Summary
Background:
Given the lack of long-term prospective studies, it is challenging for clinicians to make informed decisions about screening and treatment decisions regarding the risk of hepatocellular carcinoma (HCC) in patients with non-alcoholic steatohepatitis (NASH) who do not have cirrhosis.
Aims:
To characterise the pooled risk of HCC in the non-cirrhosis population.
Methods:
Published studies were identified through April 2016 in MEDLINE, Scopus, Science Citation Index, AMED and the Cochrane Library. Two independent reviewers screened citations and extracted data. Random effect odds ratios (OR) were calculated to obtain aggregate estimates of effect size between NASH and non-NASH groups. Between-study variability and heterogeneity were assessed.
Results:
Nineteen studies with 168 571 participants were included. Eighty-six per cent of included subjects had cirrhosis. The prevalence of HCC in non-cirrhotic NASH was 38.0%; among other aetiologies in non-cirrhotics, it was 14.2% (P < 0.001). Non-cirrhotic NASH subjects were at greater odds of developing HCC than non-cirrhotic subjects of other aetiologies (OR 2.61, 95% CI 1.27–5.35, P = 0.009). When examining all NASH subjects either with or without cirrhosis, those with NASH as the underlying liver disease did not have a significantly increased risk of HCC (OR 1.43, 95% CI 0.77-2.65, P = 0.250).
Conclusions:
In non-cirrhotic subjects, those with NASH have a higher risk of HCC compared to other aetiologies of liver disease. Further study investigating the risk factors of HCC among non-cirrhotic NASH patients is needed.
1 |. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease worldwide.1–3 Typically associated with the metabolic syndrome (hypertension, hyperglycemia, central obesity and dyslipidemia), the pathogenesis of NAFLD is characterised by a dysfunctional central adipose tissue compartment and insulin resistance leading to the release of inflammatory cytokines, as well as down regulation of adiponectin, an anti-inflammatory molecule.4,5 The downstream effects of this imbalance lead to lipid deposition in hepatocytes, causing lipotoxicity which interferes with intracellular signaling.6 In addition, fatty acid oxidation yields free radicals, resulting in oxidative stress.7 The subsequent hepatic inflammatory response leads to non-alcoholic steatohepatitis (NASH) in 25% of patients.8 Prolonged damage may ultimately lead to cirrhosis in approximately 20% of NASH patients (about 4%-5% of NAFLD patients).2,9
The prevalence of NAFLD has increased dramatically over the past several decades. In the USA, 30%-40% of adults have NAFLD4 and the prevalence in Japan is between 20% and 35% of the adult population.1,9,10 These figures are predicted to rise exponentially, as the World Health Organization estimates that the global percentage of overweight adults will increase from 35% to 57.8% by 2030.11 There is also mounting evidence that the prevalence of NASH is underestimated and that patients with cryptogenic cirrhosis may primarily have underlying NASH.12,13
NASH has gained particular attention over the last decade because of its association with hepatocellular carcinoma (HCC). This is largely driven by both lipotoxicity and insulin resistance, ultimately leading to increased fibrogenesis, inflammation and abnormal cellular proliferation in addition to alteration of cell death via apoptosis, necroptosis and autophagy, are associated with HCC.5 The incidence of HCC has increased by more than threefold over the past 30 years from 1.5 to 4.9 per 100 000, and HCC is now the 5th most common cancer worldwide and 3rd leading cause of cancer-related death.14 Rates of HCC are also increasing in the USA with nearly a fourfold increase from 1.5 to 6.2 per 100 000 persons since 1973.15 Notably, there has been a stark increase in the percentage of HCC arising from a nonviral aetiology. The Japanese NOBLESSE study group found that this more than doubled from 10% in 1991 to 24.1% in 2010.16 While HCC often arises in the presence of cirrhosis (with an exception being chronic hepatitis B given the direct hepatotoxic nature of the virus), NASH-associated HCC may develop in both the presence and absence of cirrhosis.5,17,18 For this reason, there may be a substantial number of higher risk patients who are not captured by routine HCC screening per current guidelines from the American Association for the Study of Liver Diseases (AASLD).19
Given the potential for NASH to become a leading cause of HCC in the future,20 estimating its substantial morbidity and mortality is important. Tokushige et al9 demonstrated that 11.3% of patients with NASH cirrhosis developed HCC within 5 years, on par with a 12.5% rate for patients with alcoholic cirrhosis; however, survival is lower.21 In addition, NASH cirrhosis is currently the second leading indication for liver transplantation (LT) in the USA.22 However, transplant candidates with NASH cirrhosis and HCC are less likely to undergo LT, perhaps in part because of the more advanced stage of their tumours at the time of diagnosis and an increased burden of medical comorbidity in the setting of advanced age.23 While the literature clearly demonstrates an association between HCC and NASH on an individual study level, large scale studies are lacking in this patient population as we are aware of only one other meta-analysis investigating this subject incorporating studies from 2012 and before which found that HCC risk was largely limited to NASH cirrhosis patients.24 This makes informed decisions regarding HCC screening, surveillance and treatment difficult for the clinician. We aimed to determine the pooled risk of HCC in patients with NASH both in the presence and absence of cirrhosis using the available literature.
2 |. METHODS
2.1 |. Search strategy and study selection
We performed a systematic review of the medical literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Trained study investigators systematically searched the medical literature for publications that contained information allowing for the comparison of HCC risk in NASH patients both with and without cirrhosis. Published studies were identified through electronic databases; MEDLINE, Scopus, Science Citation Index, AMED and the Cochrane Library were utilised. The search criteria included all publications through April 2016 with English language and human subject restriction. Electronic search criteria included the following terms or keywords: “nonalcoholic steatohepatitis,” “hepatocellular carcinoma,” “cirrhosis.” Reference lists of the included articles were reviewed in their entirety to identify any publications not identified by the initial database searches. Using the same keywords and terms, recent conference abstract lists and other relevant grey literature sources were also searched for relevant studies. Studies were excluded if there was no included control or comparison group, aetiology of liver disease was unclear, no cases of NASH were included, or if information about cirrhosis or the lack thereof was not available. Studies were not excluded based on the aetiology of liver disease in the comparator group (eg all chronic HCV). Letters to the editor and review articles were also excluded. Institutional review board approval was not required for this systematic review.
2.2 |. Data extraction
Two investigators (JS and BW) independently reviewed the titles and abstracts of all studies identified using the previously described search criteria to identify studies meeting the inclusion criteria. Each study meeting requirements of the first-round inclusion criteria then underwent a full-text independent review by both reviewers. Disagreements about inclusion between reviewers were resolved by a third clinical reviewer (PS). Two reviewers (JS and BW) independently extracted the following data from each study that met inclusion criteria: patient characteristics (age, gender, model for end-stage liver disease [MELD] score and aetiology of liver disease), study-level characteristics (author, publication year, study design, enrolment period, target population, total number of enrolled patients and percentage of patients with HCC).
2.3 |. Primary and secondary outcomes
The primary outcome of our study was the pooled risk of HCC in patients with NASH both in the presence and absence of cirrhosis when compared to all other aetiologies of liver disease, including alcoholic, chronic hepatitis B or C, autoimmune, cholestatic, Wilson’s disease and hereditary hemochromatosis. The secondary outcome of interest was the pooled HCC risk in NASH patients without cirrhosis compared to the same composite group of all other aetiologies of liver disease.
2.4 |. Study quality and bias assessment
As all included studies were observational, the quality of observational studies was assessed by the Newcastle-Ottawa Quality Assessment for Case Control and Cohort Studies scale25 and determined based on selection of study groups (maximum four stars), comparability of groups (maximum two stars) and ascertainment of outcome (maximum three stars) on an nine-star scale. The supplementary information provides the bias assessment for the included studies. No studies were excluded due to low-quality.
2.5 |. Statistical analysis
Review manager software (Rev-Man version 5.3; Copenhagen; The Nordic Cochrane Centre; The Cochrane Collaboration; 2014) was utilised to perform descriptive analysis of the studies identified, excluded, and included, and meta-analysis of the reported study effect measures. Pooled odds ratios (ORs) were estimated by the Maentel-Haenszel method. Corresponding 95% confidence intervals (CIs) were calculated using DerSimonian and Laird random-effect models, which account for both between- and within-study variabili1ty.26,27 Between-study variability was then separately assessed using the Cochran’s Q statistic (with P < 0.10 considered significant).26,27 The proportion of heterogeneity accounted for by between-study variability was estimated using the I2 index and adjudicated to be significant if I2 was >50%.26,27 A funnel plot was created post hoc to assess for the presence or absence of significant publication bias due to the tendency for studies with positive results to be published.
3 |. RESULTS
3.1 |. Included studies
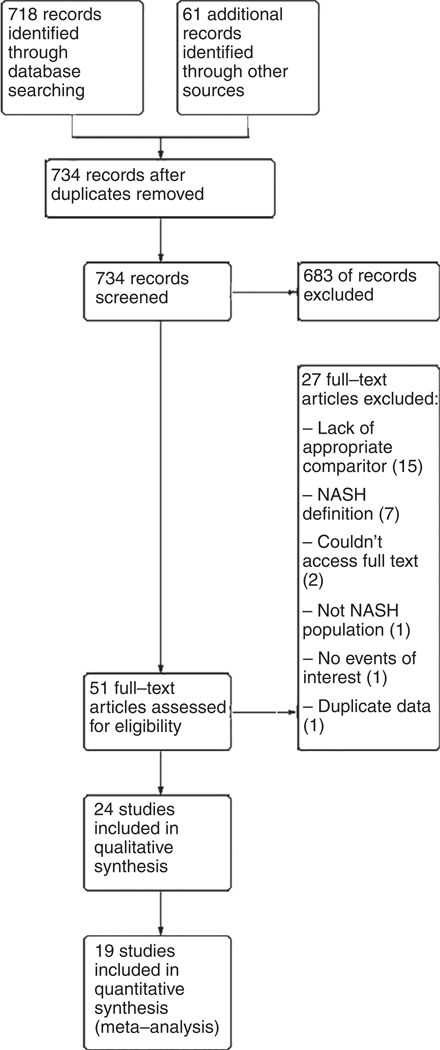
The electronic search criteria identified 734 studies. After ensuring no duplicates were present, we screened titles and abstracts, and 51 studies were assessed for eligibility through full-text review. Through the qualitative systematic review process described in detail in the methods section, 19 observational case-control or cohort studies9,16,17,23,28–42 met the inclusion criteria for quantitative synthesis in our meta-analysis. No additional studies were appropriate for inclusion based on our a priori determined inclusion and exclusion criteria.
Study level characteristics are found in Table 1. The majority of studies controlled for confounding from common comorbidities seen concurrently with NASH including diabetes and obesity, each of which has been associated with HCC risk.23,43–45 A summary of the search results is presented in Figure 1, reflecting the reporting standards of the PRISMA.46
TABLE 1.
Study level characteristics: NASH subjects without cirrhosis compared to other aetiologies of chronic liver disease without cirrhosis
| Reference | Year published | Years enrolled | Study design | NASH definition | Confounders adjusted for | ALD definition | NASH (%) | HCV (%) | ALD (%) | HBV (%) | Other/Not specified (%)a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ertle et al42 | 2011 | 2007–2008 | Retrospective cross-sectional | Liver histology or cryptogenic liver disease in the presence of metabolic syndrome | Diabetes Obesity |
>2 drinks/day or >6 drinks daily on weekends for >5 years | 36.4 (n = 59) | 21.6 (n = 35) | 11.7 (n = 19) | 17.9 (n = 29) | 12.3 (n = 20) |
| Reddy et al29 | 2012 | 2000–2010 | Prospective cohort (median follow-up 50 mo) | Liver histology | Diabetes Obesity |
2 drinks per day | 24.3 (n = 52) | 75.7 (n =162) | — | — | |
| Tokushige et al9 | 2013 | 2006–2009 | Prospective cohort (5 year follow-up) | Expert clinical opinion | Age Gender |
N/A | 2.0 (n = 292) | Not presented | Not presented | Not presented | 98.0 (n = 14 238) |
| Schutte et al30 | 2014 | 1994–2013 | Retrospective cohort (median follow-up not described) | Ultrasound with steatosis in the absence of other liver disease | Age Diabetes Gender Obesity |
>70 g/day of alcohol or history of alcohol abuse in medical record | 6.5 (n = 43) | 3.2 (n = 21) | 15.1 (n =100) | 4.3 (n = 29) | 71.0 (n = 471) |
| Rim et al31 | 2014 | 2005–2012 | Cross-sectional | Expert clinical opinion | Not stated | N /A | 7.9 (n = 35) | — | — | 92.1 (n = 724) | — |
| Tateishi et al16 | 2015 | 1991–2010 | Prospective cohort (up to 20 years follow-up) | Expert clinical opinion | Age Diabetes Gender Obesity |
>80 g/day without another definitive aetiology | 11.2 (n = 590) | Not presented | 27.2 (N = 1423) | Not presented | 61.5 (n = 3217) |
| Mittal et al17 | 2015 | 2004–2011 | Retrospective cohort (up to 6 years follow-up) | Liver histology or cryptogenic liver disease in the presence of metabolic syndrome | Age Ethnicity |
>3 drinks a day, alcoholism/alcohol abuse in the medical record, enrolment in substance abuse program and/or history of alcoholic hepatitis | 8.0 (n = 120) | 67.5 (n = 1013) | 19.1 (n = 286) | 4.6 (n = 69) | 0.8 (n = 12) |
Includes any combination of hepatitis B, hepatitis C (if not captured separately), alcoholic liver disease (if not captured separately) autoimmune, hemochromatosis, primary sclerosing cholangitis, primary biliary cirrhosis, Wilson’s disease, toxic liver disease.
FIGURE 1.
PRISMA diagram for search scheme for HCC risk in NASH
A total of 168 571 participants were included in our analysis. Patient level characteristics are found in the Supporting information. Patients with NASH (n = 13 345) were compared to patients with liver disease from a composite of all other non-NASH aetiologies (n = 155 226). Fourteen percent (n = 23 059) of included subjects did not have cirrhosis, of which 1191 had NASH. The prevalence of HCC in all included subjects was 14.5% (n = 24 467). Mean ages ranged from 46 to 75 years. For subjects with cirrhosis, mean laboratory MELD scores ranged from 9 to 22.
3.2 |. HCC in all NASH patients
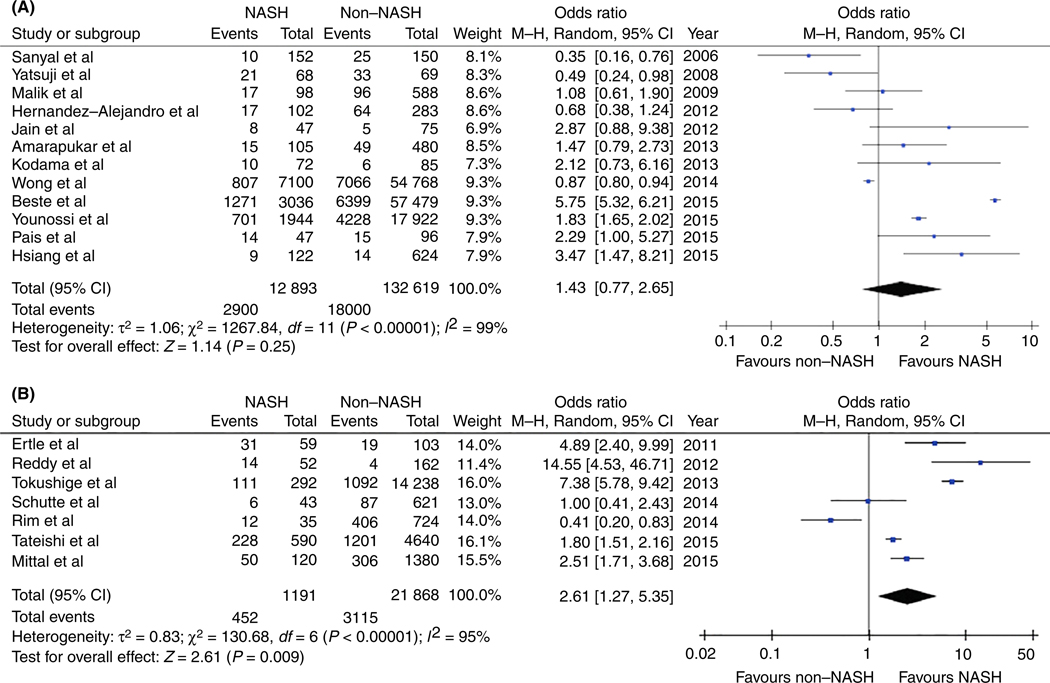
Figure 2A demonstrates the risk of HCC in NASH patients both in the presence and absence of cirrhosis. Twelve observational studies contributed 145 512 subjects of whom 20 900 (14.4%) had HCC. The prevalence of HCC in all NASH was 22.5% and among other all non-NASH subjects was 13.6%, P < 0.001 The aggregate measure of effect from the 12 included studies showed that while not statistically significant, there was a trend towards significance with increased risk of HCC when comparing NASH patients to all other aetiologies of liver disease (OR 1.43, 95% CI 0.77-2.65, P = 0.25, I2 = 99%). No publication bias was observed (Figure S1).
FIGURE 2.
A, Pooled measure of effects for NASH and HCC in all patients (either with or without cirrhosis). There was a trend towards significance with increased risk of HCC when comparing NASH patients to all other aetiologies of liver disease, however, this was not statistically significant. B, Pooled measure of effects of NASH and HCC in patients without cirrhosis. The overall pooled estimate from the seven studies indicates that patients with NASH have a 261% increased risk of HCC when compared to all other aetiologies of liver disease
3.3 |. HCC in NASH patients without cirrhosis
Figure 2B shows the risk of HCC in NASH patients without cirrhosis. Seven observational studies were included in this analysis involving 3567 cases (15.4%) of HCC in 23 059 patients. The prevalence of HCC in noncirrhotic NASH was 38.0% and among other aetiologies in noncirrhotics was 14.2%, P < 0.001. The overall pooled estimate from the seven studies combined indicated that NASH patients without cirrhosis had a nearly threefold increased risk of HCC when compared to all other aetiologies of liver disease (OR 2.61, 95% CI 1.27-5.35, P = 0.009, I2 = 95%). Data on fibrosis stage at the individual patient level could not be extracted from the included studies for sub-analysis.
4 |. DISCUSSION
This study is the most comprehensive assessment of epidemiologic data highlighting the unique association of NASH with HCC. We performed two separate meta-analyses to better quantify this relationship. Our systematic review and meta-analysis is the first study to offer a pooled estimate and quantitative assessment of the clinical risk of HCC in both patients with and without cirrhosis. We have demonstrated a highly significant increased risk of HCC in NASH subjects. This appears to be specifically pertinent to noncirrhotic NASH patients, who are estimated to have a more than 2.5-fold increased risk of HCC in the absence of cirrhosis compared to other aetiologies of noncirrhotic chronic liver disease. This effect persists when comparing all NASH patients with and without cirrhosis to a composite of all other aetiologies of liver disease, albeit the magnitude of effect is attenuated. This important finding suggests a need to reframe our understanding of the NASH-HCC association to ensure that effective surveillance measures are instituted. The poorer outcomes both pre- and post-transplantation47 in this patient population, where tumours are often discovered at a more advanced stage with larger volume and greater degrees of infiltration and in the presence of diabetes and obesity,23,43,44 only further underscores the need for improved HCC screening. Consequently, outcomes for NASH-associated HCC are, in general, inferior to other aetiologies of liver disease as lower survival rates and higher 1-year mortality rates after diagnosis of HCC have been reported.23 In fact, the majority of NASH patients with HCC die from their primary liver cancer rather than from cardiovascular disease, and rates of HCC-related mortality are greatest in the NASH population.23 On the other hand, survival rates for surgical resection in patients with NASH and HCC are similar to other liver diseases, particularly in the absence of cirrhosis, albeit in highly selected surgical candidates.18,47
Historically, NASH patients are less likely to undergo appropriate HCC screening and surveillance.48 When screening is performed, there are inherent limitations to the most widely used imaging modality as ultrasound has a lower sensitivity for detection of smaller tumours in patients with NASH, owing largely to visceral adiposity and hepatic steatosis.49,50 NASH patients also have the lowest rates of recognised liver disease, as diagnostic uncertainty about either NASH or the presence of cirrhosis often compromises effective screening; this has been cited as the leading reason medical providers do not perform HCC screening in this patient population.50
Non-alcoholic steatohepatitis patients without cirrhosis are not addressed by the current EASL-EORTC HCC guidelines and the AASLD HCC screening guidelines beyond the recognition that HCC risk is increased in noncirrhotic NAFLD but that surveillance efficacy has not yet been demonstrated.19,51 Revisiting this recommendation seems warranted based on our findings, however, given the large number of patients with NAFLD and/or NASH without cirrhosis, extending HCC screening to this patient population would substantially increase healthcare spending associated with HCC reduction and would need to be modelled extensively prior to widespread use. In fact, the AASLD HCC guidelines suggest an annual incidence of 1.5% as the threshold for effective HCC surveillance in this population and while our study was not intended to evaluate this proposition, our high prevalence rate in the noncirrhotic NASH group strongly suggest this problem may be more common than previously thought. More research is needed to better quantify the cost-effectiveness of universal screening in this increasingly recognised high-risk population to provide updated guidance in this high-risk population.
Stratification by fibrosis score may offer some further identification of the subgroup of noncirrhotic NASH most likely to benefit from screening practices; however, reports of HCC in patients with NAFLD and stage 0 fibrosis have been described.5 The effect of fibrosis on HCC risk remains unknown. Previous studies suggest the composite of stage 3 and 4 fibrosis have similar cumulative HCC incidence rates when compared to cirrhosis alone.52,53 Recommendations have been made to screen those patients with stage 3 fibrosis disease in addition to those with cirrhosis,23 although this was based on one experience and the authors’ expert opinion. To our knowledge, no direct comparison has been made between advanced fibrosis (F3-F4) and early stage fibrosis (F0-F2), although cases of HCC in early stages of fibrosis have been reported in case reports or case series in over one hundred patients.5 Further research to stratify the association between fibrosis stage and HCC risk in NASH patients without cirrhosis would aid in targeting populations at highest or comparable risks.
Several limitations need to be considered when interpreting the findings of our work. First, we excluded a large number of studies due to either missing data or a lack of an effective comparator group. Second, as with any comparative effectiveness research, information bias inherent in the primary literature can always pose a problem and while the inclusion of only high-quality observational studies was meant to protect against the introduction of bias, there are inherent limitations to retrospective study designs that should be acknowledged. There was also limited data available about the stage of fibrosis in patients without cirrhosis. In many instances, no information about time to diagnosis or differing follow-up periods in the cohort studies was able to be extracted either which could introduce either lead or lag time bias into our analysis. Our work also suffered from significant heterogeneity in the included studies which, while offset by the large number of patients included, is still worth mentioning as it precluded further meta-analysis of several potential outcomes of interest. This phenomenon may be explained in part by our use of a composite group of multiple liver diseases, especially since different aetiologies of chronic liver disease appear to have inherently different risks for HCC incidence. Our results may have been biased towards the null by our inclusion of chronic hepatitis C in the comparator group, as this population historically has one of the highest risks of HCC.23,32,54 On the other hand, inclusion of only NASH and not just NAFLD subjects may introduce bias away from the null hypothesis and exaggerate the HCC risk in the NASH population as NASH by definition includes only subjects who are most likely to have some degree of liver fibrosis.
In conclusion, taken as an isolated aetiology, individual studies suggest NASH is associated with HCC risk when compared to other aetiologies of liver disease. In pooled analysis, we found that in noncirrhotic patients, those with NASH have a higher risk of HCC compared to other aetiologies of liver disease. This important finding suggests a need for clinicians to reframe their understanding of NASH and ensure effective surveillance measures are instituted. Further studies investigating the risk factors of HCC among noncirrhotic NASH patients are needed.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Puja M. Shah MD, MSc for their help with the systematic review search process.
Declaration of funding interests: This work was supported in part by grant funding from the National Institutes of Health (grant 5T32DK007769-15). This work was also supported by a Transplant Hepatology Fellowship Award from the American Association for the Study of Liver Diseases (AASLD).
Funding information
National Institutes of Health; American Association for the Study of Liver Diseases (AASLD)
Declaration of personal interests: Mary Rinella has served as a speaker, a consultant and an advisory board member for Intercept, Genfit, Echosens, and has received research funding from Novartis. Mary Rinella has served in an advisory role for Gilead, Novartis, Immuron, NGM Bio, NuSirt, Enanta Pharmaceuticals and BMS. Stephen Caldwell has served as a consultant and an advisory board member for Shionogi, and has received research funding from Gilead, GenFit, Conatus, Galmed, Taiwan J, Intercept, Vital Therapy and Mallinckrodt. Rohit Loomba has served as a consultant for Bird Rock Bio, BMS, Coh Bar, Celgene, Civi Bio, Conatus, Enanta, Gilead, GRI Bio, Ionis, Metacrine, NGM, Receptos, Sanofi, Salix, Kowa, Median technologies; he served as a consultant for Bird Rock Bio, BMS, Coh Bar, Celgene, Civi Bio, Conatus, Enanta, Gilead, GRI Bio, Ionis, Metacrine, NGM, Receptos, Sanofi, Salix, Kowa, Median technologies; he also received grants from Nuisirt, Allergan, BMS, Boehringer Ingleheim, Eli Lily, Galectin, Galmed, GE, Genfit, Gilead, Intercept, Janssen, Madrigal, NGM, Prometheus, Siemens, Shire, Pfizer; he is also a Co-Founder of Liponexus Inc.
Footnotes
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: prevalence and impact on world health. Hepatology. 2016;64 (1):19–22. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;20(10):28785. [DOI] [PubMed] [Google Scholar]
- 4.Khan FZ, Perumpail RB, Wong RJ, Ahmed A. Advances in hepatocellular carcinoma: nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7(18):2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in nonalcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56 (6):1384–1391. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell S, Ikura Y, Dias D, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in nonalcoholic fatty liver disease patients. J Hepatol. 2005;42(4):585–591. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654.e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokushige K, Hashimoto E, Kodama K. Hepatocarcinogenesis in nonalcoholic fatty liver disease in Japan. J Gastroenterol Hepatol. 2013;28(Suppl. 4):88–92. [DOI] [PubMed] [Google Scholar]
- 10.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21(14):4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664–669. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 15.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50(3):350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594–601.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pais R, Fartoux L, Goumard C, et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46(9):856–863. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balakrishnan M, El-Serag HB. Editorial: NAFLD-related hepatocellular carcinoma – increasing or not? With or without cirrhosis? Aliment Pharmacol Ther. 2018;47(3):437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinmann A, Alt Y, Koch S, et al. Treatment and survival of nonalcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62 (6):1723–1730. [DOI] [PubMed] [Google Scholar]
- 24.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10(12):1342–1359.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 26.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14 (Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 27.Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312(2):171–179. [DOI] [PubMed] [Google Scholar]
- 28.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN.Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001-2013. Gastroenterology. 2015;149(6):1471–1482.e5. [DOI] [PubMed] [Google Scholar]
- 29.Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809–1819. [DOI] [PubMed] [Google Scholar]
- 30.Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rim MY, Kwon OS, Ha M, et al. [Clinical features of non-alcoholic fatty liver disease in cryptogenic hepatocellular carcinoma]. Korean J Gastroenterol. 2014;63(5):292–298. [DOI] [PubMed] [Google Scholar]
- 32.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–689. [DOI] [PubMed] [Google Scholar]
- 33.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K.Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24(2):248–254. [DOI] [PubMed] [Google Scholar]
- 34.Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7(7):800–806. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Alejandro R, Croome KP, Drage M, et al. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18(31):4145–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012;24(7):840–848. [DOI] [PubMed] [Google Scholar]
- 37.Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34(3):159–163. [DOI] [PubMed] [Google Scholar]
- 38.Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(Suppl. 1):E247–E252. [DOI] [PubMed] [Google Scholar]
- 39.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. [DOI] [PubMed] [Google Scholar]
- 40.Pais R, Lebray P, Rousseau G, et al. Nonalcoholic fatty liver disease increases the risk of hepatocellular carcinoma in patients with alcohol-associated cirrhosis awaiting liver transplants. Clin Gastroenterol Hepatol. 2015;13(5):992–999.e2. [DOI] [PubMed] [Google Scholar]
- 41.Hsiang JC, Bai WW, Raos Z, et al. Epidemiology, disease burden and outcomes of cirrhosis in a large secondary care hospital in South Auckland, New Zealand. Int Med J. 2015;45(2):160–169. [DOI] [PubMed] [Google Scholar]
- 42.Ertle J, Dechene A, Sowa JP, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128(10):2436–2443. [DOI] [PubMed] [Google Scholar]
- 43.Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis C cirrhosis. Am J Gastroenterol. 2016;111(11):1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63(3):827–838. [DOI] [PubMed] [Google Scholar]
- 45.Konerman M, Loomba R. Editorial: diabetes and its association with hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2015;42(1):117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 47.Wong CR, Njei B, Nguyen MH, Nguyen A, Lim JK. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;46(11–12):1061–1069. [DOI] [PubMed] [Google Scholar]
- 48.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma (hcc) in non alcoholic fatty liver disease (NAFLD): a multicenter prospective study. Hepatology. 2015;63:827–838. [DOI] [PubMed] [Google Scholar]
- 49.Singal AG, Yopp AC, Gupta S, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res. 2012;5(9):1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic steatohepatitis is the most rapidly growing indication for simultaneous liver kidney transplantation in the United States. Transplantation. 2015;100(3):607–612. [DOI] [PubMed] [Google Scholar]
- 51.European Association For The Study Of The Liver and European Organisation For Research And Treatment Of Cancer. EASL-EORTC Clinical Practice Guidelines management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 52.Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol. 2015;8(1):1–9. [DOI] [PubMed] [Google Scholar]
- 53.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54(4):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toshikuni N, Izumi A, Nishino K, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24(7):1276–1283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.