Abstract

In the present study, pore adsorption behavior of globular myoglobin (Mb) at mesoporous silicas was examined utilizing the low-temperature differential scanning calorimetry (DSC) method. The DSC method relies on a decrease in heat of fusion for the pore water upon adsorption of Mb. The amount and structure of Mb adsorbed into the mesoporous silica were examined by DSC and optical absorption spectroscopy. The results indicated that the pore adsorption behavior of Mb strongly depended on the solution pH and pore size of mesoporous silica. For the adsorption of Mb (diameter = 3.5 nm) into mesoporous silica with narrow pores (pore diameter = 3.3 nm) at a pH ranging from 7.0 to 3.7, the penetration of both folded and denatured Mb molecules was confirmed. The folded Mb could penetrate into large mesoporous silica pores (pore diameter = 5.3 and 7.9 nm), whereas the penetration of the denatured Mb molecules was completely inhibited. The distribution of folded Mb at mesoporous silica depended on the pore size; almost all folded Mb molecules located inside mesoporous silica pores of diameters 3.3 and 5.3 nm, whereas the Mb molecules distributed at bot internal and external pore surfaces of mesoporous silica with 7.9 nm in pore diameter. These pore adsorption behaviors suggest that aggregation or stacking of the Mb molecules at the pore entrance regions of the large pores affected the pore adsorption behavior.
Introduction
Inorganic nanoporous materials have been used in the chromatographic separation of protein molecules and as rigid hosts for protein encapsulation.1−6 In these applications, the location of protein molecules on the nanoporous material is a major contributor to the overall adsorption/desorption process or biocatalytic reaction. Particularly, the key factor is whether or not molecules can penetrate into the pores of the inorganic nanoporous material. The location of the adsorbed protein is, however, difficult to observe experimentally.
The effect of pore size on adsorption of a protein has been studied using surfactant-templated mesoporous silica because the mesoporous silica possesses uniform pore structures.1−6 In general, adsorption of protein molecules at mesoporous silica is performed by adding particulate mesoporous silica to a protein solution, and the total amount of protein adsorbed at the mesoporous silica is estimated by the decrease of protein molecules in the supernatant solvent phase.7−13 The total amount of protein adsorbed has often been used to approximate the adsorption of protein into the pores (pore adsorption). Nitrogen adsorption/desorption isotherm measurements provided information on the decrease in the pore volume of mesoporous silica upon protein adsorption.7 The decrease in pore volume can be used to estimate the amount of protein molecules inside the pores, but the requirement for large sample weights and thermal baking limits the application of this technique to study the pore adsorption behavior. Fluorescence microscopes have been used to observe the distribution of fluorescence-labeled proteins adsorbed at a single mesoporous silica particle when the particle size is larger than 1 μm.14,15 Spectroscopic methods based on optical interference16−18 and waveguide mode19,20 allow precise determination of the pore adsorption behavior of protein molecules, but their applications were limited for an inorganic nanoporous film.
In our previous study, it was demonstrated that differential scanning calorimetry (DSC) was useful to estimate the amounts of protein molecules adsorbed inside and outside of pores of particulate mesoporous silica.21 When a suspension of mesoporous silica powders is subjected to the DSC measurement, DSC melting peaks of water inside the pores (pore water) appear at a lower temperature than those of bulk water.22,23 Since the melting peak area of pore water decreases linearly with the increase in the amount of protein molecules inside the pore, the pore adsorption behavior of a protein can be quantitatively analyzed.21 In our previous DSC study on the adsorption of myoglobin (Mb) from water containing no electrolyte, it was confirmed that almost all Mb (diameter: 3.5 nm) molecules could penetrate into a narrow cylindrical pore (BJH pore diameter = 3.9 nm).17 On the other hand, the Mb molecules distributed both inside and outside of the pores of mesoporous silica with large pores (BJH pore diameter: 6.4 nm).21 To date, it has been considered that the penetration of protein molecules is easier for larger mesoporous silica pores.15,16 The results of our previous DSC study differed from this consideration.
Solution pH is an important factor in governing the adsorption and catalytic behaviors of protein molecules because it regulates the net surface charge of the protein and silica pore wall.9−13 For the adsorption of Mb into mesoporous silica materials, it was reported that acidic conditions were favored for the Mb adsorption. The isoelectric point (pI) of Mb is around 7,13 and pKa values of surface silanol groups at the silica surface are ≤2 (Q3 silanol) and 8.2 (Q2 silanol).24 The effective adsorption of Mb into acidic conditions has hence been explained by the electrostatic interaction between Mb and the silica surface.11−13 In these studies, the distribution and folding state of Mb molecules in mesoporous silica were not taken into account to explain the Mb adsorption. Investigation of these factors will contribute to the entire clarification of the protein adsorption.
This study was conducted to clarify the effect of the solution pH and pore size on the adsorption of a protein into mesoporous silicas (BJH pore diameters: 7.9, 5.3, and 3.3 nm). For that purpose, globular Mb was used as a model protein, because its tertiary structure can be measured by simple optical absorption spectroscopy.25,26 In addition, folded Mb molecules inside the mesoporous silica pore can be treated as a hard sphere with a diameter of 3.5 nm.26 Herein, the total amount of Mb adsorbed into mesoporous silica was estimated by a traditional adsorption assay, and the folding state of Mb adsorbed at mesoporous silica was determined by monitoring the Söret band of Mb in the visible absorption spectrum.25,26 In the DSC study, we first examined the effect of electrolytes on the DSC melting peak. The buffer electrolytes are generally required to regulate the solution pH during the Mb adsorption, but the electrolytes inside the mesoporous silica pore significantly influence melting of the pore water.27 Then, we examined the pore adsorption of Mb into mesoporous silicas by DSC.
Experimental Section
Materials and Chemicals
Myoglobin (Mb) from equine heart was purchased from Sigma-Aldrich Japan (Tokyo, Japan). The molecular weight of the Mb was 17 800 Da. Milli-Q water was used for all experiments. SBA-type mesoporous silica powders were synthesized using Pluronic P123 or Brij S10 as a template surfactant.26,28 Hereafter, the mesoporous silica prepared by the surfactant-templating approach is called as-prepared mesoporous silica. Scanning electron microscopy (SEM) images of mesoporous silicas are shown in Figure S1. A hexagonal arrangement of cylindrical pores was confirmed by small-angle X-ray (SAXS) profiles (Figure S2). The SAXS profiles were measured on a Rigaku SmartLab X-ray diffractometer with Cu Kα radiation. The pore diameters of mesoporous silicas were calculated from the adsorption branch of a nitrogen isotherm using the BJH method (Figure 1). The structural parameters of the as-prepared mesoporous silica powders obtained from the nitrogen adsorption isotherms and SAXS profiles are listed in Table 1. Since SBA-type mesoporous silica has micropores within its pore wall, the mesopore volume (Vmeso) was calculated by a t-plot analysis of the adsorption isotherm.29 Hereinafter, we designate mesoporous silica as SBAxx, where xx means the BJH pore diameter in Å. All chemicals used for the adsorption of Mb were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Figure 1.

Pore distribution plots of as-prepared mesoporous silicas (shaded circles) and mesoporous silicas after immersion in a 0.1 M phosphate buffer solution (open circles). The pH of phosphate buffer was 7.0.
Table 1. Structural Parameters of As-Prepared Mesoporous Silicas and Mesoporous Silicas After Immersion in a 0.1 M Phosphate Buffer Solution (PBS, pH 7.0).
| conditions | DBJHa/nm | ABETb/m2 g–1 | Vpc/cm3 g–1 | d100d/nm | Vmesoe/cm3 g–1 | |
|---|---|---|---|---|---|---|
| SBA79 | as-prepared | 7.9 | 763 | 1.05 | 102 | 0.60 |
| SBA53 | as-prepared | 5.3 | 651 | 0.64 | 87 | 0.40 |
| SBA39 | as-prepared | 3.9 | 920 | 1.05 | 57 | 0.99 |
| SBA79 | PBS | 7.9 | 581 | 0.87 | 102 | 0.47 |
| SBA53 | PBS | 5.4 | 420 | 0.56 | 86 | 0.38 |
| SBA39 | PBS | 3.4 | 679 | 0.64 | 56 | 0.60 |
BJH pore diameter.
Brunauer–Emmett–Teller (BET) surface area.
Single-point adsorption total pore volume of pores at around p/p0 = 0.97.
d100 spacing determined by SAXS.
Mesopore volume calculated by t-plot analysis.
Myoglobin Adsorption Assay
The particulate mesoporous silica (15 mg) was added to 3 mL of an Mb aqueous solution containing 0.1 M phosphate buffer (pH 3.7 to 7.0) at 10 °C. The initial concentrations of Mb were between 0.0 and 1.5 mg mL–1. After being shaken for 20 h at 10 °C, the mixture was centrifuged at 14 000 rpm for 10 min. The supernatant was subjected to absorption spectrum measurements to estimate the total amount of Mb adsorbed. The resulting mesoporous silica with Mb was dispersed in pure water and shaken for 1 h at 10 °C. After the centrifugation, the supernatant solution phase was removed by pipetting. This rinsing procedure was repeated three times to remove free Mb and electrolytes (sodium and phosphate ions). It should be noted that the detachment of Mb molecules during the rinsing procedure was negligibly small (less than a few percent). After the rinsing procedure, the sample suspension was subjected to optical absorption and DSC experiments.
Transmission Absorption Spectrum Measurements
The tertiary structure of Mb adsorbed at mesoporous silica was characterized by UV–vis transmission absorption spectroscopy.26,28 The sample suspension was poured into a quartz cell (1 cm × 1 cm) with a screw cap. The transmission absorption spectrum for the sample suspension was measured on a JASCO model V-570 spectrophotometer equipped with a cryostat (Unisoku Co., Ltd., CoolSpek USP-230). In the absorption spectrum experiment, an integrating sphere was set just after the output window of the cryostat, and the transmitted light collected by the integrating sphere was detected.28
DSC Measurements
After rinsing with pure water, the suspension of particulate mesoporous silica with Mb was put in an aluminum sample pan and sealed with a crimper. For the DSC measurement of the mesoporous silica without Mb, a mixture of particulate mesoporous silica and the phosphate buffer was shaken and rinsed by the same procedures as those for the Mb adsorption. DSC measurements were performed on a Rigaku Thermo-Plus DSC-8230 instrument equipped with a cooling system using liquid nitrogen. The scanning speed was fixed at 2 K min–1.21 After the DSC measurement, the sample pan was heated at 200 °C for 2 h to measure the weight of the dry MPS sample. The weight of the mesoporous silica powder in the aluminum pan was around 10 mg.
Nitrogen Adsorption/Desorption Isotherm Measurement
The particulate mesoporous silica with/without Mb was dried at 90 °C in vacuo prior to the nitrogen adsorption/desorption measurement. The nitrogen adsorption/desorption isotherm measurement was performed using a Micrometrics ASAP 2020 instrument.
Results
Mesopore Volumes of Mesoporous Silicas
The SBA-type mesoporous silica has cylindrical mesopore channels and sometimes has micropores within the silica pore wall.29 Determination of the mesopore volume is required to analyze the DSC melting peak of pore water. We hence estimated the mesopore volume by a t-plot analysis (Figure S3).29 The structural parameters of as-prepared mesoporous silicas are listed in Table 1.
The structural parameters of mesoporous silica sometimes change by hydrolysis of the silica matrix.30 In this study, we examined the effect of hydrolysis on the pore structures of mesoporous silicas as follows: after a suspension of mesoporous silica was shaken in a 0.1 M phosphate buffer solution for 20 h at 10 °C, the mesoporous silica was rinsed with pure water three times. This procedure is the same as that for the Mb adsorption assay described in the Experimental Section. The structural parameters of mesoporous silicas after rinsing are listed in Table 1. The pore diameters of SBA79 and SBA53 were maintained by the hydrolysis, whereas a decrease in pore diameter from 3.9 to 3.3 nm happened for SBA39 (Figure 1). The SAXS pattern of each mesoporous silica did not change after hydrolysis (Figure S4), indicating preservation of the hexagonal arrangement of the cylindrical mesopore channels during the hydrolysis. The differences in structural parameters were less than 8% for mesoporous silicas hydrolyzed in phosphate buffers (pH 7.0–3.7) and in pure water. In this study, the structural parameters of mesoporous silicas were used to analyze the DSC data with a relative uncertainty of 7% as described later. Keeping in mind the uncertainty of the DSC measurements, we considered that the effect of the solution pH for the hydrolysis was not significant. The structural parameters obtained for the neutral phosphate buffer (pH 7.0) listed in Table 1 were thus used for the analysis of DSC results.
Adsorption of Mb at Mesoporous Silicas
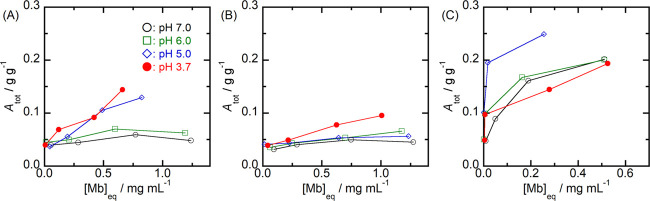
Figure 2 shows the effects of solution pH on the total amount of Mb adsorbed (Atot) at each mesoporous silica. Since the precipitation of Mb was observed for concentrated Mb solutions (≥2 mg mL–1) at pH less than 5.0, the initial concentration of Mb in the solution phase ranged from 0.25 to 1.5 mg mL–1. In the SBA79 system, Atot values found for pH 5.0 were larger than those for other pH conditions. On the other hand, the largest adsorption of Mb on SBA53 was observed at pH 3.7. In the SBA39 system, the adsorption of Mb tended to be larger with a decrease in solution pH. The effect of pH on the adsorption of Mb was thus different depending on the pore size of mesoporous silica.
Figure 2.
Plots of the total amount of Mb adsorbed (Atot) as a function of the equilibrium concentration of Mb ([Mb]eq) for (A) SBA39, (B) SBA53, and (C) SBA79. The initial concentration of Mb in the phosphate buffer solution ranged from 0.25 to 1.5 mg mL–1.
The tertiary Mb structures adsorbed at mesoporous silicas were evaluated by observing the Söret band peak of Mb.25,26 In the bulk solution phase, a sharp Söret band peak of Mb was seen for Mb in the phosphate buffer solutions at a pH ranging from 7.0 to 5.0 (Figure 3A), indicating the folded state of Mb in those buffer solutions. Weak and broad Söret band peaks for Mb at pH 3.7 indicate acid denaturation of Mb in the bulk solution. The absorption spectra for Mb adsorbed at mesoporous silicas are shown in Figure 3B–D. In each mesoporous silica system, sharp Söret band peaks are observed for Mb adsorbed at pH 7.0 and 6.0, indicating that Mb molecules preserve their globular structure during the adsorption process under these pH conditions. On the other hand, Mb adsorbed at pH 5.0 exhibited broad Söret band peaks. As shown in Figure 3A, Mb in bulk water at pH 5.0 exhibits a sharp Söret band peak, so it can be considered that the denaturation occurs during the adsorption process. The broad Söret band peak for Mb adsorbed at pH 3.7 indicates the adsorption of acid-denatured Mb at pH 3.7.
Figure 3.
Transmission absorption spectra of (A) Mb in 0.1 M phosphate buffer solutions and Mb adsorbed at (B) SBA39, (C) SBA53, and (D) SBA79. The adsorption of Mb was realized by adding mesoporous silicas to a 0.1 M phosphate buffer solution containing 1.5 mg mL–1 Mb. After the adsorption of Mb, mesoporous silica was rinsed with pure water three times and then was dispersed in pure water for the spectral measurement.
There are two silanol groups on a silica surface. The pKa of the Q3 silanol is ≤2 and that of the Q2 silanol is around 8.2.24 Keeping in mind these pKa values, it is expected that the negative surface charge at the silica pore wall is mainly due to deprotonated Q3 silanols at a pH ranging from 7.0 to 3.7. This means that the negative surface charge of the silica pore wall is almost constant at those pH ranges. In contrast, the surface charge of Mb (pI 7 to 7.3)13 becomes more positive with the decrease in the solution pH from 7.0 to 3.7. It therefore can be expected that the stronger electrostatic interactions between positively charged Mb and the negatively charged silica pore wall induce the deformation of Mb during the adsorption process at pH 5.0.
DSC Study in a Pure Water System
When a suspension of mesoporous silica powder in pure water (deionized water containing no electrolyte) was used for the DSC measurement, freezing and melting of pore water occurred below 0 °C in the DSC curve. The DSC melting peaks for SBA53 with/without Mb adsorbed are shown in Figure S5. Onset melting temperatures (Tm), heat of fusion of pore water (ΔH), and the total amount of Mb adsorbed (Atot) are summarized in Table 2. After the adsorption of Mb at SBA53, both the melting temperature depression and decrease in ΔH were observed (W/W systems in Table 2). The experimental scheme for the W/W system is shown in Figure S6A. These changes in melting peak are attributed to the pore adsorption of Mb at SBA53.21
Table 2. Total Amounts of Mb Adsorbed, and Melting Temperatures and Heats of Fusion of Pore Water Obtained for SBA53a.
| system | [Mb]e/mg mL–1 | Atotf/g g–1 | Tmg/°C | ΔHh/J g–1 |
|---|---|---|---|---|
| W/Wb | 0 | –22.4 | –47 | |
| PB/PBc | 0 | –24.2 | –33 | |
| PB/Wd | 0 | –22.2 | –50 | |
| W/Wb | 1.5 | 0.056 | –22.9 | –34 |
| PB/PBc | 1.5 | 0.052 | –25.6 | –34 |
| PB/Wd | 1.5 | 0.055 | –23.5 | –36 |
The experimental scheme for each system is shown in Figure S6.
Pure water was used for shaking, rinsing, and the DSC experiment.
Phosphate buffer solution was used for shaking, rinsing, and the DSC experiment.
Phosphate buffer solution was used for shaking, and pure water was used for rinsing and the DSC experiment.
Initial concentration of Mb in the phosphate buffer or pure water used for shaking.
Total amount of adsorbed Mb (per gram of mesoporous silica) derived from the adsorption assay.
Onset melting temperature of pore water.
Heat of fusion of pore water.
The mesoporous silica has cylindrical mesopore channels and micropores within the silica pore wall. Water within the micropores can be regarded as unfreezable water due to the small size of micropores (<2 nm).22,23 Inside the mesopore channel, water in the vicinity of the channel wall is also regarded as an unfreezable water layer with thickness tnf.22,23 This interfacial water layer does not contribute to the endothermic melting peak on the DSC curve. The melting peak is thus due to the frozen pore water in the interior of the mesopore channel. The Mb molecules adsorb in the mesopore channel, and the adsorbed Mb excludes both the freezable pore water in the interior of mesopore channel and the unfreezable water layer at the surface of the mesopore channel (Figure S7). Herein, the volume fraction of the freezable pore water replaced by Mb molecules is defined by ϕ. On the assumption that the enthalpy of fusion of water and the density of water/ice are not affected by the pore adsorption of Mb, the change in the melting peak area (heat of fusion) upon adsorption of Mb, ΔΔH, is described by
| 1 |
where ΔH0 and ΔHads are heats of fusion in the absence and presence of Mb inside the mesopore channel, respectively.21 When the volume of the freezable pore water excluded by one Mb molecule is defined by vex, the volume fraction (ϕ) of the freezable pore water replaced by Mb molecules is given by
| 2 |
where Apore, NA, and MMb are, respectively, the amount of Mb inside the pores per gram of mesoporous silica (g g–1), Avogadro’s number, and the molecular weight of Mb.21 The BJH pore radius estimated from the nitrogen adsorption branch (RBJH in nm) was used to define the size of the mesopore channel (Table 1). The densities of water and ice inside the mesopore channel were assumed to be the same (1 g cm–3).31 The thickness of the unfreezable water layer, tnf, was assumed to be 0.4 nm.22,23
As listed in Table 2 (W/W system), the adsorption of Mb induced a decrease of −ΔH (−47 to −34 J g–1) and lowering of Tm (−22.4 to −22.9 °C). The volume fraction of the freezable pore water (ϕ) replaced by Mb molecules was calculated to be 0.28 by inserting ΔΔH into eq 1. When this ϕ value was inserted into eq 2, we found that the amount of Mb located inside the pores (Apore) was almost equal to the total amount of Mb adsorbed as described later. This result confirms that almost all Mb molecules are located inside the pores of SBA53.
When the DSC measurement of mesoporous silica in pure water was repeated, the relative uncertainties in ΔH and ΔΔH, determined by three individual DSC measurements, were 5 and 7%, respectively. This uncertainty was used for discussion of the following DSC results.
Effect of Electrolytes on the DSC Melting Peak of Pore Water
Recently, Meissner et al. reported that freezing/melting behaviors of the pore water depended on the concentration of an electrolyte.27 Herein, the effect of the buffer electrolytes (phosphate and sodium ions) on the DSC melting peak of pore water was examined by DSC measurements for three different systems: (i) W/W, (ii) PB/PB, and (iii) PB/W (Figure S6). W and PB denote pure water and 0.1 M phosphate buffer (pH 7.0), respectively. The DSC curves obtained for each system are summarized in Figure S5. In the W/W system, SBA53 powder was added to pure water, and the mixture was shaken for 20 h (Figure S6A). After centrifugation, excess water was removed from pipetting. Then, we repeated rinsing of SBA53 by pure water three times. Finally, sample suspension after removing excess rinsing water was used for the DSC experiment. In the PB/PB system, the phosphate buffer solution was used for all of the above procedures. The sample suspension for the PB/PB system contains buffer electrolytes, which were used to regulate the solution pH (Figure S6B). In the PB/W system, the phosphate buffer solution was used for the 20 h shaking process. The pure water was used for the rinsing of SBA53 and for the DSC experiment. After rinsing with pure water, it was expected that the buffer electrolytes could be removed from the sample suspension prior to the DSC measurement (Figure S6B). The DSC parameters obtained from the DSC curves in Figure S5 are summarized in Table 2.
By comparing the DSC results for SBA53 in the W/W and PB/PB systems, it was found that the presence of phosphate buffer in the sample suspension induced both melting temperature depression (−22.4 to −24.2 °C) and decrease in the melting peak area (−47 to −33 kJ g–1) of pore water (Table 2). These results indicate that the buffer electrolytes inside the SBA53 pore significantly change the DSC melting peak of the pore water. We thus examined the PB/W system.
The melting peak obtained for the PB/W system was essentially the same as that for the W/W system (Figure S5). Tm and ΔH obtained for both systems were almost in agreement (Table 2). This agreement indicates that the buffer electrolyte (phosphate and sodium ions) can be removed from not only the solution phase but also silica mesopores by rinsing with pure water three times (Figure 6B). It was also confirmed that the removal of the buffer electrolyte was not affected by the solution pH for the initial shaking of SBA53. The difference in the ΔH values obtained for SBA53 shaken at pH ranging from 7.0 to 3.7 was 6%, which was close to the relative uncertainty in ΔH (5%).
Figure 6.

Plausible schematic illustration for the distribution of Mb adsorbed at (A) SBA39, (B) SBA53, and (C) SBA79.
When the adsorption assay and DSC measurement were performed in the W/W system, Atot and ΔΔH were estimated to be 0.056 g g–1 and −13 J g–1, respectively (Table 2). The presence of the buffer electrolyte did not significantly affect the adsorption of Mb on SBA53; Atot obtained for the phosphate buffer was 0.052 g g–1. In the PB/PB system, the ΔΔH value was estimated to be −2 J g–1 (Table 2), which was much smaller than that observed for the W/W system. In the PB/W system, on the other hand, the ΔΔH value (−14 J g–1) agreed well with that for the W/W system. These results indicate that rinsing with pure water three times removes the buffer electrolyte inside the pores even though Mb is present (Figure S6B). That is, eqs 1 and 2 can be applied to analyze the pore adsorption of Mb after the removal of buffer electrolytes.
DSC Study on Mb Adsorption into Neutral Solutions (pH 7.0 and 6.0)
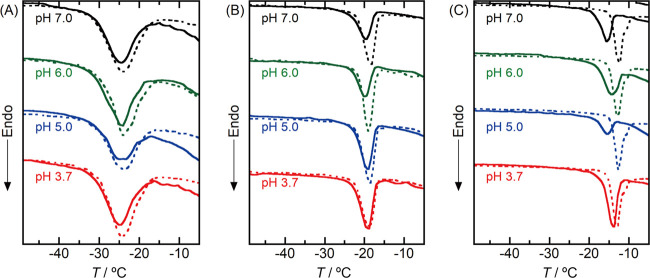
The samples for the following DSC results were prepared by adding the particulate mesoporous silica to a 1.5 mg mL–1 Mb solution, which was the maximum concentration for the adsorption assay (see Figure 2). Figure 4 shows changes in the DSC melting peaks upon the adsorption of Mb in mesoporous silicas. The volume fractions of the freezable pore water replaced by the Mb molecules (ϕ) estimated by inserting ΔH into eq 1 are summarized in Figure 5, together with Atot derived from the adsorption assay.
Figure 4.
DSC melting peaks for a sample suspension containing mesoporous silicas (dashed lines) and mesoporous silicas with Mb adsorbed (solid lines): (A) SBA39, (B) SBA53, and (C) SBA79. The mesoporous silica with Mb was prepared by adding mesoporous silica to a 0.1 M phosphate buffer solution containing 1.5 mg mL–1 Mb. After adsorption of Mb, mesoporous silica was rinsed with pure water three times and then was dispersed in pure water for the DSC measurement.
Figure 5.
pH dependencies on Atot and ϕ for (A) SBA39, (B) SBA53, and (C) SBA79. The mesoporous silica with Mb was prepared by adding mesoporous silica to a 0.1 M phosphate buffer solution containing 1.5 mg mL–1 Mb.
In neutral conditions (pH 7.0 and 6.0), a decrease in the melting peak area upon the adsorption of Mb was observed for each mesoporous silica (Figure 4). Since Mb molecules adsorbed from the neutral solutions exhibit a sharp Söret absorption band (Figure 3), the decreases in the melting peak area indicate that the folded Mb molecules penetrate into each mesoporous silica pore without structural deformation. The preservation of the folded structure of Mb was also confirmed by a small-angle neutron scattering study.26 The Mb was hence treated as a hard sphere (3.52 nm)26 to evaluate the amount of Mb inside the mesoporous silica pores. On the assumption of Atot being equal to Apore, volumes of the freezable pore water excluded by one Mb molecule (vex) were calculated by inserting Atot and ϕ into eq 2. The calculated vex values are listed in Table S1, together with the parameters obtained from the adsorption assay and the DSC experiment.
The vex values were estimated to be 44 nm3 for SBA39 and 39 nm3 for SBA53. These values are larger than 22.8 nm3 of a single spherical Mb.26 The estimated large vex values can be explained by the hydration shell around an Mb, because the hydrated water around a protein is unfreezable or noncrystalline water.32 Recent THz spectroscopic and molecular dynamics simulation studies have estimated the thickness of the dynamic hydration shell around a protein to be 1 to 2 nm.33−36 We hence calculated the thickness of the unfreezable hydration shell around the confined Mb from the vex values. The geometrical model for this calculation is shown in Figure S7. As a result, the thicknesses of the hydration shell were calculated to be 1.8 nm for SBA39 and 0.5 nm for SBA53, respectively (Table S1). These thicknesses agree with the reported types.33−36 This agreement suggests that almost all Mb molecules adsorbed are located inside the pores of SBA39 and SBA53 as schematically shown in Figure 6A,B. In addition, it is also suggested that intermediate water (water loosely bound to Mb)37 does not contribute to the DSC melting peaks shown in Figure 4. The pore adsorption behaviors for SBA39 and SBA53 are consistent with the pore adsorption of Mb into mesoporous silica (pore diameter: 3.9 nm) from Mb solutions containing no electrolytes.21
For the Mb adsorption at SBA79, the vex value was calculated to be around 14 nm3, which was much smaller than those for other mesoporous silicas (Table S1). This small vex value indicates that Mb molecules are located at both the pore interior and the outer surface of SBA79. A similar Mb distribution was confirmed for the adsorption of Mb in the mesoporous silica with a pore diameter of 6.4 nm.21 As schematically shown in Figure 6C, the Mb molecules (diameter: 3.5 nm) are expected to aggregate or stack at pore entrances, the diameters of which are about twice those of the Mb dimensions. The molecular aggregation and/or stacking would hinder the successive penetration of the Mb molecules into the pore interior.
DSC Study on Mb Adsorption from Acidic Solutions (pH 5.0 and 3.7)
The absorption spectral experiment confirmed denaturation of Mb molecules adsorbed from acidic solutions (pH 5.0 and 3.7). Since the volumes of the denatured Mb with the hydration shell have not been defined, eq 2 cannot be simply applied to estimate the amount of Mb molecules inside the pores. Herein, the ϕ values are used for qualitative discussion of the pore adsorption of Mb.
In the SBA39 system, the ϕ values obtained for the acidic solutions (pH 5.0 and 3.7) are larger than those for the neutral solutions (pH 7.0 and 6.0). This tendency indicates that the pore adsorption of Mb takes place under all pH conditions, that is, the denatured Mb molecules can penetrate into the narrow pores of SBA39 (Figure 6A).
In the SBA53 system, significant decreases in the melting peak area were not found for the adsorption of Mb at pH 5.0 and 3.7 (Figure 4B). The ϕ values were estimated to be almost zero for the adsorption of Mb at pH 5.0 and 3.7 (Figure 5B), indicating that the penetration of Mb molecules into the SBA53 pores is inhibited in these pH ranges. The absorption spectral experiment revealed that folded Mb molecules were denatured during the adsorption process at pH 5.0 (Figure 3B). It hence can be considered that the Mb molecules denatured at the pore entrance and the outer surface of SBA53 at pH 5.0, and the denatured Mb molecules stay at the outer surface region (Figure 6B). The Mb molecules denatured at the outer surface region would aggregate with each other38 and inhibit the successive penetration of the Mb molecules into the pore interior. At pH 3.7, the acid-denatured Mb molecules would also aggregate at the outer surface region (Figure 6B).
The inhibition of the pore adsorption is supported by the results of nitrogen adsorption/desorption isotherm experiments. For the adsorption of Mb at pH 7.0, a significant decrease in nitrogen adsorption upon adsorption of Mb was observed (Figure S8). The total pore volumes with and without Mb were 0.56 and 0.48 cm3 g–1, respectively. The decrease of the total pore volume indicates the pore adsorption of Mb at pH 7.0. In contrast, the adsorption of Mb at pH 3.7 did not induce a significant decrease in the nitrogen adsorption (Figure S8). The total pore volumes with and without Mb were, respectively, 0.56 and 0.54 cm3 g–1.
In the SBA79 system, the pore adsorption of Mb at pH 3.7 was also not indicated by the DSC experiments. As shown in Figure 5C, the ϕ value at pH 3.7 was estimated to be almost zero, indicating the inhibition of the penetration of the acid-denatured Mb molecules into the SBA79 pores (Figure 6C). For the adsorption of Mb at pH 5.0, on the other hand, the pore adsorption of Mb was clearly confirmed by large ϕ (Figure 5C). This result suggests that the folded Mb molecules penetrate into the SBA79 pores, and their denaturation occurs at the inner pore surface (Figure 6C). Bearing in mind the distribution of Mb adsorbed into the neutral solutions, we consider that the denatured Mb molecules are located at both the pore interior and the outer surface region at pH 5.0 (Figure 6C). Although penetration of Mb was completely rejected at pH 3.7, the total amounts of Mb adsorbed at pH 3.7 were almost the same as those at pH 7.0 (Figures 2C and 5C). These results suggest that multilayer deposition of the acid-denatured Mb molecules took place at the external surface of SBA79 at pH 3.7.
Discussion
In the present study, the absorption spectral and DSC experiments were performed for mesoporous silicas with Mb adsorbed from the concentrated Mb solution (1.5 mg mL–1). The folded state and distribution of Mb are shown in Figure 6. We discuss the effects of solution pH and pore size on the adsorption behaviors of Mb into mesoporous silicas.
For the Mb adsorption at pH 7.0 and 6.0, as schematically shown in Figure 6, the globular Mb molecules are located inside the pores of SBA39 and SBA53. On the other hand, the globular Mb molecules are located at both the pore interior and the outer surface region of SBA79. In our previous DSC study on the adsorption of Mb into mesoporous silicas in unbuffered solutions, the adsorption of Mb into pores was also confirmed for the mesoporous silica with a pore diameter of 3.9 nm, whereas Mb molecules located at both the pore interior and the outer surface region of mesoporous silica with a pore diameter of 6.4 nm.21 Therefore, the Mb molecules (diameter: 3.5 nm) are expected to aggregate or stack at pore entrances, the diameters of which are about twice those of the Mb dimensions. The several Mb molecules simultaneously adsorbed at the entrance of large pores (7.9 nm) will be able to aggregate or stack because intermolecular electrostatic repulsion of Mb molecules (pI ∼ 7)13 is weak at pH 7.0 and 6.0. The aggregated or stacked Mb molecules would hinder the successive penetration of the Mb molecules into the pore interior. Since two globular Mb molecules cannot simultaneously locate at the entrance of small pores (diameter: less than 5.3 nm), the Mb molecules would penetrate into the pore interiors of SBA39 and SBA53 without the aggregation at the pore entrance.
The pore adsorption behaviors at pH 5.0 and 3.7 are also explained by the aggregation at the pore entrance. In SBA53 and SBA79, the flexible denatured Mb molecules would be able to locate at the pore entrance region of the large pores (5.3 and 7.9 nm), resulting in aggregation at the pore entrance. Although the Mb molecules (pI ∼ 7)13 are positively charged at pH 5.0 and 3.7, the electrostatic repulsion between these charged Mb molecules can be shielded under high ionic strength conditions (0.1 M phosphate buffer).39 In SBA39, the significant aggregation of denatured Mb molecules was not indicated by the DSC experiment, and the denatured Mb molecules could penetrate into the small pore of SBA39. We consider that the denatured Mb molecules could not simultaneously locate at the pore entrance of SBA39 due to the small pore size (3.3 nm).
On the basis of the above adsorption schemes, we classify the adsorption isotherms derived from the total amount of Mb adsorbed (Atot) shown in Figure 2. In SBA39, the analysis of the DSC melting peak confirms that the majority of the folded Mb molecules locate inside the pore interior. The pore adsorption of denatured Mb is also indicated. It hence can be considered that all adsorption isotherms for SBA39 (Figure 2A) are described by the adsorption model based on the inner pore surface as one major adsorption site. Since similar pore adsorption of folded Mb molecules at SBA53 is determined, the adsorption isotherms for SBA53 at pH 7.0 and 6.0 (Figure 2B) can also be described by the adsorption at the inner pore surface. On the other hand, the pore adsorption of denatured Mb molecules at pH 5.0 and 3.7 is completely inhibited due to aggregation of the denatured Mb molecules at the pore entrance region. The isotherms for SBA53 at these pH ranges (Figure 2B) thus are described by adsorption at the outer surface region. The molecular aggregation and/or intermolecular interaction should be taken into account for the adsorption at the outer surface region.
In SBA79, the distribution of folded Mb molecules at both the pore interior and the outer surface region is determined (Figure 6C). This suggests that the adsorption isotherms obtained at a pH ranging from 7.0 to 6.0 (Figure 2C) should be described by the adsorption model considering the two adsorption sites. The adsorption isotherm at pH 5.0 would be described by the adsorption of folded Mb at the two adsorption sites. The denaturation of Mb after the adsorption should be also taken into account for the Mb adsorption at pH 5.0. Since no penetration of the acid-denatured Mb is determined, the isotherm for pH 3.7 (Figure 2C) is described by adsorption and aggregation at the outer surface region.
The adsorption isotherm of a protein is usually obtained by measuring the total amount of protein in a porous material. This isotherm has been discussed by electrostatic and van der Waals interactions, ion exchange, and others.9 The present study revealed that the distribution of Mb adsorbed at mesoporous silica strongly depended on the pore size, solution pH, and folded state of protein. This result indicates that the determination of the adsorption site is required to choose an appropriate model describing protein adsorption in an inorganic nanoporous material.40
Conclusions
In the present study, the effects of pore size and solution pH on the pore adsorption behavior of Mb into mesoporous silicas were examined utilizing the DSC method. The DSC method relies on observing the decrease in the melting peak area of the pore water upon adsorption of Mb. Since the decrease in the melting peak area is proportional to the volume of pore water excluded by Mb molecules, the amount of Mb located inside the pores can be estimated. Herein, we first examined the effect of buffer electrolyte, which was used to adjust the solution pH during the Mb adsorption, on the melting behavior of pore water. The results confirmed that the buffer electrolyte significantly influenced the melting behavior of pore water, and prevented the estimation of pore adsorption of Mb by the DSC method. The removal of the buffer electrolyte from the mesoporous silica pores was required for the DSC study on the pore adsorption of Mb.
The DSC experiments clearly indicated that the pore adsorption behavior of Mb strongly depended on the solution pH and pore size of mesoporous silica. For the adsorption of Mb (3.5 nm) at pH 7.0 and 6.0, predominant pore adsorption of globular Mb into mesoporous silicas with small pores (pore diameter: 3.3 and 5.3 nm) was confirmed, whereas the globular Mb molecules are located at both the pore interior and outer surface regions of mesoporous silica with large pores (pore diameter: 7.9 nm). For the adsorption of Mb at pH 3.7, pore adsorption of denatured Mb was confirmed for the smallest pore (3.3 nm), whereas the pore adsorption of denatured Mb was inhibited for the mesoporous silica with large pores (5.3 and 7.9 nm). This inhibition was likely explained by the aggregation of denatured Mb molecules at pore entrance regions of the large pores.
Acknowledgments
This work was supported in part by JSPS Kakenhi Grant nos. 16H04160 and 17K19022.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02602.
SEM images of mesoporous silicas; SAXS data; nitrogen adsorption/desorption data; DSC curves for SBA53; schematic illustration of Mb with a hydration shell; and results of the adsorption assay and DSC experiments (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Martin T.; Galarneau A.; Di Renzo F.; Brunel D.; Fajula F. Great improvement of chromatographic performance using MCM-41 spheres as stationary phase in HPLC. Chem. Mater. 2004, 16, 1725–1731. 10.1021/cm030443c. [DOI] [Google Scholar]
- Kim J.; Grate J. W.; Wang P. Nanostructures for Enzyme Stabilization. Chem. Eng. Sci. 2006, 61, 1017–1026. 10.1016/j.ces.2005.05.067. [DOI] [Google Scholar]
- Brennan J. D. Biofriendly Sol-Gel Processing for the Entrapment of Soluble and Membrane-Bound Proteins: Toward Novel Solid-Phase Assays for High-Throughput Screening. Acc. Chem. Res. 2007, 40, 827–835. 10.1021/ar6000268. [DOI] [PubMed] [Google Scholar]
- Gu Z.; Biswas A.; Zhao M.; Tang Y. Tailoring Nanocarriers for Intracellular Protein Delivery. Chem. Soc. Rev. 2011, 40, 3638–3655. 10.1039/c0cs00227e. [DOI] [PubMed] [Google Scholar]
- Ariga K.; Vinu A.; Yamauchi Y.; Ji Q.; Hill J. P. Nanoarchitectonics for mesoporous materials. Bull. Chem. Soc. Jpn. 2012, 85, 1–32. 10.1246/bcsj.20110162. [DOI] [Google Scholar]
- Rimola A.; Costa D.; Sodupe M.; Lambert J.-F.; Ugliengo P. Silica surface features and their role in the adsorption of biomolecules: computational modeling and experiments. Chem. Rev. 2013, 113, 4216–4313. 10.1021/cr3003054. [DOI] [PubMed] [Google Scholar]
- Itoh T.; Ishii R.; Ebina T.; Hanaoka T.; Fukushima Y.; Mizukami F. Encapsulation of myoglobin with a mesoporous silicate results in new capabilities. Bioconjugate Chem. 2006, 17, 236–240. 10.1021/bc050238i. [DOI] [PubMed] [Google Scholar]
- Sang L.-C.; Coppens M.-O. Effects of surface curvature and surface chemistry on the structure and activity of proteins adsorbed in nanopores. Phys. Chem. Chem. Phys. 2011, 13, 6689–6698. 10.1039/c0cp02273j. [DOI] [PubMed] [Google Scholar]
- Moerz S. T.; Huber P. Protein adsorption into mesopores: A combination of electrostatic interaction, counterion release, and van der Waals forces. Langmuir 2014, 30, 2729–2737. 10.1021/la404947j. [DOI] [PubMed] [Google Scholar]
- Sang L.-C.; Vinu A.; Coppens M.-O. General description of the adsorption of proteins at their iso-electric point in nanoporous materials. Langmuir 2011, 27, 13828–13837. 10.1021/la202907f. [DOI] [PubMed] [Google Scholar]
- Lynch M. M.; Liu J.; Nigra M.; Coppens M.-O. Chaperonin-inspired pH protection by mesoporous silica SBA-15 on myoglobin and lysozyme. Langmuir 2016, 32, 9604–9610. 10.1021/acs.langmuir.6b02832. [DOI] [PubMed] [Google Scholar]
- Moerz S. T.; Huber P. pH-dependent selective protein adsorption into mesoporous silica. J. Phys. Chem. C 2015, 119, 27072–27079. 10.1021/acs.jpcc.5b09606. [DOI] [Google Scholar]
- Essa H.; Magner E.; Cooney J.; Hodnett B. K. Influence of pH and ionic strength on the adsorption, leaching and activity of myoglobin immobilized onto ordered mesoporous silicates. J. Mol. Catal., B 2007, 49, 61–68. 10.1016/j.molcatb.2007.07.005. [DOI] [Google Scholar]
- Schlipf D. M.; Rankin S. E.; Knutson B. L. Pore-size dependent protein adsorption and protection from proteolytic hydrolysis in tailored mesoporous silica particles. ACS Appl. Mater. Interfaces 2013, 5, 10111–10117. 10.1021/am402754h. [DOI] [PubMed] [Google Scholar]
- Clemments A.; Botella P.; Landry C. C. Spatial mapping of protein adsorption on mesoporous silica nanoparticles by stochastic optical reconstruction microscopy. J. Am. Chem. Soc. 2017, 139, 3978–3981. 10.1021/jacs.7b01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deere J.; Serantoni M.; Edler K. J.; Hodnett B. K.; Wall J. G.; Magner E. Measurement of the adsorption of cytochrome c onto external surface of a thin-film mesoporous silicate by ellipsometry. Langmuir 2004, 20, 532–536. 10.1021/la035358y. [DOI] [PubMed] [Google Scholar]
- Chen M. Y.; Sailor M. J. Charge-gated transport of proteins in nanostructured optical films of mesoporous silica. Anal. Chem. 2011, 83, 7186–7193. 10.1021/ac201636n. [DOI] [PubMed] [Google Scholar]
- Karlsson L. M.; Tengvall P.; Lundström I.; Arwin H. Penetration and loading of human serum albumin in porous silica layers with different pore sizes and thicknesses. J. Colloid Interface Sci. 2003, 266, 40–47. 10.1016/S0021-9797(03)00595-2. [DOI] [PubMed] [Google Scholar]
- Hotta K.; Yamaguchi A.; Teramae N. Nanoporous waveguide sensor with optimized nanoarchitectures for highly sensitive label-free biosensing. ACS Nano 2012, 6, 1541–1547. 10.1021/nn204494z. [DOI] [PubMed] [Google Scholar]
- Arafune H.; Hotta K.; Itoh T.; Teramae N.; Yamaguchi A. Nanoporous waveguide spectroscopy for the estimation of enzyme adsorption on mesoporous silica. Anal. Sci. 2017, 33, 473–476. 10.2116/analsci.33.473. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A.; Taki K.; Kijima J.; Edanami Y.; Shibuya Y. Characterization of myoglobin adsorption into mesoporous silica pores by differential scanning calorimetry. Anal. Sci. 2018, 34, 1393–1399. 10.2116/analsci.18P371. [DOI] [PubMed] [Google Scholar]
- Kittaka S.; Ishimaru S.; Kuranishi M.; Matsuda T.; Yamaguchi T. Enthalpy and interfacial free energy changes of water capillary condensed in mesoporous silica, MCM-41 and SBA-15. Phys. Chem. Chem. Phys. 2006, 8, 3223–3231. 10.1039/b518365k. [DOI] [PubMed] [Google Scholar]
- Jähnert S.; Cháves F. V.; Schaumann G. E.; Schreiber A.; Schönhoff M.; Findenegg G. H. Melting and freezing of water in cylindrical silica nanopores. Phys. Chem. Chem. Phys. 2008, 10, 6039–6051. 10.1039/b809438c. [DOI] [PubMed] [Google Scholar]
- Rosenholm J. M.; Czuryszkiewicz T.; Kleitz F.; Rosenholm J. B.; Lindén M. On the nature of the Bronsted acidic groups on native and functionalized mesoporous siliceous SBA-15 as studied by benzylamine adsorption from solution. Langmuir 2007, 23, 4315–4323. 10.1021/la062450w. [DOI] [PubMed] [Google Scholar]
- Pace C. N.; Vanderburg K. E. Determining globular protein stability: guanidine hydrochloride denaturation of myoglobin. Biochemistry 1979, 18, 288–292. 10.1021/bi00569a008. [DOI] [PubMed] [Google Scholar]
- Kijima J.; Shibuya Y.; Katayama K.; Itoh T.; Iwase H.; Fukushima Y.; Kubo M.; Yamaguchi A. Structural characterization of myoglobin molecules adsorbed within mesoporous silica. J. Phys. Chem. C 2018, 122, 15567–15574. 10.1021/acs.jpcc.8b04356. [DOI] [Google Scholar]
- Meissner J.; Prause A.; Findenegg G. H. Secondary confinement of water observed in eutectic melting of aqueous salt systems in nanopores. J. Phys. Chem. Lett. 2016, 7, 1816–1820. 10.1021/acs.jpclett.6b00756. [DOI] [PubMed] [Google Scholar]
- Shibuya Y.; Itoh T.; Matsuura S.; Yamaguchi A. Structural stability of light-harvesting protein LH2 adsorbed on mesoporous silica supports. Anal. Sci. 2015, 31, 1069–1074. 10.2116/analsci.31.1069. [DOI] [PubMed] [Google Scholar]
- Galarneau A.; Cambon H.; Di Renzo F.; Fajula F. True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature. Langmuir 2001, 17, 8328–8335. 10.1021/la0105477. [DOI] [Google Scholar]
- Michaux F.; Carteret C.; Stébé M. J.; Blin J. L. Hydrothermal stability of mesostructured silica prepared using a nonionic fluorinated surfactant. Microporous Mesoporous Mater. 2008, 116, 308–317. 10.1016/j.micromeso.2008.04.018. [DOI] [Google Scholar]
- Liu D.; Zhang Y.; Chen C.-C.; Mou C.-Y.; Poole P. H.; Chen S.-H. Observation of the density minimum in deeply supercooled confined water. Proc. Natl. Acad. U.S.A. 2007, 104, 9570–9574. 10.1073/pnas.0701352104. [DOI] [Google Scholar]
- Doster W.; Bachleintner A.; Dunau R.; Hiebl M.; Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys. J. 1986, 50, 213–219. 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laage D.; Elsaesser T.; Hynes J. T. Water dynamics in the hydration shells of biomolecules. Chem. Rev. 2017, 117, 10694–10725. 10.1021/acs.chemrev.6b00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus S.; Kim S. J.; Heyden M.; Yu X.; Heugen U.; Gruebele M.; Leitner D. M.; Havenith M. An extended dynamical hydration shell around proteins. Proc. Natl. Acad. U.S.A. 2007, 104, 20749–20752. 10.1073/pnas.0709207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti Nibali V.; Havenith M. New insights into the role of water in biological function: Studying solvated biomolecules using terahertz absorption spectroscopy in conjunction with molecular dynamics simulations. J. Am. Chem. Soc. 2014, 136, 12800–12807. 10.1021/ja504441h. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A.; Edanami Y.; Yamaguchi T.; Shibuya Y.; Fukaya N.; Kohzuma T. Effect of cavity size of mesoporous silica on type I copper site geometry in Pseudoazurin. Bull. Chem. Soc. Jpn. 2020, 93, 630–636. 10.1246/bcsj.20190355. [DOI] [Google Scholar]
- Tanaka M.; Kobayashi S.; Murakami D.; Aratsu F.; Kashiwazaki A.; Hoshiba T.; Fukushima K. Design of polymeric biomaterials: The “intermediate water concept”. Bull. Chem. Soc. Jpn. 2019, 92, 2043–2057. 10.1246/bcsj.20190274. [DOI] [Google Scholar]
- Lynch I.; Dawson K. A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. 10.1016/S1748-0132(08)70014-8. [DOI] [Google Scholar]
- Israelachvili J. N.Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, 2011. [Google Scholar]
- Swenson H.; Stadie N. P. Langmuir’s theory of adsorption: a centennial review. Langmuir 2019, 35, 5409–5426. 10.1021/acs.langmuir.9b00154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.