Abstract
Pheochromocytomas are rare catecholamine-secreting neuroendocrine tumors that can occasionally progress to life-threatening disease, including a multisystem crisis. Patients with Neurofibromatosis type 1 (NF1) may develop pheochromocytomas, and the consequent chronic elevation of plasma catecholamine levels could further complicate various cardiovascular and pulmonary manifestations they may have. A 37-year-old African American female with NF1 presented with severe dyspnea, chills, myalgia, vomiting, and abdominal pain. Within several hours of hospital admission, she developed progressive agitation and died from circulatory collapse. An autopsy revealed disseminated histoplasmosis with necrotizing granulomatosis in her lungs, mediastinum, liver, and spleen, as well as bilateral pheochromocytomas with one tumor showing marked hemorrhage. Additionally, she had cardiac hypertrophy, myocarditis, pulmonary edema, apical bullae, features of pulmonary hypertension and interstitial fibrosis. Disseminated histoplasmosis caused by the fungal organism Histoplasma capsulatum is rarely described in immunocompetent individuals. This case is presented to illustrate that chronic hypercatecholaminemia caused by pheochromocytomas may potentially mask disseminated fungal infections which in turn could induce pheochromocytoma multisystem crisis in susceptible patients with neurofibromatosis.
Keywords: Forensic pathology, Neurofibromatosis type 1, Pheochromocytoma, Histoplasmosis, Sudden, Death
Introduction
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen neurofibromatosis, is a dominantly inherited genetic disorder caused by mutations in the NF1 gene and has an incidence of approximately 1 in 3000 (1). It demonstrates highly variable clinical manifestations among which multiple skin lesions (cutaneous neurofibromas, café-au-lait macules, and axillary/inguinal freckling) and iris hamartomas (Lisch nodules) are characteristic of the disease. Patients with NF1 can also develop interstitial lung disease, apical bullae, cardiac structural defects, essential and pulmonary hypertension, and bone and neurologic abnormalities (2 -6). They are prone to developing both benign and malignant tumors including pheochromocytomas, optic pathway gliomas, spinal and plexiform neurofibromas, breast cancer, and soft tissue sarcomas such as malignant peripheral nerve sheath tumors, rhabdomyosarcomas, and gastrointestinal stromal tumors. Pheochromocytomas are seen in 0.1% to 5.7% of NF1 patients and bilateral pheochromocytomas and extra-adrenal paragangliomas are extremely rare (7,8).
Secretion of catecholamines such as epinephrine, norepinephrine, and dopamine by a pheochromocytoma can further aggravate some of the clinical manifestations in NF1 patients. While sustained or paroxysmal hypertension, flushing, and palpitations are well-known to occur, catecholamine-mediated myocarditis and Takotsubo-like cardiomyopathy are poorly understood entities also affecting the cardiovascular system in patients with pheochromocytoma (9 -11). Whether catecholamine excess in NF1 has any effect on patient immunity and susceptibility to infection is not well-known.
The autopsy findings of bilateral pheochromocytomas and unexpected disseminated histoplasmosis in an NF1 patient who likely presented with acute pheochromocytoma multisystem crisis (PMSC) and succumbed after multiple episodes of cardiac arrest are reported herein.
Care Report
A 37-year-old African American female with a medical history of NF1, hypertension, schizophrenia, and seizure disorder presented with upper respiratory symptoms, nausea, and myalgia. She tested negative for influenza and was treated symptomatically. Two weeks later, she returned to the emergency department with dyspnea, chills, myalgia, nausea, emesis, and abdominal pain of 2 days duration. She was well-oriented but found to be hypothermic, tachycardic, and in respiratory distress. A chest X-ray demonstrated opacities in the lower lung lobes concerning for pulmonary edema and aspiration pneumonia. She was started on intravenous fluids, broad-spectrum antibiotics, and high flow ventilation. However, she became restless with worsening tachycardia (up to 160 beats/min) and tachypnea (35-40 breaths/min), and also developed severe emesis resistant to antiemetics.
Computerized tomography (CT) of the chest revealed extensive bibasilar ground-glass opacities with areas of conglomeration, interlobular septal thickening, emphysematous cysts, and a 2.5 cm, mass-like consolidation in the perihilar region of the left lower lobe. Abdomen/pelvis CT with contrast revealed a 1.5 cm right adrenal mass and a 5.3 cm heterogeneously enhancing mass in the left adrenal which was highly suspect for a pheochromocytoma with internal hemorrhage. The patient was admitted to the intensive care unit where she became progressively agitated and later, hypotensive and unresponsive requiring cardiopulmonary resuscitation. Less than an hour later, she became unresponsive again and was revived with maximum pressor support. Soon afterward, she developed cardiac arrest for a third time and could not be resuscitated.
Her laboratory investigations revealed an elevated white blood cell count of 18.9 × 109/L as well as elevations in liver enzymes, serum lactate, and serum creatinine. Blood and urine cultures returned negative. Plasma catecholamines showed marked elevations over 50 times the upper normal values; metanephrines 25.20 nmol/L (0-0.49) and normetanephines 49.70 nmol/L (0-0.89).
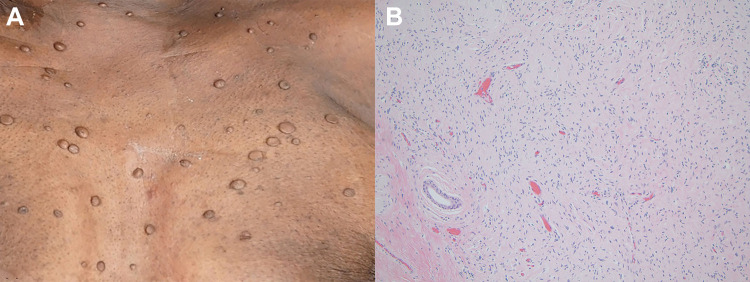
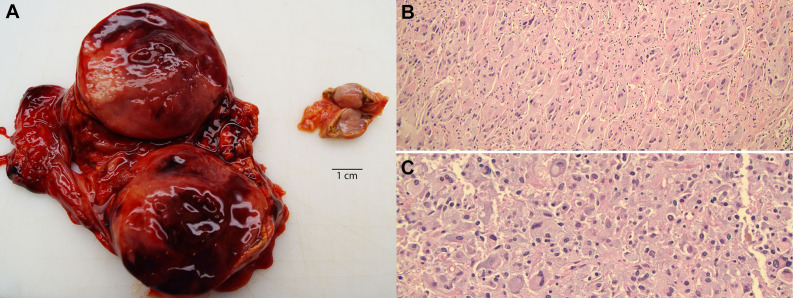
At autopsy, the skin of the decedent was notable for numerous cutaneous nodules ranging in size from 0.2 to 4.5 cm and a few macules ( Image 1A ). The right and left lungs were heavy with pulmonary edema and congestion (weighing 1175 g and 1200 g, respectively) and showed multiple areas of apical subpleural blebbing. On cut sections of the left lower lobe, an area of consolidation and a 1.2 cm cavitary lesion with necrosis were identified. The left hilar lymph nodes appeared modestly enlarged and soft. In the heart, the left ventricular wall was hypertrophied (1.8 cm thick; expected 1.0-1.5 cm) and the tricuspid and mitral valves were thickened with myxoid change. The liver was diffusely congested and a 0.8 cm, tan-yellow lesion was identified in the right lobe. The spleen had multiple tan-red nodules ranging in size from 0.3 to 0.6 cm. There was a 5.3 cm, solid hemorrhagic mass in the left adrenal gland and a 1.5 cm, oval, tan nodule in the right adrenal gland (Image 2A) . Gross examination of the unfixed brain revealed cerebral edema.
Image 1.
Neurofibromatosis. A, Skin with numerous flesh-colored soft nodules of varying sizes. B, Histologic section of a representative skin nodule shows fascicles of spindled cells with elongated, wavy nuclei and ample cytoplasm, consistent with a neurofibroma. Hematoxylin and eosin, ×100.
Image 2.
Adrenal tumors with bilateral pheochromocytoma. A, Photomicrograph of the enlarged, hemorrhagic adrenal mass (left) and a smaller adrenal nodule (right). Note the bright yellow rim of adrenal cortex around both tumors. B, Histologic sections from the right adrenal tumor show a neoplastic cell proliferation in Zellballen pattern. C, Sections of the larger left adrenal tumor show sheet-like proliferation of neoplastic cells. These cells demonstrate oval, hyperchromatic nuclei, mild to moderate nuclear pleomorphism, and abundant amphophilic granular cytoplasm. Hematoxylin and eosin, ×100.
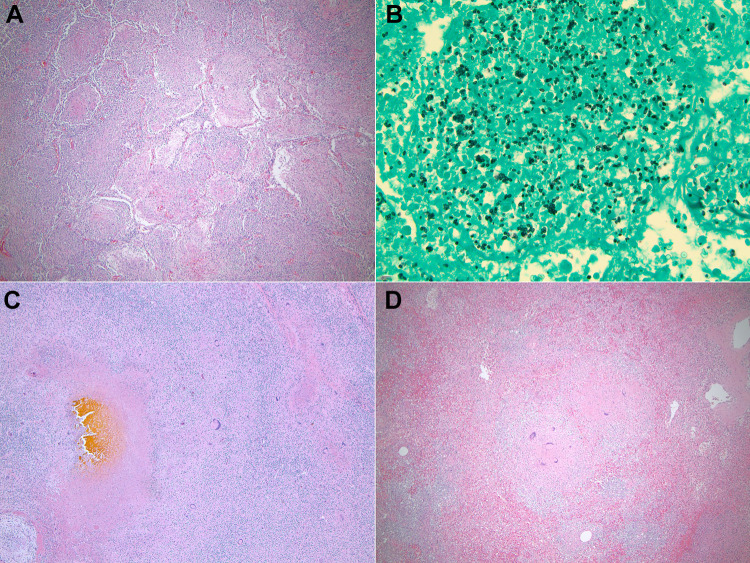
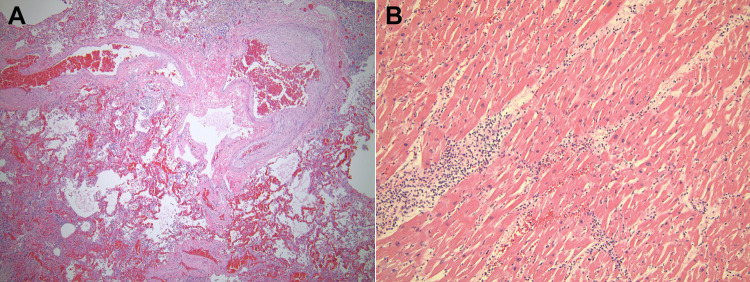
Histologic sections of a representative skin nodule showed fascicles of spindled cells with elongated, wavy nuclei and ample cytoplasm, consistent with a neurofibroma ( Image 1B ). The most notable findings were present in the lungs. Multiple epithelioid granulomas with multinucleated giant cells were found bilaterally, predominantly in the lower lobes. They occupied alveolar spaces and formed sizable conglomerates (Image 3A) . One such conglomerate of necrotizing granulomas in the left lower lobe formed a central cavitary lesion containing necroinflammatory material. A silver stain highlighted abundant small fungal yeast forms of varying sizes (2-6 microns in diameter) present in the necrotic granulomas. They were morphologically consistent with histoplasmosis ( Image 3B ). A left enlarged hilar lymph node also showed similar necrotizing granulomatous disease with numerous fungi effacing the normal nodal architecture. A Ziehl-Neelson stain was negative for acid-fast organisms in the lung and nodal lesions. Additionally, the lung sections showed vascular congestion, pleural edema, and emphysematous subpleural blebbing involving both upper lobes. There was evidence of pulmonary hypertension in the bilateral lungs with hypertrophied muscular vessels with intimal fibrosis and medial hypertrophy, dilated vessels, and rare thrombosed small vessels ( Image 4A ). Patchy interstitial fibrosis was also present.
Image 3.
Granulomatosis with histoplasmosis. A, Section of the left lung lobe demonstrates a conglomerate of epithelioid granulomas; Hematoxylin and eosin, ×40. B, Gomori methenamine silver (GMS) stain highlights small intra- and extracellular fungal organisms, morphologically consistent with histoplasmosis; ×400. C, Right liver lobe lesion demonstrates necrotizing granulomatosis. Note the bile staining in the center of the necrotic lesion and the surrounding multinucleated giant cells and chronic inflammation. This lesion contained GMS-positive fungal organisms (not shown). D, Histologic section of the spleen demonstrates nonnecrotizing granulomas with multinucleated giant cells. C and D, Hematoxylin and eosin, ×40.
Image 4.
Pulmonary changes and myocarditis. A, Section of the lung shows blood vessels with medial hypertrophy and dilatation. The background shows vascular congestion and pulmonary edema. Hematoxylin and eosin, ×40. B, Section of the left ventricle shows lymphocytes and neutrophils infiltrating the myocardium. Also note the enlarged cardiomyocyte nuclei; Hematoxylin and eosin, ×100.
Cardiomyocyte hypertrophy and myocarditis were identified in both ventricles and the septum of the heart. Myocarditis featured lymphocytes and neutrophils infiltrating among the cardiomyocytes from the epicardium to the endocardium (Image 4B) . There was no evidence of myocardial infarction, fibrosis, or coronary artery occlusion. The liver showed vascular congestion, mild bile stasis, and many small, non-necrotizing epithelioid granulomas scattered throughout the parenchyma. The macroscopic lesion in the right liver lobe corresponded to necrotizing granulomas with abundant fungal organisms, consistent with histoplasmosis ( Image 3C ). The spleen contained multiple small granulomas with multinucleated giant cells and no significant necrosis or fungal organisms ( Image 3D ). Multiple remote microinfarctions were present in the cerebellum. However, there was no evidence of disseminated infection to the brain.
The bilateral adrenal masses were composed of neoplastic cells with oval, hyperchromatic nuclei, mild to moderate nuclear pleomorphism, and abundant amphophilic granular cytoplasm. These cells were arranged in alveolar nests/Zellballen pattern (Image 2B) . In the larger left adrenal mass, these neoplastic cells appeared more sheet-like with prominent hemorrhage but with no necrosis (Image 2C). A rim of preserved adrenal cortical cells was present around both masses. No granulomas or significant mitoses were noted in either of these tumors. The histologic findings were consistent with bilateral pheochromocytomas. The cause of death in this patient with NF1 was determined to be disseminated histoplasmosis and PMSC.
Discussion
The mortality of NF1 patients is primarily due to cardiovascular diseases and malignant neoplasms resulting in a shortened life expectancy by 8 to 15 years (12). Sudden death in these patients warrants an autopsy investigation as detailed reports of the findings in such scenarios are sparse in the literature (13,14). Takamiya et al. recently reported the sudden death of a 33-year-old Japanese male with NF1 who initially presented with nonspecific abdominal symptoms and headache. Postmortem examination revealed a unilateral pheochromocytoma and myocarditis with elevated catecholamine levels detected in the blood. The authors attributed the cause of death to pheochromocytoma (adrenal) crisis (14). Our case also demonstrated similar biochemical and histopathological findings in a NF1 patient who had a rapid course of clinical deterioration.
Pheochromocytoma multisystem crisis is a rare, acute, and severe complication of these tumors. Lability in blood pressures with hypertension and/or hypotension, cardiogenic shock, signs of acute coronary syndrome, high fever, sepsis, hemoptysis, encephalopathy, acute abdominal pain, vomiting, and abnormal liver function are some of the presenting features which are often nonspecific and vague (15 -17). Hence, the diagnosis of a PMSC can be significantly delayed unless the clinical suspicion for an underlying pheochromocytoma is high.
In the presented case, the decedent showed multiple manifestations of NF1 including bilateral pheochromocytomas which are extremely unusual. Evidence of acute hemorrhage into the larger pheochromocytoma with corresponding markedly elevated serum catecholamines, and the overall clinical findings supported PMSC in this patient. The association between NF1 and structural defects in the cardiovascular and pulmonary systems are known (2 -4). Her autopsy revealed multiple lung and heart pathologies including pulmonary hypertension, apical bullae, interstitial fibrosis, cardiac hypertrophy, and myocarditis. It is believed that catecholamine excess resulting from pheochromocytoma plays a role in the pathogenesis of myocarditis and certain cardiomyopathies (10,14).
The postmortem finding of disseminated histoplasmosis in the decedent was unexpected. Histoplasmosis, endemic to the Mississippi and Ohio River valleys, is caused by the fungal organism Histoplasma capsulatum. Symptomatic disease is uncommon and acute pulmonary histoplasmosis, where patients may present with fever, malaise, headache, weakness, and myalgia is often self-limiting (18,19). Clinical manifestations of chronic cavitary pulmonary histoplasmosis and disseminated histoplasmosis are extremely rare (<1%). The former is known to associate with preexisting lung diseases such as emphysematous bullae, and the latter in immunocompromised states. In severe cases, patients can present with a sepsis-like clinical picture with hypotension, disseminated intravascular coagulation, renal failure, and acute respiratory distress (18). Apart from having NF1, the decedent appeared otherwise immunocompetent. It can be speculated that the catecholamine excess contributed to masking of the severity and dissemination of her infection. While histoplasmosis superimposed on already compromised lungs may explain her presenting respiratory symptoms, the findings of necrotizing and cavitary granulomatous disease involving multiple organs indicated the chronicity of the infection that appeared disproportionate to the patient’s stated relatively short duration of her illness.
What triggers a pheochromocytoma to enlarge, hemorrhage, and lead to PMSC is not completely understood. Drugs including metoclopramide and certain anesthetic agents, as well as abdominal trauma are some of the causative factors (20). Sparse reports describe that bacterial infections and sepsis could trigger catecholamine release from a pheochromocytoma that could then develop into a PMSC (21 -23). Interestingly, pro-inflammatory cytokines such as interleukin-6 are secreted in high amounts from pheochromocytomas involved in PMSC (24). The same cytokines are induced by systemic infections as well. Our histologic examination of the adrenal tumors did not reveal direct involvement by histoplasmosis or granulomatous disease. Serum or immunohistochemical evidence of tumor cytokines was also not tested. However, it is very likely that pro-inflammatory cytokines from the patient’s disseminated infection played a significant role in causing the pheochromocytoma to grow and hemorrhage. Ours is the first report of a disseminated fungal infection which potentially induced PMSC.
Conclusion
This case underscores the value of a comprehensive postmortem examination in NF1 patients with sudden death. Although there are no clear recommendations for screening of pheochromocytomas in patients with NF1, this report emphasizes the importance of close clinical follow-up of these patients especially in the setting of unexplained hypertension. Early detection and removal of an adrenal tumor can avert severe complications of pheochromocytomas. Our case also illustrates that pheochromocytomas can potentially mask infections causing them to progress into severe disease.
AUTHORS
Brannon G. Broadfoot, MD, Department of Pathology, University of Arkansas for Medical Sciences
Roles: Manuscript draft preparation, image acquisition, final edits, and reviews.
Asangi R. Kumarapeli, MD, PhD, Department of Pathology, University of Arkansas for Medical Sciences
Roles: Case selection for manuscript, editing of draft, final edits, and reviews.
Footnotes
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects.
Statement of Informed Consent: No identifiable personal data was presented in this manuscript.
Disclosures & Declaration of Conflicts of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
ORCID iD: Asangi R. Kumarapeli https://orcid.org/0000-0003-3628-8248
References
- 1. Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004 doi:10.1038/nrdp.2017.4 PubMed PMID: 28230061. [DOI] [PubMed] [Google Scholar]
- 2. Tedesco MA, Di Salvo G, Natale F, et al. The heart in neurofibromatosis type 1: an echocardiographic study. Am Heart J. 2002;143(5):883–888. PubMed PMID: 12040353. [DOI] [PubMed] [Google Scholar]
- 3. Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J. 2007;29(1):210–214. doi:10.1183/09031936.06.00044006 PubMed PMID: 16870664. [DOI] [PubMed] [Google Scholar]
- 4. Gumbiene L, Petrulioniene Z, Rucinskas K, et al. Pulmonary hypertension: a fatal complication of neurofibromatosis type 1. Respir Care. 2011;56(11):1844–1848. doi:10.4187/respcare.01030 PubMed PMID: 21605478. [DOI] [PubMed] [Google Scholar]
- 5. Jutant EM, Girerd B, Jais X, et al. Pulmonary hypertension associated with neurofibromatosis type 1. Eur Respir Rev. 2018;27(149):12 doi:10.1183/16000617.0053-2018 PubMed PMID: 30158278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engel PJ, Baughman RP, Menon SG, Kereiakes DJ, Taylor L, Scott M. Pulmonary hypertension in neurofibromatosis. Am J Cardiol. 2007;99(8):1177–1178. doi:10.1016/j.amjcard.2006.11.072 PubMed PMID: 17437753. [DOI] [PubMed] [Google Scholar]
- 7. Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. Von Recklinghausen’s disease and pheochromocytomas. J Urol. 1999;162(5):1582–1586. PubMed PMID: 10524872. [PubMed] [Google Scholar]
- 8. Gruber LM, Erickson D, Babovic-Vuksanovic D, Thompson GB, Young WF, Jr, Bancos I. Pheochromocytoma and paraganglioma in patients with neurofibromatosis type 1. Clin Endocrinol (Oxf). 2017;86(1):141–149. doi:10.1111/cen.13163 PubMed PMID: 27460956. [DOI] [PubMed] [Google Scholar]
- 9. Gagnon N, Mansour S, Bitton Y, Bourdeau I. Takotsubo-like cardiomyopathy in a large cohort of patients with pheochromocytoma and paraganglioma. Endocr Pract. 2017;23(10):1178–1192. doi:10.4158/EP171930.OR PubMed PMID: 28704094. [DOI] [PubMed] [Google Scholar]
- 10. Ferreira VM, Marcelino M, Piechnik SK, et al. Pheochromocytoma is characterized by catecholamine-mediated myocarditis, focal and diffuse myocardial fibrosis, and myocardial dysfunction. J Am Coll Cardiol. 2016;67(20):2364–2374. doi:10.1016/j.jacc.2016.03.543 PubMed PMID: 27199060. [DOI] [PubMed] [Google Scholar]
- 11. Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am. 2011;40(2):295–311, vii doi:10.1016/j.ecl.2011.02.002 PubMed PMID: 21565668; PubMed Central PMCID: PMCPMC3094542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stewart DR, Korf BR, Nathanson KL, Stevenson DA, Yohay K. Care of adults with neurofibromatosis type 1: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20(7):671–682. doi:10.1038/gim.2018.28 PubMed PMID: 30006586. [DOI] [PubMed] [Google Scholar]
- 13. Byard RW. Forensic considerations in cases of neurofibromatosis—an overview. J Forensic Sci. 2007;52(5):1164–1170. doi:10.1111/j.1556-4029.2007.00512.x PubMed PMID: 17645486. [DOI] [PubMed] [Google Scholar]
- 14. Takamiya M, Niitsu H, Saigusa K. An autopsy case of sudden death in neurofibromatosis type 1 with pheochromocytoma and myocarditis. Am J Forensic Med Pathol. 2018;39(1):78–81. doi:10.1097/PAF.0000000000000367 PubMed PMID: 29210711. [DOI] [PubMed] [Google Scholar]
- 15. Lassnig E, Weber T, Auer J, Nomeyer R, Eber B. Pheochromocytoma crisis presenting with shock and tako-tsubo-like cardiomyopathy. Int J Cardiol. 2009;134(3):e138–e140. doi:10.1016/j.ijcard.2008.03.012 PubMed PMID: 18579235. [DOI] [PubMed] [Google Scholar]
- 16. Newell KA, Prinz RA, Pickleman J, et al. Pheochromocytoma multisystem crisis. A surgical emergency. Arch Surg. 1988;123(8):956–959. PubMed PMID: 2899426. [DOI] [PubMed] [Google Scholar]
- 17. Fred HL, Allred DP, Garber HE, Retiene K, Lipscomb H. Pheochromocytoma masquerading as overwhelming infection. Am Heart J. 1967;73(2):149–154. PubMed PMID: 6017565. [DOI] [PubMed] [Google Scholar]
- 18. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–132. doi:10.1128/CMR.00027-06 PubMed PMID: 17223625; PubMed Central PMCID: PMCPMC1797635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knox KS, Hage CA. Histoplasmosis. Proc Am Thorac Soc. 2010;7(3):169–172. doi:10.1513/pats.200907-069AL PubMed PMID: 20463244. [DOI] [PubMed] [Google Scholar]
- 20. Leonard JB, Munir KM, Kim HK. Metoclopramide induced pheochromocytoma crisis. Am J Emerg Med. 2018;36(6):1124 e1-e2 doi:10.1016/j.ajem.2018.03.009 PubMed PMID: 29534916. [DOI] [PubMed] [Google Scholar]
- 21. Abe I, Nomura M, Watanabe M, et al. Pheochromocytoma crisis caused by campylobacter fetus. Int J Urol. 2012;19(5):465–467. doi:10.1111/j.1442-2042.2011.02950.x PubMed PMID: 22221008. [DOI] [PubMed] [Google Scholar]
- 22. Tanriver Y, Betz MJ, Nibbe L, Pfluger T, Beuschlein F, Strowski MZ. Sepsis and cardiomyopathy as rare clinical manifestations of pheochromocytoma—two case report studies. Exp Clin Endocrinol Diabetes. 2010;118(10):747–753. doi:10.1055/s-0030-1253413 PubMed PMID: 20539976. [DOI] [PubMed] [Google Scholar]
- 23. Myers MG, Arshinoff SA. Infection and pheochromocytoma. JAMA. 1977;237(19):2095–2096. PubMed PMID: 576892. [PubMed] [Google Scholar]
- 24. Minetto M, Dovio A, Ventura M, et al. Interleukin-6 producing pheochromocytoma presenting with acute inflammatory syndrome. J Endocrinol Invest. 2003;26(5):453–457. doi:10.1007/BF03345202 PubMed PMID: 12906374. [DOI] [PubMed] [Google Scholar]