Abstract

Phospho-N-acetylmuramoyl-pentapeptide translocase (MraYAA) from Aquifex aeolicus is the binding target for the nucleotide antibiotic muraymycin D2 (MD2). MraYAA in the presence of the MD2 ligand has been crystallized and released, while the interactions between the ligand and active-site residues remain less quantitatively and qualitatively defined. We characterized theoretically the key residues involved in noncovalent interactions with MD2 in the MraYAA active site. We applied the quantum theory of atoms in molecules and natural bond orbital analyses based on the density functional theory method on the solved crystal structure of MraY with the MD2 to quantitatively estimate the intermolecular interactions. The obtained results revealed the presence of multiple hydrogen bonds in the investigated active site with strength ranging from van der Waals to covalent limits. Lys70, Asp193, Gly194, Asp196, Gly264, Ala321, Gln305, and His325 are key active-site residues interacting with MD2. Conventional and unconventional hydrogen bonds in addition with charge–dipole and dipole–dipole interactions contribute significantly to stabilize the MD2 binding to the MraYAA active site. It was also found that water molecules inside the active site have substantial effects on its structure stability through hydrogen-bonding interactions with MD2 and the interacting residues.
1. Introduction
Bacterial peptidoglycan biosynthesis is vital for bacterial survival, in which phospho-N-acetylmuramoyl-pentapeptide translocase (MraY) is one of the key players.1 MraY transfers phospho-N-acetylmuramoyl-pentapeptide (P-MurNAc-pp) from UDP-MurNAc-pentapeptide (UM5A) to the carrier lipid undecaprenyl phosphate (C55P) to yielding uridine monophosphate and undecaprenyl-pyrophosphoryl-MurNAc-pp, also named lipid I, during an Mg2+-dependent group-transfer reaction.2,3 MraY is one of the members of the polyisoprenyl-phosphate N-acetylglucosaminosugar-1-phosphate-transferase superfamily4 and is a promising drug target for natural-product nucleoside antibiotics, such as muraymycins (Figure 1).5,6 Muraymycins are one subclass of nucleoside antibiotics which act as competitive inhibitors for the natural substrate UM5A of MraY.7,8
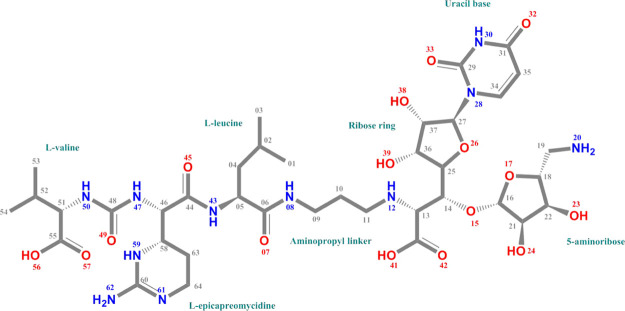
Figure 1.
Chemical structure and atomic numbering scheme for MD2. MD2 is constituted by a nucleoside portion (uracil base, ribose ring, and 5-aminoribose) and a peptide portion (l-leucine, l-epicapreomycidine, and l-valine) connected together by an aminopropyl linker.
Structurally, muraymycins contain a common core of a 5′-C-glycyluridine (GlyU) nucleoside attached with a 5″-amino-5″-deoxyribose (ADR) moiety via a standard O-glycosidic bond (ADR–GlyU disaccharide). The disaccharide is extended to a linear oligopeptide speared with an aminopropyl linker.9 Variations within this peptide are a basis for dividing muraymycin congeners into four different series (A–D).7,9 If the peptide of muraymycins is l-leucine and there is a hydroxyl group in the 2″-position of the ADR moiety, muraymycin will be classified as muraymycin D2 (MD2) (Figure 1).9
MraY genes expressed in the nonpathogenic Gram-negative bacterium Aquifex aeolicus (MraYAA) and then purified MraYAA in complex with MD2 was crystallized.6 The static structure showed that MD2 binding to MraYAA leads to significantly conformational changes of the active site (a nucleoside-binding pocket and a peptide-binding site with constitutes).6 Noncovalent intermolecular interactions, consisting of van der Waals, electrostatic, and hydrogen-bonding (H-bonding) interactions,13,14 play central roles in the conformational stabilization, where MD2 outcompetes UM5A for binding through Coulombic interactions.10−12
Computational methods are needed for accurate description of the intermolecular interactions in a protein–ligand complex. Nowadays, the physical nature of the intermolecular interactions can be provided by experimental and theoretical methods with high accuracy. Zhao and Truhlar investigated theoretically the reliability of the M06 family of density functional theory (DFT) to study hydrogen-bonded systems.15−17 The quantum theory of atoms in molecules (QTAIM) is an extremely helpful theoretic method to characterize, classify, and analyze intra- and intermolecular interactions based on the electron density distribution function, ρ(r).14,18−20 Natural bond orbital (NBO) analysis is another reliable approach to meticulously describe the hydrogen bond (H-bond) nature.21−24 Our purpose of this study is to characterize the intermolecular interaction nature between MD2 and residues of the MraYAA–MD2 active site using the QTAIM and NBO analyses in the DFT treatment. The information gained from the study can be very useful for the design of new generations of nucleoside antibiotics.
2. Computational Details
The crystal structure of MraYAA–MD2 solved at a 2.95 Å resolution was released in 2016 and is available in the Protein Data Bank (PDB) with accession code 5CKR.6 As per the crystallization conditions of this protein complex, the MraYAA–MD2 crystals were grown in a water-based crystallization solution containing Na cacodylate (0.1 M, pH 5.6), MgCl2 (0.050 M), and PEG400 (40% w/v) under special thermodynamic conditions and under conditions governed by the physicochemical laws. Before forming the crystal lattice, all protein molecules dissolved in this crystallization solution experienced the solvent effects of total water molecules existing in protein solution drop and then formed a regular three-dimensional array in the unit cells through the noncovalent intermolecular interactions with its aqueous environment. It is worth mentioning that the water molecules around this protein exerted their solvent effects on the individual protein atoms not only in the solution state (protein drop) but also in the crystal solid state.
Although Matthews coefficient25−27 estimated the MraYAA–MD2 crystal solvent content to be about 69.22%, the electron density map obtained from X-ray diffraction could detect only nine constant water molecules of total water molecules existing in the MraYAA–MD2 crystal structure in this context.6 Therefore, to get a realistic picture of solvation, we added possible missing water molecules to this crystal structure employing the VMD program.28 A periodic rectangular box containing TIP3P water molecules29 was created in a distance of 12 Å from the surface of this box to the closest atom of the MraYAA–MD2 crystal structure using the CHARMM36 force field.30 Because molecular dynamics (MD) simulation of this membrane protein–ligand complex is computationally very expensive and difficult, only water molecules of this protein–water system were simulated in the NPT ensemble at T = 310 K and P = 1 atm with a time step of 1 fs in 200 ps to achieve the classical equilibrium simulation utilizing NAMD software.31,32 During the MD simulation, all atoms of the MraYAA–MD2 complex were kept frozen.
After the initial simulation of this protein–water system, its calculations were continued at the quantum mechanic level. The crystal structure of MraYAA in complex with MD2 shows that its active site at a distance of 6 Å away from the substrate consists of amino acid residues Lys70, Thr75, Met76, Asn190, Leu191, Asp193, Gly194, Leu195, Asp196, Gly197, Asn255, Phe262, Met263, Gly264, Asp265, Ser268, Val302, Gln305, Ile306, Arg316, Phe318, Lys319, Arg320, Ala321, Pro322, His325, and Glu334.6 To gain physical insights into the nature of the intermolecular interactions between MD2 and the MraYAA–MD2 active site, a structural model was designed for this active site based on its specified residues in addition with MD2. The first structural model (model I) was thereby constructed, as shown in Figure 2. As already mentioned above, nine water molecules were detected in this crystal structure, in which four of them are located adjacent to residues Leu191, Asp193, Gly197, Leu198, Ala199, Ile200, Phe251, Asn255, Met263, His324, and His325 as well as MD2. The second structural model (model II) was planned to identify the nature of the intermolecular interactions among the water molecules, these residues, and MD2 (Figure 3).
Figure 2.

Active site residues of MraYAA–MD2 at a distance of 6 Å away from the substrate (structural model I) surrounding the uracil base (a), the 5-aminoribose (b), and the peptide portion of MD2 (c).
Figure 3.

Water molecules are enclosed by MD2 and active-site residues (structural model II).
Because the structural coordinates of oxygen, carbon, nitrogen, and sulfur atoms were determined directly from X-ray diffraction with the solvent effects present in this crystal structure, while the coordinates of its hydrogen atoms (with only one electron) in the electron density map were not not resolved, hydrogen atoms of each structural model underwent a partly geometry optimization using a hybrid meta–GGA density functional (M06-2X)15,16 with the standard 6-31G* basis set. During the geometry optimization, the coordinates of the whole nonhydrogen atoms were constrained to their initial coordinates because optimization of the hydrogens should be done without any change on the positions of the nonhydrogen atoms. Each optimized structural model also experienced the vibrational frequency calculations by the same method. Because the IR spectra of these optimized structures lacked any imaginary frequency, each structure achieved a true minimum on its potential energy surface.
The QTAIM analysis provides useful insights into the physical nature of intra- and intermolecular interactions in terms of the topological properties of the electron density distribution function, ρ(r), the bond path (BP), and the bond critical point (BCP).18,19 The magnitude and sign of the electron density at a BCP, ρBCP(rcp), its Laplacian at this point, ∇2ρBCP(rcp), and the electronic energy density, HBCP, contain the useful information regarding the nature and strength of the interaction between two atoms as shared (covalent bond) or closed-shell (such as van der Waals, ionic, H-bonding, H–H bonding, and so forth).33,34
The NBO analysis is another helpful approach to recognize the H-bonding interaction based on the charge-transfer (CT) interaction between the lone electron pair orbital of an electron donor (proton acceptor), nB, and the valence antibonding orbital of an electron acceptor (proton donor), σA–H*. The energy of the CT interaction, nB → σA–H, known as the second-order stabilization energy, E(2), is evaluated by the second-order perturbation theory according to the equation below21,22,35
| 1 |
⟨nB|F|σA–H*⟩ is the Fock matrix element, while εσA–H – ε(nB) is the energy difference between the donor and the acceptor orbitals. We performed the QTAIM and NBO analyses on each optimized structural model at the M06-2X/6-31G** level.
All DFT calculations were performed by means of Gaussian 09 software,36 and the QTAIM results were calculated employing AIM 2000 package.37
3. Results and Discussion
MD2 is a peptidyl nucleoside antibiotic. Its peptide includes l-leucine, l-valine, and l-epicapreomycidine, arising from the oxidative cyclization of the l-arginine side chain, and its nucleoside consists of two components of GlyU and 5-aminoribose. The peptide portion is linked to the nucleoside portion by an aminopropyl spacer (Figure 1).7 The first structural model shows that the backbones and the side chains of residues Lys70, Gly194, Leu195, Asp196, Gly197, Asn255, phe262, and Glu334 are located around the uridine moiety of the MD2 nucleoside and residues Thr75, Met76, Asn190, Asp193, Met263, Gly264, Asp265, and Ser268 are placed in the neighboring site of its 5-aminoribose (Figure 2a,b). On the other hand, the backbones and the side chains of residues Lue191, Val302, Gl305, Ile306, Arg316, Phe318, Lys319, Arg320, Ala321, Pro322, and His325 are arranged around the peptide portion of MD2 (Figure 2c). Because the positions of all atoms of the MraYAA–MD2 complex remained fixed during the MD simulation, no changes were observed in the location of the active site residues as well as MD2 in the simulated protein–ligand complex. In the following sections, the nature of the intermolecular interactions between MD2 and these residues is quantitated by the QTAIM and NBO analyses.
3.1. Characterization of H-Bonding Interactions of MD2 with the Neighboring Residues by the QTAIM Analysis
All data related to the topological parameters of the QTAIM analysis of the first structural model are collected in Table 1. According to Bader’s topological theory,18,34 if ρBCP is lower than 0.10 a.u. and its ∇2ρBCP is a low positive value, the closed-shell interactions will appear in the BCP between two atoms. For the appearance of the H-bonding interaction, Koch and Popelier proposed that electron density at the BCP between the hydrogen atom and proton acceptor (H···B BCP) must be in the range of 0.002–0.040 a.u. and its Laplacian at this point must lie within the 0.020–0.150 a.u. range.38 As can be seen from Table 1, the ρ and ∇2ρ values at the various hydrogen BCPs (HBCPs) exceed the proposed range by Koch and Popelier, indicating the presence of very strong H-bonds within this active site.
Table 1. Structural and Topological Parameters of ρBCP(r) in the Various H···B BCPs Detected in Mur–Residue Pairs Were Computed at the M06-2X/6-31G** Levela.
| proton donor | proton acceptor | H-bond | d | ∠ | ρBCP | ∇2ρBCP | HBCP | |EHB| |
|---|---|---|---|---|---|---|---|---|
| Mur | Thr75 | O24–H24···Oγ1 | 2.03 | 124.67 | 0.0220 | 0.0733 | –0.0204 | 25.84 |
| Mur | Asn190 | C10–H102···O | 2.67 | 131.61 | 0.0066 | 0.0235 | –0.0034 | 5.53 |
| Mur | Asn190 | C19–H192···Oδ1 | 2.78 | 119.48 | 0.0049 | 0.0208 | –0.0024 | 4.38 |
| Mur | Asn190 | N20–H201···Oδ1 | 2.29 | 136.40 | 0.0117 | 0.0432 | –0.0087 | 12.33 |
| Mur | Asp193 | C11–H111···O | 2.58 | 151.00 | 0.0075 | 0.0251 | –0.0042 | 6.46 |
| Mur | Asp193 | C35–H35···Oδ1 | 2.69 | 115.71 | 0.0067 | 0.0259 | –0.0030 | 5.47 |
| Mur | Asp193 | C22–H22···Oδ2 | 2.32 | 130.01 | 0.0148 | 0.0455 | –0.0111 | 14.67 |
| Mur | Asp193 | N20–H202···Oδ2 | 2.37 | 121.00 | 0.0127 | 0.0444 | –0.0092 | 12.94 |
| Mur | Asp193 | C19–H191···Oδ2 | 2.30 | 119.09 | 0.0169 | 0.0550 | –0.0127 | 17.13 |
| Mur | Asp196 | N30–H30···Oδ1 | 1.38 | 157.28 | 0.1033 | 0.1891 | –0.1794 | 177.72 |
| Mur | Phe262 | C37–H37···Cε2 | 2.43 | 154.11 | 0.0129 | 0.0397 | –0.0064 | 9.90 |
| Mur | Ser268 | N20–H201···Oγ | 2.21 | 123.86 | 0.0155 | 0.0557 | –0.0127 | 17.23 |
| Mur | Ala321 | N47–H47···O | 1.45 | 124.07 | 0.0821 | 0.3493 | –0.1132 | 137.31 |
| Mur | Ala321 | N50–H50···O | 2.22 | 118.87 | 0.0175 | 0.0606 | –0.0145 | 19.29 |
| Mur | His325 | C64–H641···Nδ1 | 2.59 | 147.73 | 0.0095 | 0.0273 | –0.0051 | 7.46 |
| Lys70 | Mur | Nζ–Hζ2···O33 | 1.76 | 156.86 | 0.0361 | 0.1168 | –0.0274 | 36.72 |
| Gly194 | Mur | Cα–Hα2···O26 | 1.84 | 138.81 | 0.0371 | 0.1356 | –0.0340 | 44.60 |
| Gly194 | Mur | Cα–Hα2···N28 | 2.18 | 129.54 | 0.0209 | 0.0836 | –0.0152 | 22.43 |
| Asp196 | Mur | Cα–Hα···O32 | 2.50 | 163.44 | 0.0113 | 0.0322 | –0.0078 | 10.33 |
| Gly194 | Mur | Cα–Hα3···O42 | 2.66 | 133.49 | 0.0068 | 0.0245 | –0.0034 | 5.69 |
| Asn255 | Mur | Nδ2–Hδ22···O32 | 2.34 | 165.74 | 0.0098 | 0.0322 | –0.0072 | 9.84 |
| Phe262 | Mur | Cβ–Hβ3···O23 | 2.32 | 164.71 | 0.0136 | 0.0406 | –0.0105 | 13.67 |
| Phe262 | Mur | Cβ–Hβ3···O24 | 2.57 | 122.42 | 0.0092 | 0.0330 | –0.0056 | 8.53 |
| Gly264 | Mur | N–H···O23 | 1.56 | 127.07 | 0.0621 | 0.2736 | –0.0712 | 92.26 |
| Gln305 | Mur | Nε2–Hε21···O56 | 1.59 | 117.42 | 0.0596 | 0.3020 | –0.0657 | 90.50 |
| Ala321 | Mur | N–H···H50–N50 | 1.91 | 0.0127 | 0.0500 | –0.0079 | 12.39 | |
| Pro322 | Mur | Cδ−Hδ2···O45 | 2.14 | 129.96 | 0.0193 | 0.0661 | –0.0150 | 20.35 |
H-bond length (d) in angstrom (Å), H-bond angle (∠) in degree (°). Whole ρBCP, ∇2ρBCP, and HBCP parameters are in atomic units (a.u.). |EHB| is in kJ/mol.
The QTAIM analysis reveals the presence of two BPs beginning from the O32 and H30 nuclei of the uracil base of uridine of MD2 nucleoside and terminating at the Hα and Oδ1 nuclei of Asp196, respectively. Its O32 is also joined to Hδ22 in Asn255 by Hδ22···O32 BCP and Hζ2···O33 BCP lies between Lys70 and the uracil base. Their BCP properties signify the formation of the conventional N30–H30···Oδ1 and the unconventional Cα–Hα···O32 H-bonds in Mur–Asp196 pair and two conventional H-bonds of Nδ2–Hδ22···O32 and Nζ–Hζ2···O33 in Mur–Asn255 and Mur–Lys70 pairs, respectively (Figure 4 and Table 1). To estimate the H-bond strength, there is an appropriate energetic criterion called a H-bonding interaction energy (EHB) that is correlated with the electronic potential energy density at the BCP, VBCP, according to Espinosa’s expression39,40
| 2 |
Figure 4.

Residues Lys70, Asp196, and Asn255 participate in H-bonding interactions with the uracil base. Gly194 interacts with the uracil base and the ribose ring. The aromatic ring of Phe262 is involved in the π···π stacking interaction with the uracil ring.
The moduli of the H-bond energies, |EHB|, of the different Mur–residue pairs are also presented in Table 1.
Conventionally, if the extent of H-bond energies exceeds 15 kcal/mol (62.76 kJ/mol), these bonds are considered as strong H-bonding interactions, whereas the strength of moderate (normal) H-bonds is in the range of 4–15 kcal/mol (16.74–62.76 kJ/mol). The strength of weak H-bonds varies between 1 and 4 kcal/mol (4.18 and 16.74 kJ/mol).13,34 It is worth mentioning that the H-bond strength also depends on its length and angle.34 Geometrically, an ideal H-bond forms when the angle among three atoms is 180° and the H-bond length becomes shortened.13
As shown in Table 1, the greatest |EHB| value (177.72 kJ/mol) corresponds to the N30–H30···Oδ1 H-bond with a length of 1.38 Å and an angle of 157.28°. Additionally, the values of ρBCP (0.1033 a.u.) and ∇2ρBCP (0.1891 a.u.) at the H30···Oδ1 BCP are highest in comparison with those of other bonds. Indeed, it is the strongest H-bond that MD2 forms within the active site of MraYAA. It is reasonable to suggest that Asp196 is one of the crucial residues of MraYAA–MD2 and it plays an essential role in MD2 binding to its active site through this very strong H-bond. This finding supports the result of Asp196 mutation.6 Nζ–Hζ2···O33 H-bond with an |EHB| of 36.72 kJ/mol and a length of 1.76 Å is a moderate H-bond, whereas the other two are treated as weak H-bonds.
Figure 4 shows that Hα2 of Gly194 is simultaneously connected to O26 of the ribose ring of uridine and N28 of its uracil base by two HBCPs. Moreover, Hα3···O42 BCP exists between Gly194 and the aminopropyl linker of MD2 (Figure 5). Cα–Hα2···O26 with an |EHB| of 44.60 kJ/mol and a length of 1.84 Å is the strongest H-bonding interaction of the C–H···B kind, B can be C, O, or N, where MD2 forms inside this active site because its ρBCP, ∇2ρBCP, and |EHB| values are largest in comparison with the other unconventional H-bonds (Table 1). Although these types of H-bonds are of less concern in biological systems, their influences on the conservation and the stabilization of MD2 binding to the MraYAA active site are irrefutable.
Figure 5.

Gly194 interacts with the aminopropyl linker. The 5-aminoribose participates in conventional and unconventional H-bonds with residues Thr75, Asn190, Asp193, Phe262, Gly264, and Ser268.
As depicted in Figure 2a, the uracil base ring is placed in a position of face-to-face (stacked) conformation relative to the aromatic ring of Phe262. The QTAIM analysis explicates that this particular geometrical arrangement leads to the creation of a ring critical point (RCP) in the interior of each ring and a cage critical point (CCP) in the enclosed space between these two rings. The RCPs and CCP denote the occurrence of the electrostatic interaction of the π···π stacking type between the π electron clouds of these rings (Figure 4). The modulus of the sum of π···π stacking interaction energies between these rings is 65.07 kJ/mol. Thus, the face-to-face π-stacking interaction of the aromatic ring of Phe262 with the uracil base of MD2, tunicamycin, capuramycin, 3′-hydroxymureidomycin A, and carbacaprazamycin influences significantly their stacking inside this active site.41 In addition to these three critical points, H37···Cε2 BCP exists between the ribose ring and the aromatic ring of Phe262. Consequently, they are involved in a weak unconventional C37–H37···Cε2 H-bond together (Table 1). On the other hand, the side chain of Phe262 is extended in the position adjacent to the 5-aminoribose of the MD2 nucleoside (Figure 2a). Two HBCPs of Hβ3···O23 and Hβ3···O24 are identified between them (Figure 5). Because the |EHB| (13.67 kJ/mol) of Cβ–Hβ3···O23 is greater than that (8.53 kJ/mol) of Cβ– Hβ3···O24, the former H-bond is stronger than the latter H-bond.
As displayed in Figure 5, the nitrogen atom of the 5-aminoribose donates its two hydrogens to Oδ1, Oδ2, and Oγ of Asn190, Asp193, and Ser268, respectively. It also formed H24···Oγ1 BCP with Thr75 and H···O23 BCP with Gly264. Therefore, five intermolecular HBCPs are created among these nuclei. Because the |EHB| (25.84 kJ/mol) of O24–H24···Oγ1 in the Mur–Thr75 pair is larger than that (17.23 kJ/mol) of N20–H201···Oγ in the Mur–Ser268 pair, the former H-bond is stronger than the latter H-bond. The |EHB| (12.33 kJ/mol) of N20–H201···Oδ1 in the Mur–Asn190 pair is almost equal to that (12.94 kJ/mol) of N20–H202···Oδ2 in Mur–Asp193; therefore, the strengths of these two H-bonds are nearly identical. The values of ρBCP (0.0621 a.u.), ∇2ρBCP (0.2736 a.u.), and |EHB| (92.26 kJ/mol) of H···O23 BCP demonstrate that N–H···O23 in the Mur–Gly264 pair is a strong H-bonding interaction, and it has a great importance in MD2 binding to the MraYAA active site. As a consequence, Gly264 is not only another key residue in this active site but also a crucial amino acid in complex with capuramycin.41
As indicated in Table 1, 5-aminoribose formed three BCPs with the side chains of Asn190 and Asp193. One of them exists in the path connecting H192 and Oδ1 of Asn190 and the other two are in the path joining Oδ2 of Asp193 with H191 and H22. Furthermore, two BPs begin from carbonyl oxygens of Asn190 and Asp193 and terminate at H102 and H111 of the aminopropyl linker, respectively, and H35···Oδ1 BCP is between the uracil base and Asp193 (Figure 4). Of these, C19–H191···Oδ2 with an |EHB| of 17.13 kJ/mol is a moderate H-bond, whereas the other unconventional H-bonds are weak interactions.
The QTAIM analysis reveals that the nitrogen backbones of l-epicapreomycidine (N47) and l-valine (N50) of the peptide portion of MD2 are proton donors to carbonyl oxygen of Ala321 (Figure 6). The values of ρBCP and ∇2ρBCP in H47···O and H50···O BCPs demonstrate that the N47–H47···O H-bond is much stronger than the N50–H50···O H-bond (Table 1). Indeed, N47–H47···O with a length of 1.45 Å, an angle of 124.07°, and an |EHB| of 137.31 kJ/mol is the second strongest H-bond of MD2 in this active site. In addition to these H-bonds, the H···H50 BP is also formed between them. Interaction derived from linking two hydrogen atoms is characteristic of a specific type of van der Waals interaction called hydrogen–hydrogen bonding (H–H bonding) interaction.18 Furthermore, the O45 and H641 nuclei of l-epicapreomycidine form two BPs of Hδ2···O45 and H641···Nδ1 with Pro322 and His325, respectively. The |EHB| (20.35 kJ/mol) of Cδ−Hδ2···O45 is higher than that (7.46 kJ/mol) of C64–H641···Nδ1. Thus, l-epicapreomycidine has stronger H-bonding interaction with Pro322 than His325. The QTAIM analysis recognizes a BCP between O56 of l-valine and Hε21 of Gln305. The values of ρBCP (0.0596 a.u.), ∇2ρBCP (0.3020 a.u.), and |EHB| (90.50 kJ/mol) of this BCP are indicative of the formation of the strong Nε2–Hε21···O56 H-bond in the Mur–Gln305 pair (Figure 6). Although mutation results did not prove the importance of the role of Gln305 in MD2 binding to the MraYAA active site,6 our computational findings demonstrate that the residues accompanying Ala321 are other two crucial residues that play key roles in the binding stability of its peptide portion to this active site through very strong H-bonds.
Figure 6.

Residues Gln305, Ala321, Pro322, and His325 provide the peptide-portion binding to the MraYAA active site through unconventional and conventional H-bonds.
As already mentioned above, the small positive value of ∇2ρBCP implies the presence of a closed-shell interaction in that BCP. Rozas et al.42 reported that the positive ∇2ρBCP and the negative HBCP values reflect a partly covalent character of a pertinent H-bond. Our results indicate that the HBCP values of the various HBCPs become negative (Table 1). Thus, the fundamental natures of these interactions must be considered as the intermediate between covalent and electrostatic characters. Based on the ρHBCP values, we suggest that the strong H-bonds of MD2 have to be included that are basically covalent and partially electrostatic in nature, whereas its weak H-bonds have a basically electrostatic character. The moderate H-bonds of MD2 are a mixture of both covalent and electrostatic contributions where weakening of the H-bond is accompanied by the decrease of the covalent part.
Generally, the covalent character of the putative H-bonds in the different Mur–residue pairs in terms of the |EHB| magnitude decreases as follows: N30–H30···Oδ1 (Mur–Asp196) > N47–H47···O (Mur–Ala321) > N–H···O23 (Mur–Gly264) > Nε2–Hε21···O56 (Mur–Gln305) > Cα–Hα2···O26 (Mur–Gly194) > Nζ–Hζ2···O33 (Mur–Lys70) > O24–H24···Oγ1 (Mur–Thr75) > Cα–Hα2···N28 (Mur–Gly194) > Cδ−Hδ2···O45 (Mur–Pro322) > N50–H50···O (Mur–Ala321) > N20–H201···Oγ (Mur–Ser268) ≅ C19–H191···Oδ2 (Mur–Asp193) > C22–H22···Oδ2 (Mur–Asp193) > Cβ–Hβ3···O23 (Mur–Phe262) > N20–H202···Oδ2 (Mur–Asp193) ≅ N20–H201···Oδ1 (Mur–Asn190) > Cα–Hα···O32 (Mur–Asp196) > C37–H37···Cε2 (Mur–Phe262) ≅ Nδ2–Hδ22···O32 (Mur–Asn255) > Cβ–Hβ3···O24 (Mur–Phe262) > C64–H641···Nδ1 (Mur–His325) > C11–H111···O (Mur–Asp193 > Cα–Hα3···O42 (Mur–Gly194) ≅ C10–H102···O (Mur–Asn190) ≅ C35–H35···Oδ1 (Mur–Asp193) > C19–H192···Oδ1 (Mur–Asn190).
It is worthy to mention that the whole H-bonds that are detected inside the MraYAA–MD2 active site by the QTAIM analysis are in agreement with the predicted H-bonds for this active site by experimental findings.6 Based on our results, the active site residues formed most of the H-bonds with uracil and 5-amino ribose; therefore, the nucleoside portion of MD2 is involved in much more H-bonding interactions than its peptide portion.
3.2. Exploration of the Intermolecular Orbital Partners in the H-Bonds of Mur–Residue Pairs by the NBO Analysis
The H-bonding interactions of MD2 with partner residues are grouped into three classes including strong, moderate, and weak H-bonds using the QTAIM analysis. In this section, the strengths of the local orbitals of partner atoms at these H-bonds will be evaluated by the second-order orbital energies obtained from the NBO analysis. The calculated NBO results of the CT interactions in the corresponding Mur–residue pairs are tabulated in Table 2.
Table 2. NBO Results of Local Orbitals Involved in CT Interactions in Mur–Residue Pairs Were Computed at the M06-2X/6-31G** Level.
| electron donor | electron acceptor | CT | E(2) (kJ/mol) | ||
|---|---|---|---|---|---|
| Thr75 | Mur | nOγ1 → σO24–H24* | 21.88 | 0.0085 | |
| Asn190 | Mur | nOδ1 → σN20–H201* | 10.75 | 0.0029 | |
| Asp193 | Mur | nO → σC11–H111* | 2.64 | 0.0008 | |
| Asp193 | Mur | nOδ2 → σC22–H22* | 8.12 | 0.0047 | |
| Asp193 | Mur | nOδ2 → σN20–H202* | 5.86 | 0.0017 | |
| Asp193 | Mur | nOδ2 → σC19–H191* | 3.26 | 0.0015 | |
| Asp196 | Mur | nOδ1 → σN30–H30* | 349.62 | 0.1219 | |
| Ser268 | Mur | nOγ → σN20–H201* | 13.47 | 0.0040 | |
| Ala321 | Mur | nO → σN47–H47* | 112.63 | 0.0300 | |
| Ala321 | Mur | nO → σN50–H50* | 7.99 | 0.0036 | |
| His325 | Mur | nNδ1 → σC64–H641* | 1.84 | 0.0007 | |
| Mur | Lys70 | nO33 → σNζ–Hζ2* | 65.56 | 0.0226 | |
| Mur | Gly194 | nO26 → σCα–Hα2* | 24.81 | 0.0094 | |
| Mur | Asp196 | nO32 → σCα–Hα* | 8.28 | 0.0038 | |
| Mur | Gly194 | nO42 → σCα–Hα3* | 2.76 | 0.0012 | |
| Mur | Asn255 | nO32 → σNδ2–Hδ22* | 8.95 | 0.0026 | |
| Mur | Phe262 | nO23 → σCβ–Hβ3* | 9.25 | 0.0037 | |
| Mur | Phe262 | nO24 → σCβ–Hβ3* | 2.13 | 0.0009 | |
| Mur | Gly264 | nO23 → σN–H* | 106.06 | 0.0312 | |
| Mur | Gln305 | nO56 → σNε2–Hε21* | 73.51 | 0.0194 | |
| Mur | Pro322 | nO45 → σCδ−Hδ2* | 4.77 | 0.0014 |
The NBO analysis indicates that electrons of lone pair Oδ2 of Asp193 are simultaneously transferred to the antibonding orbitals of σC19–H191*, σC22–H22, and σN20–H202* in 5-aminoribose and a CT also occurred from its nO into σC11–H111 in the aminopropyl linker. It is clearly evident that the electron-transfer process decreases the electronic occupancy of an electron donor and increases the occupation number of an electron acceptor. The amount of CT, qCT, between the interacting orbitals is approximately proportional to the associated stabilization energy, eq 1, and is calculated as22
| 3 |
As shown in Table 2, the qCT and E(2) values of these CT interactions are very small; therefore, the cited orbitals weakly interact with each other. Moreover, the CT interactions of nO42 → σCα–Hα3*, nO32 → σCα–Hα, nO32 → σNδ2–Hδ22*, nO23 → σCβ–Hβ3, and nNδ1 → σC64–H641* in Mur–Gly194, Mur–Asp196, Mur–Asn255, Mur–Phe262, and Mur–His325 pairs, respectively, have very low qCT and E(2) values. Thus, similar to the QTAIM results, the NBO analysis also emphasizes that they are weak interactions.
The NBO analysis reveals that σN20–H201* accepts separately the qCT values of 0.0040 and 0.0029 e from the lone pair orbitals of Oγ in Ser268 and Oδ1 in Asn190, respectively. E(2) (13.47 kJ/mol) of the nOγ → σN20–H201 interaction is greater than that (10.75 kJ/mol) of the nOδ1 → σN20–H201* interaction. Thus, 5-aminoribose has a stronger CT interaction with Ser268 than Asn190. As seen in Table 2, the Nζ–Hζ2···O33 H-bond is the result of a CT (0.0226 e) from the nO33 of the uracil base into σNζ–Hζ2 of Lys70.
The great E(2) value (65.56 kJ/mol) of the nO33 → σNζ–Hζ2* interaction is indicative of the existence of a relatively strong attractive interaction between these orbitals. O24–H24···Oγ1 in the Mur–Thr75 pair arises from the CT interaction of nOγ1 → σO24–H24 with E(2) of 21.88 kJ/mol. The nO26 of the ribose ring overlaps with σCα–Hα2* of Gly194, resulting in the formation of Cα-Hα2···O26 in the Mur–Gly194 pair. The nO26 → σCα–Hα2 interaction is the strongest CT interaction of the C–H···B kind because its E(2) value (24.81 kJ/mol) is highest in comparison with the other unconventional interactions (Table 2). This finding is in agreement with the |EHB| prediction for this interaction.
As mentioned earlier (Section 3.1), MD2 forms the strongest H-bond with Asp196 in this active site. The N30–H30···Oδ1 H-bond occurs as a consequence of electron transfer from the nOδ1 of Asp196 into σN30–H30* of the uracil base. The nOδ1 → σN30–H30 CT interaction has the largest values of qCT (0.1219 e) and E(2) (349.62 kJ/mol) compared to other interactions. The nO →σN47–H47*, nO23 → σN–H, and nO56 → σNε2–Hε21* interactions in Mur–Ala321, Mur–Gly264, and Mur–Gln305 pairs, respectively, also have very large qCT and E(2) values (Table 2). Consequently, there are very strong attractive interactions between the interacting local orbitals in each cited Mur–residue pair, resulting in the formation of these four very strong H-bonds in this active site. Accordingly, we suggest that the CT interactions of residues Asp196, Gly264, Gln305, and Ala321 with MD2 play roles to some extent in its stabilization and conservation inside this binding site.
3.3. Characterization of Other Noncovalent Interactions in Mur–Residue Pairs
The QTAIM and NBO analyses established only some residues of the first structural model that are capable of forming H-bonds with MD2. As known, a single proton (positive charge) becomes localized on the Nζ atom of Lys and the imidazole ring of His is partially protonated. There is a single delocalized electron (negative charge) between Oδ1 and Oδ2 atoms of Asp. Therefore, Mur–Asp193 and Mur–Asp196 are negatively charged pairs, whereas Mur–Lys70 and Mur–His325 are positively charged pairs. Hence, the electrostatic interactions of charge–dipole and dipole–dipole types are expected to take place in these charged Mur–residue pairs. Besides, Mur–Thr70, Mur–Asn190, Mur–Asn255, Mur–Ser268, and Mur–Gln305 are polar Mur–residue pairs because of the presence of side-chain hydroxyl groups in Thr and Ser as well as the side-chain carboxamide group in Gln and Asn. Consequently, the dipole–dipole interaction makes an important contribution to the intermolecular interactions of each polar pair. The nonpolar side chains of residues Gly194, Phe262, Gly264, Ala321, and Pro322 can be induced by the dipole moment of MD2, resulting in a dipole–induced-dipole interaction contribution to intermolecular interactions of each Mur–residue pair.
Our results indicated the other residues of the first structural model incapable of forming H-bonds with MD2. However, it is expected that their side chains can interact with different parts of MD2 through other types of noncovalent interactions. Because Arg possesses a guanidine group, the Mur–Arg320 pair is a positively charged pair. Hence, residues Asp265 and Arg320 can interact with Mur through charge–dipole and dipole–dipole interactions. Nonpolar side chains of residues Leu191, Leu195, Met263, Val302, and Ile306 can participate in dipole–induced–dipole interactions as well as van der Waals interactions of the hydrophobic type with MD2.
Finally, we conclude that not only H-bonding interactions but also van der Waals and electrostatic interactions cooperatively locate MD2 inside this active site because these interactions concomitant with H-bonds play very important roles in conserving MD2 binding to MraYAA and stabilizing its active site.
3.4. QTAIM and NBO Analyses on the Second Structural Model
As mentioned earlier (Section 2), the MraYAA–MD2 complex was simulated in a water box under periodic boundary conditions. Our MD outcomes showed that the positions of the added water molecules to around and inside this protein complex were changed in the different frames of simulation, but the locations of the key crystallographic water molecules, however, were kept nearby the MraYAA–MD2 active site without canceling out during the MD process, implicating that these water molecules play critical roles in MD2 binding. The first, third, sixth, and eighth molecules of water are situated near the active site and are surrounded by some of its residues as well as the nucleoside portion of MD2 (model II in Figure 3). These molecules play important roles in maintaining and stabilizing the positions of MD2 and residues inside this active site because they are able to form H-bonds with both of them. The QTAIM and NBO results corresponding to the formation of H-bonds in the second structural model are gathered in Tables 3 and 4, respectively.
Table 3. Structural and Topological Parameters of ρBCP(r) in the Various H···B BCPs Detected in Wt–Residue Pairs, Mur–Wt Pairs, and Wt–Wt Pair Were Calculated at the M06-2X/6-31G** Levela.
| proton donor | proton acceptor | H-bond | d | ∠ | ρBCP | ∇2ρBCP | HBCP | |EHB| |
|---|---|---|---|---|---|---|---|---|
| Wt8 | Leu191 | O–H1···O | 2.49 | 148.25 | 0.0065 | 0.0263 | –0.0040 | 6.34 |
| Wt1 | Asp193 | O–H2···Oδ1 | 1.23 | 123.31 | 0.1519 | 0.1256 | –0.3910 | 355.92 |
| Wt3 | Asp193 | O–H1···Oδ2 | 2.23 | 172.62 | 0.0148 | 0.0351 | –0.0115 | 13.91 |
| Wt8 | Asp193 | O–H1···O | 2.04 | 149.37 | 0.0189 | 0.0600 | –0.0155 | 20.12 |
| Wt6 | Asn255 | O–H2···Oδ1 | 1.86 | 130.73 | 0.0291 | 0.1145 | –0.0257 | 34.99 |
| Ala199 | Wt1 | Cβ–Hβ2···O | 2.44 | 110.43 | 0.0134 | 0.0511 | –0.0081 | 12.65 |
| Ala199 | Wt1 | N–H···O | 2.00 | 152.43 | 0.0267 | 0.0940 | –0.0227 | 30.13 |
| Met263 | Wt3 | N–H···O | 1.58 | 146.95 | 0.0593 | 0.2194 | –0.0603 | 76.74 |
| Met263 | Wt3 | Cβ–Hβ3···O | 1.99 | 119.79 | 0.0275 | 0.1066 | –0.0237 | 32.42 |
| Ile200 | Wt6 | N–H···O | 2.22 | 146.91 | 0.0149 | 0.0457 | –0.0123 | 15.78 |
| Ile200 | Wt6 | Cβ–Hβ···O | 2.50 | 128.75 | 0.0096 | 0.0334 | –0.0061 | 8.99 |
| Met263 | Wt6 | Cε–Hε3···O | 2.41 | 136.36 | 0.0124 | 0.0394 | –0.0087 | 11.97 |
| His324 | Wt8 | Nε–Hε2···O | 2.64 | 130.90 | 0.0066 | 0.0258 | –0.0036 | 6.00 |
| His325 | Wt8 | Nε–Hε2···O | 2.05 | 162.16 | 0.0206 | 0.0584 | –0.0178 | 21.97 |
| Wt1 | Mur | O–H2···O32 | 2.52 | 171.29 | 0.0065 | 0.0233 | –0.0040 | 6.01 |
| Wt3 | Mur | O–H2···O32 | 2.01 | 158.90 | 0.0216 | 0.0635 | –0.0182 | 22.87 |
| Wt8 | Mur | O–H2···O42 | 2.30 | 145.45 | 0.0110 | 0.0385 | –0.0081 | 11.31 |
| Mur | Wt3 | O23–H23···O | 2.64 | 170.93 | 0.0051 | 0.0203 | –0.0026 | 4.49 |
| Mur | Wt3 | C35–H35···O | 2.68 | 131.29 | 0.0064 | 0.0239 | –0.0030 | 5.22 |
| Wt6 | Wt1 | O–H1···O | 1.99 | 112.61 | 0.0277 | 0.1101 | –0.0240 | 33.15 |
| Wt1 | Wt6 | O–H1···O | 1.85 | 124.61 | 0.0333 | 0.1556 | –0.0315 | 44.59 |
H-bond length (d) in angstrom (Å), H-bond angle (∠) in degree (°). All ρBCP, ∇2ρBCP, and HBCP parameters are in atomic units (a.u.). |EHB| is in kJ/mol.
Table 4. NBO Results of Local Orbitals Involved in CT Interactions in Wt–Residue Pairs, Mur–Wt Pairs, and Wt–Wt Pair Were Computed at the M06-2X/6-31G** Level.
| electron donor | electron acceptor | CT | E(2) (kJ/mol) | ||
|---|---|---|---|---|---|
| Leu191 | Wt8 | nO → σO–H1* | 4.69 | 0.0013 | |
| Asp193 | Wt1 | nOδ1 → σO–H1* | 241.50 | 0.1148 | |
| Asp193 | Wt3 | nOδ2 → σO–H1* | 24.39 | 0.0126 | |
| Asp193 | Wt8 | nO → σO–H2* | 22.76 | 0.0066 | |
| Asn255 | Wt6 | nOδ1 → σO–H2* | 37.57 | 0.0108 | |
| Wt1 | Ala199 | nO → σCβ–Hβ2* | 2.30 | 0.0008 | |
| Wt1 | Ala199 | nO → σN–H* | 30.79 | 0.0129 | |
| Wt3 | Met263 | nO → σN–H* | 142.21 | 0.0433 | |
| Wt3 | Met263 | nO → σCβ–Hβ3* | 21.38 | 0.0078 | |
| Wt6 | Ile200 | nO → σN–H* | 19.54 | 0.0072 | |
| Wt6 | Ile200 | nO → σCβ–Hβ* | 4.73 | 0.0016 | |
| Wt6 | Met263 | nO → σCε–Hε3* | 8.16 | 0.0028 | |
| Wt8 | His324 | nO → σNε2–Hε2* | 2.68 | 0.0009 | |
| Wt8 | His325 | nO → σNε2–Hε2* | 43.68 | 0.0143 | |
| Mur | Wt1 | nO32 → σO–H2* | 1.97 | 0.0005 | |
| Mur | Wt3 | nO32 → σO–H2* | 25.82 | 0.0113 | |
| Mur | Wt8 | nO42 → σO–H2* | 4.14 | 0.0011 | |
| Wt3 | Mur | nO → σO23–H23* | 5.23 | 0.0019 | |
| Wt3 | Mur | nO → σC35–H35* | 3.05 | 0.0010 | |
| Wt1 | Wt6 | nO → σO–H1* | 19.50 | 0.0086 | |
| Wt6 | Wt1 | nO → σO–H2* | 39.29 | 0.0139 |
The QTAIM analysis demonstrates that the first water molecule (Wt1) participates in an unconventional H-bond with Ala199 and two conventional H-bonds with Asp193 and Ala199 (Figure 7). The amounts of ρBCP, ∇2ρBCP, and |EHB| at the paths of H···O and Hβ2···O in Wt1–Ala199 pair confirm that N–H···O is a moderate H-bond, whereas Cβ–Hβ2···O is a weak interaction (Table 3). The topological parameters on H2···Oδ1 BCP in the Wt1–Asp193 pair, namely, ρBCP = 0.1519 a.u., ∇2ρBCP = 0.1256 a.u., and HBCP = −0.3910 a.u., signify the presence of a very strong H-bond with a covalent character in this pair. O–H1···Oδ1 emerges as a consequence of a great CT (0.1148 e) from the nOδ1 of Asp193 into antibonding σO–H1* of Wt1. It is evident from the results in Tables 3 and 4 that the highest values of |EHB| (355.92 kJ/mol) and E(2) (241.50 kJ/mol) belong to O–H1···Oδ1 H-bond and nOδ1 → σO–H1 CT interaction, respectively. Hence, it is the strongest H-bonding interaction in the second structural model and has a main contribution to the MraYAA–MD2–water interaction.
Figure 7.

Water molecules participate in H-bonding interactions with residues of the active site as well as with the nucleoside portion of MD2.
The oxygen lone pair of the third water molecule (Wt3) overlaps with the antibonding orbitals of σN–H* and σCβ–Hβ3 in Met263. There is also an orbital overlap between its σO–H1* and nOδ2 in Asp193. These orbital overlaps result in the formation of the H-bonds of N–H···O and Cβ–Hβ3···O in Wt3–Met263 pair as well as O–H1···Oδ2 in Wt3–Asp193 pair (Figure 7). Topological parameters and stabilization energies indicate that Wt3 has the strongest H-bonding interaction with Met263 because N–H···O has the largest values of |EHB| (76.74 kJ/mol) and E(2) (142.21 kJ/mol) with respect to other interactions of Wt3 (Tables 3 and 4). This strong H-bond with a length of 1.58 Å is predominantly covalent in nature. The |EHB| (32.42 kJ/mol) of Cβ–Hβ3···O is more than twice that (13.91 kJ/mol) of O–H1···Oδ2, hence the unconventional H-bond is stronger than the conventional H-bond. As a result, Met263 plays a critical role in MraYAA–MD2–Wt3 interaction through these two H-bonds.
The QTAIM analysis finds a BCP in the path connecting oxygen of the sixth water molecule (Wt6) to Hε3 of Met263. The Hε3···O BCP has the small values of ρBCP (0.0124 a.u.), ∇2ρBCP (0.0394 a.u.), and |EHB| (11.97 kJ/mol). Therefore, Met263 weakly interacts with Wt6. The oxygen of Wt6 is also linked to the nuclei of H and Hβ in Ile200 by two HBCPs. Although both H-bonds in the Wt6–Ile200 pair are weak interactions, N–H···O is somewhat stronger than Cβ–Hβ···O because the |EHB| (15.78 kJ/mol) of conventional H-bond is larger than that (8.99 kJ/mol) of unconventional H-bond. Besides, Oδ1 of Asn255 acts as a proton acceptor in the interaction with H2 of Wt6. The relatively large values of ρBCP (0.0291 a.u), ∇2ρBCP (0.1145 a.u), and |EHB| (34.99 kJ/mol) of H2···Oδ1 BCP affirm that Wt6 has the strongest H-bonding interaction with Asn255. This H-bond is the result of the CT interaction of nOδ1 → σO–H2* with a qCT of 0.0108 e and an E(2) of 37.57 kJ/mol.
In addition to Wt1 and Wt3, the eighth water molecule (Wt8) also interacts with Asp193 through the moderate H-bond of O–H1···O (Table 3). The appearance of this H-bond is due to the CT interaction of nO → σO–H2* with an E(2) of 22.76 kJ/mol and a qCT of 0.0066 e. The antibonding σO–H1 of Wt8 weakly attracts the nO of Leu191 and H-bond of O–H1···O with an |EHB| of 6.34 kJ/mol is formed between them (Figure 7). There are two HBCPs that connect the oxygen nucleus of Wt8 to the Hε2 nuclei in His324 and His325 (Table 3). These H-bonds are the results of the CTs of 0.0009 e and 0.0143 e from the nO of Wt8 into the antibonding orbitals of σNε2–Hε2* in His324 and His325, respectively. The |EHB| (21.97 kJ/mol) of Nε2–Hε2···O in the Wt8–His325 pair is more than 3 times larger than that (6.00 kJ/mol) in the Wt8–His324 pair. Consequently, Wt8 has the stronger H-bonding interaction with His325 than His324.
The O32 lone pair of the uracil base transfers the charges of 0.0005 and 0.0113 e into the antibonding orbitals of σO–H2* in Wt1 and Wt3, respectively. There are also two CT interactions of nO → σO23–H23 and nO → σC35–H35* between MD2 and Wt3. The nO42 of the aminopropyl linker overlaps with the antibonding σO–H2 in Wt8. Accordingly, MD2 is involved in three H-bonds of O23–H23···O, O–H2···O32, and C35–H35···O with Wt3 as well as two H-bonds of O–H2···O32 and O–H2···O42 with Wt1 and Wt8, respectively (Figure 7). Among these H-bonds, O–H2···O32 in the Mur–Wt3 pair is the strongest H-bonding interaction because it has the largest values of ρBCP (0.0216 a.u.), ∇2ρBCP (0.0635 a.u.), and |EHB| (22.87 kJ/mol) in comparison with four weak interactions of MD2 (Table 3).
Our results also demonstrate that Wt1 plays concurrently both donor and acceptor roles in the formation of two moderate O–H···O H-bonds with Wt6 (Figure 7). O–H1···O that is the consequence of the nO → σO–H2* interaction with a qCT of 0.0139 e and an E(2) of 39.29 kJ/mol is stronger than O–H1···O with an |EHB| of (33.15 kJ/mol) and an E(2) of 19.50 kJ/mol.
In addition to the H-bonding interactions, intermolecular interactions in Wt1–Asp193, Wt3–Asp193, Wt8–Asp193, Wt8–His324, and Wt8–His325 pairs also include charge–dipole and dipole–dipole interactions. Intermolecular interactions of other Wt–residue pairs as well as Mur–Wt pairs consist of dipole–dipole and dipole–induced–dipole interactions as well. Thus, we conclude that these water molecules play fundamental roles in positioning residues and locating the conserved nucleoside portion of MD2 within this active site by electrostatic, conventional, and unconventional H-bonding interactions.
4. Conclusions
In summary, MraYAA–MD2 has been computationally studied to identify the physical natures of the intermolecular interactions involved in its active site at the M06-2X/6-31G** level. According to the site mutations in the active site, ITC, and kinetic results, our computational analysis data shed new light on critical residues. The QTAIM and NBO analyses have been performed on this active site for better understanding of intermolecular interactions. Our results specified that Asp196 is one of the critical residues of MraYAA–MD2 that interact strongly with the uracil base of MD2 through an H-bond with dominantly covalent in nature. Gly264 is another important residue that forms a strong H-bond with 5-aminoribose of MD2. Two very strong H-bonds of MD2 with residues Gln305 and Ala321 are mainly responsible to stabilize its peptide portion in this active site. The π···π interaction of the aromatic ring of Phe262 plays an important role in stacking uracil base of MD2 within this active site. Uridine participates in two moderate H-bonds with Gly194 where both are the strongest unconventional H-bonding interactions of MD2.
Electrostatic and van der Waals interactions are of great importance in this active site stabilization. Residues Asp265 and Arg320 are unable to participate in CT interactions with MD2, but they can ensure its binding to MraYAA mainly by electrostatic interactions, especially charge–dipole and dipole–dipole. Nonpolar side chains of residues Leu191, Gly194, Leu195, Phe262, Met263, Gly264, Val302, Ile306, Ala321, and Pro322 can interact with the different parts of MD2 via dipole–induced–dipole and hydrophobic interactions. In addition to H-bonds, residues Lys70, Asp193, Asp196, and His325 are also involved in charge–dipole and dipole–dipole interactions via MD2.
It was also found that the H-bond between Asp193 and Wt1 is the strongest protein–water H-bond in this active site and this interaction has significant effects on its structure stability. Met263 plays a role in MraYAA–MD2–Wt3 interactions through a strong N–H···O H-bond and a moderate Cβ–Hβ3···O H-bond. Wt6 has three weak H-bonding interactions with Met263 and Ile200 and a moderate H-bond with Asn255. Wt1 and Wt6 molecules assist in stabilizing the active site through two moderate water–water interactions. Wt8 forms two weak H-bonds with Leu191 and His324 and two moderate H-bonds with Asp193 and His325. The intermolecular interactions of water molecules with the nucleoside portion of MD2 help its binding stability within this active site.
Finally, our results detected most of the H-bonds around the nucleoside portion of MD2 that cannot be directly palpated from a static crystal complex. This finding indicates that the nucleoside portion plays more important roles in MD2 positioning in this active site than the peptide portion.
Acknowledgments
We thank the support of computation facilities of theoretical high-performance computing (HPC) cluster at Academia Sinica. This work was supported by funds from the Ministry of Science and Technology (MOST), Taiwan (MOST-108-2113-M-001-021-MY3 and MOST-108-3114-Y-001-002) and Academia Sinica (AS-KPQ-109-BioMed and AS-SUMMIT-109).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01551.
Initial atomic coordinates of the first and second structural models, which underwent the partial geometry optimization at the M06-2X/6-31G* level by using Gaussian 09 program package and interacting residues with MD2 in these optimized structures visualized by means of the GaussView 06 program (PDF)
Author Contributions
¶ S.M.Z. and E.K.A. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Boyle D. S.; Donachie W. D. mraY Is an Essential Gene for Cell Growth in Escherichia coli. J. Bacteriol. 1998, 180, 6429–6432. 10.1128/.180.23.6429-6432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve W. G.; Neuhaus F. C. Evidence for an initial acceptor of UDP-NAc-muramyl-pentapeptide in the synthesis of bacterial mucopeptide. Biochem. Biophys. Res. Commun. 1965, 18, 6–12. 10.1016/0006-291x(65)90873-9. [DOI] [PubMed] [Google Scholar]
- Anderson J. S.; Matsuhashi M.; Haskin M. A.; Strominger J. L. Lipid-phosphoacetylmuramyl-pentapeptide and Lipid-phosphodisaccharide-pentapeptide: Presumed Membrane Transport Intermediates in Cell Wall Synthesis. Biochemistry 1965, 53, 881–889. 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A. A family of UDP-GIcNAc/MurNAc: polyisoprenol-P GlcNAc/MurNAc- 1-P transferases. Glycobiology 1994, 4, 768–771. 10.1093/glycob/4.6.768. [DOI] [PubMed] [Google Scholar]
- Hakulinen J. K.; Hering J.; Brändén G.; Chen H.; Snijder A.; Ek M.; Johansson P. MraY-antibiotic complex reveals details of tunicamycin mode of action. Nat. Chem. Biol. 2017, 13, 265–267. 10.1038/nchembio.2270. [DOI] [PubMed] [Google Scholar]
- Chung B. C.; Mashalidis E. H.; Tanino T.; Kim M.; Matsuda A.; Hong J.; Ichikawa S.; Lee S.-Y. Structural insights into inhibition of lipid i production in bacterial cell wall synthesis. Nature 2016, 533, 557–560. 10.1038/nature17636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann D.; Koppermann S.; Wirth M.; Niro G.; Leyerer K.; Ducho C. Muraymycin nucleoside-peptide antibiotics: uridine-derived natural products as lead structures for the development of novel antibacterial agents. Beilstein J. Org. Chem. 2016, 12, 769–795. 10.3762/bjoc.12.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanino T.; Al-Dabbagh B.; Mengin-Lecreulx D.; Bouhss A.; Oyama H.; Ichikawa S.; Matsuda A. Mechanistic analysis of muraymycin analogues: a guide to the design of MraY inhibitors. J. Med. Chem. 2011, 54, 8421–8439. 10.1021/jm200906r. [DOI] [PubMed] [Google Scholar]
- Cui Z.; Wang X.; Liu X.; Lemke A.; Koppermann S.; Ducho C.; Rohr J.; Thorson J. S.; Van Lanen S. G. Self-Resistance during Muraymycin Biosynthesis: a Complementary Nucleotidyltransferase and Phosphotransferase with Identical Modification Sites and Distinct Temporal Order. Antimicrob. Agents Chemother. 2018, 62, e00193-18 10.1128/aac.00193-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politzer P.; Murray J. S.; Clark T. Mathematical modeling and physical reality in noncovalent interactions. J. Mol. Model. 2015, 21, 52. 10.1007/s00894-015-2585-5. [DOI] [PubMed] [Google Scholar]
- Clark T.; Murray J. S.; Politzer P. A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys. Chem. Chem. Phys. 2018, 20, 30076–30082. 10.1039/c8cp06786d. [DOI] [PubMed] [Google Scholar]
- Politzer P.; Murray J. S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals 2019, 9, 165. 10.3390/cryst9030165. [DOI] [Google Scholar]
- Karshikoff A.Non-covalent Interactions in Proteins; Imperial College Press: London, U.K., 2006. [Google Scholar]
- Astani E. K.; Chen N.-C.; Huang Y.-C.; Ersali S.; Lin P.-J.; Guan H.-H.; Lin C.-C.; Chuankhayan P.; Chen C.-J.; Chen C. J. Characterization of Dimeric Interactions within Protrusion-Domain Interfaces of Parallel and X-Shaped Conformations of Macrobrachium rosenbergii Nodavirus: A Theoretical Study Using the DFT Method along with QTAIM and NBO Analyses. ACS Omega 2020, 5, 3428–3443. 10.1021/acsomega.9b03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Zhao Y.; Schultz N. E.; Truhlar D. G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. 10.1021/ct0502763. [DOI] [PubMed] [Google Scholar]
- Li R.; Zheng J.; Truhlar D. G. Density functional approximations for charge transfer excitations with intermediate spatial overlap. Phys. Chem. Chem. Phys. 2010, 12, 12697. 10.1039/c0cp00549e. [DOI] [PubMed] [Google Scholar]
- Bader R. F. W.Atoms in Molecules-A Quantum Theory; Clarendon Press: Oxford, U.K., 1990. [Google Scholar]
- Bader R. F. W.; Cheng C. Properties of atoms in molecules: electrophilic aromatic substitution. J. Phys. Chem. 1989, 93, 2946–2956. 10.1021/j100345a020. [DOI] [Google Scholar]
- Astani E. K.; Heshmati E.; Chen C.-J.; Hadipour N. L. A theoretical study on the characteristics of the intermolecular interactions in the active site of human androsterone sulphotransferase: DFT calculations of NQR and NMR parameters and QTAIM analysis. J. Mol. Graphics Modell. 2016, 68, 14–22. 10.1016/j.jmgm.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Reed A. E.; Curtiss L. A.; Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. 10.1021/cr00088a005. [DOI] [Google Scholar]
- Weinhold F.; Landis C. R.. Discovering Chemistry with Natural Bond Orbitals. John Wiley & Sons, Inc: Wisconsin, 2012. [Google Scholar]
- Astani E. K.; Hadipour N. L.; Chen C.-J. Molecular interactions investigated with DFT calculations of QTAIM and NBO analyses: An application to dimeric structures of rice α-amylase/subtilisin inhibitor. Chem. Phys. Lett. 2017, 672, 80–88. 10.1016/j.cplett.2017.01.047. [DOI] [Google Scholar]
- Astani E. K.; Chen N.-C.; Huang Y.-C.; Bahrami A.; Chen L.-Y.; Lin P.-R.; Guan H.-H.; Lin C.-C.; Chuankhayan P.; Hadipour N. L.; Chen C.-J. DFT, QTAIM, and NBO studies on the trimeric interactions in the protrusion domain of a piscine betanodavirus. J. Mol. Graphics Modell. 2017, 78, 61–73. 10.1016/j.jmgm.2017.09.020. [DOI] [PubMed] [Google Scholar]
- Kantardjieff K. A.; Rupp B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 2003, 12, 1865–1871. 10.1110/ps.0350503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. X-ray crystallographic studies of proteins. Annu. Rev. Phys. Chem. 1976, 27, 493–523. 10.1146/annurev.pc.27.100176.002425. [DOI] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Mahoney M. W.; Jorgensen W. L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 2000, 112, 8910–8922. 10.1063/1.481505. [DOI] [Google Scholar]
- Best R. B.; Zhu X.; Shim J.; Lopes P. E. M.; Mittal J.; Feig M.; MacKerell A. D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalé L.; Skeel R.; Bhandarkar M.; Brunner R.; Gursoy A.; Krawetz N.; Phillips J.; Shinozaki A.; Varadarajan K.; Schulten K. NAMD2: Greater Scalability for Parallel Molecular Dynamics. J. Comput. Phys. 1999, 151, 283–312. 10.1006/jcph.1999.6201. [DOI] [Google Scholar]
- Phillips J. C.; Braun R.; Wang W.; Gumbart J.; Tajkhorshid E.; Villa E.; Chipot C.; Skeel R. D.; Kalé L.; Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski S. J. What Is the Covalency of Hydrogen Bonding?. Chem. Rev. 2011, 111, 2597–2625. 10.1021/cr800346f. [DOI] [PubMed] [Google Scholar]
- Matta C. F.Hydrogen Bonding—New Insights; Springer, 2006. [Google Scholar]
- Alabugin I. V.; Manoharan M.; Peabody S.; Weinhold F. Electronic basis of improper hydrogen bonding: A subtle balance of hyperconjugation and rehybridization. J. Am. Chem. Soc. 2003, 125, 5973–5987. 10.1021/ja034656e. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam N. J.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford CT, 2009.
- Biegler-König F.; Schonbohm J. J.; Bayles D. J. AIM2000-a program to analyze and visualize atoms in molecules. J. Comput. Chem. 2001, 22, 545–559. . [DOI] [PubMed] [Google Scholar]
- Koch U.; Popelier P. L. A. Characterization of C-H···O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. A 1995, 99, 9747–9754. 10.1021/j100024a016. [DOI] [Google Scholar]
- Espinosa E.; Molins E.; Lecomte C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. 10.1016/s0009-2614(98)00036-0. [DOI] [Google Scholar]
- Espinosa E.; Lecomte I.; Rozas I.; Elguero J.; Molins E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. 10.1016/s0009-2614(01)00178-6. [DOI] [Google Scholar]
- Mashalidis E. H.; Kaeser B.; Terasawa Y.; Katsuyama A.; Kwom D. Y.; Lee K.; Hong J.; Ichikawa S.; Lee S. Y. Chemical logic of MraY inhibition by antibacterial nucleoside natural products. Nat. Commun. 2019, 10, 2917. 10.1038/s41467-019-10957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas I.; Alkorta I.; Elguero J. Behavior of Ylides Containing N, O, and C Atoms as hydrogen bond acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. 10.1021/ja0017864. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.