Abstract
The N-terminal von Willebrand domain of Ku80 supports interactions with a Ku binding motif (KBM) that has been identified in at least three other DNA repair proteins: the non-homologous end joining (NHEJ) scaffold APLF, the modulator of retrovirus infection, MRI, and the Werner syndrome protein (WRN). A second, more recently identified Ku binding motif present in XLF and several other proteins (KBMX) has also been reported to interact with this domain. The isolated Ku80 von Willebrand antigen domain (vWA) from Xenopus laevis has a sequence that is 60% identical with the human domain, is readily expressed and has been used to investigate these interactions. Structural characterization of the complexes formed with the KBM motifs in human APLF, MRI, and WRN identify a conserved binding site that is consistent with previously-reported mutational studies. In contrast with the KBM binding site, structural studies indicate that the KBMX site is occluded by a distorted helix. Fluorescence polarization and 19F NMR studies of a fluorinated XLF C-terminal peptide failed to indicate any interaction with the frog vWA. It was hypothesized that availability of this binding site is conditional, i.e., dependent on specific experimental conditions or other repair factors to make the site available for binding. Modulating the fraction of KBMX-accessible binding site mutationally demonstrated that the more open site is capable of binding the KBMXXLF motif peptide. It is suggested that the conditional nature of KBMX binding limits formation non-productive complexes so that activation-dependent site availability can more optimally support advancing the synapsis process.
Keywords: Ku80 von Willebrand factor A domain, Fluorine-19 NMR, X-ray crystallography, Ku binding motif, conditional binding site
Background
Repair of damaged DNA generally requires multiple enzymatic steps that are coordinated through formation of repair complexes. The Ku70/Ku80 heterodimer is the major component of the DNA repair complex responsible for non-homologous end joining (NHEJ), forming a complex with each end of a DNA double strand break [1, 2]. Each subunit of the heterodimer contains an acidic, N-terminal von Willebrand Factor A-like domain (vWA) of approximately 240 residues, also referred to as the α domain [3] that mediates interactions with other repair factors [4–6]. A Ku binding motif (KBM) that is recognized by the Ku80 von Willebrand domain has been identified in the protein aprataxin and PNKP-like factor (APLF), which functions as a scaffold for the assembly of NHEJ repair factors [6, 7], as well as two other proteins: the modulator of retrovirus infection (MRI) – also referred to as the cell cycle regulator of NHEJ (CYREN) [8, 9], and the Werner syndrome protein (WRN), which contains KBMs at both the N- and C-termini [5]. As a result of the use of a common binding motif, none of these interactions is constitutive, so that these ligands bind competitively.
In addition to the identification of Ku80 vWA-interacting Ku binding motifs in other repair proteins, a second Ku binding motif located at the C-terminus of XLF has also been identified [10], and is also believed to be present in several other repair proteins [5]. A recent crystallographic study reported that the binding site for this XLF Ku binding motif (KBMX) is also located in the Ku80 vWA, contralateral to the KBM binding surface and near the interface with the remainder of the Ku80 molecule [11]. Surprisingly, structural determinations of the unliganded structures by several groups indicate that this site is unavailable for ligand interactions, so that KBMX binding requires a rotation of the vWA domain away from the remaining Ku80 structure to unblock the binding site [3, 11, 12]. The interaction of a flexible peptide ligand that adopts an ensemble of conformations with a binding site that is normally blocked represents a significant energetic challenge, leading us to further investigate whether such an interaction can also be observed in the isolated domain. To further complicate the picture, previous attempts to measure the binding interaction using fluorescein-labeled KBMX peptides failed to indicate high affinity, i.e., low micromolar Kd, binding [5].
In order to gain further insight into the structural basis for these interactions, identify specific interactions that can influence competitive binding affinities, and to better understand how binding occurs to the occluded KBMX site, we have investigated the structural basis for KBM and KBMX binding. As an alternative to investigation of the intact Ku70/Ku80 heterodimer, these studies utilized the isolated Ku80 von Willebrand A-like domain (xlKu80 vWA) that is present in the Ku80 subunit of Xenopus laevis. As noted previously [5], both the Ku70/Ku80 heterodimer and the KBMs are highly conserved among vertebrate species, including X. laevis, which exhibits 60% sequence identity with its human homolog.
Materials and Methods
Peptides.
Peptides used in these studies were obtained from Genscript and are listed in Table S1.
Expression of the isolated Xenopus laevis Ku80 von Willebrand domain
The Ku80 N-terminal domain has been referred to as an α/β domain [3], and the von Willebrand Factor A domain [13]. Attempts to express various constructs corresponding to the human Ku80 von Willebrand domain (vWA), residues 1–243, resulted in poor yields and in the formation of inclusion bodies, although small amounts of the isolated domain could be obtained. We attribute this to several possible characteristics including the poorly conserved and partially disordered loop (residues 170–190) connecting betaE (βE) with alpha6 (α6) (secondary structure identifications based on Walker et al. [3]), and to the presence of a buried glutamic acid residue at position 133. It was hypothesized that these features might require a more sophisticated chaperone system than is available in the Escherichia coli (E. coli) strain used to produce the isolated domain. Although Glu133 is generally well conserved, sequence comparisons indicated that in Xenopus species, this residue is replaced by alanine. As noted above, the Ku binding motifs are highly conserved in all vertebrates including Xenopus - the human and xenopus Ku80 von Willebrand domains share 60 % sequence identity. Since the presence of the disordered loop is on a side of the domain distal to residues identified as part of the Ku binding region [6], a codon-optimized gene containing an N-terminal His-tag was obtained in which the loop corresponding to residues 171–188 in the xlKu80 sequence was deleted and a C190S mutation was introduced (Figure S1). The gene was cloned into a pET30a vector (NdeI/HindIII) by GenScript and transformed into E. coli strain BL21(DE3). The N-terminal His-tagged domain was expressed by autoinduction [14] overnight at 25 °C using ZYM-5052 media. The harvested cells were disrupted by sonication and centrifuged at 40,000 x g for 20 m at 4 °C. The supernatants were applied to a Nickel-charged NTA column (prepacked 5 ml HisTrap NTA HP, GE healthcare) and eluted with a gradient of 25 to 500 mM imidazole. The highly purified xlKu80 vWA was then obtained by gel filtration (Superdex 75 prep grade, GE healthcare) in a buffer composed of 25 mM HEPES pH 7.5, 150 mM NaCl, 1 mM TCEP. The QuikChange site-directed mutagenesis kit (Agilent) was used to create an expression vector for the xlKu80 vWA S229A variant. Expression and purification of the variant protein were the same as for the wild-type.
Although we did not obtain sufficient yields of the isolated human Ku80 vWA domain for structural studies, we did obtain small quantities that were used for binding studies. His-MBP-human Ku80vWA (1–240 aa) (pDEST566-hKu80vWA) containing a TEV protease recognition site between the MBP and Ku80vWA was transformed into Rosetta2 (DE3) E. coli cells. Four surface-exposed hydrophobic residues were mutated (F47Q, F88Q, L234Q, and F237Y) to improve solubility. Other procedures were identical to those described above, except that an anion exchange column (prepacked 5 ml HiTrap Q HP, GE healthcare) was utilized after Ni-NTA, prior to gel filtration, and the sample was subsequently treated with TEV protease overnight at 4 °C.
Crystallization, data collection and structure determination
The human Ku-binding motif (KBM) peptides from APLF, MRI, and WRN (KBMAPLF, KBMMRI, and KBMWRN) and the KBMX peptide from XLF (KBMXXLF) were obtained from GenScript. Peptide concentrations were determined using the extinction coefficient values at 205 nm [15]. Each peptide suspended in H2O was mixed with xlKu80vWA (10.4 mg/ml; MW= 26 kD; 0.4 mM) at a 1:1 (peptide:protein) ratio and incubated at room temperature for 1 h. Crystallization screening of xlKu80vWA/KBM peptides was carried out using sitting drop vapor diffusion with 250 nL sample mixed with 250 nL screening buffer per each condition, and incubated at 22 °C. Crystals from xlKu80vWA complexed with KBMAPLF were obtained in 0.1 M HEPES pH 7.5, 20 % (w/v) PEG4000 (condition 3 in JBScreen Nuc-Pro 2, Jena Bioscience), with KBMMRI in 0.2 M potassium formate, 20 % (w/v) PEG3350 (condition 70 in PEGs suite, Qiagen), and with KBMWRN in 0.1 M Na-HEPES pH 7.5, 25 % (w/v) PEG3000. Crystals of the mutated xlKu80vWA(S229A) complexed with KBMAPLF were obtained in 20 % (w/v) PEG3350. Crystals for the xlKu80 vWA(S229A) bound to KBMXXLF and to both KBMAPLF and KBMXXLF were obtained with 0.1 M HEPES NaOH pH 7.5, 25 % (w/v) PEG3350. Diffraction grade crystals were grown within 24 hours and no further optimization of conditions was required. All samples were subsequently frozen in liquid nitrogen using the crystallization buffer plus an additional 15 % or 20 % ethylene glycol as a cryoprotectant. Data for xlKu80vWA(S229A) complexed with both KBMAPLF and KBMXXLF were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-BM beamline at the Advanced Photon Source, Argonne National Laboratory. Other data were collected using a 007HF Rigaku X-ray generator equipped with either a Saturn944 CCD detector or a DECTRIS P200K detector.
Data were processed using HKL200 or HKL3000 [16]. Phasing was performed by molecular replacement (MR) with PHASER [17] using the von Willebrand domain coordinate of Ku80 (PDB: 1JEQ) for xlKu80vWA/KBMWRN, which was subsequently used as the search model in MR for xlKu80vWA/KBMMRI and xlKu80vWA/KBMAPLF complexes. The crystal structures of xlKu80vWA(S229A) complexed with KBM and or KBMX peptides were solved by MR using xlKu80vWA/KBMAPLF as a search model. The structures were refined using PHENIX [18] and iterative cycles of model building using Coot [19]. The final model structures exhibited favorable geometry with 100 % of the residues in the allowed region of the Ramachandran plot. Crystallographic statistics are summarized in Table 2.1. All structure figures were prepared using PyMOL v2.0.
Table 2.1.
Selected crystallographic data.
| Data Collection | XlKu80-KBMAPLF (PDB: 6TYZ) |
XlKu80-KBMMRI (PDB:6TYU) |
XlKu80-KBMWRN (PDB:6TYV) |
|---|---|---|---|
| Space Group | P212121 | P212121 | P212121 |
| Unit Cell [a,b,c] | 43.8, 71.2, 79.5 | 44.6, 71.4, 79.2 | 44.6, 71.4, 79.2 |
| Wavelength [Å] | 1.54 | 1.54 | 1.54 |
| X-ray Source | Cu | Cu | Cu |
| Resolution [Å] (Highest shell) | 50–1.51 (1.54–1.51) | 50–1.47 (1.50–1.47) | 50–1.92 (1.95–1.92) |
| No. of Reflections [unique] | 234,283 (39,709) | 212,709(43,410) | 94,595 (18,919) |
| Completeness [%] | 99.8 (98.2) | 99.1 (88.3) | 99.5 (99.0) |
| Rsyma [%] | 4.6 (45.5) | 4.4 (47.2) | 6.4 (41.4) |
| I/σ | 50.0 (2.5) | 53.0 (2.3) | 34.3 (3.2) |
| Redundancy | 5.9 (3.3) | 4.9 (2.1) | 5.0 (3.2) |
| Refinement | |||
| Resolution [Å] (Highest shell) | 29.5–1.51 (1.55–1.51) | 37.8–1.47 (1.50–1.47) | 37.52–1.92(2.02–1.92) |
| Molecules per AU | 1 | 1 | 1 |
| No. of a.a.(XlKu80)/AU | 209 | 209 | 207 |
| No. of a.a.(Peptide)/AU | 12 | 11 | 13 |
| No. of waters/AU | 172 | 192 | 113 |
| Rworking b [%] | 17.8 | 20.2 | 19.0 |
| Rfree c [%] | 20.4 | 22.0 | 22.2 |
| Average B factor [Å2] | |||
| Protein | 28.9 | 30.0 | 45.3 |
| Peptide | 26.8 | 27.9 | 53.5 |
| Water | 37.3 | 40.8 | 46.3 |
| RMS deviations | |||
| Bonds [Å] | 0.007 | 0.008 | 0.008 |
| Angles [°] | 0.831 | 0.906 | 0.831 |
| Ramachandran [%] | |||
| Favored, Allowed, Outlier | 98.2, 1.8, 0 | 98.2, 1.8, 0 | 96.4, 3.4, 0 |
Rsym = ΣΣ|I- 〈I〉|/ ΣΣ|I|, where I is the observed intensity and <I> is the average intensity.
Rworking = Σ||Fo|-|Fc||/ Σ|Fo, where Fo and Fc are the observed and calculated structure factors, respectively.
Rfree = Σ||Fo|-|Fc||/ Σ|Fo| for 5 % of the data not used at any stage of the structural refinement.
Fluorescence polarization assay
Apparent peptide dissociation constants were determined based on fluorescence polarization (FP) measurements performed on a POLARstar Omega microplate reader (BMG Labtech) using FITC-labeled peptides as described previously [20]. Titrations were performed using serial dilutions of xlKu80vWA in a mixture with a final concentration of 100 nM FITC-labeled peptide in FP buffer (25 mM HEPES, 150 mM NaCl, 1 mM EDTA, 2 mM DTT, 0.05 % Tween20, 0.1 % BSA, pH 7.4). 50 µL volumes of the binding reactions were pipetted into wells of a 96-well black flat-bottomed plate (Corning, ME) and fluorescence polarization was read at room temperature, using excitation at 485 nm and emission at 520 nm. Data for the interaction of a peptide with xlKu80vWA were fit to a single-site binding equation as described previously [20]. All experiments were at least triplicated and data represent the mean. Fluorescein-labeled peptides are listed in Table S1.
Fluorine-19 NMR studies
Fluorine-19 NMR studies were performed on an Agilent DD2 600 NMR spectrometer operating at a 19F frequency of 564.279 MHz. Measurements were performed at 25 °C using a triple resonance 1H–19F (1H–19F, 13C) 5 mm PFG variable temperature probe. 0.2 mM of either xlKu80 vWA, or the muted xlKu80 vWA(S229A) form of protein was titrated with fluorinated XLF KBMX peptide: KPKKKAKGLF*M, where F* = 4-fluoro-L-phenylalanine. The NMR buffer contained 150 mM NaCl, 25 mM HEPES pH 7.5, 1 mM TCEP, 1 mM EDTA, and 10 % D2O. Protease inhibitor cocktail (Ameresco) was added to our peptide stock solution to protect the peptide from proteolysis. Fluorine-19 chemical shifts are referenced to external TFA, using 0.2 mM 4-fluorobenzamide (Sigma-Aldrich) as an internal standard (δ = −34.9 ppm).
Results
Interaction of human APLF, WRN, and MRI Ku binding motifs with the Xenopus Ku80 von Willebrand domain
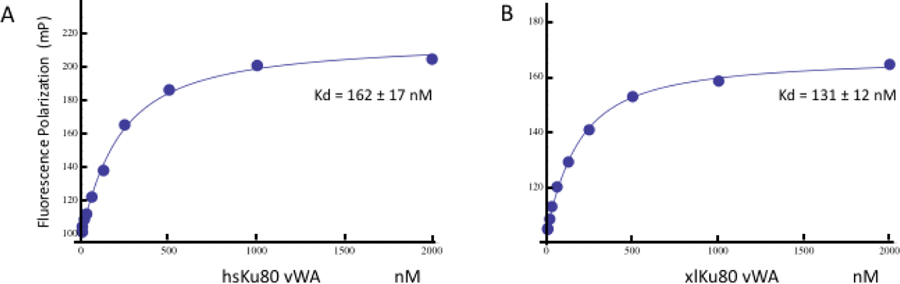
In order to ascertain that the isolated xlKu80 vWA provides a useful model for studying the basis of KBM interaction, we performed fluorescence polarization measurements comparing the binding of the fluorescein-labeled human APLF KBM peptide (sequence in Table 1) to human and Xenopus vWA domains (Figure 1). Although we obtained insufficient quantities of the human domain for more detailed studies, the approach described in Methods was sufficient for binding studies. As indicated in Figure 1, the human peptide exhibited similar affinities for both homologs. The binding affinities of the MRI and WRN N-terminal KBM peptides previously have been reported to be approximately half the value of the APLF KBM [5].
Table 1.
Human and Xenopus KBM sequences
| Protein | Start | KBM Sequencea | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | |||||||||||||||||
| −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||||
| hAPLF | 179 | L | A | E | R | K | R | I | L | P | T | W | M | L | A | E | H |
| xAPLF | 185 | F | V | Q | R | K | R | V | L | P | D | W | M | L | H | D | D |
| hMRI | 6 | S | E | T | K | T | R | V | L | P | S | W | L | T | A | Q | V |
| xMRI | 31 | S | K | G | K | K | R | V | L | P | E | W | M | K | E | E | N |
| WRN-NTb | 8 | T | T | A | Q | Q | R | K | C | P | E | W | M | N | V | Q | N |
| xWRN-NT | 1 | M | T | S | L | Q | R | K | L | P | E | W | M | S | V | K | Q |
| hWRN-CTb | 1400 | S | A | E | R | K | R | R | L | P | V | W | F | A | K | G | |
| xWRN-CT | 1398 | T | T | R | P | R | R | R | L | P | E | W | Q | S | T | K | |
Fully conserved residues are underlined
WRN contains KBM sequences at both its N-terminus (NT) and C-terminus (CT)
Figure 1. Fluorescence polarization studies of the binding of human APLF Ku binding motif peptide with the human and frog Ku80 von Willebrand domains.
Fluorescence polarization of: A) fluorescein-labeled hsAPLF KBM peptide as a function of the hsKu80 vWA. B) fluorescent labeled hsAPLF KBM peptide as a function of the xlKu80 vWA. The measurements were performed at T = (give buffer, etc.) giving Kd = 162 ± 17 nM (panel A) and 131 ± 12 nM (panel B).
Structure of the Xenopus laevis Ku80 vWD and comparison with the human enzyme.
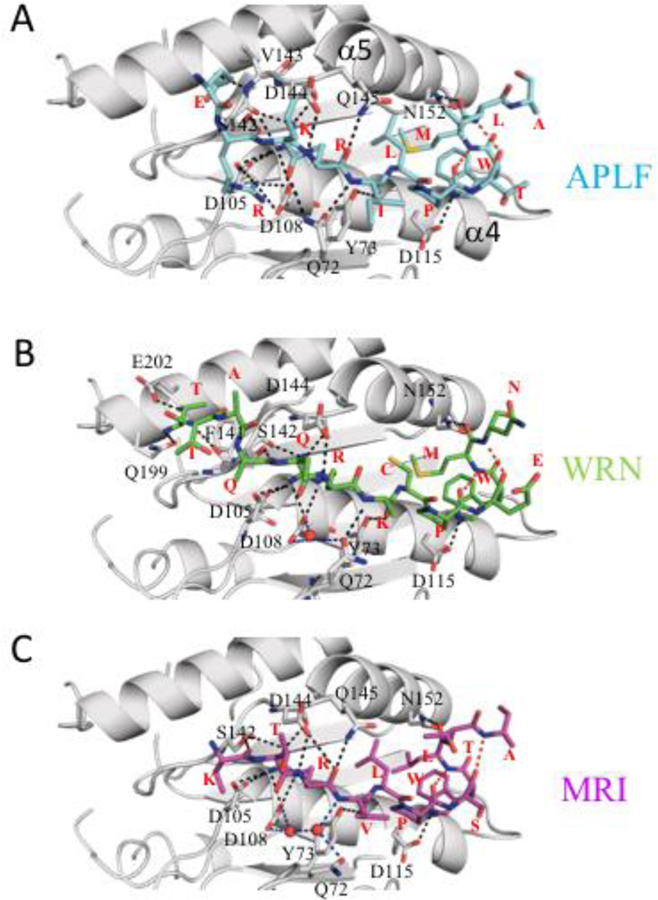
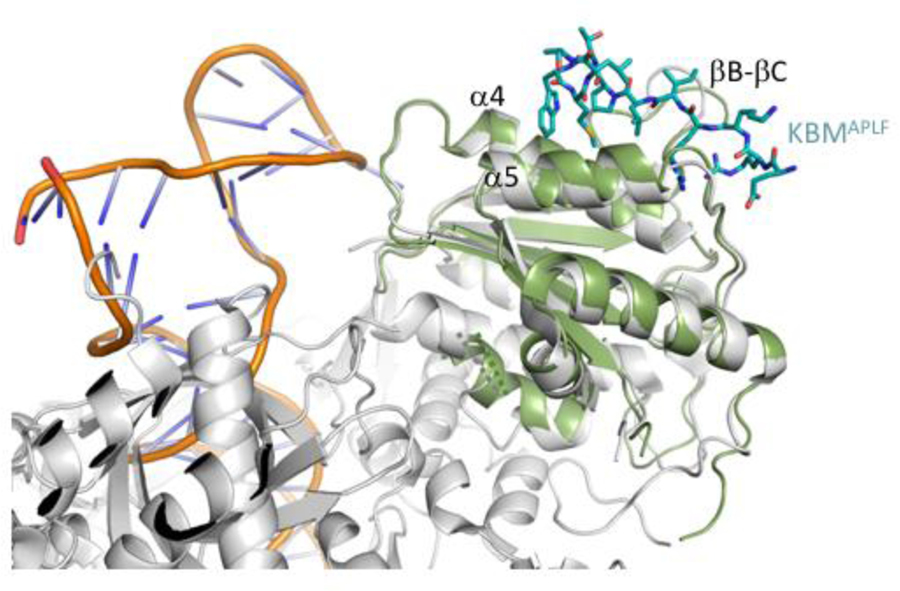
Crystal structures of xlKu80 vWA complexed with Ku binding motif (KBM) from APLF, MRI and WRN were determined at a resolution of 1.5, 1.5 and 1.9 Å, respectively (Table 2.1). XlKu80 vWA is a globular protein composed of a Rossman-like fold with seven alpha helices and six beta strands with an anti-parallel βC and parallel βA, βB, βD, βE and βE’ (Figure S1A). The central β-sheet is flanked on both sides by alpha helices (Figure S1B). All of the secondary structure motifs identified in the human vWA are present in the frog construct studied (Figure S1). APLF, MRI and WRN peptides occupy the hydrophobic groove formed between α4 and α5 and flanked by the βB-βC loop and βD-α5 loop (Figure 2). These structures overlay well with the Ku80 vWA domain in previously reported structures of human K70/Ku80 in various complexes with DNA and DNA-PK catalytic subunit (DNA-PKcs) [3, 11, 12], with RMSD values ranging from 0.149–0.720 Å (Table S2) for the X-ray structures and larger values for the cryo-EM structure. Further, the KBM binding surface does not conflict with the DNA interaction surface [3, 11] or the DNA-PKcs binding surface [12] (Figure 3). The xlKu80 vWA domains in the three complexes superimpose well with a very low RMSD (Table S2), indicating that KBM peptides interact with xlKu80 vWA similarly. This conclusion is also supported by the similar dissociation constants previously reported [5]. Electron densities defining the three bound KBM ligands are shown in Figure S2.
Figure 2. Detailed views of the KBM-Ku80 vWA structures showing H-bonds.
Hydrogen bonding between xlKu80 vWA (gray) and (A) APLF (cyan), (B) WRN (green), and (C) MRI KBM peptide (magenta) is indicated by black dotted lines. Interacting vWA residues are labeled, and each peptide residue type is indicated in red. In some cases, disordered sidechains have been truncated. Interpeptide H-bonds are shown as red dotted lines, and H-bonds mediated by water molecules are in blue.
Figure 3. Position of the KBM binding surface.
Overlaid cartoon representations of the human Ku70/Ku80-DNA complex (gray protein, DNA orange phosphate backbone, PDB: 1JEY) with the frog Ku80 vWA (green) complexed with the APLF KBM peptide (cyan stick representation). The KBM binding surface does not conflict with the DNA binding region.
In the poorly conserved loop segment, aa 171–188, that was deleted in order to improve the suitability of the construct for crystallization, the nearest observed residues resolved in the structure are Asp169 and Gly191 in APLF and MRI complexes and Val168 and Pro192 in the WRN complex. We conclude that deletion of this segment does not significantly perturb the domain structure and, as shown in Figure 1, apparently has little to no effect on peptide binding, as the Kd values determined for binding of the human KBMAPLF peptide to the human and frog proteins were 0.162 nM and 0.131 nM, respectively. Comparison with the cryo-EM structure of the DNA-PKcs-Ku70/Ku80-DNA complex further demonstrates no conflict with the DNA-PKcs binding surface (PDB: 5Y3R; [12]). The RMSD values of APLF peptide in the human Ku70/Ku80 DNA complex (PDB: 6ERF, chain Q) aligned to the APLF, MRI, and WRN peptides in our xlKu80 vWA-KBM structures are 0.25 Å, 0.36 Å, and 0.34 Å for the 9 Cα atoms found in the binding pocket, respectively. Some C-terminal residues are not observed in the structures, presumably due to disorder.
Structure of the bound KBM
The KBM binding site identified in these studies is consistent with the location that was initially identified on the basis of mutational studies to involve hsKu80 residues Leu68 and Tyr74 on the βB-βC loop, and Ile112 on α-helix-4, where the secondary structure element identifications are those given by [3]. The corresponding xl residues are Leu67, Tyr73, and Ile111. These residues contribute to the hydrophobic binding pocket that interacts primarily with the conserved Pro residue in the KBM, while Tyr73 also forms an H-bond with the carbonyl oxygen of KBM residue 3 (See Table 1 for KBM numbering and Tables S3, S4 and S5 for hydrogen bonding). The bound KBM in all 3 structures adopt an extended structure that terminates in a short 310 helix stabilized by a pair of 4→1 H bonds that involve the +7 and +4 (Pro) and the +8 and +5 residues (Figure 3). The KBM motif may be defined as: R1XhP4XW6h where R1, P4, and W6 are required for high affinity binding, and there is a preference but not a requirement for aliphatic residues at positions 3 and 7. Each of the KBM peptides forms extensive H-bond interactions with the vWA domain that involve both the backbone and sidechain groups (Figure 3). The conserved Arg, Pro, and Trp residues that define the binding motif form analogous interactions in each of the three complexes. The Arg sidechain makes up to 6 H-bonds with Asp105, Asp108, and the carbonyl oxygens from Ser142 and Asp144. In each case +6 Trp NHε is H-bonded to an Asp115 carboxylate oxygen. In the KBMAPLF complex, the Q72 sidechain forms H-bonds with both the carbonyl of the residue at position 0 and the NH of the +2 residue. In the WRN complex, there is a direct interaction only with the +1 NH, while in the MRI complex there are only indirect, water mediated interactions. In the KBMMRI complex, the sidechain of the −1 Lys also supports binding, while in KBMWRN, the sidechain of the −1 Gln does not interact with the domain.
Binding of flexible ligands that generally can adopt an ensemble of conformations leads to a conformational selection process that is determined by the binding site. The cluster of hydrophobic sidechains at positions 3,4,6, and 7 not only interact with the binding site, but probably contribute significantly toward stabilizing the active conformation of the uncomplexed peptide, reducing the conformational adjustments required to convert the uncomplexed peptide to the bound form. Similarly, prediction of the conformation of the KBM sequence using the PhD secondary structure server [21] indicates that the peptide favors a helical rather than an extended geometry if any residue other than Pro is substituted at position 4 (Table S6), that would need to unfold prior to binding. By disrupting helical structure N-terminal to the proline, this residue could pre-organize the binding motif to facilitate peptide-protein association, and is consistent with the high affinities that have been measured.
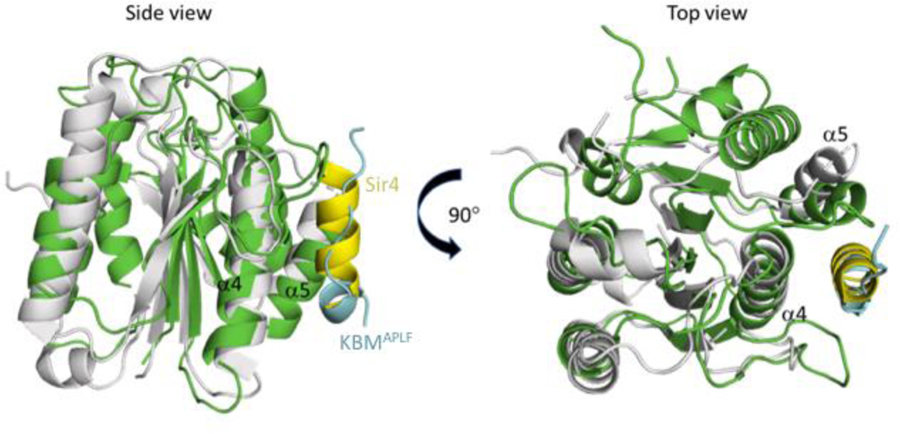
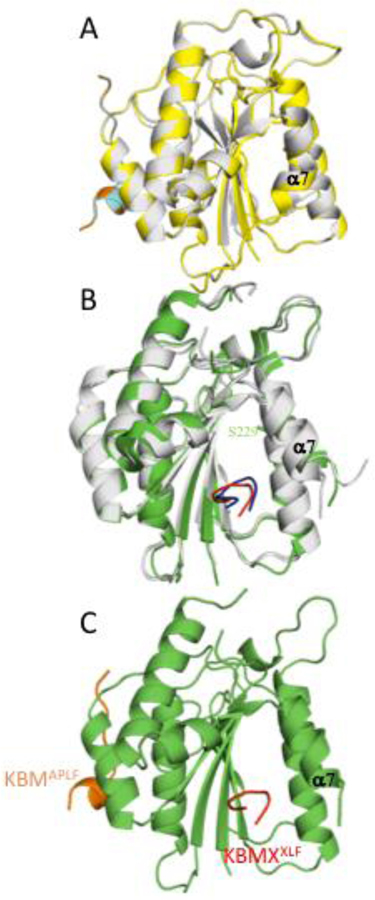
Comparison with the yeast Ku80 von Willebrand domain
The Ku heterodimer plays an essential role in telomere maintenance [22]. In the yeast Saccharomyces cerevisiae, the Ku80 von Willebrand domain interacts with a K80 binding motif that is present in the Sir4 deacetylase [23]. Structural analysis demonstrates that this interaction involves the same binding surface that is used in vertebrates to support the binding of APLF, WRN, and MRI. The structure of the yeast Ku80 von Willebrand domain complex with a Sir4 Ku binding motif peptide (PDB: 5Y59) has been reported by Chen et al. [23]. Despite a low sequence identity level of 20 %, the structures are fairly similar – even in regions of the protein with limited identity (RMSD = 3.201 Å over 111 Cα atoms calculated by PyMol). Most surprisingly, although the peptide binding surfaces for both Ku80 von Willebrand domains largely overlap, the structural selectivity of these surfaces is quite different, as the bound Sir4 Ku80 binding peptide adopts a helical structure (Figure 4). The hydrophobic groove formed between α4 and α5 that forms the KBM binding surface is not well conserved in the yeast protein, with many of the aligned residues exhibiting non-conservative substitutions, consistent with the low sequence identity. Further, the βB-βC loop in the frog/human that contains important KBM interacting residues is larger than the corresponding loop in the yeast vWD and likely plays a major role in peptide recognition. However, the yeast Ku80 vWA domain does interact with several hydrophobic sidechains in the Sir4 helix; the interaction is supported by shallow hydrophobic pockets into which the yeast Sir4 Leu107, Leu110 and Leu111 residues fit, while the Sir4 Lys106 and Lys114 make salt bridges with the domain, and the Lys114 carbonyl forms a hydrogen bond with a Gln sidechain. In contrast with the interaction surface in the yeast Ku80 vWA, the binding site in the frog and human domains creates a much larger hydrophobic cavity into which four residues in the Ku binding motif at the +3, +4 (conserved Pro), +6 (conserved Trp), and +7 positions all fit.
Figure 4. Comparison of yeast (Ku80 vWA-Sir4) and frog (Ku80 vWA-KBM) complexes.
ScKu80 vWA (gray) with bound Sir4 peptide (yellow) (PDB: 5Y59) is superimposed onto xlKu80 vWA (green) with APLF KBM peptide (cyan). Both Sir4 and APLF interact through the hydrophobic pocket formed between α4 and α5.
The interaction with the XLF Ku binding motif (“KBMX”)
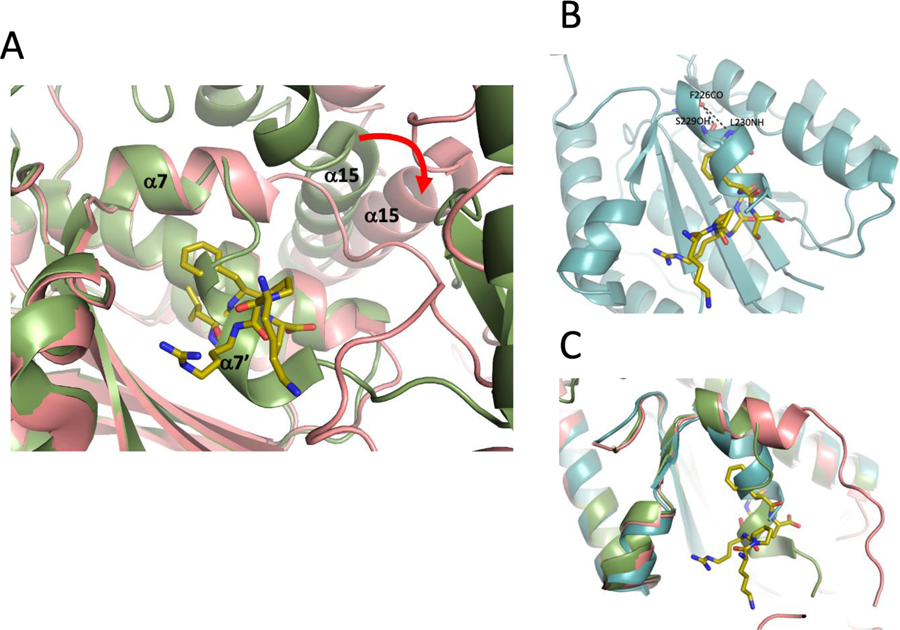
The Ku70/Ku80-DNA complex also has been reported to interact with the repair factor XLF (Cernunnos) in a manner that is dependent on the presence of its C-terminal residues (Table 3) [5, 10, 11]. A recent crystallographic study demonstrated that this “KBMX” motif at the C-terminus of XLF interacts with a site that is unavailable in the absence of XLF, being blocked by the discontinuous C-terminal α7 helix of the domain, which makes a sharp turn into the binding site due in part to steric conflict with helix α15 (Figure 5A). A hydrogen bond between the protonated Glu133 carboxylate and Val236 carbonyl is indicated by proximity of these groups in the structure [3, 11] and supports the bent path of helix α7. In order to accommodate the XLF peptide, the entire von Willebrand domain needs to rotate away from the remaining Ku80 structure, and this positional change allows further extension of α7 which then exposes the hydrophobic KBMX binding pocket. In the isolated xlKu80 vWA domain, the KBMX site is also blocked, however in this case, change in helix α7 direction is somewhat more gradual (Figures 5B and 5C). Thus, in contrast with the KBM binding site which is fully exposed on a surface of the vWA domain that does not conflict with other known interactions (Figure 2), the KBMX site is unavailable in all of the reported structures of Ku70/Ku80 in the absence of the ligand (PDB: 1JEY, 1JEQ [3]; PDB: 5Y3R [12]; PDB: 6ERF [11]) and in the structure reported here for the isolated xlKu80 vWA domain (Figure 5B). A comparison of the KBMX binding sites in the unliganded human and frog structures with more open structure and a bound KBMX peptide is shown in Figure 5C.
Table 3.
XLF-like C-terminus Ku binding motifs (KBMX) in human and frog
| protein | Positions | KBMX Sequences |
|---|---|---|
| hsXLF | 282–299 | QRPQLSKVKRKKPRGLFS |
| xlXLF | 294–311 | RPPAGASKPKKKAKGLFM |
| hsWRN | 1415–1432 | SDTSKKLMDKTKRGGLFS |
| xlWRN | 1419–1436 | RCIQESKNLGEEKGSFFD |
| hsMRI | 140–157 | EEEEEEDVLKYVREIFFS |
| xtMRI | 141–158 | DSDSDDDALRLIREIFFT |
The frog sequences correspond to Xenopus laevis (xl) or, if not available, Xenopus tropicalis (xt).
Fully conserved residues are underlined
Figure 5. Occlusion of the XLF KBMX binding site in Ku80 vWA.
A) Overlaid cartoon representations of the KBMX binding site in human Ku80 in the absence (green, PDB: 1JEY) or presence (pink, PDB: 6ERG) of the XLF C-terminal peptide (shown in gold stick representation). Opening of the binding site results from extension of helix α7, which requires movement relative to helix α15 and other structure elements in full length Ku80. B) Overlaid representation of the xlKu80 vWA structure (cyan cartoon) with the bound XLF KBMX peptide (gold stick representation). C) Comparison of the human (green) and frog (cyan) vWA structures in the absence of the KBMX peptide with the human Ku80 vWA domain when the KBMX peptide is bound (pink).
The interaction of a flexible peptide ligand with the KBMX site presents a challenging problem. In addition to the need to supply additional energy for rotation of the vWA domain required to make the binding site accessible, an entropic penalty is associated with the selection of a specific, possibly rare peptide conformation by the binding site [24]. Such interactions are unusual and typically require a dynamic structure in that alternates between open and closed conformations. Nemoz et al. proposed that the buried Glu133 residue plays a role in destabilizing the closed conformation, thus helping to form the open, peptide-accessible KBMX site conformation. The presence of an Ala residue at the corresponding position in the frog vWA domain would then appear to preclude or greatly reduce such binding, however as noted by Grundy et al. [5], the KBMX motifs are present in frogs and other vertebrates, suggesting that Glu133 is not an absolute requirement for KBMX recognition and binding.
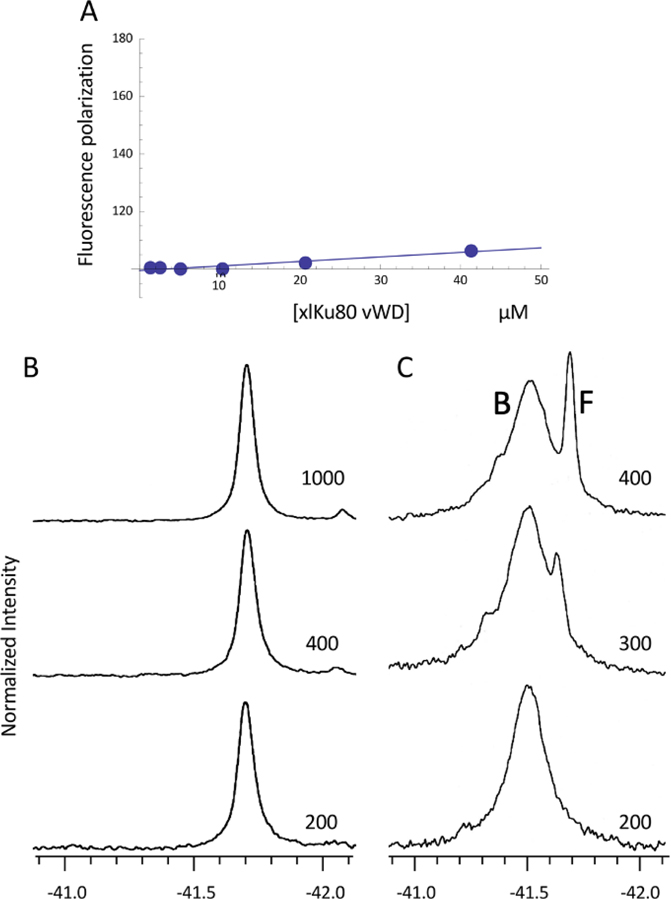
The interaction of the XLF derived KBMX peptides with xlKu80 vWA were evaluated using both fluorescence polarization and 19F NMR (Figure 6A–C). The first approach utilized fluorescein-labeled peptides, while the second approach used peptides in which the penultimate Phe residue was replaced by a p-fluoroPhe (Table S1). Although we were sufficiently confident of the conserved nature of the KBM to evaluate binding of human KBM peptides to the xlKu80 vWA, the interaction of the KBMX-containing peptides with the xlKu80 vWA utilized sequences found in the frog. Fluorescence polarization measurements using fluorescein-tagged Xenopus KBMXXLF failed to indicate high affinity (Kd ≤ µM) binding to the isolated domain (Figure 6A), a result similar to that reported by Grundy et al. using a similar approach with the human Ku70/Ku80 complex [5].
Figure 6. Fluorescence polarization and 19F NMR studies of KBMX peptide - vWA domain binding.
A) Fluorescence polarization studies performed by titration the xlKu80 vWA into solutions containing 100 nM FITC-GG-KPKKKAKGLFM. B) 19F NMR spectra of a samples containing 0.2 mM xlKu80 vWA domain plus 0.2 (bottom), 0.4 (middle), or 1.0 (top) mM of the KBMXXLF peptide: KPKKKAKGLF*M, where F* = 4-fluoro-L-phenylalanine. C) 19F NMR spectra of a samples containing 0.2 mM xlKu80 vWA(S229A) plus 0.2 (bottom), 0.3 (middle), or 0.4 (top) mM of the fluorinated KBMXXLF peptide. Resonances arising from the bound and free KBMXXLF peptide are labeled. The spectra were obtained at 25 °C. Sample buffer: 25 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM TCEP, 1 mM EDTA, 0.25 mM NaN3, 10 % D2O for the lock.
The 19F NMR linewidths of a fluorinated APLF KBMX peptide at concentrations of 0.2, 0.4, and 1.0 mM were measured in the presence of 0.2 mM xlKu80 vWA domain (Figure 6B). Assuming a Kd value of 5 µM similar to values reported by Nemoz et al. [11], the peptide should be more than 87% bound at a 0.2 mM concentration and 20 % bound at 1 mM peptide. The linewidth increased to 40 Hz in the presence of the vWA domain, however this effect was independent of the peptide concentration and is attributed to non-specific binding and/or an increase in sample viscosity. Binding of the peptide to the globular xlKu80 vWA (MW ~ 27 kD) would be expected to significantly broaden the 19F resonance or, if the exchange rate is sufficiently slow, to result in resonances for the vWA-complexed and uncomplexed peptide, analogous to previous studies using this approach, e.g. [25]. Similar results were obtained using a 4-fluoroPhe substituted MRI KBMX peptide (not shown).
Mutational evaluation of conditional binding
Based on the results summarized in the previous section, we hypothesized that the availability of the KBMX binding site in Ku80 vWA is conditional, i.e., contingent on particular experimental conditions or other binding factors that make the KBMX site more available to incoming ligands. As shown in Figure 5B, in the xlKu80 vWA domain the KBMX site is blocked by the α7 helix that bends at residue Ser229. In general serine residues disfavor standard helical geometry [26] and in this case, the Ser229 hydroxyl makes an H-bond with Phe226 carbonyl, replacing an H-bond between the carbonyl and Leu230 NH that would be present in an extended α-helix. In order to evaluate the conditional binding hypothesis, we investigated the interaction of the 19F-labeled KBMX peptide studied above, with the xlKu80 vWA(S229A) mutant. This mutation is predicted to increase the relative open/closed conformational ratio of the site, but will not necessarily lock the helix in an open position. In contrast with the results shown in Figure 6B, the 19F resonance of the fluorinated XLF KBMX peptide in the presence of the xLKu80 vWA(S229A) mutant, shows a linewidth of 109 Hz at a 1:1 ratio with the xlKu80 vWA (Figure 6C). Further additions of the fluorinated KBMX peptide resulted in the appearance of a second, much sharper resonance at the position of the uncomplexed peptide (Figure 6C). The observation of slow exchange between the 19F resonances of the bound and free peptide is consistent with a dissociation constant in the low micromolar range, as was reported for the human Ku70/Ku80 heterodimer by Nemoz et al. [11]. We therefore conclude that the binding affinity of the KBMXXLF peptide for the isolated frog von Willebrand domain is negligible, but increases dramatically as a result of the S229A mutation predicted to decrease the stability of the blocked KBMX site.
Crystallographic confirmation of binding of the XLF KBMX peptide to xl Ku80 vWA(S229A).
Binding of the KBMXXLF peptide to the mutated xlKu80 vWA(S229A) domain was further evaluated using X-ray crystallography (Figure 7 and Table 2.2). As shown in Figure 7A, xlKu80 vWA and xlKu80 vWA(S229A) complexed with KBMAPLF superimpose with an RMSD value of 0.6 Å over 163 alpha carbon atoms, indicating that there is no significant perturbation of the overall structure. Therefore, the effect of the mutation is rather subtle, increasing the open/closed ratio that exists in solution under typical experimental conditions but not providing sufficient destabilization of the closed conformation to make the open form predominate under the conditions of the crystallographic study. Since the 19F NMR study discussed above indicate that the mutated vWA exhibits a dramatically increased affinity for the KBMXXLF peptide, we also crystallized the xlKu80 vWA(S229A) domain in the presence of a KBMXXLF peptide (Table S1). Figure 7B shows a cartoon diagram of the xlKu80 vWA(S229A)-KBMXXLF complex overlaid with the corresponding region of hsKu80 bound to the human XLF KBMX peptide (PDB: 6ERG). These results are consistent with the conclusion that the effect of the S229A mutation is fairly modest, creating a more dynamic structure in which the KBMX site is more available some fraction of time, but not locking the helix into an open (unblocked) conformation. As is apparent from the figure, the two KBMX peptides, although differing slightly in sequence and bound to the vWA domains from two different sources (Tables 2.2 and S1), follow a nearly identical path. A crystal structure of xlKu80 vWA(S229A) complexed with both the KBMAPLF and KBMXXLF peptides was also obtained (Table 2.2 and Figure 7C). The KBM and KBMX motifs bind to opposite sides of the Ku80 vWA and there appears to be no significant conformationally-mediated interaction. A potential cooperative interaction between the KBM and KBMX motifs near the C-terminus of WRN was not observed by Grundy et al. [5], and is made further unlikely by the variable length of the connecting sequences among different vertebrates (Grundy et al. [5]).
Figure 7. Structural features of the KBMX binding site in a mutated xlKu80 vWA domain.
A) Cartoon rendering showing the helix α7 region of xlKu80 vWA(S229A) (yellow), overlaid with the xlKu80 vWA (gray) bound to the KBMAPLF peptide, colored in cyan and orange, respectively. B) Cartoon rendering of the xlKu80 vWA(S229A) domain (green) bound to a KBMXXLF peptide (red) overlaid with the corresponding structures of the human proteins from PDB: 6ERG, chains B (gray) and F (blue). C) Cartoon rendering of the xlKu80 vWA(S229A) domain (green) bound to KBMAPLF (orange) and KBMXXLF (red) simultaneously. Apparently, the S229A mutation does not alter the conformation in the crystal structure, but nevertheless in solution creates a sufficiently dynamic state to support peptide binding.
Table 2.2.
Selected crystallographic data.
| Data Collection | XlKu80(S229A)-KBMAPLF (PDB: 6TYW) |
XlKu80(S229A)-KBMXXLF (PDB: 6TYX) |
XlKu80(S229A)-KBMAPLF/KBMXXLF (PDB: 6TYT) |
|---|---|---|---|
| Space Group | P212121 | P21 | C2221 |
| Unit cell | |||
| a,b,c [Å] | 43.8, 71.4, 80.0 | 44.8, 71.6, 74.6 | 43.6, 79.4, 146.2 |
| α,β,γ [°] | 90, 90, 90 | 90, 98.3, 90 | 90, 90, 90 |
| Wavelength [Å] | 1.54 | 1.54 | 1.0 |
| X-ray Source | Cu | Cu | 22-BM |
| Resolution [Å] (Highest shell) | 50–1.70 (1.73–1.70) | 50–1.90 (1.93–1.90) | 50–2.35 (2.39–2.35) |
| No. of Reflections [unique] | 112,216 (28,054) | 149,026(36,348) | 60,482 (10,611) |
| Completeness [%] | 98.8 (86.3) | 98.6 (94.9) | 97.0 (79.3) |
| Rsyma [%] | 5.2 (11.8) | 4.5 (26.9) | 4.3 (65.3) |
| I/σ | 50.6 (6.9) | 39.7 (3.1) | 43.0 (1.4) |
| Redundancy | 4.0 (2.1) | 4.1 (2.3) | 5.7 (4.5) |
| Refinement | |||
| Resolution [Å] (Highest shell) | 22.8–1.70 (1.76–1.70) | 40.7–1.90 (1.95–1.90) | 34.9–2.40(2.49–2.40) |
| Molecules per AU | 1 | 2 | 1 |
| No. of a.a. (XlKu80S229A)/AU |
218 | 427 | 206 |
| No. of a.a.(Peptide)/AU | 13 | 12 | 17 |
| No. of waters/AU | 219 | 228 | 4 |
| Rworking b [%] | 17.8 | 18.4 | 22.3 |
| Rfree c [%] | 22.0 | 22.8 | 26.0 |
| Average B factor [Å2] | |||
| Protein | 31.6 | 46.6 | 82.6 |
| Peptide | 25.7 | 63.0 | 97.9 |
| Water | 36.5 | 52.5 | 62.7 |
| RMS deviations | |||
| Bonds [Å] | 0.009 | 0.004 | 0.002 |
| Angles [°] | 0.914 | 0.647 | 0.378 |
| Ramachandran [%] | |||
| Favored, Allowed, Outlier | 98.3, 1.7, 0 | 97.9, 2.1, 0 | 96.3, 3.7, 0 |
Rsym = ΣΣ|I- 〈I〉|/ ΣΣ|I|, where I is the observed intensity and <I> is the average intensity.
Rworking = Σ||Fo|-|Fc||/ Σ|Fo, where Fo and Fc are the observed and calculated structure factors, respectively.
Rfree = Σ||Fo|-|Fc||/ Σ|Fo| for 5 % of the data not used at any stage of the structural refinement.
Discussion
Optimal repair of DNA lesions typically requires multiple enzymes that can be recruited and organized by scaffold proteins. As a result of the variability of DNA damage, these interactions can involve multiple binding partners that compete for common recognition sites, allowing the system to explore alternate partners in order to select one that is optimally suited to repair a particular form of damage. The structural basis for competition by the Ku binding motifs contained in APLF, WRN and MRI are elucidated in the present study. Competitive binding by APLF and WRN may have a straightforward interpretation. Werner syndrome protein (WRN) contains helicase and exonuclease domains that resect the DNA ends [27, 28]. APLF is a scaffold that recruits XRCC4-Ligase4 to complete NHEJ repair. Since end resection must precede ligation, the WRN-APLF competition may help to limit premature attempts at ligation. The ability of WRN to displace APLF is further supported by its ability to interact with both the Ku70 and Ku80 subunits [28]. Premature ligation can lead to the formation of abortive DNA adducts [29, 30] requiring additional steps to correct. Hence, it is advantageous for the repair system to chronologically order the end processing and ligation steps.
MRI/CYREN is a small, largely unstructured protein that has been reported to support NHEJ [31, 32], while another recent study described its activities as inhibiting classical non-homologous end-joining during parts of the cell cycle in which sister chromatids are present [8]. The study by Hung et al. [32] indicates that MRI is able to support classical NHEJ in the absence of XLF, a factor that supports the synapsis process [35, 36]. The structure of the xlKu80 vWA domain – KBMMRI complex reported here demonstrates the mode of direct binding to Ku80, and the putative C-terminal KBMXMRI motif suggests that it may additionally form either a bipartite intramolecular complex or an intermolecular complex with a second Ku80 molecule.
In addition to the protein interactions that are mediated by the Ku binding motif, a second Ku-binding peptide motif located at the C-terminus of XLF as well as several other proteins has recently been identified [5, 10, 37]. Nemoz et al. [11] have mapped the binding site of this KBMX motif to a Ku80 vWA domain site located on the opposite face of the domain relative to that involved in KBM binding. Surprisingly, this binding site is occluded in four different reported structures of the human Ku70/Ku80 complex – PDB: 1JEY, 1QEY, 5Y3R, and 6ERF, as well as the structure of the isolated xlKu80 vWA determined in this study, and hence not readily available for binding (Figure 5). In the human Ku70/Ku80 heterodimer, binding of the XLF peptide required a rotation of the vWA domain away from the remaining Ku80 structure in order to allow helix α7 to extend so that it does not block the KBMX site. The need for peptide binding to induce this large conformational change in combination with the entropic penalty associated with the selection of a specific bound conformation from the conformational ensemble that typically characterizes peptides in solution makes such an interaction challenging. In contrast with the binding data obtained by Nemoz et al., a previous effort using fluorescein-labeled KBMX peptides failed to observe high affinity binding [5]. In the present study, both fluorescence polarization measurements using a fluorescein-labeled KBMXXLF peptide as well as 19F NMR studies of a fluorine-labeled KBMXXLF peptide failed to indicate high affinity, micromolar Kd binding to the isolated xlKu80 vWA domain. The ability of a ligand to interact with a site that is blocked in its absence is not unprecedented; one well studied example involves non-nucleoside RT inhibitor (NNRTI) binding to HIV-1 reverse transcriptase [38–40]. In that case, the occasional availability of the NNRTI site that presumably arises due to dynamic fluctuations allows rapid association by NNRTIs that have been optimized to target this site. In contrast, the XLF peptide that targets the KBMX site of the Ku80 vWA presumably exists as an ensemble of conformations.
Our failure to observe KBMX peptide binding to the isolated xlKu80 vWA even at a 1 mM peptide concentration led us to conclude that the recently identified KBMX binding interaction is conditional, i.e. dependent on a specific set of experimental conditions and/or other repair factors that increase the probability of having an open, ligand-accessible KBMX binding site. The S229A mutation introduced into the xlKu80 vWA to increase the relative probability of the open conformation has such a modest effect that no difference in the structure of the protein determined by X-ray crystallography in the absence of a KBMX peptide (Figure 7A) is observed, and the KBMX site remains blocked. However, in contrast with the results for the unmodified xlKu80 vWA, 19F NMR studies showed a dramatic increase in binding affinity of the fluorinated KBMXXLF peptide resulting from introduction of the S229A mutation into the xlKu80 vWA domain. The binding interaction of the XLF KBMX peptide with the xlKu80 vWA domain was further confirmed by X-ray crystallography (Figure 7B), revealing a structure that is closely analogous to that of the human Ku80-KBMXXLF peptide complex (PDB: 6ERG, [11]). We conclude that the S229A mutation is sufficient to enhance the open/closed equilibrium of the binding site, so that it becomes sufficient to support KBMX peptide binding.
Although extrapolation of the results obtained for the isolated xlKu80 vWA interaction with the KBM peptides to the human Ku heterodimer is straightforward, the implications of the KBMX studies are more indirect. As illustrated in Figure 5, the KBMX binding pocket is blocked in both the frog and human domains, however the details of this blockage differ. These differences can in principle result from several factors, including the absence of a deleted segment in our xlKu80 vWA construct (Figure S1), differences in behavior between the human and frog Ku80, or as a result of differences in behavior between the isolated Ku80 vWA and the Ku70/Ku80 heterodimer. Of these possibilities, we believe the latter explanation to be the most likely. The deleted segment is not directly involved in binding either the KBM or KBMX peptide motifs, and the ends of the disordered segment are found in similar positions despite the deletion. As to the second possibility, there is a high level of residue homology between human and frog Ku80 - even in the α7 helices which as shown in Figure 5 behave differently. In contrast, there are many additional interactions between the Ku80 vWA domain and the remaining Ku80 structure that can influence conformation, including but not limited to those illustrated in Figure 5A. Although the details of the conformational changes that block and unblock the XLF binding site differ, the primary driver for blocking the site is likely the general tendency to minimize solvent exposure of hydrophobic residues that line the KBMX site.
The conditional nature of the KBMX motif binding observed here is consistent with the earlier observations of Grundy et al. [5] on the lack of binding by a fluorescein-labeled KBMX peptide to the human Ku70/Ku80 heterodimer, leading to the question as to the specific conditions that were responsible for the XLF peptide binding data obtained by Nemoz et al. [11]. Nemoz et al. proposed a role for Glu133 in opening the occluded KBMX site, however the XLF, WRN, and MRI proteins in the frog also contain conserved KBMX sequences while the corresponding xlKu80 vWA has an Ala rather than a Glu residue at the corresponding position. The present study further demonstrates that analogous complexes can form with the xlKu80 vWA domain (Figure 7B). In the Ku70/Ku80 heterodimer, KBMX site availability appears to depend on a reorientation of the entire Ku80 vWA domain [11], allowing extension of α-helix 7 (Figure 7). In the structures of the KBMX peptide complexes determined by Nemoz et al., PDB: 6ERG and 6ERH, the N-terminal His-TEV tag tethers the domain N-terminus to the bound DNA, and thus might have contributed to domain repositioning and opening of the KBMX site.
The structure shown in Figure 7C reveals how closely spaced KBM and KBMX motifs, as occur at the C-terminus of WRN, might bind cooperatively to XlKu80 vWA. Grundy et al. (2016) reported that a WRN C-terminal peptide containing both motifs failed to show evidence of cooperative binding, while in vivo studies indicated that cooperative binding was occurring. These results are consistent with the requirement for an additional interaction, present in the in vivo experiment, to open the KBMX site.
XLF plays a central role in the synapsis process, supporting the transition from an initial, flexible connection of the blunt DNA ends to a close synaptic state [35], and this transition appears to be dependent on the C-terminal KBMX-Ku/DNA interaction [10, 35, 36, 41]. The conditional nature of KBMX binding may be dependent on sensing that the set of repair proteins and/or DNA required for NHEJ is sufficient to allow the process to advance. This could involve a steric clash with the Ku80 vWA domain in the unrotated position, leading to domain reorientation. Another interesting possibility arises from consideration of vWA residues ~ 180–194 that are located at the interface between the Ku80 vWA domain and the remaining Ku80 structure. In the intact Ku70/Ku80 heterodimer structures corresponding to PDB: 1JEQ, 1JEY, 6ERF, 5Y3R, these residues are ordered and contribute to the Ku80 vWA domain interface with the remaining structure. Alternatively, in the structures of the Ku70/Ku80-KBMX complexes reported by Nemoz et al., PDB: 6ERG, 6ERH, these residues become disordered. Consequently, additional direct or indirect interactions of this segment with other proteins might alter its involvement at the domain interface, facilitating the orientational change of the vWA domain that appears to be required for opening the KBMX site in the Ku70/Ku80 heterodimer. We note that these residues, many of which were deleted in our construct of the isolated vWA domain, do not interact directly with the bound KBM or KBMX peptides, so the effect on the KBMX site is mediated by indirect interactions in the full molecule. This potential mechanism is amenable to further investigation.
Supplementary Material
Highlights.
Structures of APLF, MRI, WRN and XLF peptides bound to frog Ku80 vWA are reported
A ternary complex of Ku80 vWA bound to APLF and XLF peptides has been characterized
The Ku80 site that binds the XLF C-terminal motif is normally closed
Binding of the XLF motif is conditional, requiring additional unblocking factors
Acknowledgements:
We are grateful to Dr. Juno Krahn in the X-ray crystallography core for many helpful discussions, and to Dr. Matt Schellenberg and Andrea Kaminski for insightful comments on the manuscript.
FUNDING
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health [ZIA-ES050111 to R.E.L., ZIA-ES102645 to L.C.P.]; KK was supported by the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea. Crystallographic data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advanced Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. SER-CAT is supported by its member institutions (see www.ser-cat.org/members.html).
References
- [1].Davis AJ, Chen DJ, DNA double strand break repair via non-homologous end-joining, Transl Cancer Res, 2 (2013) 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grundy GJ, Moulding HA, Caldecott KW, Rulten SL, One ring to bring them all--the role of Ku in mammalian non-homologous end joining, DNA Repair (Amst), 17 (2014) 30–38. [DOI] [PubMed] [Google Scholar]
- [3].Walker JR, Corpina RA, Goldberg J, Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair, Nature, 412 (2001) 607–614. [DOI] [PubMed] [Google Scholar]
- [4].Fell VL, Schild-Poulter C, The Ku heterodimer: function in DNA repair and beyond, Mutat Res Rev Mutat Res, 763 (2015) 15–29. [DOI] [PubMed] [Google Scholar]
- [5].Grundy GJ, Rulten SL, Arribas-Bosacoma R, Davidson K, Kozik Z, Oliver AW, Pearl LH, Caldecott KW, The Ku-binding motif is a conserved module for recruitment and stimulation of non-homologous end-joining proteins, Nat Commun, 7 (2016) 11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grundy GJ, Rulten SL, Zeng Z, Arribas-Bosacoma R, Iles N, Manley K, Oliver A, Caldecott KW, APLF promotes the assembly and activity of non-homologous end joining protein complexes, EMBO J, 32 (2013) 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shirodkar P, Fenton AL, Meng L, Koch CA, Identification and functional characterization of a Ku-binding motif in aprataxin polynucleotide kinase/phosphatase-like factor (APLF), J Biol Chem, 288 (2013) 19604–19613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arnoult N, Correia A, Ma J, Merlo A, Garcia-Gomez S, Maric M, Tognetti M, Benner CW, Boulton SJ, Saghatelian A, Karlseder J, Regulation of DNA repair pathway choice in S and G2 phases by the NHEJ inhibitor CYREN, Nature, 549 (2017) 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Agarwal S, Harada J, Schreifels J, Lech P, Nikolai B, Yamaguchi T, Chanda SK, Somia NV, Isolation, characterization, and genetic complementation of a cellular mutant resistant to retroviral infection, Proc Natl Acad Sci U S A, 103 (2006) 15933–15938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yano K, Morotomi-Yano K, Lee KJ, Chen DJ, Functional significance of the interaction with Ku in DNA double-strand break recognition of XLF, Febs Lett, 585 (2011) 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nemoz C, Ropars V, Frit P, Gontier A, Drevet P, Yu J, Guerois R, Pitois A, Comte A, Delteil C, Barboule N, Legrand P, Baconnais S, Yin Y, Tadi S, Barbet-Massin E, Berger I, Le Cam E, Modesti M, Rothenberg E, Calsou P, Charbonnier JB, XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining, Nat Struct Mol Biol, 25 (2018) 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yin X, Liu M, Tian Y, Wang J, Xu Y, Cryo-EM structure of human DNA-PK holoenzyme, Cell Res, 27 (2017) 1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aravind L, Koonin EV, Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system, Genome research, 11 (2001) 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Studier FW, Protein production by auto-induction in high density shaking cultures, Protein Expr Purif, 41 (2005) 207–234. [DOI] [PubMed] [Google Scholar]
- [15].Anthis NJ, Clore GM, Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm, Protein Sci, 22 (2013) 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Otwinowski Z, Minor W, Processing of X-ray diffraction data collected in oscillation mode, Method Enzymol, 276 (1997) 307–326. [DOI] [PubMed] [Google Scholar]
- [17].McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, Phaser crystallographic software, Journal of applied crystallography, 40 (2007) 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH, PHENIX: a comprehensive Python-based system for macromolecular structure solution, Acta crystallographica. Section D, Biological crystallography, 66 (2010) 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Emsley P, Cowtan K, Coot: model-building tools for molecular graphics, Acta crystallographica. Section D, Biological crystallography, 60 (2004) 2126–2132. [DOI] [PubMed] [Google Scholar]
- [20].Kirby TW, Gassman NR, Smith CE, Pedersen LC, Gabel SA, Sobhany M, Wilson SH, London RE, Nuclear Localization of the DNA Repair Scaffold XRCC1: Uncovering the Functional Role of a Bipartite NLS, Sci Rep, 5 (2015) 13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rost B, Sander C, Schneider R, PHD--an automatic mail server for protein secondary structure prediction, Comput Appl Biosci, 10 (1994) 53–60. [DOI] [PubMed] [Google Scholar]
- [22].Boulton SJ, Jackson SP, Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing, EMBO J, 17 (1998) 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen H, Xue J, Churikov D, Hass EP, Shi S, Lemon LD, Luciano P, Bertuch AA, Zappulla DC, Geli V, Wu J, Lei M, Structural Insights into Yeast Telomerase Recruitment to Telomeres, Cell, 172 (2018) 331–343 e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khan AR, Parrish JC, Fraser ME, Smith WW, Bartlett PA, James MN, Lowering the entropic barrier for binding conformationally flexible inhibitors to enzymes, Biochemistry-Us, 37 (1998) 16839–16845. [DOI] [PubMed] [Google Scholar]
- [25].Gabel SA, DeRose EF, London RE, XRCC1 interaction with the REV1 C-terminal domain suggests a role in post replication repair, DNA Repair (Amst), 12 (2013) 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Malkov SN, Zivkovic MV, Beljanski MV, Hall MB, Zaric SD, A reexamination of the propensities of amino acids towards a particular secondary structure: classification of amino acids based on their chemical structure, J Mol Model, 14 (2008) 769–775. [DOI] [PubMed] [Google Scholar]
- [27].Li B, Comai L, Functional interaction between Ku and the werner syndrome protein in DNA end processing, J Biol Chem, 275 (2000) 28349–28352. [DOI] [PubMed] [Google Scholar]
- [28].Karmakar P, Snowden CM, Ramsden DA, Bohr VA, Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus, Nucleic acids research, 30 (2002) 3583–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC, The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates, Nature, 443 (2006) 713–716. [DOI] [PubMed] [Google Scholar]
- [30].Tumbale P, Williams JS, Schellenberg MJ, Kunkel TA, Williams RS, Aprataxin resolves adenylated RNA-DNA junctions to maintain genome integrity, Nature, 506 (2014) 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Slavoff SA, Heo J, Budnik BA, Hanakahi LA, Saghatelian A, A human short open reading frame (sORF)-encoded polypeptide that stimulates DNA end joining, J Biol Chem, 289 (2014) 10950–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hung PJ, Johnson B, Chen BR, Byrum AK, Bredemeyer AL, Yewdell WT, Johnson TE, Lee BJ, Deivasigamani S, Hindi I, Amatya P, Gross ML, Paull TT, Pisapia DJ, Chaudhuri J, Petrini JJH, Mosammaparast N, Amarasinghe GK, Zha S, Tyler JK, Sleckman BP, MRI Is a DNA Damage Response Adaptor during Classical Non-homologous End Joining, Mol Cell, 71 (2018) 332–342 e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ropars V, Drevet P, Legrand P, Baconnais S, Amram J, Faure G, Marquez JA, Pietrement O, Guerois R, Callebaut I, Le Cam E, Revy P, de Villartay JP, Charbonnier JB, Structural characterization of filaments formed by human Xrcc4-Cernunnos/XLF complex involved in nonhomologous DNA end-joining, Proc Natl Acad Sci U S A, 108 (2011) 12663–12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu Q, Ochi T, Matak-Vinkovic D, Robinson CV, Chirgadze DY, Blundell TL, Non-homologous end-joining partners in a helical dance: structural studies of XLF-XRCC4 interactions, Biochem Soc Trans, 39 (2011) 1387–1392, suppl 1382 p following 1392. [DOI] [PubMed] [Google Scholar]
- [35].Zhao B, Watanabe G, Morten MJ, Reid DA, Rothenberg E, Lieber MR, The essential elements for the noncovalent association of two DNA ends during NHEJ synapsis, Nat Commun, 10 (2019) 3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Andres SN, Vergnes A, Ristic D, Wyman C, Modesti M, Junop M, A human XRCC4-XLF complex bridges DNA, Nucleic acids research, 40 (2012) 1868–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rulten SL, Grundy GJ, Non-homologous end joining: Common interaction sites and exchange of multiple factors in the DNA repair process, Bioessays, 39 (2017). [DOI] [PubMed] [Google Scholar]
- [38].Hsiou Y, Ding J, Das K, Clark AD, Hughes SH, Arnold E, Structure of unliganded HIV-1 reverse transcriptase at 2.7 angstrom resolution: Implications of conformational changes for polymerization and inhibition mechanisms, Structure, 4 (1996) 853–860. [DOI] [PubMed] [Google Scholar]
- [39].Rodgers DW, Gamblin SJ, Harris BA, Ray S, Culp JS, Hellmig B, Woolf DJ, Debouck C, Harrison SC, The structure of unliganded reverse transcriptase from the human immunodeficiency virus type 1, Proc Natl Acad Sci U S A, 92 (1995) 1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Das K, Lewi PJ, Hughes SH, Arnold E, Crystallography and the design of anti-AIDS drugs: conformational flexibility and positional adaptability are important in the design of non-nucleoside HIV-1 reverse transcriptase inhibitors, Prog Biophys Mol Biol, 88 (2005) 209–231. [DOI] [PubMed] [Google Scholar]
- [41].Bhargava R, Sandhu M, Muk S, Lee G, Vaidehi N, Stark JM, C-NHEJ without indels is robust and requires synergistic function of distinct XLF domains, Nat Commun, 9 (2018) 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.