Abstract

In the current context, the development of bio-based and high-performance materials is one of the main research priorities. This study aims to combine the outstanding properties of cellulose nanofibrils (CNFs) or nanocrystals (CNCs) with those of bio-based poly(lactic acid) (PLA). Three-phase multilayered materials (TMLs) were built up by complexing a dry CNF- or CNC-based film with two PLA sheets, using a heat-pressing process. Before the preparation of the nanocellulosic films, CNFs and CNCs were modified by the adsorption of a rosin-based nanoemulsion. The rosin mixture as a natural compound is of interest because of its low cost, renewability, hydrophobicity, and its antimicrobial and antioxidant properties. After demonstrating the efficiency of the complexing procedure, we investigated the barrier properties of the multilayered materials against both oxygen and water vapor, with highly encouraging results. In fact, the presence of nanocellulose as an inner layer between the two PLA films significantly enhanced the oxygen barrier, with a decrease in oxygen permeability comprised between 84 and 96% and between 44 and 50% for neat nanocelluloses and nanocelluloses with rosins as the inner layer, respectively. On the other hand, the antioxidant properties of the final multilayered materials including rosins were highlighted, with a highly encouraging radical scavenging activity close to 20%. Because of the simplicity and the efficiency of the proposed method, this study paves the way toward the development of hybrid multimaterials that could be highly attractive for food packaging applications.
1. Introduction
Sustainability, biodegradability, renewability, and abundance are the main exceptional advantages of a remarkable category of cellulosic materials: the nanocelluloses. These nanoscale materials, whether cellulose nanofibrils (CNFs) or nanocrystals (CNCs), have been the focus of numerous studies in recent decades. Their outstanding intrinsic properties (for example, low density compared to other organic materials, high specific surface area, surface chemical reactivity, high tensile strength, barrier properties against oxygen)1−5 make them materials of choice for many different applications (among others, hydrogels, composites, conducting materials, piezoelectric materials, biosensors, pharmaceuticals, biomedical devices).2,6 In these applications, the use of nanocelluloses in the buildup of multiphasic materials (nanocomposites or multilayers) is highly promising, especially for bio-based food packaging applications. Moreover, conferring active functionalities to them, like antimicrobial or antioxidant properties, could increase their appeal in such applications.7,8 In the literature, numerous publications focused on this topic, with the use of various active molecules and different procedures for their binding with nanocelluloses.9−11
Among exploitable and tunable natural materials, rosins are highly attractive because of their abundance, sustainability, and low cost. They are the main products of conifers, with the pine resin derived from pine trees being the most important. More than one million metric tons of rosin are produced each year.12,13 Abietic- and pimaric-type acids are the main components of a natural rosin mixture. Application of rosins can be found in a number of products, mostly in cosmetics, varnishes, paints, coatings, medicines, paper-sizing agents, and emulsions.13−15 It is worth noting that rosin is often used in growth polymerization to produce low-molecular-weight polymeric materials that include rosin as a natural compound and that these polymerizations are generally performed via atom-transfer radical polymerization (ATRP).14 For example, Wang et al.12 used ATRP to polymerize rosin monomers on the surface of lignin for surface functionalization.
Many researchers have elucidated the antimicrobial activity of rosin. For example, Moustafa et al.13 recently showed that rosin could be used in packaging materials as a shelf-life extender for food products. They performed esterification of rosin on organoclay and introduced the modified organoclays in a blend of poly(lactic acid) (PLA)/poly(butylene adipate-co-terephthalate). They demonstrated the antibacterial activity of the produced nanocomposites and their usefulness in food packaging applications. de Castro et al.11 performed efficient green esterification of rosin on cellulose nanocrystals and highlighted the hydrophobic and antimicrobial behaviors of the resulting grafted CNC. One of the limitations of natural rosin is its insolubility in water and, therefore, rosin can be used in the form of a suspension or emulsion.16
An emulsion results from mixing two immiscible liquids using both mechanical shear and a surfactant.17 A nanoemulsion is defined as the dispersion of nano-sized particles (typically between 20 and 200 nm)18 prepared by mechanical forces. Nanoemulsions are clear and thermodynamically stable. Their main application is drug delivery in various systems, as widely described in the literature.18,19 The combination of both nanocelluloses and natural rosins has only recently been proposed in the literature. In 2016, de Castro et al.11 studied the green esterification of rosins on the surface of the CNCs, while in 2018, Niu et al.20 followed a similar protocol on cellulose nanofibers and introduced a modified CNF in the bulk of a poly(lactic acid)/chitosan polymeric matrix. Both studies highlighted the antimicrobial activity brought about by the presence of rosin in the prepared systems. Although these grafting methods aimed to limit the use of organic and toxic solvents, they required time-consuming multistep procedures. In this sense, the adsorption of rosin nanoemulsions on the nanocellulose surface can be considered an improvement, which decreases the use of chemicals and reaction steps.
With the current awareness of the need for the use of biodegradable materials, the packaging industry, and especially that of food packaging, has been continuously evolving for several years. Among bio-based and biodegradable materials, poly(lactic acid) (PLA) is highly attractive because of its production from natural resources, as well as its transparency, mechanical properties, and printability, making it a polymer of choice for replacing fossil-based plastics generally used in the food packaging industry.21,22 However, the thermal and barrier properties of PLA are generally not sufficient for food packaging applications. Its glass transition temperature is approximately 60 °C, and it is highly permeable to water vapor and oxygen, compared to the other polymers traditionally used in food packaging. Inserting cellulosic nanostructures into PLA-based packaging makes it possible, by combining their respective intrinsic properties, to produce packaging that is more efficient in terms of mechanical and barrier properties, and still biodegradable. The studies describing the preparation of such multiphasic materials in the literature show that the main type of process is based on the buildup of bulk nanocomposites. However, this is highly challenging, as the compatibility between the hydrophilic nanocelluloses and the generally hydrophobic polymer matrix needs to be significantly improved. Many research groups proposed different procedures to enhance this compatibility.23−26 Another strategy consists of the incorporation of one or multiple layers to improve the final barrier and mechanical properties of the multilayered system.
Multilayered materials can be built up using different methods (among others, layer-by-layer deposition, electrodynamic processing, microlayer coextrusion, bar coating),27 which all aim to deposit a layer of a specific material on a polymer substrate. A multilayered material is composed of at least two layers, but the thinnest layer is generally covered with another polymer layer, which may or may not be different from the initial one. The compatibility between the layers is generally challenging, and that explains the frequent use of adhesive layers, especially in the packaging industry. Only a few studies deal with the preparation of such PLA-based multilayered materials. In 2013, Aulin et al.28 were among the first to perform the layer-by-layer deposition of CNFs combined with cationic polyethyleneimine (PEI) on a PLA substrate. They demonstrated the efficiency of the deposition of the nanocellulose/PEI mixture onto the PLA substrate and highlighted the improvement in both oxygen and water vapor barrier properties of the final coated materials by 94 and 50%, respectively. This promising study was confirmed by the work of Meriçer et al.,29 who produced multilayered PLA-based materials including microfibrillated cellulose (MFC) by evaporation casting of both layers, with improved mechanical (increase in Young’s modulus by 60%) and barrier properties (decrease in oxygen permeability of one order of magnitude). Similarly, Hosseini et al.30 prepared multilayered films based on PLA and fish gelatin by successive solvent-casting procedures, with oxygen and water vapor barrier properties enhanced by 87 and 91%, respectively, compared to pristine PLA. Furthermore, specific active compounds can be added inside the multilayered structure, to be released through the packaging or to scavenge some specific molecules from food products. In a multilayer strategy, the presence of both nanocelluloses and active compounds inside the active layer can aid their entrapment or their possible release. In the literature, CNCs have been mixed with silver nanoparticles31 or with carvacrol32 to confer antibacterial or antioxidant properties to them, respectively.
Therefore, in this study, PLA-based multilayered materials were shaped via a two-step procedure. Rosin-based nanoemulsions were first adsorbed on CNFs and CNCs, leading to the preparation of neat or modified CNF- and CNC-based self-standing films. Then, the dry nanocellulose films were complexed between two commercial PLA sheets by heat pressing. After the optimization of the process, the multiphase materials were characterized, and their structural, mechanical, barrier, and antioxidant properties were investigated.
2. Results and Discussion
2.1. Nanometric Size of Rosin Particles
The preparation of a rosin nanoemulsion was based on the alkylketene dimer (AKD)-based nanoemulsion production method patented by Missoum et al.33 By definition, nanoemulsions are nano-sized stable colloidal emulsions.34 A rosin nanoemulsion was prepared following the protocol of mixing two immiscible liquids (water and an organic solvent) in the presence of a surfactant in the water phase. The chemical structure of the surfactant was composed of a quaternary ammonium as the head group and a long aliphatic chain as the tail group. Rosin was dissolved in the organic phase, added to the water phase, and sonicated to obtain a dispersed oil-in-water single-phase emulsion. The organic solvent was then totally evaporated by heating the emulsion to obtain a stable colloidal dispersion of rosin in water. To verify the aggregation state and get the proper nanometric size of rosin particles in the prepared emulsion, a dynamic light scattering (DLS) measurement was performed on a diluted sample. The size distribution of the intensity of rosin particles obtained with the CUMULANT method is presented in Figure 1. The size distribution of particles is Gaussian, with the particle diameter size equal to 186 nm measured by DLS, confirming the nanometric character of the rosin particles in the emulsion.
Figure 1.
Size distribution of the relative intensity obtained from a DLS measurement performed on a rosin nanoemulsion freshly prepared (rosin (T0)) and 6 months later (rosin (T0 + 6)).
The DLS measurement has been performed directly after the nanoemulsion preparation (rosin (T0)) and 6 months later (rosin (T0 + 6)). The DLS profile and values were 191 ± 6 and 195 ± 9 nm for rosin (T0) and rosin (T0 + 6), respectively. These results are very similar and prove the stability of the nanoemulsions. Hence, these were used for further adsorption on both CNFs and CNCs.
2.2. Efficiency of the Rosin Adsorption on CNFs and CNCs
Several acids are present in a natural rosin mixture, the most predominant of which are shown in Figure 9. Both CNFs and CNCs contain many hydroxyl groups (−OH) at their surface, which can easily create hydrogen bonds with carboxyl groups in the acid mixture from rosin particles. To confirm the presence of rosin molecules on both CNF and CNC, Fourier transform infrared (FTIR) analyses were performed on commercial rosin as well as neat CNF, CNF–rosin, neat CNC, and CNC–rosin films. The resulting spectra are presented in Figure 2.
Figure 9.

Main carboxylic acids found in rosin. Reprinted and adapted from ref (11). Copyright © (2016), with permission from Elsevier.
Figure 2.
FTIR spectra of commercial rosin (powder), and neat CNF, CNF–rosin, neat CNC, and CNC–rosin films.
In the spectrum of the supplied neat rosin, an intense peak at 1695 cm–1 can be related to many carboxyl groups (−COOH) from the different acids composing the rosin mixture. Moreover, a large peak between 2900 and 3000 cm–1 can be attributed to the −CH carbons from alkene bonds of different acids. The spectra related to neat CNFs and CNCs both exhibit peaks classically observed for cellulosic samples, with, among others, peaks at 1105, 1059, and 1028 cm–1 linked to the vibrations of C–O bonds, and a large peak at approximately 3000–3600 cm–1 linked to the stretching vibration of bonds of primary and secondary hydroxyl groups. In both CNF–rosin and CNC–rosin spectra, respectively, the peaks at 1692 and 1691 cm–1 are attributed to the carboxyl groups from the rosin mixture present in the samples. Note that this peak is more intense in the case of CNCs, which could be explained by the loss of the unadsorbed rosin nanoemulsion during the filtration process of a CNF-based film preparation. Moreover, CNCs have negative charges on their surface as they were prepared by sulfuric acid hydrolysis of cellulose pulp and rosin nanoemulsion is stabilized by a surfactant with positive head groups. Thus, electrostatic interactions occur between CNCs and the rosin nanoemulsion, resulting in higher adsorption of rosin onto CNCs compared to CNFs as the CNF surface is barely negatively charged. However, from these FTIR spectra, it is possible to qualitatively conclude about the presence of rosin in both CNF–rosin and CNC–rosin samples. Modified CNF–rosin and CNC–rosin were thus efficiently produced, with a weight ratio of rosins to CNC or CNF close to 0.11.
2.3. Water Contact Angles on Nanocellulosic Films
Water contact angle measurements were performed on both unmodified CNF and CNC films, as well as on modified CNF–rosin and CNC–rosin films. Neat CNC and CNF films exhibit water contact angles equal to 54 ± 1 and 36 ± 6°, respectively, and these values are consistent with the values found in the literature.35,36 After adsorption of rosin nanoparticles on CNC, the CNC–rosin cast film shows a visible decrease in the contact angle down to 32 ± 1.5°, probably due to the presence of hydrophilic properties of rosin molecules. The contact angle of the modified CNF–rosin film is equal to 42 ± 5°. Taking into account the standard deviation of this value and the uncertainty of the neat CNF film contact angle, it is difficult to reach any conclusions about the evolution of the water contact angle, although a similar trend was observed for the CNC–rosin sample.
2.4. Multilayered Materials Built Up by Complexing
Multilayered materials (TML) based on both PLA and nanocellulose were built up via the complexing of three dry films: two commercial PLA films and a single neat or modified CNC or CNF self-standing film in between. As previously described, neat (or modified) CNC (or CNC–rosin) and CNF (or CNF–rosin) self-standing films were prepared by solvent-casting or filtration processes, respectively, and were then heat-pressed between two PLA films (25 μm thick each), leading to TML–CNC (or TML–CNC–rosin) and TML–CNF (or TML–CNF–rosin) multilayered materials. After several trials, we determined the optimal conditions for the protocol of heat pressing of multilayered materials. We found that setting the temperature at 170 °C allowed the preparation of homogeneous and uncolored samples. The temperature needs to be higher than the melting temperature of the PLA (approximately 150 °C) to permit the creep of the semicrystalline polymer throughout the multilayered system and to ensure good adhesion and sealing between the three layers. Table 1 summarizes all of the samples with their respective references, as well as their determined basis weight (in g/m2), and their theoretical and experimental thicknesses (in μm).
Table 1. Sample References with Corresponding Basis Weight (in g/m2), and Theoretical and Experimental Thicknesses.
| sample | basis weight (g/m2) | theoretical thickness (μm) | experimental thickness (μm) | |
|---|---|---|---|---|
| single films | PLA | 30 | 25 | 25 |
| CNC | 67 | 40 | 40 | |
| CNC–rosin | 61 | 40 | 44 | |
| CNF | 29 | 30 | 35 | |
| CNF–rosin | 32 | 30 | 38 | |
| heat-pressed multilayered (TML) materials | TML–PLA | 90 | 75 | 86 |
| TML–CNC | 100 | 90 | 82 | |
| TML–CNC–rosin | 121 | 94 | 94 | |
| TML–CNF | 90 | 85 | 78 | |
| TML–CNF–rosin | 93 | 88 | 75 | |
By comparing the theoretical and experimental thicknesses presented in Table 1 for multilayers (TML) prepared with an inner layer composed of CNC or CNF, it is worth noting that the experimental thicknesses tend to be statistically lower than the theoretical ones (except for the TML–CNC–rosin sample). This discrepancy can be explained by the potential impregnation of the melted PLA through the CNF or CNC nanoporous films during the heat-compression process and/or the pressure applied during the heat-pressing process that might flatten the TML materials, leading to a decrease in the total thickness of the material.
2.5. Structural and Mechanical Properties of Complexed Multilayered Materials
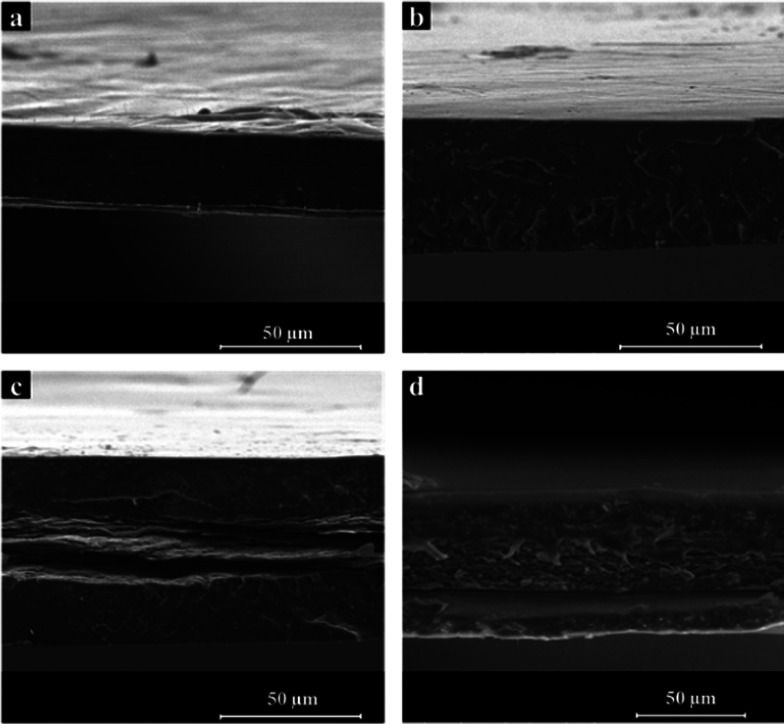
To confirm the assumption made about the impregnation of the PLA layers within the cellulosic layer, the structure of the multilayered (TML) materials was investigated using scanning electron microscopy (SEM) imaging. The cross-sectional images are presented in Figure 3.
Figure 3.
SEM images of (a) a commercial PLA heat-pressed film, (b) reference TML–PLA multilayered material, (c) multilayered material prepared with an inner CNF film, and (d) and multilayered material prepared with an inner CNC film.
In Figure 3a,b, the cross sections of the commercial PLA film built up under the same heat-pressing conditions as multilayered materials, and of the TML–PLA multilayered system, are, respectively, presented. Some defects can be observed on the PLA cross section in Figure 3a, which might have been generated by the heat-pressing process. The corresponding multilayered TML–PLA material (Figure 3b), composed of three heat-pressed PLA films, seems to be homogeneous, and no separation between the three layers can be observed. However, some defects and cracks also appear, indicating that heat pressing can affect the structure of the PLA-based materials. Figure 3c shows the cross section of the TML–CNF. The inner CNF layer can be distinctly observed, although it seems to be impregnated with the top PLA layer. The boundary between the CNF and the lower PLA layer can be clearly distinguished, showing the poor compatibility between neat CNF and PLA. A similar boundary can be observed in the cross section of the TML–CNC presented in Figure 3d. The top PLA layer seems to be impregnated in the inner CNC layer, whereas the boundary between the CNC and the lower PLA layer is visible. Figure 3a,b are interesting since they allow drawing conclusions about the efficiency of the heat-pressing method to combine several PLA sheets together. The process is not entirely homogeneous, probably because of a difference in temperature and pressure in the heat-press device, and it requires further optimization. However, both TML–CNF and TML–CNC materials are self-standing and uniform, without delamination between the layers. Sanyang et al.37 prepared starch/PLA bilayered materials via a casting procedure and, in the SEM analysis, they found an interface between the two layers, probably due to the poor compatibility between the two components. However, they found an enhancement in mechanical and barrier properties in their bilayered materials.
Regarding the transparencies of the different materials studied in this work, we measured their transmittances at the wavelength of 550 nm (T550 nm), corresponding to the visible light wavelength, and the values are shown in Figure 4c. The commercial film of PLA exhibits a transmittance at 550 nm equal to 91%, which is consistent with the values found in the literature.38
Figure 4.

Photographs of films prepared by (a) solvent casting of a suspension of modified CNC–rosin, (b) filtration of a suspension of a modified CNF–rosin suspension, and (c) transmittance values of single films (PLA, CNC, CNC–rosin, CNF, CNF–rosin) and corresponding multilayered materials.
CNC and modified CNC–rosin films have transmittances of 61 and 82%, respectively. The value obtained for neat CNC is consistent with the literature, although this value is highly dependent on the dispersion state and chirality of the CNC inside the film.39,40 The transmittances of CNF and CNF–rosin films are equal to 15 and 11%, respectively, and highlight the fact that CNF-based films are less transparent than CNC films. These values are consistent with the values found in the literature41 for similar CNF. In both cases, the presence of rosin nanoparticles in the film does not significantly influence the optical transparency of the CNC or CNF film. Figure 4 shows photographs of prepared films of modified CNC–rosin (Figure 4a) and modified CNF–rosin (Figure 4b) and highlights their self-standing and transparency properties. The transmittance values of multilayers indicate that their transparency decreases by incorporating a PLA, CNC, or CNC–rosin layer between two PLA films using the heat-pressing process. The transmittances of TML–PLA, TML–CNC, and TML–CNC–rosin are very close to each other and equal to 47, 47, and 50%, respectively. Despite the lower transmittance values of CNC-based films compared to a pure PLA film, the incorporation of the nanocellulosic films did not influence the entire multilayered material transmittance. However, by incorporating a CNF or CNF–rosin layer in the multilayered system, transmittance slightly increases up to 21 and 17%, respectively. This result could be explained by the fact that the pores present in both CNF and CNF–rosin films are closed and covered by transparent PLA during the heat-pressing procedure, leading to an increase in the transparency. Meriçer et al.29 combined one layer of PLA with a top layer of CNF through a casting procedure and reported a similar trend in the transmittance values. For food packaging applications, transparency of materials is essential for esthetic, marketing, and consumer satisfaction.27,42
We also investigated the mechanical properties of both single- and multilayered materials. The values of Young’s modulus and of both elongation and stress at break are presented in Figure 5.
Figure 5.
Young’s modulus values (a), strain at break (b), and stress at break (c) measured on single films (PLA, CNC, CNC–rosin, CNF, CNF–rosin) and corresponding multilayered materials.
According to these values, a commercial PLA film exhibits a Young’s modulus equal to 3.0 GPa. Although this value is highly dependent on the type of PLA and its processing, it is consistent with the literature values.21,43 Young’s modulus of CNC and CNF films is not affected by the presence of adsorbed rosin nanoparticles at the nanocellulose surface. In fact, from the values presented in Figure 5a, Young’s modulus values of CNC and CNC–rosin are equal to 17 and 13 GPa, respectively, and CNF and CNF–rosin have modulus values equal to 8.1 and 8.4 GPa, respectively. These values are consistent with the values found in the literature for CNF films, although the values obtained for the CNC films are somewhat higher.1,44−46 Note that these values are highly linked to the nature of the nanocelluloses, as well as to their dispersion and orientation. Nevertheless, Young’s modulus values of multilayered materials are lower than those of corresponding single films, probably due to the heat-pressing process inducing some defects in the material structure and thus a relative fragility, as suggested by SEM images in Figure 3. However, the presence of a nanocellulosic film between the two PLA sheets clearly improves the final mechanical properties. In fact, in each case, the Young’s modulus of the multilayered material (TML–CNC, TML–CNC–rosin, TML–CNF, or TML–CNF–rosin) is at least 2.5 times higher than that of the TML–PLA sample. It can be explained by the higher Young’s modulus of the inner nanocellulosic layer compared to the pure PLA film, or by the relatively good adhesion between the three layers. Regarding the values of elongation at break of the samples (Figure 5b), neat PLA exhibits an elongation at break at ambient temperature equal to 2.1%, average four times higher than those of CNC and CNF, as expected in the literature.9,40,43,47 Moreover, the presence of rosins in the nanocellulose sample seems to decrease the elongation at break, resulting in reduction of the elasticity of the materials, although the results must be interpreted with caution due to the relatively large standard deviations. Nevertheless, corresponding multilayered materials have higher elongations at break, which is logically explained by the preponderant presence of PLA. Disparities are observed between the values of TML–CNC and TML–CNF, the latter being equal to the elongation at break of neat PLA. It could be explained by the covering of PLA in the numerous pores of the CNF film during the heat-pressing process that would induce the increase in the elongation at break of the final multilayered material. This hypothesis is coherent with the previous results obtained in the transmittance of the CNF-based multilayered material in comparison with the neat CNF one. The stress at break values (Figure 5c) follows the same tendency. Neat PLA exhibits stress at break value at ambient temperature equal to 69 MPa, on average two times higher than those of CNC and CNF, the presence of rosins in the nanocellulose sample seems to decrease the stress at break, despite large standard deviations, and the TML–CNF multilayered material displays the highest value.
The evaluation of the mechanical properties is crucial when thinking about packaging applications, and especially food packaging since one of the purposes of the food packaging material is to protect the product during its transportation and handling, while ensuring its mechanical integrity until it reaches the consumer.30,47 Thus, in terms of mechanical properties, food packaging mainly requires high elongation at break with high stress at break, especially for the manufacturing process by thermoforming, while the Young’s modulus must remain high too. In this sense, the TML–CNF multilayered material shows the best performance for use as food packaging.
2.6. Barrier and Antioxidant Properties
While focusing on food packaging applications, we investigated the barrier properties of single nanocellulosic films, multilayered materials (TML), and a commercial PLA film. The values of oxygen and water vapor permeability are presented in Figure 6. As expected, CNC and CNF films have a high barrier against oxygen (with values equal to 0.02 and 0.01 × 1018 m3·m/(m2·s·Pa), respectively) but low barrier toward water vapor (with values equal to 8.9 ± 0.3 and 14.3 ± 0.2 × 1016 kg·m/(m2·s·Pa), for CNC and CNF films, respectively). These values are expected for such nanocelluloses. In fact, the dense and entangled network of nanomaterials created in the dry films causes their high oxygen barrier properties, whereas their hydrophilic behavior explains their high water vapor transmission rate. Furthermore, a commercial single PLA film, different from the one used in the preparation strategy for wet multilayered materials, exhibits oxygen and water vapor permeabilities equal to 1.8 × 1018 m3·m/(m2·s·Pa) and 3.7 ± 0.4 × 1016 kg·m/(m2·s·Pa), respectively, which is consistent with the values found in the literature.21,48 The water vapor permeability value is still much higher in our study, which can be linked to the degradation of the commercial PLA under ambient conditions since its purchase (2 years ago).
Figure 6.

Oxygen and water vapor permeabilities of a neat commercial PLA film and multilayered materials with PLA, CNC, CNC–rosin, CNF, or CNF–rosin films as inner layers (TML–PLA, TML–CNC, TML–CNC–rosin, TML–CNF, or TML–CNF–rosin, respectively).
As can be seen in Figure 6, oxygen and water vapor permeability values of the multilayered TML–PLA material increased to 8.1 × 1018 m3·m/(m2·s·Pa) and 6.1 ± 0.6 × 1016 kg·m/(m2·s·Pa), respectively. This increase is attributed to the heat-pressing process, during which some cracks or pores are created in the materials, inducing a preferential path for both oxygen and water vapor molecules. Note that the PLA crystallinity might also be influenced by the heat-pressing step. The presence of a nanocellulosic layer between the two PLA films clearly decreases the oxygen permeability of the multilayered materials. Indeed, TML–CNC and TML–CNF exhibit oxygen permeability equal to 0.1 × 1018 m3·m/(m2·s·Pa) and 0.3 × 1018 m3·m/(m2·s·Pa), respectively. The oxygen permeability is thus reduced 80 and 27 times, respectively, which is highly promising. However, the presence of rosin on the nanocellulose surface seems to increase these values up to 0.9 × 1018 m3·m/(m2·s·Pa) and 1.2 × 1018 m3·m/(m2·s·Pa) for TML–CNC–rosin and TML–CNF–rosin, respectively. This increase in oxygen permeability after the adsorption of rosin onto nanocellulosic materials could screen the hydrogen interactions, resulting in the spacing of the nanocrystals or the nanofibrils away from each other, thus inducing a more porous cellulosic structure. The values obtained for water vapor permeability are equal to (2.3 ± 0.2), (2.9 ± 0.2), (4.5 ± 0.5), and (3.2 ± 0.5) × 1016 kg·m/(m2·s·Pa) for TML–CNC, TML–CNC–rosin, TML–CNF, and TML–CNF–rosin, respectively. Water vapor permeability is enhanced in both cases with the introduction of nanocelluloses in the materials compared to the TML–PLA material, although any trend can be drawn for the presence of rosin in the sample. In addition, as the molecules composing the gum-rosin mixture are hydrophilic, their presence in both TML–CNC–rosin and TML–CNF–rosin should not positively influence the barrier properties against water vapor.
However, it is interesting to position the multilayered materials prepared in this study in relation to the polymers classically used in the food packaging industry, as shown in Figure 7. Note that in Figure 7, neither oxygen nor water vapor permeability values are normalized with respect to their thickness, unlike what has been done for the permeability values presented in Figure 6. Moreover, values of oxygen permeability were measured at 0% RH, whereas all other materials in Figure 7 were characterized at 50% RH. However, since only a general trend is required here, this does not affect the conclusions arrived at from Figure 7. As expected, the commercial PLA film used in this part is not a strong enough barrier against oxygen and water vapor to compete with the other synthetic polymers in terms of the food packaging application requirements.
Figure 7.

Oxygen and water vapor transmission rate values required for food packaging materials according to specific foodstuffs and TML multilayered materials (TML–PLA, TML–CNC, TML–CNC–rosin, TML–CNF, and TML–CNF–rosin) positioning. Adapted from ref (49). Copyright © (2012).
Moreover, the multilayered TML–PLA material, prepared with an inner PLA layer, exhibits somewhat interesting properties that are, in fact, close to those required for packaging of low-fat and dry food. The multilayered materials prepared with a nanocellulosic inner layer come closer, in terms of barrier properties, to meat packaging materials. However, only the material containing a neat CNC-cast film (TML–CNC) could be used as such a meat packaging material. Other materials (TML–CNC–rosin, TML–CNF, and TML–CNF–rosin) are not strong barriers against oxygen and water vapor, although they could also be used as materials for low-fat and dry food packaging. In addition, it could be highly interesting to investigate the sorption properties of the materials containing rosins in relation to aromatic compounds. Indeed, the aromatic groups in rosin molecules could interact with aromatic rings of volatile compounds50 and thus enhance some specific sorption properties, interesting for special food packaging applications, as already studied in the literature.51
In addition to their antimicrobial properties, widely demonstrated in the literature,11,20 we investigated the antioxidant properties of natural rosins. Indeed, oxidation is one of the main degradation processes occurring during food degradation. Several methods exist to characterize this antioxidant activity, as presented in the review of Gómez-Estaca et al.52 Among these methods, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay is classically used to determine the efficiency of antioxidant-active materials. Crouvisier-Urion et al.53 performed the DPPH assay on chitosan/lignin composite films. Lignin is a known antioxidant compound, and the authors investigated the kinetics of the radical scavenging activity (RSA) of the films. They found that, for any antioxidant compound, RSA kinetics always decreases and generally reaches a plateau, with the value of this plateau a characteristic of the antioxidant. In our study, a similar protocol as that proposed by Crouvisier-Urion et al.53 was performed on commercial rosins for the first time. In Figure 8, the RSA evolution of the rosins is plotted as a function of time, reaching a plateau leading to up to a 90% decrease in the DPPH radical. It highlights the high antioxidant capacity of the rosin mixture.
Figure 8.

Evolution of the radical scavenging activity (RSA) as a function of time for different samples: neat CNC and CNF films, CNC–rosin and CNF–rosin films, TML–CNC–rosin and TML–CNF–rosin, and a rosin mixture.
In this study, the DPPH test was performed on neat CNC, neat CNF, modified CNC–rosin, and modified CNF–rosin films as well as on TML–CNC–rosin and TML–CNF–rosin multilayered materials. The resulting RSA kinetics are plotted as a function of time in Figure 8. The antioxidant capacity of CNC–rosin and CNF–rosin films is clearly observed, with the RSA values at the plateau equal to 21 and 20%, respectively. The CNC and CNF films were studied as a negative reference, and the observed unstable values can be directly linked to the fact that nanocellulose films were slightly disintegrated in the ethanol medium. However, it is noteworthy that neither CNCs nor CNFs exhibit any antioxidant capacity, as expected. In this sense, it is assumed that rosin nanoemulsions present in CNC–rosin and CNF–rosin films are still active against oxidation.
Multilayered materials TML–CNC–rosin and TML–CNF–rosin were also analyzed through the DPPH test. This experiment may be controversial and may need some further explanations. In the DPPH assay, antioxidant compounds need to be in direct contact with radicals. An additional DPPH test was performed on samples fully sealed (no direct contact between the inner layer and the DPPH solution), and no significant decrease in RSA was observed. In this experiment, the inner cellulosic layer of the multilayered materials is in direct contact with the DPPH solution, leading to the RSA values at the plateau equal to 29 and 43% for TML–CNC–rosin and TML–CNF–rosin samples, respectively. These results highlight the activity of the rosin nanoparticles inside the multilayered materials. However, it is difficult to deduce anything about the possible migration of the rosin nanoparticles through the film. Future experiments should be performed on TML–CNC–rosin and TML–CNF–rosin samples, as well as on CNC–rosin and CNF–rosin films, to determine whether the antioxidant properties are due to the surface activity of the films or not. Moreover, according to the type of application, different antioxidant phenomena can be envisaged. Nevertheless, at this stage of the study, it can be assumed that TML–CNC–rosin and TML–CNF–rosin films are antioxidants by direct contact with the inner layer, which emphasizes the role of rosin nanoparticles in the films. Although they do not significantly influence the barrier properties against oxygen and water vapor molecules, they confer the antioxidant behavior to the prepared films, which is highly sought after for many food packaging applications.
3. Conclusions
This study highlights an effective process for the development of PLA-based multilayered materials containing cellulosic nanostructures. This method, consisting of the complexing of a dry CNC or CNF self-standing film modified with natural rosins between two PLA sheets by heat pressing, clearly shows a significant improvement in oxygen barrier properties of the multilayered materials. Moreover, the presence of rosin nanoparticles in the inner cellulosic layer confers antioxidant properties to the materials. This procedure makes it possible to incorporate nanocelluloses—modified or not—into a PLA matrix quickly and easily, which paves the way toward the development of hybrid materials containing nanostructures and active compounds for, among others, food packaging applications.
4. Experimental Section
4.1. Materials
Spray-dried powder of cellulose nanocrystals (CNC) extracted from wood pulp was supplied by CelluForce© (Canada). Cellulose nanofibril aqueous suspension at 2 wt % (with viscosity equal to 1 Pa·s at 100 s–1) and the rosin-based nanoemulsion (with approximately 10 wt % of rosin content) were provided by the startup INOFIB (Saint-Martin d’Hères, France). Figure 9 presents the main components found in the commercial mixture of natural gum rosin (Sigma-Aldrich Chimie, France) used for the preparation of the rosin-based nanoemulsion. Ethanol (95%) was purchased from Revol (France) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Chimie (France). Earthfirst PLA film (Indegeo) was supplied by Sidaplax (France) company.
4.2. Preparation of Neat CNF and Modified CNF–Rosin Films by Filtration
The previously prepared rosin nanoemulsion (1 g) was added to 47 g of a 2% (wt/wt) aqueous suspension of CNF to obtain a rosin/CNF weight ratio equal to 0.11. The formulated suspension was then mixed under mechanical stirring for 30 min at room temperature to adsorb the nanomicelles of rosin onto the CNF surface. The CNF–rosin suspension was then stored in a hermetically sealed vial at 4 °C for further usage.
Both 47 g of the CNF suspension and 48 g of the formulated CNF–rosin suspension were then diluted to 0.5 wt % and mixed for 10 min under magnetic stirring. The diluted suspensions were then filtered through a 1 μm cutoff Nylon web and dried at 90 °C for 12 min under vacuum to obtain the CNF and CNF–rosin films. More precisely, 20 cm diameter sheets were prepared at 30 g/m2, using a Rapid–Köthen hand sheet former. This method, described in the literature,35 was adapted to the preparation of CNF sheets and could be transferred to the industrial scale.
4.3. Preparation of Neat CNC and Modified CNC–Rosin Films by Solvent Casting
Rosin nanoparticles were adsorbed on CNC, as previously described for CNF. Briefly, an aqueous suspension of CNC at 1% (wt/wt) was prepared by adding a correct amount of spray-dried CNC in water and by applying an ultrasonic treatment to the suspension (2.5 kJ/g of dried CNC). Rosin nanoemulsion (0.35 g) was then introduced in 31.4 g of the 1 wt % CNC suspension by keeping a rosin/CNC weight ratio equal to 0.11. Magnetic stirring at room temperature was performed for 30 min to adsorb the rosin nanoemulsion onto the CNC surface. The recovered CNC–rosin suspension was then stored in a hermetically sealed vial at 4 °C for further usage.
Both neat CNC and modified CNC–rosin suspensions were diluted at 0.5 wt % and sonicated (2.5 kJ/g of dried CNC). Both 62.8 g of CNC suspension and 63.2 g of formulated CNC–rosin suspensions were introduced in 10 cm diameter Petri dishes by keeping the basis weight of targeted films equal to 40 g/m2. For the preparation of CNC and CNC–rosin films, cast suspensions were then kept in a fume hood for 7 days at room temperature to evaporate water.54 In this case, the method of film preparation was adapted to CNC material from the literature but was different from that used for CNF because the preparation of CNC films was not feasible by filtration.
4.4. Complexing of Multilayered Materials by Heat Pressing
The multilayered materials were prepared by incorporating one neat or modified CNF or CNC film between two PLA sheets. The three-layer sandwich system was then heat-pressed (heat press device, Saint-Eloi Mécanique Outillage, France) between two metallic plates and two protective nonadhesive paper sheets, under 0.5 MPa pressure, for 10 min at 170 °C. Different thermopressed multilayered (TML) materials were thus prepared with an inner layer of PLA (TML–PLA) as a reference, or with CNF (TML–CNF), modified CNF–rosin (TML–CNF–rosin), CNC (TML–CNC), and modified CNC–rosin (TML–CNC–rosin).
4.5. Characterization Methods
4.5.1. Rosin Nanoemulsion Characterization by Dynamic Light Scattering (DLS)
As reported in the literature,55 the size of the rosin nanoparticles from the nanoemulsion was determined by DLS, using a VASCO Cordouan nanosizer. More precisely, three droplets of the nanoemulsion were introduced in the device with the dual-thickness controller (DTC) in the up position, diluted with distilled water, and roughly homogenized. The laser intensity and the correlator settings were adjusted at 5 μs as the time interval and 600 as the number of channels. Data were recorded using NanoQ software at 25 °C for 15 acquisitions of 30 s with a noise/signal ratio limit inferior to 0.5% (dual limits settings). Both the Pade–Laplace and the CUMULANT method were used for data processing. At least triplicate measurements were performed.
4.5.2. CNC and CNF Film Characterization by Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared spectroscopy was performed on both unmodified and modified nanocellulose films using a PerkinElmer spectrum 65. Films were analyzed through 16 scans and with a resolution of 2 cm–1. The baseline of the FTIR spectra was corrected, and the data were tuned up. Each spectrum containing CNC or CNF was normalized at 1110 cm–1 (related to pure cellulose). Each measurement was at least duplicated, and most representative spectra were plotted.
4.5.3. Contact Angle Measurements
Contact angle measurements were carried out on neat and modified nanocellulose films by depositing 5 μL water droplets on the film surface at room temperature. An OCA20 DataPhysics (DataPhysics Instrument) system, equipped with a CCD camera, was used to record the angles between the solvent and the substrate. The acquisition of contact angle and drop volume was performed for the first 60 s after deposition. At least five measurements were performed for each sample.
4.5.4. Scanning Electron Microscopy (SEM)
A Quanta200 device was used to produce SEM images of PLA films and sections of multilayered materials obtained by cryofracture. The working distance during the acquisition was between 9.8 and 10.1 mm, with a voltage of 10 kV and at a magnitude of 1000×.
4.5.5. UV–Visible Spectrophotometric Direct Transmission Analysis
Three samples of each film were prepared with dimensions equal to 15 mm × 50 mm. Direct transmittance, at a wavelength of 550 nm (T550 nm), was measured at least three times for each sample, using a UV–vis spectrophotometer (UV-1800, Shimadzu).
4.5.6. Tensile Tests
Mechanical properties of the films were determined under specific conditions (23 °C, 50% RH) using an Instron Universal Testing Machine Model 4507 (Instron Engineering Corporation, Canton, MA) equipped with pneumatic jaws. After its average thickness was measured, each sample (100 mm × 15 mm) endured a tensile cross-head speed of 10 mm/min, according to the French standard NF Q 03-004 (July 1986). The samples were preconditioned at 23 °C and 50% RH at least 1 day before the experiment. At least five tests were carried out for each sample.
4.5.7. Oxygen Permeability
The oxygen permeability of the materials was investigated at 23 °C and 0% RH or 50% RH, using a Systech Illinois M8001 Oxygen Permeation Analyzer. Data were processed according to the ASTM-F 1927 standard. For each sample, masks with a specific exchange surface of 6.2 cm2 were prepared and placed in the device chamber. Pure oxygen was used for the measurement. The final value of the oxygen transmission rate (OTR) was determined when a plateau was reached. The oxygen permeability coefficient (OP) was calculated by eq 1, according to the ASTM-F 1927 standard, where l is the average thickness of the sample in mm. Experiments were conducted in duplicate.
| 1 |
4.5.8. Water Vapor Permeability
Water vapor permeability of the materials was determined at 23 °C and 50% RH, according to the T448 om-09 TAPPI standard. For each sample, masks with a specific exchange surface of 6.2 cm2 were prepared and mounted on specific test dishes filled with dried CaCl2 as desiccant material. Regular weighing (every 4 h on the first day, then every 12 h) of the whole system was performed and the water vapor transmission rate (WVTR) in g/(m2·day) was calculated by eq 2 according to the T448 om-09 TAPPI standard.
| 2 |
where x is the gain, in grams for the period y, in hours, and A is the exposed area of the sample (6.2 cm2 in our study). The water vapor permeability coefficient (WVP) was calculated by eq 3.
| 3 |
where l is the average thickness of the sample, in mm. Each experiment has been at least duplicated.
4.5.9. Antioxidant Activity Characterization
The DPPH assay (free radical 2,2-diphenyl-1-picrylhydrazyl DPPH•) was performed to determine the radical scavenging activity (RSA) of the samples. The method was adapted according to Crouvisier-Urion et al.53 Samples of 100 mg of material were prepared and placed in a closed vial containing 10 mL of a 50 mg/L solution of DPPH in ethanol and were left under magnetic stirring in the dark and at room temperature. Aliquots of the medium solutions were collected at regular time intervals, and absorbance at 515 nm was measured (Asample), using a UV–vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Note that in ethanol, DPPH• is purple and turns yellowish when it is in direct contact with an antioxidant molecule. The collected solutions were returned to the corresponding vial after each measurement. Absorbance measurement of the DPPH solution (ADPPH) in ethanol was performed at the same time to correct the autodegradation of the DPPH• radical. From the experimental data, the radical scavenging activity at time t of each sample was calculated by eq 4
| 4 |
For each sample, the measurements of the RSA (in %) were duplicated and plotted as a function of the reaction time until a plateau was visible.
Acknowledgments
This work was supported by the French National Agency (ANR) as part of the program ANR-16-CE08-0040. LGP2 is part of the LabEx Tec 21 (Investissements d’Avenir—Grant Agreement No. ANR-11-LABC-0030) and of PolyNat Carnot Institute (Investissements d’Avenir—Grant Agreement No. ANR-16-CARN-0025-01). This research was made possible thanks to the facilities of the TekLiCell platform funded by the Région Rhône-Alpes (ERDF: European Regional Development Fund). The authors would like to thank Bertine Khelifi (LGP2, Grenoble, France) for the SEM experiments.
Author Present Address
∥ GenesInk, 39 Avenue Gaston Imbert, 13790 Rousset Cedex, France.
Author Present Address
⊥ INRAE, UR BIA, F-44316 Nantes, France.
Author Present Address
# Nestle Research Center, 1010 Lausanne, Switzerland.
Author Contributions
M.L.G. and B.D. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Dufresne A.Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; de Gruyter: Berlin, 2018. [Google Scholar]
- Kargarzadeh H.; Mariano M.; Gopakumar D.; Ahmad I.; Thomas S.; Dufresne A.; Huang J.; Lin N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. 10.1007/s10570-018-1723-5. [DOI] [Google Scholar]
- Natterodt J. C.; Petri-Fink A.; Weder C.; Zoppe J. O. Cellulose Nanocrystals: Surface Modification, Applications and Opportunities at Interfaces. Chim. Int. J. Chem. 2017, 71, 376–383. 10.2533/chimia.2017.376. [DOI] [PubMed] [Google Scholar]
- Klemm D.; Kramer F.; Moritz S.; Lindström T.; Ankerfors M.; Gray D.; Dorris A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem., Int. Ed. 2011, 50, 5438–5466. 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- Nechyporchuk O.; Belgacem M. N.; Bras J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. 10.1016/j.indcrop.2016.02.016. [DOI] [Google Scholar]
- Kontturi E.; Laaksonen P.; Linder M. B.; Nonappa; Gröschel A. H.; Rojas O. J.; Ikkala O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779 10.1002/adma.201703779. [DOI] [PubMed] [Google Scholar]
- Hubbe M. A.; Ferrer A.; Tyagi P.; Yin Y.; Salas C.; Pal L.; Rojas O. J. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. BioResources 2017, 12, 2143–2233. 10.15376/biores.12.1.2143-2233. [DOI] [Google Scholar]
- Ferrer A.; Pal L.; Hubbe M. Nanocellulose in Packaging: Advances in Barrier Layer Technologies. Ind. Crops Prod. 2017, 95, 574–582. 10.1016/j.indcrop.2016.11.012. [DOI] [Google Scholar]
- Bideau B.; Bras J.; Saini S.; Daneault C.; Loranger E. Mechanical and Antibacterial Properties of a Nanocellulose-Polypyrrole Multilayer Composite. Mater. Sci. Eng. C 2016, 69, 977–984. 10.1016/j.msec.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Missoum K.; Sadocco P.; Causio J.; Belgacem M. N.; Bras J. Antibacterial Activity and Biodegradability Assessment of Chemically Grafted Nanofibrillated Cellulose. Mater. Sci. Eng. C 2014, 45, 477–483. 10.1016/j.msec.2014.09.037. [DOI] [PubMed] [Google Scholar]
- de Castro D. O.; Bras J.; Gandini A.; Belgacem N. Surface Grafting of Cellulose Nanocrystals with Natural Antimicrobial Rosin Mixture Using a Green Process. Carbohydr. Polym. 2016, 137, 1–8. 10.1016/j.carbpol.2015.09.101. [DOI] [PubMed] [Google Scholar]
- Wang J.; Yao K.; Korich A. L.; Li S.; Ma S.; Ploehn H. J.; Iovine P. M.; Wang C.; Chu F.; Tang C. Combining Renewable Gum Rosin and Lignin: Towards Hydrophobic Polymer Composites by Controlled Polymerization. J. Polym. Sci., Part A: Polym. Chem. 2011, 49, 3728–3738. 10.1002/pola.24809. [DOI] [Google Scholar]
- Moustafa H.; El Kissi N.; Abou-Kandil A. I.; Abdel-Aziz M. S.; Dufresne A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. 10.1021/acsami.7b05557. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Yao K.; Lee J.; Chandler D.; Wang J.; Wang C.; Chu F.; Tang C. Well-Defined Renewable Polymers Derived from Gum Rosin. Macromolecules 2010, 43, 5922–5924. 10.1021/ma101071p. [DOI] [Google Scholar]
- Arrieta M. P.; Samper M. D.; Jiménez-López M.; Aldas M.; López J. Combined Effect of Linseed Oil and Gum Rosin as Natural Additives for PVC. Ind. Crops Prod. 2017, 99, 196–204. 10.1016/j.indcrop.2017.02.009. [DOI] [Google Scholar]
- Belgacem M. N., Ed. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, 2008. [Google Scholar]
- Kale S. N.; Deore S. L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. 10.5530/srp.2017.1.8. [DOI] [Google Scholar]
- Fornaguera C.; Dols-Perez A.; Calderó G.; García-Celma M. J.; Camarasa J.; Solans C. PLGA Nanoparticles Prepared by Nano-Emulsion Templating Using Low-Energy Methods as Efficient Nanocarriers for Drug Delivery across the Blood–Brain Barrier. J. Controlled Release 2015, 211, 134–143. 10.1016/j.jconrel.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Anton N.; Benoit J.-P.; Saulnier P. Design and Production of Nanoparticles Formulated from Nano-Emulsion Templates—A Review. J. Controlled Release 2008, 128, 185–199. 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Niu X.; Liu Y.; Song Y.; Han J.; Pan H. Rosin Modified Cellulose Nanofiber as a Reinforcing and Co-Antimicrobial Agents in Polylactic Acid/Chitosan Composite Film for Food Packaging. Carbohydr. Polym. 2018, 183, 102–109. 10.1016/j.carbpol.2017.11.079. [DOI] [PubMed] [Google Scholar]
- Castro-Aguirre E.; Iñiguez-Franco F.; Samsudin H.; Fang X.; Auras R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Delivery Rev. 2016, 107, 333–366. 10.1016/j.addr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Fiori S.Chapter 13. Industrial Uses of PLA. In Polymer Chemistry Series; Jiménez A.; Peltzer M.; Ruseckaite R., Eds.; Royal Society of Chemistry: Cambridge, 2014; pp 315–333. [Google Scholar]
- Oksman K.; Aitomäki Y.; Mathew A. P.; Siqueira G.; Zhou Q.; Butylina S.; Tanpichai S.; Zhou X.; Hooshmand S. Review of the Recent Developments in Cellulose Nanocomposite Processing. Composites, Part A 2016, 83, 2–18. 10.1016/j.compositesa.2015.10.041. [DOI] [Google Scholar]
- Chi K.; Catchmark J. M. Enhanced Dispersion and Interface Compatibilization of Crystalline Nanocellulose in Polylactide by Surfactant Adsorption. Cellulose 2017, 24, 4845–4860. 10.1007/s10570-017-1479-3. [DOI] [Google Scholar]
- Lin N.; Dufresne A. Physical and/or Chemical Compatibilization of Extruded Cellulose Nanocrystal Reinforced Polystyrene Nanocomposites. Macromolecules 2013, 46, 5570–5583. 10.1021/ma4010154. [DOI] [Google Scholar]
- Espino-Pérez E.; Bras J.; Almeida G.; Plessis C.; Belgacem N.; Perré P.; Domenek S. Designed Cellulose Nanocrystal Surface Properties for Improving Barrier Properties in Polylactide Nanocomposites. Carbohydr. Polym. 2018, 183, 267–277. 10.1016/j.carbpol.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Cerqueira M. A. P. R.; Lagaron J. M.; Castro L. M. P.; de Oliveira Soares Vicente A. A. M., Eds. Nanomaterials for Food Packaging: Materials, Processing Technologies and Safety Issues. In Micro & Nano Technologies Series; Elsevier: Amsterdam, 2018. [Google Scholar]
- Aulin C.; Karabulut E.; Tran A.; Wågberg L.; Lindström T. Transparent Nanocellulosic Multilayer Thin Films on Polylactic Acid with Tunable Gas Barrier Properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. 10.1021/am401700n. [DOI] [PubMed] [Google Scholar]
- Meriçer Ç.; Minelli M.; Angelis M. G. D.; Baschetti M. G.; Stancampiano A.; Laurita R.; Gherardi M.; Colombo V.; Trifol J.; Szabo P.; Lindström T. Atmospheric Plasma Assisted PLA/Microfibrillated Cellulose (MFC) Multilayer Biocomposite for Sustainable Barrier Application. Ind. Crops Prod. 2016, 93, 235–243. 10.1016/j.indcrop.2016.03.020. [DOI] [Google Scholar]
- Hosseini S. F.; Javidi Z.; Rezaei M. Efficient Gas Barrier Properties of Multi-Layer Films Based on Poly(Lactic Acid) and Fish Gelatin. Int. J. Biol. Macromol. 2016, 92, 1205–1214. 10.1016/j.ijbiomac.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Fortunati E.; Armentano I.; Zhou Q.; Iannoni A.; Saino E.; Visai L.; Berglund L. A.; Kenny J. M. Multifunctional Bionanocomposite Films of Poly(Lactic Acid), Cellulose Nanocrystals and Silver Nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. 10.1016/j.carbpol.2011.09.066. [DOI] [Google Scholar]
- Luzi F.; Fortunati E.; Giovanale G.; Mazzaglia A.; Torre L.; Balestra G. M. Cellulose Nanocrystals from Actinidia Deliciosa Pruning Residues Combined with Carvacrol in PVA_CH Films with Antioxidant/Antimicrobial Properties for Packaging Applications. Int. J. Biol. Macromol. 2017, 104, 43–55. 10.1016/j.ijbiomac.2017.05.176. [DOI] [PubMed] [Google Scholar]
- Missoum K.; Bras J.; Belgacem N.. Method for Forming a Hydrophobic Layer. U.S. Patent US20160168696A12016.
- Jaiswal M.; Dudhe R.; Sharma P. K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhanari K.; Syverud K.; Chinga-Carrasco G.; Paso K.; Stenius P. Reduction of Water Wettability of Nanofibrillated Cellulose by Adsorption of Cationic Surfactants. Cellulose 2011, 18, 257–270. 10.1007/s10570-010-9482-y. [DOI] [Google Scholar]
- Dankovich T. A.; Gray D. G. Contact Angle Measurements on Smooth Nanocrystalline Cellulose (I) Thin Films. J. Adhes. Sci. Technol. 2011, 25, 699–708. 10.1163/016942410X525885. [DOI] [Google Scholar]
- Sanyang M. L.; Sapuan S. M.; Jawaid M.; Ishak M. R.; Sahari J. Development and Characterization of Sugar Palm Starch and Poly(Lactic Acid) Bilayer Films. Carbohydr. Polym. 2016, 146, 36–45. 10.1016/j.carbpol.2016.03.051. [DOI] [PubMed] [Google Scholar]
- Pinto A. M.; Cabral J.; Tanaka D. A. P.; Mendes A. M.; Magalhães F. D. Effect of Incorporation of Graphene Oxide and Graphene Nanoplatelets on Mechanical and Gas Permeability Properties of Poly(Lactic Acid) Films: Incorporation of GO and GNP in PLA Films. Polym. Int. 2013, 62, 33–40. 10.1002/pi.4290. [DOI] [Google Scholar]
- Beck S.; Bouchard J.; Chauve G.; Berry R. Controlled Production of Patterns in Iridescent Solid Films of Cellulose Nanocrystals. Cellulose 2013, 20, 1401–1411. 10.1007/s10570-013-9888-4. [DOI] [Google Scholar]
- Lizundia E.; Urruchi A.; Vilas J. L.; León L. M. Increased Functional Properties and Thermal Stability of Flexible Cellulose Nanocrystal/ZnO Films. Carbohydr. Polym. 2016, 136, 250–258. 10.1016/j.carbpol.2015.09.041. [DOI] [PubMed] [Google Scholar]
- Xiong R.; Han Y.; Wang Y.; Zhang W.; Zhang X.; Lu C. Flexible, Highly Transparent and Iridescent All-Cellulose Hybrid Nanopaper with Enhanced Mechanical Strength and Writable Surface. Carbohydr. Polym. 2014, 113, 264–271. 10.1016/j.carbpol.2014.06.069. [DOI] [PubMed] [Google Scholar]
- Arrieta M. P.; Samper M. D.; Aldas M.; López J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008 10.3390/ma10091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlotta D. A. Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. 10.1023/A:1020200822435. [DOI] [Google Scholar]
- Hänninen A.; Sarlin E.; Lyyra I.; Salpavaara T.; Kellomäki M.; Tuukkanen S. Nanocellulose and Chitosan Based Films as Low Cost, Green Piezoelectric Materials. Carbohydr. Polym. 2018, 202, 418–424. 10.1016/j.carbpol.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Reising A. B.; Moon R. J.; Youngblood J. P. Effect of Particle Alignment on Mechanical Properties of Neat Cellulose Nanocrystal Films. J. Sci. Technol. For. Prod. Processes 2012, 2, 32–41. [Google Scholar]
- Bras J.; Viet D.; Bruzzese C.; Dufresne A. Correlation between Stiffness of Sheets Prepared from Cellulose Whiskers and Nanoparticles Dimensions. Carbohydr. Polym. 2011, 84, 211–215. 10.1016/j.carbpol.2010.11.022. [DOI] [Google Scholar]
- Arora A.; Padua G. W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. 10.1111/j.1750-3841.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- Colomines G.; Ducruet V.; Courgneau C.; Guinault A.; Domenek S. Barrier Properties of Poly(Lactic Acid) and Its Morphological Changes Induced by Aroma Compound Sorption. Polym. Int. 2010, 59, 818–826. 10.1002/pi.2793. [DOI] [Google Scholar]
- Schmid M.; Dallmann K.; Bugnicourt E.; Cordoni D.; Wild F.; Lazzeri A.; Noller K. Properties of Whey-Protein-Coated Films and Laminates as Novel Recyclable Food Packaging Materials with Excellent Barrier Properties. Int. J. Polym. Sci. 2012, 2012, 562381 10.1155/2012/562381. [DOI] [Google Scholar]
- Espino-Pérez E.; Bras J.; Almeida G.; Relkin P.; Belgacem N.; Plessis C.; Domenek S. Cellulose Nanocrystal Surface Functionalization for the Controlled Sorption of Water and Organic Vapours. Cellulose 2016, 23, 2955–2970. 10.1007/s10570-016-0994-y. [DOI] [Google Scholar]
- Auras R.; Harte B.; Selke S. Sorption of Ethyl Acetate and D-Limonene in Poly(Lactide) Polymers. J. Sci. Food Agric. 2006, 86, 648–656. 10.1002/jsfa.2391. [DOI] [Google Scholar]
- Gómez-Estaca J.; López-de-Dicastillo C.; Hernández-Muñoz P.; Catalá R.; Gavara R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- Crouvisier-Urion K.; Bodart P. R.; Winckler P.; Raya J.; Gougeon R. D.; Cayot P.; Domenek S.; Debeaufort F.; Karbowiak T. Biobased Composite Films from Chitosan and Lignin: Antioxidant Activity Related to Structure and Moisture. ACS Sustainable Chem. Eng. 2016, 4, 6371–6381. 10.1021/acssuschemeng.6b00956. [DOI] [Google Scholar]
- Majoinen J.; Kontturi E.; Ikkala O.; Gray D. G. SEM Imaging of Chiral Nematic Films Cast from Cellulose Nanocrystal Suspensions. Cellulose 2012, 19, 1599–1605. 10.1007/s10570-012-9733-1. [DOI] [Google Scholar]
- Calderó G.; García-Celma M. J.; Solans C. Formation of Polymeric Nano-Emulsions by a Low-Energy Method and Their Use for Nanoparticle Preparation. J. Colloid Interface Sci. 2011, 353, 406–411. 10.1016/j.jcis.2010.09.073. [DOI] [PubMed] [Google Scholar]