1-E2 glycoproteins heterodimer, and these spikes form a T=4 quasi-icosahedral symmetry (13–16). E1 is a class II pH-triggered membrane fusion protein that is positioned at the base of the spike, and the top of E1 is covered by a “protector” protein, E2, which is located on the distal end of the spike (17). As such, E2 is an antigen easily accessed and recognized by anti-CHIKV antibodies, and in fact, most of the reported CHIKV-neutralizing antibodies (NAbs) recognize E2 proteins (18). The membrane surface region of the E2 protein contains 6 segments: an N-linker, domain A, a primary β-ribbon connector, domain B, a secondary β-ribbon connector, and domain C (16). Human NAbs recognizing E2 domain A, E2 domain B, or the spanning epitope of E2 (consisting of domain A and domain B) could inhibit binding to target cells (19, 20), and it has been thought that such NAbs could inhibit the binding to an arthritogenic Alphavirus receptor, MXRA8 (21). In addition to binding inhibition, some NAbs inhibit low pH-activated membrane fusion (19, 22), possibly by recognizing the spanning epitope consisting of domain A and B and then inhibiting protrusion of the fusion loop of E1 by cross-linking and fixing the flexible E2 domain B. On the other hand, some NAbs exhibit not only the inhibition of entry to target cells but also the inhibition of virion release from the CHIKV-infected cells (23–25).
Recently, we obtained several monoclonal antibodies (MAbs) from a mouse immunized with purified virus particles of the CHIKV Thai#16856 strain as antigen and identified one NAb that efficiently inhibited native CHIKV infection. This NAb, named CHE19, showed inhibition activity for membrane fusion via CHIKV envelope glycoprotein (CHIKV-E) as well as inhibition activity for virion release from CHIKV-infected cells. Here, we determined the model of the binding of CHE19 to CHIKV-E by using comprehensive protein-protein docking simulation and then confirmed the inhibition mechanisms of viral membrane fusion and virus release from infected cells experimentally.
RESULTS
Generation and characterization of anti-CHIKV MAbs.
Enzyme-linked immunosorbent assay (ELISA) screening identified seven hybridoma clones, CHE15, CHE19, CHE22, CHE24, CHE29, CHE34, and CHE35, as secreting anti-CHIKV antibodies (data not shown). All MAbs were grouped into three IgG isotypes, IgG1, IgG2a, or IgG2b, and had the kappa light chain (Table 1, 2nd and 3rd columns).
TABLE 1.
Characteristics of anti-CHIKV MAbs
| MAb | IgG subclass | Light chain (λ/κ) | ELISA endpoint titer | Reaction of 293T cells of the indicated linear epitope with the indicated strain or under the indicated condition |
Region (type of recognition) | Neutralization activity of Thai#16856 strain on: |
Neutralization activity of Ross strain on: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHIKV-E (E3-E2-6k-E1)a
|
E2-6ka |
6k-E1a |
CHIKV-E Thai#16856 (capsid E3-E2-6k-E1)b
|
|||||||||||
| Thai#16856 strain | Ross strain | Thai#16856 strain | Reducing conditions | Nonreducing conditions | Native virus | VSV pseudovirus | Native virus | VSV pseudovirus | ||||||
| CHE15 | IgG1 | κ | 2,048,000 | +c | + | + | − | + | + | E2 (linear) | − | − | − | − |
| CHE19 | IgG2b | κ | 256,000 | +d | − | − | − | − | − | E2 (conformational)e | +f | + | − | − |
| CHE22 | IgG1 | κ | 64,000 | + | + | − | + | − | + | E1 (conformational) | − | + | − | + |
| CHE24 | IgG2b | κ | 32,000 | + | + | + | − | − | + | E2 (conformational) | − | ± | − | − |
| CHE29 | IgG2a | κ | 512,000 | + | + | + | − | + | + | E2 (linear) | − | − | − | − |
| CHE34 | IgG1 | κ | 2,048,000 | + | − | + | − | − | + | E2 (conformational) | − | − | − | − |
| CHE35 | IgG1 | κ | 256,000 | + | + | + | − | − | + | E2 (conformational) | − | − | − | − |
Detected by IFA.
Detected by Western blotting.
+, positive; −, negative.
Mean fluorescence intensity of CHE19 detection (measured by ImageJ software) was less than 20% of that for the other MAbs, as shown in Fig. 1.
The region of recognition of CHE19 was determined by in silico analysis in this study.
The IC50 of CHE19 against CHIKV Thai#16856 was 0.182 μg/ml by PRNT assay in this study.
ELISA using native virion as an antigen showed that CHE15 and CHE34 had reactivity to the Thai#16856 strain as high as 1:2,048,000, while CHE24 had low reactivity of 1:32,000 (Table 1, 4th column). CHE19 showed a higher titer than CHE22 or CHE24 in the ELISA, but in the indirect immunofluorescence assay (IFA), CHE19 showed lower reactivity to the cells transfected with the CHIKV-E glycoprotein (CHIKV-E3-E2-6k-E1) expression plasmid, pCAGGS/CHIKV-E Thai#16856 (pCAGGS/CHIKV-E3-E2-6k-E1 Thai16856), than either CHE22 or CHE24 (Fig. 1A), suggesting that the CHE19 lost most of its affinity to the E glycoprotein-expressing cells that were fixed with paraformaldehyde (PFA) (Table 1 and Fig. 1A). All the MAbs reacted with 293T cells transfected with the CHIKV-E glycoprotein expression vector plasmid, but CHE19 and CHE34 only reacted with E glycoprotein of the Thai strain (Table 1, 5th and 6th columns). Our results showed that 6 clones reacted with the CHIKV-E2 antigen, 1 clone (clone CHE22) reacted with the E1 antigen, 5 clones recognized the conformational epitope, and 2 clones (CHE15 and CHE29) reacted with the linear epitope (Table 1, 5th to 10th columns, and Fig. 1B). The reactivity is summarized in Table 1, 11th column. Although CHE19 did not recognize the capsid-E3-E2-6k-E1 protein under either the reducing or nonreducing conditions in Western blotting, this MAb was shown to recognize the conformational E2 protein by in silico analysis (see Fig. 6C). Next, we examined the neutralization activities of these MAbs by using a replication-deficient luciferase-expressing recombinant VSV pseudotype bearing the CHIKV envelope glycoproteins, VSVΔG-luci (CHIKV-E). We found that the CHE19 had strong neutralizing activity against VSVΔG-luci (CHIKV-E Thai#16856) but not against VSVΔG-luci (CHIKV-E Ross). CHE22 had weak neutralizing activity against both VSVΔG-luci (CHIKV-E Thai#16856) and VSVΔG-luci (CHIKV-E Ross), and CHE24 had slightly neutralizing activity against VSVΔG-luci (CHIKV-E Thai#16856) (Fig. 1C). CHE19 and CHE22 also showed similar neutralizing activities against the retrovirus pseudotype bearing the CHIKV-E (Fig. 1D). However, among the 7 MAbs, only CHE19 exhibited neutralization activity against native Thai strain CHIKV infection (Fig. 1E; Table 1), and its 50% inhibitory concentration (IC50) was 0.182 μg/ml in the plaque reduction neutralization test (PRNT) assay. Neither CHE22 nor CHE24 exhibited any neutralizing activity against native CHIKV Thai#16856 or the Ross strain (Fig. 1E).
FIG 1.
Specificity of the MAbs against CHIKV-E protein. (A) MAb reactivity by IFA of 293T cells transfected with a pDisplay empty vector, pCAGGS/CHIKV-E Thai#16856, pCAGGS/CHIKV-E Ross, pDisplay/E2-6k, or pDisplay/6k-E1 was probed with the respective MAbs and anti-CHIKV rabbit serum as a positive control. (B) Cell lysates of the 293T cells transfected with or without pCAGGS/CHIKV-E Thai#16856 were subjected to SDS-PAGE under nonreducing or reducing conditions, and CHIKV-E was detected by Western blot analysis using the anti-CHIKV rabbit serum or the anti-CHIKV MAbs. (C and D) Neutralizing activity of anti-CHIKV MAbs detected by CHIKV pseudovirus neutralization assay. (C) The VSVΔG-luci (CHIKV-E Thai#16856) pseudotype and VSVΔG-luci (CHIKV-E Ross) pseudotype were treated with the respective MAbs (final concentration, 5 μg/ml) for 30 min at 37°C and inoculated onto the Vero cell culture. (D) The pMX-luci (CHIKV-E Thai#16856), pMX-luci (CHIKV-E Ross), and pMX-luci (VSVG) retrovirus pseudotypes were treated with the respective MAbs (final concentrations, 0.04, 0.2, and 1.0 μg/ml, respectively) for 30 min at 37°C and inoculated onto the U251MG cell culture. The infectivityof the pseudotypes was determined by measuring luciferase activities as described in Materials and Methods. Data are the mean ± standard error of the mean (SEM) of three independent experiments. (E) Neutralizing activity of anti-CHIKV MAb, CHE19, CHE22, and CHE24 detected by PRNT as described in Materials and Methods. Serially diluted MAbs were mixed with about 100 PFU of CHIKV Thai#16856 for 30 min at 37°C before inoculation. Each data point is the average of two independent experiments performed in duplicate. Data are the mean ± SEM (n = 4). Statistical significance was evaluated by an unpaired two-tailed t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
FIG 6.
Binding model of CHE19 to the CHIKV spike protein. (A) Amino acid (aa) sequence alignment (1 to 300 aa) using Blosum62 for E2 (423 aa) from the CHIKV Thai#16856, Thai/ES245-7, Ross, and Thai E2 mutants. Domains of E2 determined from the crystal structure of the E2/E1 heterodimer (16) are shown. Domains A, B, and C are shown in black with underlining, while the N-linker segment and 2 β-ribbon connectors, β-ribbon (1) and β-ribbon (2) are shown in purple. The amino acid residues of Thai#16856 came into contact within 4 Å from the heavy atoms of the paratopes of the CHE19, as in panel A-2, are shown in red. The positions where residues are replaced by alanine of the respective Thai E2 mutants, YKATR/AAATA, Thai/TTTDK/AAADA, HKKW/AAAA, and RQGK/AQGA, are shown in light blue. (B) Nucleotide and amino acid sequences of the Fab fragment region of the CHE19 light chain and heavy chain. PCR primer sequences are shown in red (sense) and blue (antisense). The signal peptide sequence of each chain is highlighted in yellow. Variable regions of the light chain and heavy chain are highlighted in light blue and blue, respectively. (C-1) Deduced binding model no. 16 of CHE19 to the CHIKV spike protein. Ribbon drawings of the spike with the bound Fab fragment of CHE19 and whole model of CHE19. The E1 glycoprotein is shown in red, the E2 glycoprotein in green, and the CHE19 Fab fragment region or whole Fab fragment plus Fc structure is shown in blue for the heavy chain and light blue for the light chain. The ribbon diagram of the spike protein was reconstructed by HMHC using PDB ID 6NK7, and the ribbon diagrams of CHE19 Fab fragment and Fab fragment-Fc were reconstructed using PDB IDs 5TL5 and 5TL5 to 1IGT, respectively. (C-2) Blowup of the binding location with the amino acids (shown as spheres) that are in contact within 4 Å from the heavy atoms of the paratopes of the CHE19 region. (C-3) The binding of CHE19 Fab fragment (translucent surface drawings: blue for the heavy chain and light blue for the light chain) on the E2-E1 heterodimer (E2, green ribbon; E1, red ribbon) overlapped the position where the E3 glycoprotein (pink ribbon) was originally occupied. The fusion loop located at the distal end of the E1 glycoprotein is shown in yellow. The ribbon diagrams of the E2-E1 heterodimer in panels C-2 and C-3 were reconstructed by HMHC using PDB ID 3N42. (C-4) Structural image showing that the CHE19 (surface drawings: blue for the heavy chain and light blue for the light chain) connects the CHIKV virion by binding to the spike protein (brown ribbon) existing on two different virions. The ribbon diagrams of structural proteins are reconstructed by HMHC using PDB ID 6NK7. The ratio between virion size and spike size was determined with reference to the mature CHIKV structure model (51). These images were visualized using the UCSF Chimera software (panels C-1, C-3, and C-4) and the PyMOL software (panel C-2). (D) Expression of CHIKV mutant E proteins and their detection by the respective MAbs. (D-1) MAb reactivities by IFA of 293T cells transfected with pCAGGS/CHIKV-E Thai#16856, pCAGGS/CHIKV-E Thai/ES245-7, and four pCAGGS/CHIKV mutant E expression plasmids, pCAGGS/CHIKV-E Thai/YKATR/AAATA, pCAGGS/CHIKV-E Thai/TTTDK/AAADA, pCAGGS/CHIKV-E Thai/HKKW/AAAA, and pCAGGS/CHIKV-E Thai/RQGK/AQGA, were probed with the respective MAbs and anti-CHIKV rabbit serum as a positive control. (D-2) Expression of CHIKV mutant E proteins in 293T cells transfected with the respective CHIKV-E-expressing vectors was detected by Western blotting using anti-CHIKV rabbit serum and two anti-CHIKV mouse IgGs, CHE15 and CHE22. The cellular beta-actin as an internal control was probed by anti-beta-actin mouse MAb (Sigma-Aldrich). (E) VSVΔG-luci pseudoviruses bearing the CHIKV-E protein of the Thai#16856, Thai/ES245-7, and Thai E2 mutants (YKATR/AAATA, TTTDK/AAADA, HKKW/AAAA, and RQGK/AQGA) were examined for their infectivity to Vero cells and their reactivity against CHE19. (E-1) Pseudotype viruses except VSVΔG-luci (CHIKV-E Thai E2 HKKW/AAAA) were produced, and their infectivity was inhibited by anti-CHIKV rabbit serum treatment (1:100). The infectivity of the respective pseudotype virus is shown as a percentage of the VSVΔG-luci (CHIKV-E Thai#16856) infectivity. (E-2) VSVΔG-luci (CHIKV-E Thai E2 YKATR/AAATA) was completely resistant against the CHE19 treatment (10 μg/ml), and VSVΔG-luci (CHIKV-E Thai E2 RQGK/AQGA) was partially resistant, but VSVΔG-luci (CHIKV-E Thai E2 TTTDK/AAADA) was neutralized to the same level as VSVΔG-luci (CHIKV-E Thai#16856). The infectivity of each pseudovirus is shown as a percentage of the infectivity of the respective pseudovirus treated with the Ctrl IgG. Data are the mean ± SEM (n = 6) of three independent experiments. Statistical significance was evaluated by an unpaired two-tailed t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To determine the mechanism of neutralization by the anti-CHIKV antibody, we examined the inhibitory activity of CHE19 and CHE22 against the binding of native CHIKVs to the target cells (Fig. 2A). These NAbs, CHE19 and CHE22, did not inhibit viral binding to the target Vero cells, which was measured as the number of cell-bound viral RNAs, and CHE19 actually enhanced the binding of CHIKV to the Vero cells at 4°C (Fig. 2B). As a result, this enhanced binding activity of the CHIKV-CHE19 complex resulted in an increased plaque number (Fig. 2C and D). Although we observed neutralization activity of CHE19 at 37°C (Fig. 2C and D), this activity achieved a lower level of inhibition compared with the conventional PRNT assay shown in Fig. 1E. In this assay (Fig. 2), target cells inoculated with the virus-antibody complex were washed after 1 h of incubation, and then the medium containing methylcellulose was overlaid. Therefore, the concentration of antibody may have been decreased at viral internalization, which could have allowed the virus infections to proceed more readily than in the conventional PRNT assay.
FIG 2.
Enhancement of binding efficiency of CHIKV treated with a NAb CHE19. (A) Protocol for binding assay. CHIKV Thai#16856 (>200 PFU) was incubated with the respective MAbs (final concentration, 10 μg/ml) for 30 min at 37°C, and then the CHIKV Thai#16856-antibody mixtures were inoculated onto Vero cells (each in 2 wells of a 12-well plate) and incubated for 1 h at 4°C or 37°C. After 1 h of incubation, each well was washed twice with medium. The cells of one well of each were subjected to extract RNA for RT-qPCR detecting for cell-bound CHIKV (B), and the other well was overlaid with medium containing 1% (wt/vol) methylcellulose and 2% FBS and then cultured at 37°C for an additional 2 days for PRNT (C and D). (B) The cell-bound CHIKV RNA copy number determined by RT-qPCR is shown as a percentage of that in Vero cells inoculated with CHIKV-Ctrl IgG mixtures. (C and D) Cells were fixed by 10% formaldehyde and stained with crystal violet. Relative plaque numbers are shown as a percentage of the plaque numbers observed in Vero cells inoculated with CHIKV-Ctrl IgG mixtures at 37°C. Data are the mean ±SEM (n = 6) of three (panel B) and six (panel D) independent experiments. Statistical significance was evaluated by an unpaired two-tailed t test. *, P < 0.05; **, P < 0.01.
Next, we tried to examine the viral membrane-fusion inhibition by CHE19 and CHE22. We found that CHE19 and CHE22 had the ability to inhibit the membrane fusion via CHIKV-E at low pH in the Vero cells. In particular, we found that no multinuclear giant cells were produced in the presence of CHE19 (Fig. 3A). To quantify the effects of inhibition of membrane fusion, we performed a dual split protein (DSP)-based fusion assay (26–28) and detected the viral membrane fusion inhibition activity of CHE19 and CHE22. In this assay, the cell-to-cell fusions by CHIKV-E at low pH were reduced by CHE19 and CHE22 to approximately 1/100th and 1/10th, respectively, of the cell-to-cell fusion without antibody (Fig. 3B).
FIG 3.
Effect of MAbs on CHIKV membrane-fusion induced by low pH. Low pH-induced membrane fusion on the cells transfected with pCAGGS/CHIKV-E Thai#16856 was detected by membrane-fusion inhibition assays as described in Materials and Methods. (A) The number of low pH-induced multinuclear giant cells (arrow) observed in the Vero cells transfected with pCAGGS/CHIKV-E Thai#16856 was decreased in the cells in the presence of CHE19 and CHE22. Of note, no multinuclear giant cells were observed on the cells treated with CHE19. (B) Quantitative detection of cell-to-cell fusion detected by DSP-based fusion assay. The activities of the intact Renilla luciferase, which was constituted in the HEK cells fused via CHIKV-E, were detected at pH 7 and pH 6, as described in Materials and Methods. The fusion activity was calculated as a percentage of the Renilla luciferase activity in the cells without antibody treatment (MAb-) at pH 6. Low pH-induced membrane fusions were inhibited by CHE19 and CHE22 but not CHE29. Data are the mean ± SEM (n = 4) of two independent experiments. Statistical significance was evaluated by an unpaired two-tailed t test. ***, P < 0.001.
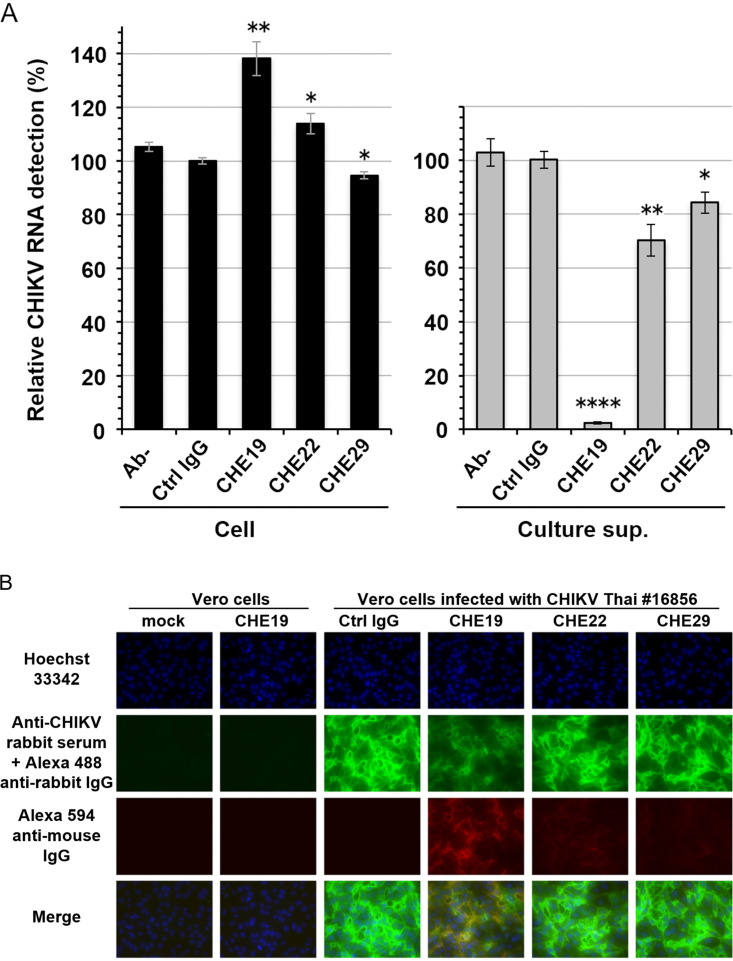
Several studies have reported that anti-CHIKV antibodies inhibited the release of virion from infected cells (23–25). Therefore, we also examined the potency of anti-CHIKV MAbs against the CHIKV virion release. We found that CHE22 and CHE29 partially inhibited virion release, while CHE19 strongly inhibited virion release (Fig. 4A). In contrast to the decreased level of viral RNA detected in the culture supernatant, the level of cell-associated viral RNA was increased in CHE19-treated cells (and slightly increased in CHE22-treated cells). In these CHIKV-producing cells, we could detect the presence of the CHIKV antigen and retention of CHE19 on the cell surface, showing that CHE19—but not the other MAbs—efficiently remained on the cell surface, and cell surface CHIKV antigens were reduced in the CHE19-treated cells (Fig. 4B).
FIG 4.
Effect of MAbs on CHIKV release from infected cells. (A) Vero cells infected with CHIKV Thai#16856 were incubated with anti-CHIKV MAbs for 3 h at 37°C. Then, the cells and the culture supernatants were harvested, and the viral RNAs were extracted and subjected to RT-qPCR as described in Materials and Methods. The amount of viral RNA is shown as a percentage of the amount of viral RNA (viral RNA in the culture supernatant or cell-associated viral RNA) from the Ctrl IgG-treated cells, which was taken as 100%. Data are the mean ± SEM (n = 6) of three independent experiments. Statistical significance was evaluated by an unpaired two-tailed t test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. (B) The CHIKV-infected cells were incubated with anti-CHIKV MAbs for 30 min at 37°C, and then the cells were washed with medium and fixed with PBS containing 4% PFA. Next, the fixed cells were incubated with the anti-CHIKV rabbit serum (1:500). The CHIKV antigen and anti-CHIKV mouse MAbs were detected by Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 594-conjugated goat anti-mouse IgG (1:500) (Invitrogen, Carlsbad, CA), respectively, for 2 h at RT. Cell nuclei were stained with Hoechst 33342 (1 μg/ml). Images were taken using a fluorescence microscope (Nikon Eclipse Ti; Nikon Instruments Inc., Tokyo) (×40 objective).
Structural localization of the antigenic regions recognized by NAb CHE19.
Next, to identify the epitope on the CHIKV-E protein recognized by CHE19, we tried to obtain neutralization escape mutant viruses against CHE19. CHIKV Thai#16856 was continuously passaged in the presence of CHE19. CHE19-resistant escape mutant viruses obtained after the 4th passage were selected. The E protein of this CHE19-resistant escape mutant virus lost its reactivity against CHE19, but the reactivities against other MAbs (CHE15, CHE22, and CHE29) remained (Fig. 5 and Fig. 6D). Sequence analysis of the E protein region revealed that 6 nucleotides were deleted in the E2 region, resulting in the deletion of 2 amino acids (245N and 246A) and the substitution of 1 amino acid (247E→K) (Fig. 6A). Therefore, we gave this CHE19-resistant escape mutant virus the name CHIKV Thai/ES245-7. Interestingly, the same nucleotide deletions have been reported in the isolate BNI236, which escapes serum neutralization in cell culture (GenBank accession no. GU434101.1). The deleted nucleotides of these escape mutants are located in the hairpin loop structure (data not shown), and this structure may facilitate the production of these deletions.
FIG 5.
Reactivity of monoclonal antibodies (MAbs) against the neutralization escape mutant, CHIKV Thai/ES245-7. Reactivity of the NAb CHE19 neutralization escape mutant, CHIKV Thai/ES245-7, was examined by IFA as described in Materials and Methods. Reactivity of the CHE19 against CHIKV Thai/ES245-7 was lost both in virus-infected Vero cells and CHIKV-E-expressing 293T cells.
The protein-protein docking models between the NAb CHE19 Fab fragment region (Fig. 6B) and the E2-E1 heterodimer of three types of CHIKVs, the Thai#16856 strain, Thai/ES245-7, and the Ross strain, were analyzed comprehensively. Table 2 shows the ranking of the Thai#16856 strains E2-E1 heterodimer-CHE19 reaction complex structure and its docking scores for the top 20 docking modes. In MEGADOCK, the larger the docking score, the more stable the docking mode is. In docking model nos. 4 and 16, parental Thai#16856 showed a larger docking score than did Thai/ES245-7 or Ross. These docking scores did not contradict the finding of the IFA that the CHE19 could not bind to the E glycoprotein of Thai/ES245-7 (Fig. 5). Between these two docking models, no. 16 showed that the paratope of the antibody interacts with the antigen (Fig. 6C), but no. 4 did not. In the docking model no. 4, the side face of the antibody heavy chain of the Fab fragment region interacts with the antigen (data not shown), suggesting that this model is an artifact of the algorithm of the software. Therefore, we selected no. 16 as the model of docking of the neutralization antibody CHE19 to the CHIKV-E Thai#16856 strain, as shown in Fig. 6C-1.
TABLE 2.
Docking score valuesa
| Model no. (rank)b | Docking score for strain: |
||
|---|---|---|---|
| Thai#16856 | Thai/ES245-7 (minus the Thai#16856 score) | Ross (minus the Thai#16856 score) | |
| 1 | 9,021.27 | 9,043.75 (22.48) | 9,373.75 (352.48) |
| 2 | 7,677.84 | 7,693.70 (15.86) | 7,638.00 (−39.84) |
| 3 | 7,546.56 | 7,544.96 (−1.60) | 6,662.70 (−883.86) |
| 4 | 7,475.72 | 7,368.76 (−106.96) | 7,124.27 (−351.45) |
| 5 | 7,113.87 | 7,075.79 (−38.08) | 7,436.48 (322.61) |
| 6 | 6,981.13 | 7,053.19 (72.06) | 6,291.41 (−689.72) |
| 7 | 6,910.97 | 6,898.84 (−12.13) | 6,345.33 (−565.64) |
| 8 | 6,902.30 | 6,905.40 (3.10) | 6,830.27 (−72.03) |
| 9 | 6,870.87 | 6,865.84 (−5.03) | 6,728.34 (−142.53) |
| 10 | 6,780.89 | 6,772.06 (−8.83) | 6,566.59 (−214.30) |
| 11 | 6,717.30 | 6,734.56 (17.26) | 6,703.43 (−13.87) |
| 12 | 6,656.75 | 6,661.57 (4.82) | 6,227.20 (−429.55) |
| 13 | 6,634.85 | 6,632.91 (−1.94) | 6,506.06 (−128.79) |
| 14 | 6,630.66 | 6,628.14 (−2.52) | 6,526.84 (−103.82) |
| 15 | 6,617.90 | 6,633.34 (15.44) | 6,277.46 (−340.44) |
| 16c | 6,600.38 | 6,384.01 (−216.37) | 6,229.08 (−371.30) |
| 17 | 6,595.01 | 6,593.61 (−1.40) | 6,736.42 (141.41) |
| 18 | 6,579.37 | 6,521.14 (−58.23) | 5,889.87 (−689.50) |
| 19 | 6,555.58 | 6,555.58 (0.00) | 6,410.71 (−144.87) |
| 20 | 6,507.16 | 6,442.43 (−64.73) | 6,693.30 (186.14) |
Antigen-antibody reaction obtained from comprehensive protein-protein docking simulation. In MEGADOCK software, larger scores indicate more stable docking.
The top 20-ranked docking models between the E2-E1 heterodimer of the Thai#16856 strain and CHE19 MAb are shown.
Docking model no. 16 was adopted in this study.
Figure 6C-2 shows the amino acid residues in the E2-E1 heterodimer complex that are in contact within 4 Å from the heavy atoms of the paratopes of the heavy chain and the light chains of the CHE19 region. These candidates of the epitopes of CHE19 in the E2-E1 heterodimer complex consist of the amino acid residues located in 4 sites of E2, namely, the N-linker region (Lys-10 and Ala-11), domain B region (Leu-210 and Thr-212), the beginning of the second β-ribbon region (Lys-233 and Trp-235), and the center of the second β-ribbon region (Arg-251, Gln-252, and Gly-253), as shown in Fig. 6A. To confirm that these sites contributed to the affinity of CHE19 to CHIKV-E Thai#16856 in the docking model 16, we tried to construct 4 kinds of CHIKV-mutant E (E3-E2 mutant-6k-E1)-expressing plasmid vectors containing alanine substitutions in these regions: Thai/YKATR/AAATA, TTTDK/AAADA, HKKW/AAAA, and RQGK/AQGA (Fig. 6A). The newly constructed CHIKV-E mutants were transfected to the 293T cells, and their expressions were confirmed by IFA using anti-CHIKV rabbit serum and anti-CHIKV mouse MAbs. The expression of one CHIKV-E mutant, CHIKV-E Thai/HKKW/AAAA, was confirmed using anti-CHIKV antibodies (CHE15 and CHE22) but not MAb CHE29 (Fig. 6D-1), suggesting that the amino acid residues His-232, Lys-233, Lys-234, and Trp-235 of E2 may be comprised of the epitope of CHE29. Reactivities of CHE19 to the 3 kinds of CHIKV E2 mutant, Thai/YKATR/AAATA, HKKW/AAAA, and RQGK/AQGA, were lost in IFA, but the reactivity to the mutant CHIKV-E Thai/TTTDK/AAADA remained (Fig. 6D-1).
293T cells transfected with the mutant CHIKV-E-expressing plasmid were used to produce VSVΔG-luci pseudotypes bearing the mutant CHIKV-E. Infectivity of the VSVΔG-luci pseudotypes bearing the mutant CHIKV-E and escape mutant (Thai/ES245-7) CHIKV-E were confirmed, but that of the VSVΔG-luci (CHIKV-E Thai/HKKW/AAAA) was not (Fig. 6E-1). This is probably the reason that the production of the VSVΔG-luci (CHIKV-E Thai/HKKW/AAAA) was very low. That is, the expression of CHIKV-E Thai/HKKW/AAAA in 293T cells transfected with the pCAGGS/CHIKV-E Thai/HKKW/AAAA vector was lower than that in the 293T cells transfected with other vectors (Fig. 6D-2). The VSVΔG-luci pseudotype bearing the mutant CHIKV-E, VSVΔG-luci (CHIKV-E Thai/YKATR/AAATA), showed the same level of resistance against CHE19 neutralization as VSVΔG-luci (CHIKV-E Thai/ES245-7), but VSVΔG-luci (CHIKV-E Thai/RQGK/AQGA) showed less resistance. On the other hand, VSVΔG-luci (CHIKV-E Thai/TTTDK/AAADA) was neutralized by CHE19 (Fig. 6E-2). Taken together, these results led us to conclude that the manner of binding between CHE19 and the E protein is docking mode no. 16, and the possible residues responsible for the binding include Lys-10, Ala-11, Lys-233, Trp-235, Arg-251, Gln-252, and Gly-253, although the roles of Lys-233 and Trp-235 remain controversial for the low expression of CHIKV-E Thai/HKKW/AAAA. The amino acid residues that are in close contact with antibody, Lys-10, Ala-11, Lys-233, Trp-235, Arg-251, Gln-252, and Gly-253, comprised the E3-E2 contact site of the p62 protein, but Leu-210 and Thr-212 did not (16). In addition, the amino acid Glu-247 of parental E2, which was mutated to Lys in the CHIKV Thai/ES245-7, also comprised the E3-E2 contact site (16). These structural analyses showed that the location of bound CHE19 Fab fragment on the E2-E1 heterodimer overlapped the position originally occupied by the E3 glycoprotein (Fig. 6C-3). In addition, the antigen-bound whole CHE19 configuration consisting of a Fab fragment and Fc showed that CHE19 connects the CHIKV spikes existing on two individual virions (Fig. 6C-4).
NAb CHE19 inhibits the degradation of CHIKV-E2.
Our binding model showed that the bound CHE19 Fab fragment overlapped the position occupied by E3, and E3 has been suggested to stabilize the E2-E1 dimer and mediate the pH protection of E1 during virus biogenesis (29, 30). Therefore, to determine whether CHE19 plays the same role as E3, we validated the stability of CHIKV-E of virions treated with antibodies. CHIKV Thai#16856 bound Vero cells at 4°C for 1 h was followed by incubation with anti-CHIKV MAbs at 37°C for 1 h, and their E proteins were detected by Western blotting. As a result, most of E2 but not E1 was lost during the incubation at 37°C. In contrast, the E2 protein of the CHIKV treated with the NAb CHE19 was less diminished after the incubation than the others, suggesting that CHE19 inhibits the degradation of E2 from the virion after binding (Fig. 7).
FIG 7.
Dissociation between CHIKV-E1 and E2 was inhibited by NAb CHE19. Vero cells were incubated with CHIKV Thai#16856 at an approximate MOI of 50 at 4°C for 1 h for binding, and then the unbound virions were washed out by medium, a new medium containing the respective antibody (10 μg/ml) was added, and the cells were further incubated for 1 h at 37°C. Then the cells were lysed and subjected to Western blot analysis. CHIKV-E1 antigens were probed by anti-CHIKV MAb (clone 6A11; EMD Millipore) and detected with HRP-conjugated rat anti-mouse Ig Mouse TrueBlot Ultra (Rockland, Gilbertsville, PA), CHIKV-E2 antigens were probed by anti-CHIKV rabbit serum and detected with HRP-conjugated goat anti-rabbit Ig (Dako, Glostrup, Denmark), and cellular beta-actin as an internal control was probed by anti-beta-actin mouse MAb (Sigma-Aldrich, St. Louis, MO) and detected with HRP-conjugated rat anti-mouse Ig Mouse TrueBlot Ultra, as described in Materials and Methods.
NAb CHE19 mediates CHIKV aggregation.
Because our binding model of whole CHE19 (Fig. 6C-4) showed the connection of the CHIKV spikes existing on two individual virions, we examined the possibility of the formation of viral aggregates via CHE19 (Fig. 8A). We found that approximately 50% of total CHE19-treated virions were precipitated by centrifugation at 15,000 × g for 30 min when 108 to 109 PFU/ml of CHIKV Thai#16856 was treated with CHE19 at 10 μg/ml for 30 min at 37°C, and 80% of the CHE19-CHIKV Thai#16856 complexes in the bottom fraction were trapped by a filter with 0.45-μm pores, suggesting that an aggregated form of CHIKV Thai#16856 consisting of more than 6 to 7 virions was produced by CHE19 treatment (Fig. 8B) since the diameter of native CHIKV is 65 to 70 nm. Moreover, as expected, a much larger number of CHIKV virions than of other MAbs were efficiently retrieved by immunoprecipitation (IP) using CHE19 (Fig. 8C). The increased immunoprecipitation efficiency of CHE19 also supports this antibody-mediated viral aggregation (Fig. 9).
FIG 8.
CHE19 mediated the viral aggregation. (A) Experimental conditions for samples a to f, detecting for antibody-mediated CHIKV aggregation using centrifugation and filtration as described in Materials and Methods. (B) The amount of viral RNA is shown relative to the amount of viral RNA from sample a; CHIKV treated with antibodies for 30 min at 37°C (without centrifugation and filtration) is taken as 1. Data are the mean ± SEM of two or four (Thai#16856 treatment with CHE19) independent experiments. Statistical significance was evaluated by an unpaired two-tailed t test. NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Immunoprecipitation of CHIKV Thai#16856 with mouse antibodies (10 μg/ml), Ctrl IgG, CHE19, CHE22, or CHE29 and rec-protein G-Sepharose 4B conjugate as described in Materials and Methods. Immunoprecipitated samples were subjected to Western blotting. Mouse antibodies were detected by HRP-conjugated goat anti-mouse Ig (Dako), and CHIKV antigens were probed with anti-CHIKV rabbit serum and detected by HRP-conjugated goat anti-rabbit Ig (Dako).
FIG 9.
NAb CHE19-mediated CHIKV aggregation affecting the CHIKV binding and releasing. The nature of the CHE19 mediation of virion aggregation suggests the mechanism of immunoprecipitation enhancement, binding enhancement, and release inhibition.
DISCUSSION
An understanding of the antibody response against viruses is important for the development of potential vaccine candidates and effective prophylactic and therapeutic monoclonal antibodies or low-molecular-weight compounds as antiviral agents. In the current study, although we obtained 3 kinds of mouse monoclonal IgG (CHE19, CHE22, and CHE24) showing significant inhibitory activity (the effect of CHE24 was partial) against a vesicular stomatitis virus (VSV) pseudotype bearing CHIKV-E, only CHE19 had potency for neutralizing native CHIKV. In contrast, the native CHIKV has a spherical shape of 65 to 70 nm diameter with 80 viral envelope spikes forming a T=4 quasi-icosahedral symmetry on its surface, the VSV is a bullet-shaped virion measuring 70 × 200 nm (31), the general retrovirus is a spherical shape of 100-nm diameter, and the details of the arrangement of CHIKV-E spikes on VSV pseudotypes and retrovirus pseudotypes are not known. Therefore, CHE22 and CHE24 might be able to effectively access the epitopes on the pseudotypes but not native CHIKV, and might be able to affect the infectivity of pseudotypes. In addition, it has been reported that the virions of human immunodeficiency virus type 1 had different numbers of envelope spikes per particle, with the wild-type virions having an average of 8 to 10 trimers per virion (32, 33). Therefore, a low concentration of MAbs might be sufficient to neutralize these pseudotypes because only a small number of spike proteins might be expressed on pseudotype virions.
In this study, we elucidated the binding model of anti-CHIKV NAb CHE19, which inhibits membrane fusion mediated by CHIKV-E at low pH and inhibits the release of virion from CHIKV-infected cells. We conclude that the epitope of CHE19 was localized in the viral E2 envelope and the possible residues responsible for binding were located in two separate segments, the N-linker (Lys-10 and Ala-11) and β-ribbon connector (Lys-233, Trp-235, Arg-251, Gln-252, and Gly-253). These amino acid residues constitute an E3-E2 contact site of the p62 protein (16), and the bound Fab fragment of CHE19 on E2 overlapped the position originally occupied by the E3 (Fig. 6C-3). It has been reported that the E3 glycoprotein of Alphavirus acts as a brace, maintaining domain B of E2 in an orientation with respect to domain A of E2 such that it creates a groove accommodating the fusion loop of E1 (16), and it has been thought that the E3 stabilizes the E2-E1 dimer and mediates pH protection of E1 during virus biogenesis (29, 30). Therefore, the decreased expression of CHIKV-E containing the mutation in the E3-E2 contact site of the p62 protein, CHIKV-E Thai/YKATR/AAATA, and CHIKV-E Thai/HKKW/AAAA may be due to the loss of interaction between E3 and E2. Recently, we showed that the E2 proteins of cell-bound CHIKV were readily lost during viral internalization, and the loss of E2 was also observed in the cells that inhibited the endosome acidification by bafilomycin A1 treatment, suggesting that part of the conformational change of the CHIKV spike glycoprotein was induced without acidification before the membrane fusion step (34). In this study, we observed that CHE19 inhibited the degradation of E2 from the virion after binding (Fig. 7). We note that this result was not due to the formation of virion aggregates mediated by CHE19 as described below because CHE19 was added to the virions already bound to the target cell surface (Fig. 7). CHE19 binds an epitope consisting of two separated segments, the N-linker and the second β-ribbon connector; CHE19 may constrain the conformational change as a clamp bridging these segments and may stabilize the E2-E1 dimer, thereby protecting E2 from degradation during viral internalization. Therefore, the remaining E2 may inhibit the membrane fusion following the protrusion of the fusion loop of E1. In this study, we were able to identify the region containing the amino acid residues His-232, Lys-233, Lys-234, and Trp-235 in the second β-ribbon connector as the epitope of CHE29. Although the binding site of CHE29 partially overlapped CHE19, this epitope was linear, and CHE29 could not act as a clamp to constrain the conformational change of E2, which may be why CHE29 could not inhibit the membrane fusion. Our results indicated that antibodies such as CHE19 and/or low-molecular-weight compounds, which bind two segments (the N-linker and second β-ribbon connector segments) with the same E3 protein and bridge these segments as a clamp, would be effective prophylactics and therapeutic reagents against Alphavirus infection. In other words, the E3 binding site would be a promising target for anti-alphavirus agents. On the other hand, an as-yet-unidentified cellular factor related to E2 degradation might also be one of the targets for therapeutic reagents against Alphavirus infection in the future.
Next, the binding model of the CHE19-CHIKV spikes suggested a configuration model in which a CHE19 connects to the CHIKV spikes existing on each different virion (Fig. 6C-4), and this model suggests the formation of an aggregate consisting of CHIKV-antibody complexes that can be represented as {- CHE19 - CHIKV virion - CHE19 - CHIKV virion}n. Viral aggregation reduces the number of virions able to initiate an independent infection event and reduces the overall infectivity (35–37). We estimated that 50% of the total 107 to 108 PFU virions formed aggregated CHIKV-antibody complexes upon treatment with CHE19 at 10 μg/ml for 30 min based on the results in Fig. 8, but no obvious reduction in infectivity was detected by in vitro assay as in the case of 50% cell culture infectious doses (CCID50) (data not shown). In addition, the binding assay (Fig. 2) showed enhanced binding activity of the CHIKV-CHE19 complex. A recent study demonstrated the macropinocytosis-dependent entry of CHIKV in vitro (38). In the in vitro experiment, decreasing the concentration of antibodies around the cell-bound CHIKV-CHE19 complex after the washing may facilitate the internalization of CHIKV by macropinocytosis. However, there is a possibility that the large aggregated CHIKVs would be cleared and that this clearing would be facilitated in vivo by phagocytosis and the subsequent degradation.
Two studies reported that antibodies induced the aggregation of viruses on the cell surface (39, 40), and another study showed that the release of aggregated virions was inhibited by tethering of the individual virions themselves (41). Recent studies have shown that the release inhibitions of CHIKV from infected cells by NAbs were egress inhibition by cell surface CHIKV-E-bound NAbs (23–25), and as a result, the viral buddings were inhibited by NAbs. In the present study, we demonstrated that the inhibition of CHIKV virion release by NAb CHE19 seems to have been caused by tethering-mediated aggregation and these virion-CHE19 complexes on the cell surface may also inhibit the egress of new virions or the egress of the virions may be directly inhibited as described in the previous studies (23–25). The CHE19 seems to stay with the virions on the surface of CHIKV-producing cells, thereby effectively inhibiting the release of the new virions, as shown in Fig. 4B. In addition, the NAb retained on the cell surface might induce the antibody-dependent cellular cytotoxicity (ADCC) in vivo, which could inhibit viral production effectively during primary CHIKV infection.
We found that CHE22 has weak membrane fusion-inhibiting activity and partial release-inhibiting activity. CHE22 may inhibit the conformational change of E1 because the E1 tertiary structure recognized by CHE22 was lost during the 37°C incubation after binding (34), but the details of its inhibitory mechanism could not be determined. We also observed the partial inhibition of viral release of not only CHE22 but also CHE29 in our assay. Therefore, it is possible that some nonneutralizing anti-CHIKV-E MAb may also play a role in the inhibition of viral release from infected cells, and such a role would also affect virus clearance in vivo.
In this study, we showed that CHE19 acts as a NAb for native CHIKV, and we demonstrated the effective viral membrane fusion-inhibiting activity and release-inhibiting activity; however, the neutralizing activity of CHE19 was limited in strain CHIKV Thai#16856. Together, therefore, our results provide additional information about the interaction of anti-CHIKV antibodies with CHIKV antigen. The data reported in this study will help in the future development of prophylactic and therapeutic antibodies.
MATERIALS AND METHODS
Cells and viruses.
A baby hamster kidney fibroblast cell line (BHK-21), African green monkey kidney cells (Vero), and a human astrocytoma cell line (U251MG) were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 50 units/ml penicillin, and 50 μg/ml streptomycin. Two human embryonic kidney cell lines (HEK293 and 293T) were maintained in Dulbecco's modified minimum essential medium (DMEM) supplemented with 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin. CHIKV Thai strain #16856 (42) or Ross strain (34) was inoculated onto BHK cells and propagated. The culture supernatant containing CHIKV was clarified by low-speed centrifugation, and aliquots of the supernatant were stored at −80°C until use. Infectious titers of CHIKV supernatants were determined on Vero cells by plaque formation assay.
To obtain the MAbs against CHIKV, inactivated CHIKV particles as immunogens were prepared as follows. A 120-ml aliquot of the culture supernatant of BHK cells infected with CHIKV Thai#16856 strain was collected and clarified by centrifugation at 10,000 × g for 15 min at 4°C in an SW32 Ti rotor (Beckman Coulter, Brea, CA) to remove the cell debris. Then, the CHIKV culture supernatant was concentrated by ultracentrifugation through a 15% sucrose cushion at 100,000 × g for 2 h at 4°C. The pellet was gently resuspended in 200 μl of TNE buffer (50 mM Tris-KCl [pH 7.6], 140 mM NaCl, 5 mM EDTA [pH 8.0]) and purified by cesium chloride density gradient ultracentrifugation. The band of the viral fraction was collected and then diluted with TNE buffer and centrifuged at 100,000 × g for 2 h at 4°C. The purified viral pellets were resuspended with TNE buffer. The amount of the purified CHIKV was determined by the Bradford method as outlined by the manufacturer (Bio-Rad). Purified CHIKV was inactivated by 0.4% formalin for 1 week at 4°C, and then some of the CHIKV particles were cultured with Vero cells to confirm that they had no cytopathic effects.
Plasmids.
The CHIKV-E glycoprotein (CHIKV-E3-E2-6k-E1) expression plasmids, pCAGGS/CHIKV-E Thai#16856 (pCAGGS/CHIKV-E3-E2-6k-E1 Thai16856) and pCAGGS/CHIKV-E Ross (pCAGGS/CHIKV-E3-E2-6k-E1 Ross), were described previously (34, 42). The E2-6k region and 6k-E1 region genes of CHIKV Thai#16856 were PCR amplified from the pCAGGS/CHIKV-E Thai#16856 plasmid as a template. The restriction enzyme (BamHI or NotI) site and in-frame codons ATG, TAG (CTA), or TAA (TTA) were introduced at the 5′ end of each primer pair for the E2-6k region (BamHI-ATG-CHIKV E2-F, TCCGGATCCATGAGCACCAAGGACAACTTCAATGTC, and NotI-TAG-CHIKV 6k-R, GCTAGCGGCCGCTACGCGCTCACAGTGTGGGCACCGATGCT) and for the 6k-E1 region (BamHI-ATG-CHIKV 6k-F, TTTAGGATCCATGGCCACATACCAAGAGGCTGCGGTATAC, and NotI-TAA-CHIKV E1-R, GCTAGCGGCCGCTTAGTGCCTGCTGAACGACACGCATAG), and cloned into pDisplay mammalian expression vector (Thermo Fisher Scientific, Waltham, MA).
The pCAGGS/CHIKV-E Thai#16856 plasmid containing each mutation (YKATR/AAATA, TTTDK/AAADA, HKKW/AAAA, and RQGK/AQGA) in E2 glycoprotein (Fig. 6A) was assembled from the mutant fragments by using an In-Fusion HD Cloning Plus kit (TaKaRa Shuzo, Shiga, Japan). Primers for each mutant fragment are shown in Table 3 and 4. The constructed mutant E (E3-E2 mutant-6k-E1) expression plasmids were named as follows: pCAGGS/CHIKV-E Thai/YKATR/AAATA, pCAGGS/CHIKV-E Thai/TTTDK/AAADA, pCAGGS/CHIKV-E Thai/HKKW/AAAA, and pCAGGS/CHIKV-E Thai/RQGK/AQGA.
TABLE 3.
Primers for mutant fragments in this study
| Primer | Sequence (5′→3′) |
|---|---|
| E3-SphI-F | GGAGGAAACCCTACGCAT |
| E2-AleI-R | GCGGTGCTCTGCACG |
| E2-XhoI-R | CCCCACGTGACCTCG |
| E2-YKR13/A-R | TGCTGTGGCAGCTGCGACATTGAAGTTGTCCTTGGTG |
| E2-YKR13/A-F | GCAGCTGCCACAGCACCATACTTAGCTCACTGTCCC |
| E2-TTTK215/A-R | TGCGTCAGCTGCAGCTAGTCCTTCATTTGAGCCACC |
| E2-TTTK215/A-F | GCTGCAGCTGACGCAGTGATTAATAACTGCAAGGTTGATCA |
| E2-BstEII HKKW235/A-F | TGCCGCGGTCACCAATGCTGCAGCTGCGCAGTATAACTCCCCTCTGGTC |
| E2-RK254/A-R | AATGGCGCCTTGTGCGTCCCCAAGTTCAGCATTACG |
| E2-RK254/A-F | GCACAAGGCGCCATTCACATCCCGTTTCCG |
TABLE 4.
Fragments of the pCAGGS/CHIKV-E mutant plasmid
| pCAGGS/CHIKV-E mutation | Primer(s) used for |
||
|---|---|---|---|
| Mutant fragment 1 | Mutant fragment 2a ,b ,d | pCAGGS/CHIKV-E vector fragmentc | |
| YKATR/AAATA | E3-SphI-F, E2-YKR13/A-Ra ,b ,d | E2-YKR13/A-F, E2-XhoI-R | pCAGGS/CHIKV-E ΔSphI-AleI |
| TTTDK/AAADA | E3-SphI-F, E2-TTTK215/A-Ra ,b ,d | E2-TTTK215/A-F, E2-XhoI-R | pCAGGS/CHIKV-E ΔSphI-XhoI |
| HKKW/AAAA | E2-BstEII HKKW235/A-F, E2-XhoI-Ra,d | pCAGGS/CHIKV-E ΔBstEII-XhoI | |
| RQGK/AQGA | E3-SphI-F, E2-RK254/A-Ra ,b ,d | E2-RK254/A-F, E2-XhoI-R | pCAGGS/CHIKV-E ΔSphI-XhoI |
Amplified by PCR.
Mutant fragments 1 and 2 were assembled by using an In-Fusion HD cloning plus kit.
The pCAGGS/CHIKV-E Thai#16856 plasmid was digested with the respective restriction enzyme.
The mutant fragment was digested with the respective restriction enzyme and then ligated with the respective pCAGGS/CHIKV-E vector fragment.
The dual-functional split-reporter protein vectors used for the cell membrane fusion assay, Rluc8155-156DSP1-7 and Rluc8155-156DSP8-11, were kindly provided by Z. Matsuda (Research Center for Asian Infectious Diseases, The University of Tokyo, Tokyo, Japan) (26).
Virus titration assays, PRNT, and CHIKV pseudovirus neutralization assay.
Vero cells were seeded at 2 × 105 cells/well in 12-well plates at 1 to 2 days before inoculation. Serially diluted virus stocks were inoculated onto the cells and incubated at 37°C for 60 min, and then EMEM containing 1% (wt/vol) methylcellulose and 2% FBS was overlaid. Two days after inoculation, cells were fixed with 4% (wt/vol) paraformaldehyde (PFA) in phosphate-buffered saline (PBS), and plaques were visualized by staining with crystal violet. For PRNT, diluted CHIKV was incubated with serially diluted antibodies for 30 min at 37°C, the CHIKV (approximately 100 PFU)-antibody mixtures were inoculated onto the Vero cell culture, and then the plaques were detected as described above.
The preparation of replication-deficient luciferase-expressing vesicular stomatitis virus (VSV) pseudotypes bearing the CHIKV envelope glycoproteins, VSVΔG-luci (CHIKV-E), was described previously (34, 42). The target Vero cells were plated into a 96-well multiplate (104 cells/well) at 1 to 2 days before inoculation, and then the VSVΔG-luci (CHIKV-E) pseudotypes were inoculated. After 20 to 24 h of incubation, the infectivity of the VSVΔG-luci (CHIKV-E) was measured using a Steady-Glo luciferase assay system (Promega, Madison, WI) and a Centro LB 960 microplate luminometer (Berthold Technologies, Bad Wildbad, Germany) according to the manufacturers’ instructions.
The retrovirus vector pMX-luci was constructed by introducing the luciferase gene into the retrovirus vector, pMX (43). Three kinds of plasmid, pMX-luci, pMD-gagpol (44), and pCAGGS (CHIKV-E Thai#16856, CHIKV-E Ross, or VSVG), were cotransfected into 293T cells by using X-tremeGENE9 transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol. Each pseudotype virion contained in extracellular supernatants was harvested at approximately 48 h after transfection and clarified by low-speed centrifugation, and aliquots of the supernatants were stored at −80°C until use. The target U251MG cells were plated into a 96-well multiplate (104 cells/well) at 1 to 2 days before inoculation, and then the pMX-luci (CHIKV-E Thai#16856), pMX-luci (CHIKV-E Ross), and pMX-luci (VSVG) retrovirus pseudotypes were inoculated. After incubation for 2 days, the infectivity of the retrovirus pseudotypes was determined by measuring luciferase activities as described previously.
Generation of mouse MAbs against CHIKV.
Six-week-old female BALB/c mice were immunized subcutaneously with 5 to 10 μg of formalin-inactivated CHIKV Thai#16856 strain in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO). Two weeks after the first injection, the mice were boosted with formalin-inactivated CHIKV in complete Freund’s adjuvant, and then, 10 days after the second injection, the mice were boosted with formalin-inactivated CHIKV without adjuvant. Three days after the final boost-immunization, the spleen cells were collected and fused to P3X63Ag8 cells using polyethylene glycol. Fused cells were cultured in RPMI 1640 (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FBS, 2% hypoxanthine-aminopterin-thymidine (HAT) supplement (Gibco, Grand Island, NY), and 2% hybridoma fusion and cloning supplement (Roche, Penzberg, Germany). After the HAT selection, anti-CHIKV antibody-producing hybridomas were selected by ELISA using the CHIKV particles as an antigen and cloned by limiting dilution.
To generate ascitic fluid, BALB/c mice were intraperitoneally injected with the hybridoma clones. Ascitic fluid was purified by affinity chromatography using Ab-Capcher (ProteNova, Kagawa, Japan) according to the manufacturer’s protocol. Isotyping of MAbs was determined by using a mouse monoclonal antibody isotyping test kit (Bio-Rad Laboratories, Richmond, CA).
CHIKV RNA extraction and detection.
Extraction of CHIKV RNA from the cell culture supernatant was performed using a QIAamp viral RNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Intracellular viral RNA was extracted from the infected cells by using an RNeasy minikit (Qiagen, Valencia, CA). To detect the viral RNA, reverse transcription-quantitative PCR (RT-qPCR) was performed by using a QuantiFast SYBR green RT-PCR kit (Qiagen) and the CHIKV-specific primer pair (CHIKV 9756-9777-F, AGCTACCGTCCCTTTCCTGCTTA, and CHIKV 9843-9866-R, CAAAACAAAGGTTGCTGCTCGTT, or CHIKV 2411-F, AGACCAGTCGACGTGTTGTAC, and CHIKV 2552-R, GCATCATATTGAAGAAGCCGCA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression detected by the primer pair GAPDH-F, GCACCGTCAAGGCTGAGAAC, and GAPDH-R, ATGGTGGTGAAGACGCCAGT, was used as a reference. Quantification of viral RNA was performed using cycle threshold values and the ΔΔCT method.
Indirect immunofluorescence assay.
CHIKV-infected Vero cells or CHIKV-E antigen expression vector-transfected 293T cells were cultured for 24 to 48 h and then fixed with methanol or PBS containing 4% PFA for 20 min. The PFA-fixed cells were permeabilized with 1% Triton X-100 for 5 min and then blocked with 1% bovine serum albumin (BSA) in PBS at room temperature (RT) for 1 h. The cells were incubated with anti-CHIKV antibodies (5 to 10 μg/ml) at 37°C for 30 min, followed by Alexa Fluor 488-conjugated goat anti-mouse IgG or Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:500) (Invitrogen, Carlsbad, CA) for 30 min at 37°C. After washing with PBS, cells were observed under a fluorescence microscope (Nikon Eclipse Ti; Nikon Instruments Inc., Tokyo). Anti-CHIKV rabbit serum was obtained from a rabbit immunized with formalin-inactivated CHIKV Thai#16856 virion and used as a positive control to detect the E glycoprotein of the CHIKV Thai#16856 strain and Ross strain. This anti-CHIKV rabbit serum reacted mainly with CHIKV-E2 and slightly with CHIKV-E1 (Fig. 1A) (34).
Western blotting.
Cell-bound virus or cell-expressed viral antigens lysed by NP-40 cell lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40) were centrifuged at 5,000 rpm for 5 min, and the cytoplasmic fractions were collected and mixed with sodium dodecyl sulfate (SDS) sample buffer. Viral antigen lysates were separated on an SDS-polyacrylamide gel (SDS-PAGE) under nonreducing or reducing conditions (with 2-mercaptoethanol) and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membrane was treated overnight at 4°C with a blocking buffer, 5% skim milk (SM) in PBS containing 0.05% Tween 20 (SM-PBS-T), and incubated for 2 h at RT with anti-CHIKV rabbit serum (1:500 to 1:1,000), anti-CHIKV monoclonal antibody clones CHE15 to CHE35 (Table 1) (5 μg/ml in blocking buffer) or clone 6A11 (1:100) (EMD Millipore), and anti-beta-actin mouse monoclonal antibody (1:500) (Sigma-Aldrich, St. Louis, MO). The membranes were then washed with PBS containing 0.1% Tween 20 (PBS-T) and incubated for 1 h at RT with horseradish peroxidase (HRP)-conjugated goat anti-rabbit Ig (1:2,000 to 1:10,000) (Dako, Glostrup, Denmark), HRP-conjugated goat anti-mouse Ig (1:1,500 to 1:10,000) (Dako) or HRP-conjugated rat anti-mouse IgG Mouse TrueBlot Ultra (1:1,000) (Rockland, Gilbertsville, PA). After 4 to 5 washes with PBS-T, the glycoprotein bands were detected with a Western Lightning ECL Pro substrate (PerkinElmer Life Sciences, Boston, MA).
ELISA and immunoprecipitation.
In order to validate the binding potential of the MAbs to the cell-free CHIKV, the obtained MAbs in this study were subjected to enzyme-linked immunosorbent assays (ELISAs). Nunc MaxiSorp 96-well plates were incubated with purified CHIKV Thai#16856 in PBS (0.1 μg/ml, 100 μl/well) at 4°C overnight, and then the wells were blocked with SM-PBS-T for 1 h at 37°C. The wells were washed 4 times with PBS-T and then incubated with the 2-fold serially diluted antibody sample in SM-PBS-T for 1 h at 37°C. Unbound antibodies were removed by washing 4 times with PBS-T, and then the plates were incubated with HRP-conjugated goat anti-mouse Ig (Dako) at a dilution of 1:16,000 for 1 h at 37°C. After washing an additional 4 times, SureBlue TMB Microwell peroxidase substrate (KPL, Gaithersburg, MD) (100 μl/well) was added, and the plates were incubated for 30 min at RT in the dark. Reaction stop solution (0.6 N sulfuric acid) was then added into each well (100 μl/well), and the optical density (OD) values at 450 nm and 620 nm were measured with a Multiskan FC microplate photometer (Thermo Scientific, Rockford, IL). The titer was determined by the last dilution degree of the MAbs (1 mg/ml) that gave a positive result.
In addition, the binding potential of the MAbs to the cell-free CHIKV was also examined by immunoprecipitation. We treated 250 μl of CHIKV Thai#16856 stock (108 to 109 PFU/ml) with mouse antibodies (10 μg/ml); mouse IgG-isotype control ab37355 (Ctrl IgG) (Abcam Cambridge, Cambridge UK); CHE19, CHE22 or CHE29; and rec-protein G-Sepharose 4B conjugate (Invitrogen) with 1.85% suspension at 4°C for 1 h. Then, the protein G-Sepharose was pelleted, washed 2 times with PBS, resuspended with 2× SDS sample buffer, and subjected to Western blot analysis under a nonreducing condition.
Binding assays.
Vero cells were seeded in 12-well plates at 2 × 105 cells per well at 1 to 2 days before infection. CHIKV Thai#16856 was incubated with the respective MAbs at a final concentration of 10 μg/ml for 30 min at 37°C and then inoculated onto the Vero cells and incubated for 1 h at 4°C or 37°C. Then, the cells were washed with medium twice and overlaid with EMEM containing 1% (wt/vol) methylcellulose and 2% FBS to observe the infectivity. Cellular RNAs containing the cell-bound viral RNAs were extracted from the cells incubated at 4°C using an RNeasy minikit. Isolated RNA was analyzed by RT-qPCR using a QuantiFast SYBR green RT-PCR kit and the primer pair CHIKV 9756-9777-F, AGCTACCGTCCCTTTCCTGCTTA, and CHIKV 9843-9866-R, CAAAACAAAGGTTGCTGCTCGTT, as described above.
Membrane fusion inhibition assays.
Vero cells were transfected with pCAGGS/CHIKV-E Thai#16856. One day after transfection, cells were incubated with or without MAbs (5 μg/ml) at 37°C for 30 min, and then their culture medium was replaced with a low-pH (pH 6.0) medium (EMEM supplemented with 2% FBS, 0.014% bicarbonate and 10 mM morpholineethanesulfonic acid [MES]) containing each of the MAbs and incubated at 37°C for 2.5 h. Then the cells were fixed with 4% PFA in PBS, and multinuclear giant cells (fused cells) were visualized by crystal violet staining.
To quantify the effects of MAbs on cell-to-cell fusion by CHIKV-E, a DSP-based fusion assay was performed (26–28). HEK293 cells were seeded at 104 cells/50 μl/well in 96-well, flat, clear-bottom black polystyrene microplates (Corning Life Sciences, Acton, MA). One day later, the cells were cotransfected with Rluc8155-156DSP1-7 and pCAGGS/CHIKV-E Thai#16856. HEK293 cells were seeded at 4 × 105 cell/2 ml/well into the Nunc UpCell 6-well multiplate (Thermo Fisher Scientific-Nunc A/S, Roskilde, Denmark) and then transfected with Rluc8155-156DSP8-11 the next day. After incubation for 48 h at 37°C, the culture medium in the 96-well plate was replaced with fresh medium, while the culture medium in the UpCell 6-well plate was replaced with fresh medium containing 60 μM of the membrane-permeable substrate EnduRen (Promega) and incubated for 2 h at 37°C. Then, the HEK293 cells preloaded with EnduRen were detached from the UpCell plate by leaving them at RT. HEK293 cells in 96-well plates were overlaid with the detached HEK293 cells (∼105 cell/50 μl) with or without each antibody (final concentration, 10 μg/ml), Ctrl IgG, CHE19, CHE22, and CHE29. The cells were then incubated at 37°C for 1 h, and the culture medium was replaced with the neutral-pH (pH 7.0) or low-pH (pH 6.0) medium (EMEM supplemented with 2% FBS, 0.014% bicarbonate, 10 mM MES, and 10 μg/ml of the respective MAbs). The cells were incubated for a final 2 h at 37°C, and the Renilla luciferase activity was measured by a Centro LB 960 microplate luminometer.
Release inhibition assays.
Vero cells were seeded in 12-well plates at 2 × 105 cells per well 1 to 2 days before infection. Cells were infected with CHIKV Thai#16856 at a multiplicity of infection (MOI) of 5 for 12 h at 37°C. After washing 2 times, medium containing MAbs (2.5 μg/ml) was added, and the cells were incubated for 3 h at 37°C. Then, the culture supernatants were harvested, and the viral RNA was extracted using a QIAamp viral RNA minikit following the manufacturer’s protocol, while the cellular RNA was extracted using an RNeasy minikit. Isolated RNA was analyzed by RT-qPCR using the primer pair CHIKV 9756–9777-F, AGCTACCGTCCCTTTCCTGCTTA, and CHIKV 9843–9866-R, CAAAACAAAGGTTGCTGCTCGTT, as described above.
Escape mutant selection.
CHIKV Thai#16856 stock (1.25 × 109 PFU) was incubated with a NAb, CHE19 at 0.625 μg/ml, for 1 h at 37°C. We added 50 μl of virus-NAb mixture onto the Vero cells culture in a 96-well multiplate (plated 1 day before inoculation; 104 cells/50 μl/well) and incubated for 24 h. Then, the culture supernatant in each well was collected and incubated with 1.25 μg/ml of CHE19 for 1 h at 37°C. Then, 50 μl of the mixture was added onto a new Vero cells culture in a 96-well multiplate and incubated for 24 h. In the 3rd to 4th passages, the virus-NAb mixtures (CHE19 concentration, 1.25 μg/ml) were added to the Vero cells culture and incubated for 48 h. After the 4th passage, neutralizing escape mutant viruses were selected. Viral RNA was extracted from the supernatant using a QIAamp viral RNA minikit and reverse transcribed into cDNA with a random hexamer primer using a Superscript III reverse transcriptase kit (Invitrogen). The CHIKV structural gene region was amplified by PCR using the sense primer CHIKV 7480–7504-F, TTTGTACGGCGGTCCTAAATAGGTAC, and antisense primer CHIKV11359-11332-R, GTGTGTCTCTTAGGGGACACATATACCT, and sequenced.
Identification of hybridoma-derived monoclonal antibody variable-region sequences.
Total RNA was extracted from the CHE19-producing hybridoma cell line using an RNeasy minikit. First-strand cDNA was synthesized using a SuperScript III reverse transcriptase kit (Invitrogen) with mouse IgG gene-specific primers. A heavy-chain primer, RACEMOG2b, AGGACAGGGGTTGATTGTTGA, which primes in the hinge, and a kappa light-chain primer, MOCKFOR, CTCATTCCTGTTGAAGCTCTTGACAAT, which primes at the 3′ end of the constant region of the light chain, were used as gene-specific reverse transcription primers for the synthesis of the CHE19 variable-region cDNA (45). Then, the cDNA of the Fab fragment region of the heavy-chain gene and light-chain gene were subjected to PCR amplification using the primers described in the previous studies (45, 46). PCR-amplified fragments were purified, cloned into pGEM-T easy vector (Promega), and sequenced.
Homology modeling of MAb CHE19 and protein-protein docking analysis of CHIKV-E glycoprotein and CHE19.
Using the amino acid sequences of the H and the L chains of the Fab fragment region of CHE19 (Fig. 6B), a BLAST search was performed on all amino acid sequences registered in the PDB database. We selected the X-ray crystal structure of the mouse monoclonal antibody mAb2177 (PDB ID 5TL5; resolution, 1.8 Å) as the most appropriate three-dimensional (3D) structure for the template in the homology modeling of CHE19. To determine the whole structure of CHE19, the X-ray crystal structure of the IgG2a mouse monoclonal antibody (PDB ID 1IGT) was used as the 3D template for the whole configuration of the antibody.
To build the 3D structural models of the E2-E1 heterodimer of the CHIKV Thai#16856 strain and its escape mutant (Thai/ES245-7) and the CHIKV Ross strain, the 3D structure of the mature CHIKV envelope complex (PDB ID 3N42) (16) was used as the template.
Three-dimensional structural models were constructed by Homology Modeling Professional for HyperChem software (HMHC) (47, 48). Structural optimization calculation in the homology modeling process was performed with the AMBER99 force field without cutoff, using permittivity of 1.0, 14 scale factor of 0.833, 14 van der Waals (VDW) scale factor of 0.5, the minimization algorithm Polak-Ribière method, and a root mean square (RMS) gradient of 1.0 kcal/Å·mol as the convergence conditions. In addition, histidine, arginine, and lysine were treated as cations, and aspartic acid and glutamic acid were treated as anions. Conserved disulfide bonds were similarly created with disulfide bonds, and the N and C termini of each domain were treated as zwitterions. Sugars that are covalently bonded to amino acid residues in each of the E1 and E2 domains were omitted from the model. The E2 domain of CHIKV Thai/ES245-7 has one deletion part (Fig. 6A), and its modeling was performed by structural optimization calculation of the two residues before and after (4 residues in total). For the side-chain rotamer of amino acids that differ from those in the template, AMBER99 force field single-point calculation was comprehensively performed using the rotamer database installed in HMHC, and the most stable energy rotamer of each side chain was determined. The initial structure prepared as described above was optimized for all atoms except the main-chain heavy atoms, and the entire structure of the complex was optimized to obtain the final structure.
Protein-protein docking analyses between CHE19 Fab fragment and each of the three kinds of CHIKV (Thai#16856, Thai/ES245-7, and Ross) E2-E1 were performed using MEGADOCK 4.0.2 (49). The obtained docking models of the CHE19 Fab fragment-CHIKV E2-E1 complex were examined to confirm that the paratope of the antibody interacts with the antigen by PyMOL software (Python-based molecular visualization system; Delano Scientific, San Francisco, CA).
Structural data of the docking models were visualized using PyMOL software (https://pymol.org/2/) and UCSF Chimera software (https://www.cgl.ucsf.edu/chimera) (50).
Detection of antibody-mediated viral aggregation.
CHIKV stock (107 to 108 PFU) was incubated with the mouse antibody, Ctrl IgG, CHE19, or CHE29 at 10 μg/ml for 30 min at 37°C, and then 1,900 μl of PBS was added to 100 μl of CHIKV-antibody complex, and the mixture was divided into 2 tubes (1,000 μl/Eppendorf tube). One of these tubes was incubated for 30 min at 4°C, the other was centrifuged at 15,000 × g for 30 min at 4°C in a TMS-21 swing-out rotor (Tomy Kogyo, Tokyo), and then the upper 600-μl fraction and bottom 100-μl fraction were collected. The bottom 100-μl fraction was diluted with 400 μl of PBS (5-fold dilution). Each 400 μl of these samples of CHIKV-antibody complex was filtrated using a Minisart syringe filter with pores of size 0.45 μm (Sartorius Stedim Biotech GmbH, Goettingen, Germany) (Fig. 8A). Then 50 μl of each filtrated and nonfiltrated sample was subjected to viral RNA extraction using a QIAamp viral RNA minikit, and the viral RNA was quantified by RT-qPCR using the CHIKV-specific primer pair CHIKV 2411-F, AGACCAGTCGACGTGTTGTAC, and CHIKV 2552-R, GCATCATATTGAAGAAGCCGCA, as described above.
ACKNOWLEDGMENTS
We thank Z. Matsuda (Research Center for Asian Infectious Diseases, The University of Tokyo) for providing the dual-functional split-reporter protein vectors, Rluc8155-156DSP1-7 and Rluc8155-156DSP8-11.
This work was supported by JSPS KAKENHI (grant numbers JP16K09934 and JP19K08929), by the Japan Initiative for Global Research Network on Infectious Diseases (JGRID) (grant number 19fm0108003h0005), and by the Japan Agency for Medical Research and Development (AMED) (grant numbers JP19fk0108036 and JP18fk0108036h0002).
REFERENCES
- 1.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz O, Albert ML. 2010. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol 8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 3.Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses–an overview. Nat Rev Rheumatol 8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 4.Morrison TE. 2014. Reemergence of chikungunya virus. J Virol 88:11644–11647. doi: 10.1128/JVI.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. 2006. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis 12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver SC. 2014. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl Trop Dis 8:e2921. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 8.Laurent P, Le Roux K, Grivard P, Bertil G, Naze F, Picard M, Staikowsky F, Barau G, Schuffenecker I, Michault A. 2007. Development of a sensitive real-time reverse transcriptase PCR assay with an internal control to detect and quantify chikungunya virus. Clin Chem 53:1408–1414. doi: 10.1373/clinchem.2007.086595. [DOI] [PubMed] [Google Scholar]
- 9.Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Renia L, Leo YS, Ng LF. 2012. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis 205:1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kam YW, Lee WW, Simarmata D, Harjanto S, Teng TS, Tolou H, Chow A, Lin RT, Leo YS, Renia L, Ng LF. 2012. Longitudinal analysis of the human antibody response to chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol 86:13005–13015. doi: 10.1128/JVI.01780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitatpattana N, Kanjanopas K, Yoksan S, Satimai W, Vongba N, Langdatsuwan S, Nakgoi K, Ratchakum S, Wauquier N, Souris M, Auewarakul P, Gonzalez JP. 2014. Long-term persistence of Chikungunya virus neutralizing antibodies in human populations of North Eastern Thailand. Virol J 11:183. doi: 10.1186/1743-422X-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung JY, Ng MM, Chu JJ. 2011. Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol 2011:249640. doi: 10.1155/2011/249640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng RH, Kuhn RJ, Olson NH, Rossmann MG, Choi HK, Smith TJ, Baker TS. 1995. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80:621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jose J, Snyder JE, Kuhn RJ. 2009. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol 4:837–856. doi: 10.2217/fmb.09.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simizu B, Yamamoto K, Hashimoto K, Ogata T. 1984. Structural proteins of Chikungunya virus. J Virol 51:254–258. doi: 10.1128/JVI.51.1.254-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, Rey FA. 2010. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468:709–712. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- 17.Modis Y. 2013. Class II fusion proteins. Adv Exp Med Biol 790:150–166. doi: 10.1007/978-1-4614-7651-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin J, Simmons G. 2019. Antiviral functions of monoclonal antibodies against chikungunya virus. Viruses 11:305. doi: 10.3390/v11040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SA, Silva LA, Fox JM, Flyak AI, Kose N, Sapparapu G, Khomandiak S, Ashbrook AW, Kahle KM, Fong RH, Swayne S, Doranz BJ, McGee CE, Heise MT, Pal P, Brien JD, Austin SK, Diamond MS, Dermody TS, Crowe JE Jr. 2015. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against chikungunya virus. Cell Host Microbe 18:86–95. doi: 10.1016/j.chom.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Xiang Y, Akahata W, Holdaway H, Pal P, Zhang X, Diamond MS, Nabel GJ, Rossmann MG. 2013. Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. Elife 2:e00435. doi: 10.7554/eLife.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R, Kim AS, Fox JM, Nair S, Basore K, Klimstra WB, Rimkunas R, Fong RH, Lin H, Poddar S, Crowe JE Jr, Doranz BJ, Fremont DH, Diamond MS. 2018. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 557:570–574. doi: 10.1038/s41586-018-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. 2013. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog 9:e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox JM, Long F, Edeling MA, Lin H, van Duijl-Richter MKS, Fong RH, Kahle KM, Smit JM, Jin J, Simmons G, Doranz BJ, Crowe JE Jr, Fremont DH, Rossmann MG, Diamond MS. 2015. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 163:1095–1107. doi: 10.1016/j.cell.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, Galaz-Montoya JG, Sherman MB, Sun SY, Goldsmith CS, O'Toole ET, Ackerman L, Carlson LA, Weaver SC, Chiu W, Simmons G. 2018. Neutralizing antibodies inhibit Chikungunya virus budding at the plasma membrane. Cell Host Microbe 24:417–428. doi: 10.1016/j.chom.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin J, Liss NM, Chen DH, Liao M, Fox JM, Shimak RM, Fong RH, Chafets D, Bakkour S, Keating S, Fomin ME, Muench MO, Sherman MB, Doranz BJ, Diamond MS, Simmons G. 2015. Neutralizing monoclonal antibodies block chikungunya virus entry and release by targeting an epitope critical to viral pathogenesis. Cell Rep 13:2553–2564. doi: 10.1016/j.celrep.2015.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa H, Meng F, Kondo N, Iwamoto A, Matsuda Z. 2012. Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP. Protein Eng Des Sel 25:813–820. doi: 10.1093/protein/gzs051. [DOI] [PubMed] [Google Scholar]
- 27.Kondo N, Miyauchi K, Meng F, Iwamoto A, Matsuda Z. 2010. Conformational changes of the HIV-1 envelope protein during membrane fusion are inhibited by the replacement of its membrane-spanning domain. J Biol Chem 285:14681–14688. doi: 10.1074/jbc.M109.067090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Li X, Nakane S, Liu S, Ishikawa H, Iwamoto A, Matsuda Z. 2014. Co-expression of foreign proteins tethered to HIV-1 envelope glycoprotein on the cell surface by introducing an intervening second membrane-spanning domain. PLoS One 9:e96790. doi: 10.1371/journal.pone.0096790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjoberg M, Lindqvist B, Garoff H. 2011. Activation of the alphavirus spike protein is suppressed by bound E3. J Virol 85:5644–5650. doi: 10.1128/JVI.00130-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchime O, Fields W, Kielian M. 2013. The role of E3 in pH protection during alphavirus assembly and exit. J Virol 87:10255–10262. doi: 10.1128/JVI.01507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cureton DK, Massol RH, Whelan SP, Kirchhausen T. 2010. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog 6:e1001127. doi: 10.1371/journal.ppat.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu P, Chertova E, Bess J Jr, Lifson JD, Arthur LO, Liu J, Taylor KA, Roux KH. 2003. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A 100:15812–15817. doi: 10.1073/pnas.2634931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu P, Liu J, Bess J Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 34.Tumkosit U, Maeda Y, Kishishita N, Siripanyaphinyo U, Omori H, Chetanachan P, Sittisaman P, Jityam C, Priengprom T, Mizushima H, Wongjaroen P, Mekada E, Tatsumi M, Takeda N, Tanaka A. 2019. The use of green fluorescent protein-tagged virus-like particles as a tracer in the early phase of chikungunya infection. Virus Res 272:197732. doi: 10.1016/j.virusres.2019.197732. [DOI] [PubMed] [Google Scholar]
- 35.Brioen P, Dekegel D, Boeye A. 1983. Neutralization of poliovirus by antibody-mediated polymerization. Virology 127:463–468. doi: 10.1016/0042-6822(83)90159-9. [DOI] [PubMed] [Google Scholar]
- 36.Thomas AA, Brioen P, Boeye A. 1985. A monoclonal antibody that neutralizes poliovirus by cross-linking virions. J Virol 54:7–13. doi: 10.1128/JVI.54.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas AA, Vrijsen R, Boeye A. 1986. Relationship between poliovirus neutralization and aggregation. J Virol 59:479–485. doi: 10.1128/JVI.59.2.479-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CHR, Mohamed Hussain K, Chu JJH. 2019. Macropinocytosis dependent entry of Chikungunya virus into human muscle cells. PLoS Negl Trop Dis 13:e0007610. doi: 10.1371/journal.pntd.0007610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajihara M, Marzi A, Nakayama E, Noda T, Kuroda M, Manzoor R, Matsuno K, Feldmann H, Yoshida R, Kawaoka Y, Takada A. 2012. Inhibition of Marburg virus budding by nonneutralizing antibodies to the envelope glycoprotein. J Virol 86:13467–13474. doi: 10.1128/JVI.01896-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderplasschen A, Hollinshead M, Smith GL. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J Gen Virol 78:2041–2048. doi: 10.1099/0022-1317-78-8-2041. [DOI] [PubMed] [Google Scholar]
- 41.Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A. 2014. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One 9:e85582. doi: 10.1371/journal.pone.0085582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka A, Tumkosit U, Nakamura S, Motooka D, Kishishita N, Priengprom T, Sa-Ngasang A, Kinoshita T, Takeda N, Maeda Y. 2017. Genome-wide screening uncovers the significance of N-sulfation of heparan sulfate as a host cell factor for chikungunya virus infection. J Virol 91:e00432-17. doi: 10.1128/JVI.00432-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawakami Y, Miura T, Bissonnette R, Hata D, Khan WN, Kitamura T, Maeda-Yamamoto M, Hartman SE, Yao L, Alt FW, Kawakami T. 1997. Bruton's tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc Natl Acad Sci U S A 94:3938–3942. doi: 10.1073/pnas.94.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ory DS, Neugeboren BA, Mulligan RC. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A 93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradbury A. 2001. Cloning hybridoma cDNA by RACE In Kontermann R, Dübel S (ed), Antibody engineering. Springer, Berlin, Heidelberg, Germany. [Google Scholar]
- 46.Morrison SL. 2002. Cloning, expression, and modification of antibody V regions. Curr Protoc Immunol 47:2.12.1–2.12.17. doi: 10.1002/0471142735.im0212s47. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji M. 2007. Development of the structure-based drug design systems, HMHC and DSHC. Mol Sc 1:NP004. [Google Scholar]
- 48.Tsuji M, Shudo K, Kagechika H. 2015. Docking simulations suggest that all-trans retinoic acid could bind to retinoid X receptors. J Comput Aided Mol Des 29:975–988. doi: 10.1007/s10822-015-9869-9. [DOI] [PubMed] [Google Scholar]
- 49.Ohue M, Shimoda T, Suzuki S, Matsuzaki Y, Ishida T, Akiyama Y. 2014. MEGADOCK 4.0: an ultra-high-performance protein-protein docking software for heterogeneous supercomputers. Bioinformatics 30:3281–3283. doi: 10.1093/bioinformatics/btu532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 51.Yap ML, Klose T, Urakami A, Hasan SS, Akahata W, Rossmann MG. 2017. Structural studies of Chikungunya virus maturation. Proc Natl Acad Sci U S A 114:13703–13707. doi: 10.1073/pnas.1713166114. [DOI] [PMC free article] [PubMed] [Google Scholar]