Abstract
Nitrous oxide, often used as an anesthetic agent, is also increasingly a drug of abuse due to its euphoric and anxiolytic effects. Frequent exposure to nitrous oxide can lead to neurologic complications, including B12 deficiency and resultant subacute myeloneuropathy, as well as direct neurotoxicity. A clinical presentation of acute sensorimotor polyneuropathy mimicking Guillain-Barré syndrome after chronic nitrous oxide abuse has been reported only rarely. Here we present a 17-year-old previously healthy girl presented with 10 days of progressive ascending sensory loss and weakness in the legs. She admitted to heavy nitrous oxide abuse over a period of a year or more. Laboratory evaluation was significant for normal vitamin B12 level with elevated homocysteine. A magnetic resonance imaging (MRI) of her spine showed abnormal signal involving the bilateral dorsal columns. Nerve conduction studies were suggestive of severe axonal sensorimotor polyneuropathy. This patient demonstrates concurrent multifactorial neurologic injury as a result of nitrous oxide abuse. She had a functional vitamin B12 deficiency as indicated by the elevated homocysteine, leading to a subacute combined degeneration that was evident on the MRI. In addition, she had evidence of direct neurotoxicity leading to axonal injury and sensorimotor polyneuropathy reminiscent of Guillain-Barré syndrome. This clinical picture is a serious but seldom reported possible complication if nitrous oxide abuse and should be considered in patients presenting with a clinical picture suspicious for Guillain-Barré syndrome or its variants.
Keywords: neurotoxicity syndromes, nitrous oxide, Guillain-Barré syndrome, autoimmune diseases of, the nervous system, polyneuropathies, neuromuscular diseases
Introduction
Nitrous oxide (N2O), also called “laughing gas,” is often used medically as an anesthetic agent but can be used recreationally for its euphoric and anxiolytic effects. It can produce hallucinations, dissociation, dizziness, and loss of balance. The onset is rapid, within seconds, and the effects wear off within minutes. Recreational inhalation of N2O, sometimes referred to as “whippets,” is reported to be increasing.1,2 In the United States, the lifetime prevalence of recreational N2O abuse may be as high as 29%.2 Chronic N2O exposure is known to cause neurotoxicity. Most commonly seen is a predominant sensory subacute myeloneuropathy involving the dorsal columns of the spinal cord,1 though peripheral neuropathies and neuropsychiatric symptoms have also been reported.3,4 “Pseudo” Guillain-Barré syndrome in the context of N2O abuse has rarely been reported.5 Here, we report an adolescent girl with chronic heavy N2O abuse who presented with progressive ascending weakness of the lower limbs, areflexia, and sensory loss, resembling Guillain-Barré syndrome.
Case Report
A 17-year-old girl with no prior medical problems presented to the emergency department with 10 days of progressive leg weakness and numbness. She reported gradual onset of progressive sensory loss involving both legs ascending to the level of the abdomen, with accompanying tingling sensation on the plantar surface of both feet. Five days prior to presentation, she noticed weakness in both legs which progressed to the point that she was unable to walk and had to crawl to the bathroom at home. There was no involvement of the upper extremities. There was no urinary or bowel incontinence, no respiratory symptoms, and no dysphagia or drooling. Examination was significant for weakness in both legs which was more severe in the left, impaired sensation to light touch throughout the legs to the level of the umbilicus, impaired vibratory sensation to the level of the mid-thighs, and impaired proprioception in the feet. Reflexes were absent at the ankles and patellae bilaterally. Strength, sensation, and reflexes in the arms were normal. Mental status and cranial nerves were normal. On further history, the patient admitted to chronic heavy abuse of N2O “whippets” over a period of a year or more, using between 100 and 200 or at times even more than a thousand N2O cartridges per day.
Laboratory evaluation revealed a normal serum vitamin B12 level of 408 pg/mL (normal range 211-945 pg/mL) and an elevated homocysteine level of 81.2 µmol/L (normal range 2.5-15 µmol/L). Other laboratory evaluations, including complete blood count, electrolytes, liver function tests, hemoglobin A1C, antinuclear antibodies, syphilis screening, and HIV screening were unremarkable. Lumbar puncture was deferred. An magnetic resonance imaging of the thoracic and lumbar spine with and without contrast revealed diffuse abnormal signal involving the bilateral dorsal columns on T2 and short-TI inversion recovery (STIR) images (Figure 1).
Figure 1.
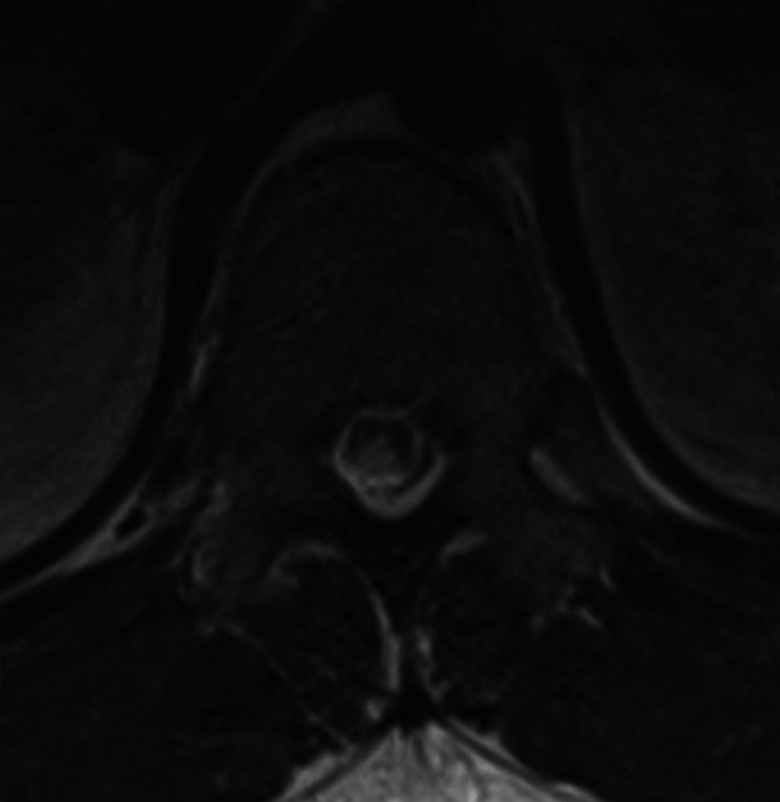
Spine magnetic resonance imaging.
Nerve conduction studies showed absent tibial motor responses bilaterally, absent right peroneal motor response, and severely reduced left peroneal motor response. The sural sensory responses were absent bilaterally. In the upper extremities, the median and ulnar sensory responses were severely reduced in amplitude but with normal motor responses. Needle electromyography (EMG) did not show clear abnormalities. The overall picture was consistent with a severe axonal sensorimotor polyneuropathy in the lower extremities as well as a sensory neuropathy in the upper extremities (Table 1).
Table 1.
Nerve Conduction Studies.
| Motor Nerves | ||||||
|---|---|---|---|---|---|---|
| Nerve/Sites | Latency (ms) | Amplitude (mV) | Segments | Distance (mm) | Velocity (m/s) | |
| Left Median—APB | ||||||
| Wrist | 3.8 | 8.3 | Wrist—APB | 80 | ||
| Elbow | 8.1 | 7.9 | Elbow—Wrist | 220 | 50.9 | |
| Left Ulnar—ADM | ||||||
| Wrist | 3.2 | 8.9 | Wrist—ADM | 80 | ||
| B.Elbow | 7.5 | 8.4 | B.Elbow—Wrist | 220 | 50.9 | |
| A.Elbow | 9.6 | 8.3 | A.Elbow—B.Elbow | 110 | 51.5 | |
| Left peroneal—EDB | ||||||
| Ankle | 6.0 | 0.3 | Ankle—EDB | |||
| Right peroneal—EDB | ||||||
| Ankle | NR | NR | Ankle—EDB | |||
| Left tibial—AH | ||||||
| Ankle | NR | NR | Ankle—AH | |||
| Right tibial—AH | ||||||
| Ankle | NR | NR | Ankle—AH | |||
| Sensory Nerves | ||||||
| Nerve/Sites | Rec. Site | Latency (ms) | Amplitude (µV) | Segments | Distance (mm) | Velocity (m/s) |
| Left sural—ankle | ||||||
| Calf | Ankle | NR | NR | Calf—Ankle | 140 | NR |
| Right sural—ankle | ||||||
| Calf | Ankle | NR | NR | Calf—Ankle | 140 | NR |
| Left median—wrist—digit II | ||||||
| Wrist | Dig II | 2.97 | 3.5 | Dig II—Wrist | 130 | 44 |
| Left ulnar—wrist—digit V | ||||||
| Wrist | Dig V | 2.55 | 3.5 | Dig V—Wrist | 110 | 43 |
Abbreviations: AH, abductor hallucis; ADM, abductor digiti minimi; APB, abductor pollicis brevis; EDB, extensor digitorum brevis; NR, no response.
The patient was treated with vitamin B12 repletion and physical therapy. A psychiatry evaluation leads to a diagnosis of previously unrecognized major depressive disorder, for which she was self-medicating by abusing whippets. She gradually regained strength and sensation in her legs over a period of weeks and was discharged to a subacute rehabilitation facility with mental health services.
Discussion
Chronic N2O exposure leads to inactivation of vitamin B12 and a functional B12 deficiency. This can result in subacute combined degeneration (SCD), a myeloneuropathy involving the dorsal columns of the spine leading to subacute onset of predominantly sensory symptoms including impaired vibratory sensation, impaired proprioception, and paresthesias. Subacute combined degeneration is the most common neurologic manifestation of N2O toxicity.6 Vitamin B12 deficiency can also lead to megaloblastic anemia, which may be quite marked, although this was not seen in our patient. It is important to note that a functional B12 deficiency can be present even with a normal serum B12 level, and therefore, other biomarkers must also be checked when B12 deficiency is suspected. It is not uncommon for individuals with chronic N2O exposure to have normal B12 levels but elevated homocysteine and/or methylmalonic acid, as seen in our patient.7
There is also evidence that N2O can have direct neuronal toxicity independent of B12 involvement.6 Paresthesias, disorders of equilibrium, gait abnormalities, weakness, and ataxia have all been described in 10% or more of individuals with N2O exposure in a recent meta-analysis.7 Progressive ascending sensorimotor neuropathy reminiscent of Guillain-Barré syndrome as a consequence of N2O exposure, as seen in our patient, has been reported only rarely.3,5,8
Our patient presented with simultaneous, multifactorial neurologic dysfunction. She had myelopathy in the setting of functional B12 deficiency as evidenced by her spinal imaging findings, pattern of sensory loss, and elevated homocysteine. Additionally, she had direct neuronal injury leading to progressive weakness and loss of deep tendon reflexes, mimicking Guillain-Barré syndrome, which was apparent on electrodiagnostic studies. In order to understand the presentation and examination findings in this patient, it was important to recognize both pathophysiologic processes as neurotoxic consequences of N2O use. Their concurrent presentation in this patient is unusual.
The exact risk factors for developing this kind of severe neurotoxicity after N2O exposure are not clear. Certainly patients with B12 deficiency at baseline are at increased risk of developing SCD.6 There also may be certain congenital disorders that predispose to N2O toxicity; adverse outcomes were reported in a child with methylenetetrahydrofolate reductase (MTHFR) deficiency who was exposed to N2O for surgical anesthesia, for example.6,9 It is possible that cumulative exposure or high doses of N2O may have played a role in this patient’s more extensive neurotoxicity. A recent meta-analysis examining 76 individuals with regular N2O exposure found a median use of 25 cartridges per day, with an interquartile range of 8 to 85 cartridges per day, and a high incidence of neurologic complications (80% or more) in this population.7 By comparison, our patient reported significantly higher use of one to two hundred, and at times up to a thousand or more, cartridges per day.
Conclusion
Vitamin B12 deficiency and resultant myeloneuropathy are well described consequences of chronic N2O exposure. Other neurotoxicity has also been described, independent of B12 status, causing paresthesias, disorders of equilibrium, gait abnormalities, weakness, and ataxia, among other symptoms. Acute sensorimotor polyneuropathy mimicking Guillain-Barré syndrome is a serious but less commonly described clinical presentation of N2O abuse that is important to recognize. This presentation can occur either independently or concurrently with the more typical myelopathy. Possible N2O exposure should be considered in the differential diagnosis for patients presenting with a clinical picture suspicious for Guillain–Barré syndrome. Additionally, further investigation into possible dose-dependent effects of N2O exposure may be warranted.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jules C. Beal, MD  https://orcid.org/0000-0003-4748-9248
https://orcid.org/0000-0003-4748-9248
References
- 1. Amsterdam JV, Nabben T, den Brink WD. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73(3):790–796. [DOI] [PubMed] [Google Scholar]
- 2. Kaar SJ, Ferris J, Waldron J, Devaney M, Ramsey J, Winstock A. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395–401. [DOI] [PubMed] [Google Scholar]
- 3. Hirvioja J, Joutsa J, Wahlsten P, Korpela J. Recurrent paraparesis and death of a patient with ‘whippet’ abuse. Oxf Med Case Reports. 2016;2016(3):41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sethi NK, Mullin P, Torgovnick J, Capasso G. Nitrous oxide ‘whippit’ abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tatum WO, Bui DD, Grant EG, Murtagh R. Pseudo-Guillain-Barre syndrome due to ‘whippet’-induced myeloneuropathy. J Neuroimaging. 2010;20(4):400–401. [DOI] [PubMed] [Google Scholar]
- 6. Sanders C, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2009;109(4):707–722. [DOI] [PubMed] [Google Scholar]
- 7. Oussalah A, Julien M, Levy J, et al. Global burden related to nitrous oxide exposure in medical and recreational settings: a systematic review and individual patient data meta-analysis. J Clin Med. 2019;8(4):pii:E551 doi:10.3390/jcm8040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris N, Lynch K, Greenberg SA. Severe motor neuropathy or neuronopathy due to nitrous oxide toxicity after correction of vitamin B12 deficiency. Muscle Nerve. 2015;51(4):614–616. [DOI] [PubMed] [Google Scholar]
- 9. Selzer RR, Rosenblatt DS, Laxova R, Hogan K: Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med. 2003;349(1):45–50. [DOI] [PubMed] [Google Scholar]


