Abstract

Spiroindenoquinoxaline pyrrolizidines (SIQPs)—7-nitro-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP I), 2′-(4-cyanophenyl)-7-nitro-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP II), and 2′-(4-methoxyphenyl)-7-nitro-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP III)—have been synthesized through a one-pot cascade Knoevenagel condensation reaction in acetonitrile (ACN) with 91, 98, and 87% yields, respectively. Structures are characterized by 1H NMR and 13C NMR spectroscopy, nuclear Overhauser enhancement spectroscopy (NOESY), Fourier transform infrared (FT-IR) and UV–vis spectroscopy, thermogravimetric analysis (TGA), high-resolution mass spectroscopy (HRTEM), fluorescence and Raman spectroscopy, and energy-dispersive analysis by X-ray (EDX) spectroscopy. SIQPs in ACN photocatalyzed methylene blue (MB) but not phenolphthalein (HIn). SIQPs distinguished the quaternary atoms and dipoles of the fluorescent dye (MB) contrary to the quinonoid HIn structure. In sunlight, SIQPs without electricity input acted as a photonic sensor to detect fluorescent dyes in waste effluents of textile, paper, dyes, and other industries. Activation energy (Ea), enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) calculated from UV–vis absorption spectra show photocatalytic reduction (PCR) activities in the order SIQP II > III > I. The N-atom of pyrrolizidine and −NO2 of nitro-indenoquinoxaline (NIQ) induced the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) electrodynamics to enable the SIQPs to catalyze biochemical activities.
1. Introduction
In nanoscience and technology, multifunctional spirochiral molecules have been in high demand due to their multiple localized chemical environments fitting within the framework of intramolecular multiple force theory (IMMFT) with multiple tentropic activities for efficient photocatalysis, biochemical activities, phase extraction, and others. Spirochirals are abundantly manufactured in nature, but their physicochemical potential like photocatalyzing activities has never been elucidated. Researchers are engaged in developing smart chiral structures with diverse constituents with extraordinary intramolecular activities having remarkable electron spins and rotational, vibrational, and translational motions. These features lead to a shorter band gap in organic chemistry, termed the highest occupied molecular orbital (HOMO) to lowest unoccupied molecular orbital (LUMO) gap, and allow semiconductor activities, which have never been reported before for reducing the methylene blue (MB) to leucomethylene blue (LMB) dye. For the first time, Huisgen et al.1 reported a class of spiroheterocyclics through the 1,3-dipolar cycloaddition reaction with complicated methods and mechanisms. Spiroheterocyclics play a vital role in biological, medicinal, and materials sciences2,3 and in the synthesis of many quinoxalines. Thus, much attention has been paid to the study of spiroheterocyclics due to their unique spirostructures with wider biological and other activities to explore their photocatalytic (PC) potential. Huang et al.4 synthesized pharmacological agents, but synthesizing spiroheterocyclics with a minimum number of steps in a shorter time using green chemistry has been a major challenge. A literature search of spiroheterocyclics shows that no attempt has been made to synthesize them using green routes despite their huge chemical relevance. Shahrestani et al.5 synthesized spiroheterocyclics with ortho–para directing groups using 4,5-dimethyl benzene-1,2-diamine as a starting material with ninhydrin. However, they could not introduce a meta-directing (MD) group (to increase activity and sites). Thus, the absence of the MD group limited their solubility and applications. To address this problem and to develop green alternatives, we replaced 4,5-dimethyl benzene-1,2-diamine with 4-nitrobenzene-1,2-diamine with ninhydrin for a new series of indenoquinoxalines (IQs). This is in contrast to researchers who conducted the reactions with ninhydrin and derivatives of phenyl 1,2-diamines. As per the literature survey, no IQ synthesis with the MD −NO2 group has been reported yet. Thus, a series of enantiomerically pure, novel chiral heterocyclic spiroindenoquinoxaline pyrrolizidines (SIQPs) with high yields via a one-pot, three-component 1,3-dipolar cycloaddition reaction under normal conditions using moieties of highly efficient azomethine ylides have been synthesized. For SIQP synthesis at RT, −NO2 was introduced in IQ, saving 80% resources with new properties and activities contrary to the IQs reported elsewhere.6,7 Introducing the −NO2 group to IQ, i.e., nitro-indenoquinoxaline (NIQ) using methanol (CH3OH) and acetic acid (CH3COOH) solvents, the SIQPs showed increased affinity toward green solvents with low activation energy (Ea), thus opening alternative ways for their applications. Efforts are initiated to conduct a similar reaction in a 10% aq CH3OH and CH3COOH medium separately for a greener synthesis. Hence, −NO2 of NIQ could catalyze the reaction, and it was complete within 3 h, giving ∼87–98% yields. NIQ with an electron-withdrawing group (EWG) extended the structural delocalization that reduced the synthesis time and Ea compared to the reported IQ. Saragi et al.8 have reported basic physiochemical and electronic properties of spiroheterocyclics, and Mahns et al.9 have reported their electronic properties, but no study has initiated their photocatalytic (PC) activities and vibrational splitting. Hongyan Xia et al.10 have reported advanced spiropyrans and their applications with fluorescent materials as per fluorescence resonance energy transfer (FRET), but their PC activities have not been discussed yet (Table 1).11−14 Barkov et al.15reported regio- and stereoselective syntheses of spiroheterocyclics using the 1,3-dipolar cycloaddition reaction. In this study, phenyl (C6H5−), cyanophenyl (Ph-CN), and methoxyphenyl (Ph-OCH3) were introduced to the pyrrolizidine ring of SIQPs to enhance their interaction and PC abilities (Figures S1–S3). Such a mechanism has never been reported yet. However, Zheng et al.16 had synthesized a few spiro compounds with restricted solubility but with many drawbacks, which encouraged us to study the solubility and PC of SIQPs. The SIQPs attain manifold physicochemical activities with stabilities in the order of SIQP II > III > I, derived from weight loss from thermogravimetric analysis (TGA). Their λmax was III λmax > II λmax > I λmax. SIQPs act as sensors at the minimum inhibition concentration (MIC) and exhibit similar other activities. Pardasani et al.17 had reported many chiral molecules to explore photocatalysis, which failed to materialize; thus, we studied the SIQPs with active chiral centers and developed a new experiment to photocatalyze the MB to LMB in visible light (Figures 1–3) and phased out the phenolphthalein (HIn) to develop a liquid–liquid phase (Figure 4). Thus, SIQPs were synthesized with various functional groups. The −CN and −OCH3 induced hydrophilic and hydrophobic interacting activities, respectively, to widen the solubility needed for drugs, biochemical dispersion, and therapeutic agents.18 SIQPs with these unique electrophobic and electrophilic constituents have never been reported. NIQ reacted with the derivate of benzylidene malononitrile and l-proline in acetonitrile (ACN) at ∼100 °C, while other researchers conducted the same reaction at a higher temperature for a longer period of time. SIQPs with quantized electron clouds lead to an extraordinary electronic transition that synchronize the PC potential. Various studies have synthesized spiroheterocyclics with varieties of chiralities to be used as drugs, but SIQPs act as green photocatalytic potential alternatives of semiconductor nanomaterials like graphene oxide (GO), TiO2, and SiO2.
Table 1. PC Activity Comparison for Reported Spiroheterocyclic Compounds and SIQPs.
Figure 1.
PC MB reduction by 1.5 mmol SIQPs I, II, and III in ACN at (a) t = 0 min, (b) after 18 min, and (c) after 120 min.
Figure 3.
PC MB reduction by 1.5 mmol SIQPs I, II, and III in EtOH at (a) t = 0 min, (b) after 30 min, and (c) after 70 min.
Figure 4.
No HIn reduction by SIQPs I, II, and III presence in the EtOH medium at (a) t = 0 min, (b) after 5 min, and (c) after 120 min.
Figure 2.
PC MB reduction by 1.5 mmol SIQPs I, II, and III in aq ACN at (a) t = 0 min, (b) after 5 min, and (c) after 95 min.
2. Results and Discussion
2.1. NO2 at the Para Position and Concentration-Driven Innovative Green Chemistry
Han19 et al. have synthesized spiroheterocyclics with selective chemical species, and a majority of the researchers have used 1,2-diamine benzene, while some of them have used 4,5-dimethyl-1,2-diamine benzene to synthesize IQ as a base material. The −CH3 at 4,5 positions of 1,2-diamine benzene20 restricts π electron delocalization due to interrupted hyperconjugation (σ–π bond interactions). The σ–π interactions immobilize the activities of spiroheterocyclics. During IQ synthesis, the −NH2 groups at 1,2 positions of benzene also hamper π delocalization due to the lone pair electron (LPE) of N-atoms. Comparatively, these electronic orientations and shifts with the σ–π and LPE−π interactions reduced the solubilization and produced a low yield in >60 min, contrary to <40 min for NIQ reported in this work (Figure S4). These electronic restrictions were tuned by placing EWG and −NO2 at the para position of NIQ to prevent interruption of hyperconjugation. It enhanced the extended delocalization, which photocatalyzed the MB to LMB, contrary to that reported for spiroheterocyclics. The −NO2 exponentially integrates the electron cloud of quinoxaline that catalyzed the partial charge on ketonic atoms (−C+–O–) of the indeno part of NIQ. The −C+–O– attracts LPE of N and H of l-proline through Coulombic interactions. Unlike previous methodologies,20,21 −NO2 electronically catalyzes azomethine ylide intermediate formation through the 1,3-dipolar cycloaddition reaction for SIQP synthesis. Thus, our strategy seems robust to synthesize chiral exponentially active SIQPs as sensors. It could further be improved by replacing l-proline with indoline-2-carboxylic acid, N-Boc-cis-4-hydroxy-d-proline methyl ester, and trans-4-hydroxy-l-proline amino acids for a new series of stable azomethine ylides as a precursor for SIQPs. However, no creative and novel routes of synthesis for extraordinary SIQP structures have been reported yet. A one-pot three-component reaction mechanism was initiated in MeOH, dichloromethane (DCM), and ACN (Table 2) to study the solvent effect on enhancing yield. ACN produced ∼87–98% yields, in contrast to other solvents. A triple bond of ACN (sp C-atom) has fascial orientation, which favors the reaction due to the −CN common ion effect. It maximized the yield in a shorter duration. Thus, ACN is better than MeOH and DCM solvents. Initially, 1.5 mmol of l-proline gave 65% SIQP yield in 4 h under the reflux condition. Under similar stoichiometric conditions, except for reducing l-proline from 1.5 to 1.0 mmol, SIQPs yielded 87–98% in 3 h. It may show stronger proline–proline interactions due to zwitterionic activities. Thus, l-proline did not react with other species except itself, and it undergoes self-aggregation. Hence, it did not react completely with other reacting species, even when the stirring rate was prolonged at RT. The 1.0 mmol l-proline acted like a sensor to enhance the yield. It attracts a relevance of degree of freedom (F) of reacting species as
| 1 |
where S indicates (number of reacting species is 4) benzylidene malononitrile, NIQ, l-proline, and ACN as dispersing agents, P (number of phases) is 1 (homogenous), and R (number of intermediate species) is 1 (azomethine ylide) at 100 °C reaction temperature T. Putting the values of S, P, R, and T coordinates, the F becomes
| 2 |
F = 4 depicts that the SIQP synthesis is controlled by four variables, i.e., the compositions of reacting species including the solvent (benzylidene malononitrile, NIQ, l-proline, and ACN) in a 1.0:1.0:1.0:8.0 ratio at constant T for SIQP I, II, and III syntheses with benzylidene malonotrile, cyano, and methoxybenzylidene malonotriles, respectively. The reactions with various compositions were studied at different temperatures, but the yields and durations were haphazard. Based on our reaction variables, the above-mentioned ratios are the most accurate and optimized with minimum time duration and maximum yield for a series of spiroheterocyclic syntheses. The reaction temperature remains constant; l-proline acted as the bridging agent, but 1.0 mmol of l-proline gave 98% yield, in contrast to 65% with 1.5 mmol. MeOH and DCM polar solvents produced a lower yield compared to ACN, which may be due to the poor solubility of the reactant, especially of l-proline. The dipole moments for ACN, MeOH, and DCM solvents show lesser values for DCM and MeOH and greater values for ACN. ACN with an electron-releasing group (ERG) (−CH3) and LPE of the N-atom expeditiously monodispersed the reacting species. ACN as a solvent robustly dispersed and induced a favorable mechanism in a reacting orientation to produce the maximum yield. Therefore, ACN was found to be a better solvent.
Table 2. SIQPs %Yield in Different Solvents under Similar Reaction Conditions (t = 3 h).
| product | MeOH% | DCM% | ACN% |
|---|---|---|---|
| SIQP I | 64 | 53 | 91 |
| SIQP II | 57 | 38 | 98 |
| SIQP III | 62 | 40 | 87 |
2.2. 1H, 13C, and Nuclear Overhauser Enhancement Spectroscopy (NOESY) NMR and Fourier Transform Infrared (FT-IR)
Two doublets in 1H NMR spectra show pyrrolizidine-ring SIQPs. Multiplets at δ 4.32 (d, 1H, J = 6.06) show the pyrrolizidine NCH proton, whereas the pyrrolizidine proton attached to a phenyl ring is deshielded with a doublet at δ = 4.81 (d, 1H, J = 5.89) (Figures S5a, S6a, and S7a). Aromatic protons produced multiplets at δ 6.93–8.27 ppm, as confirmed from the weak 13C NMR and NOE patterns (Figures S5b, S6b, and S7b). IR peaks at ∼1550 and 2260 cm–1 have −NO2 and −C≡N structures. A strong absorption band for the 1,4-disubstituted aromatic ring appears at 800–850 cm–1 (Figures S5c, S6c, and S7c). Peaks at 1176 cm–1 for C–O–C and −CH3 (sp3) stretchings at 2850–2928 cm–1 show −OCH3 in SIQP III. To determine the exact regioselectivity, connectivity of carbons is obtained by mass spectra and elemental analyses (Figures S5d, S6d, and S7d).
2.3. UV–Vis Spectral Analysis
UV–vis absorption spectra were recorded at 200–800 nm at RT. Spectral calculations for λmax, ϵmax, and emission maxima (λem) were measured in ACN (Table 3). The λmax SIQPs were 347 (I), 349 (II), and 337 (III) nm (Figures S5e, S6e, and S7e) as per Hooke’s law. Electron spectra of SIQPs were close to each other. SIQP II displays the strongest absorption (λmax = 349 nm) and εmax of 1333 M–1 cm–1. Lower εmax values for SIQPs III and I of 666 and 1200 M–1 cm–1, respectively, were found. The lowest εmax value was 666 M–1 cm–1 for SIQP III with the −OMe (ERG) substituent. The −C≡N (EWG) and −OCH3 (ERG) at the para position of the phenyl ring produced a bathochromic shift10 in the absorption band π → π* using values of SIQPs Δλ of 140 (I), 140 (II), and 139 nm (III). Higher Δλ values were found for SIQPs I and II (140) and the lowest for III (139 nm). Changes in enthalpy (ΔH), entropy (ΔS), activation energy (Ea), and Gibbs free energy (ΔG) thermodynamic parameters at 298.15 K were obtained from the binding constant.22 The SIQP molecules haphazardly moved from one point to another as they gained the kinetic energy that generated entropic disorders to favorably orient the reacting species. These orientations align the positive (h+) and negative (e–) charges for counterbalancing them in a reduction process at the cost of ΔG (J/mol). The higher entropy is associated with a lower ΔG. It indicates that the enthalpy released on reaction was not fully occupied, and hence it was expressed in terms of entropy. Thus, entropy, enthalpy, and other physical parameters are directly correlated with the PC reduction (PCR).
Table 3. UV–Vis and Fluorescent Spectral Study of SIQPs in ACN (1.5 × 10–3 M).
| UV database |
fluorescent λexc = 350 nm, Δλ = (λem – λexc) nm |
||||||
|---|---|---|---|---|---|---|---|
| product | λmax (nm) | A | ε (M–1 cm–1) | λem (nm) | Δλ (nm) | intensity (If) | quantum yield (Φ) |
| SIQP I | 347 | 1.8 | 1200 | 490 | 140 | 167 | 71.5 |
| SIQP II | 349 | 2.0 | 1333 | 490 | 140 | 13 995 | 71.5 |
| SIQP III | 337 | 1.0 | 666 | 489 | 139 | 65 | 71.6 |
2.4. Fluorescence Spectra Analysis
Fluorescence emitted wavelength (λem) values for SIQPs I, II, and III are 490, 490, and 489 nm (Figures S5f, S6f, and S7f), respectively, at 350 nm excited wavelength (λexc). Fluorescence intensity (If), Stokes shift (Δλ), and quantum yield (Φ) for SIQPs were calculated (Table 3). SIQP III has lower If and Δλ values compared with SIQPs I and II due to the ERG attached at the para position. The para position of phenyl connected to pyrrolizidine synergizes the pyrrolizidine and NIQ units with maximum HOMO → LUMO populations. ERG is unable to influence, whereas the electron–electron repulsion (EER) of −C≡N creates electron-deficient sites (EDSs) to compensate the EDS of NIQ and the pyrrolizidine generates maximum oscillations. The EWG and ERG both affect SIQPs II and III as sensors to identify the fluorescent activities. SIQP II with −C≡N produced a fluorescence intensity (If) of 13 995 au, but SIQP III (ERG −OCH3) produced 65 au. Electrons of −CH3 move toward the electronically saturated −O– atom, so it may not favorably accommodate the electron cloud of ERG and induce EER. Since the −O– atom is EWG, it attracts the sp2 electron of a phenyl ring, but two LPEs, which are already in the transition state in the electron cloud of ERG to be pumped toward the −O– atom, may not favorably accommodate the sp2 electrons. SIQP III with −OMe reduces its λem to a lower value. SIQPs with EWG and ERG covalently bonded with pyrrolizidine and NIQ structures that show a structural dependence of absorption and emission spectra. Compared to SIQP I, the ERG (−OMe) did not change If, but the EWG (−C≡N) changed the If to the highest value. The −NO2 associated with the aromatic ring causes fluorescence quenching, but our results are contrary, signifying the chemical properties of SIQPs. Quantum yields (Φ) are calculated by eq 5 at excited wavelength = 350 nm or E = 5.675 × 10–19 J, so using eq 3, the number of photons (na) absorbed is
| 3 |
From the emitted wavelength (λem) values of SIQPs I, II, and III, i.e., 490, 490, and 489 nm with E = 4.054 × 10–19, 4.054 × 10–19, and 4.063 × 10–19 J, respectively, the number of photons emitted is
 |
4 |
As quantum yield (Φ) is a ratio of the number of photons emitted to the number of photons absorbed, Φ for SIQPs I and II (eq 5) are
| 5 |
| 6 |
The highest Φ value (71.6%) is seen for SIQP III due to the greater structural rigidity (two LPEs of OMe) (Table 3). The If increases with EWR like −C≡N. Both I and III, compared to SIQP II, have negligible If values as the −C≡N initiates enormous π → π* and n → π* (Figure 7a–c). The solvent affected the absorption maxima (λmax) and the molar absorption coefficient (εmax). The high polarity of ACN caused bathochromic shifts of bands π → π* and hypsochromic shifts of bands n → π*,10 associated with groups capable of binding a free electron pair with hydrogen bonds. SIQPs possessed bathochromic shifts (Δλ = λ350 < λem), strong λem, and If (Table 3), which could widen the optical applications.23 Thus, the quantum yield, emitted wavelength (λem), and If fluorescence spectroscopy widened our research work to approve and initiate the PC activities with a unique theory, especially with the high If value of SIQP II compared to I and III.
Figure 7.

(a–c) Effect of different natures of moieties in SIQPs.
2.5. High-Resolution Mass Spectroscopy (HRTEM) and Energy-Dispersive X-ray (EDX) Studies of SIQPs
Sequentially as per Le Chatelier’s law, the Lennard-Jones potential, and London dispersive forces, SIQP III with electronically rich −OMe, −C≡N, and −NO2 optimizes its electronic clouds to a greater extent. The impacts of EWG of one −C≡N placed at the para position of the phenyl ring inhibit the ν of 2-C≡N of pyrrolizidine by ∼10 cm–1. It validates the morphology and electronic continuity established between 2-C≡N of pyrrolizidine and −NO2 of NIQ due to their electron-withdrawing nature. Their surface morphologies are illustrated in Figures 5a–c and 6a–c. The extended electronic delocalization of quinoxaline and indeno units of NIQ aligns as one-dimensional (1D) nanorods, which are illustrated by HRTEM and EDX. However, the ERG (−OMe) did not affect the ν, proving that the two LPEs of the −O– atom between −CH3 and the phenyl ring minimized the electron-releasing action due to EER. As a result, SIQP III occupies less surface energy as it has a circular shape. SIQP III may interconnect with its other similar molecular units through van der Waals forces as it is heteroatomic and has a highly asymmetric structure. The electron-rich 2-C≡N bonding with pyrrolizidine generates EER and electron–nuclei attraction to stabilize the SIQPs by gaining a higher cohesive energy with the least surface energy. Either an electrostatic dipolar interaction between −C+– and N– of the respective −C≡N groups or their delocalization seems operative. The −C≡N produced 13 995 au intensity with fluorescence. The ΦC and ΦN that develop ΨCN through a quantum dot mechanism quantized an electron cloud as it appeared in the HRTEM image (Figure 6c). Selected area electron diffraction (SAED) patterns show orbital electron distribution as no specific functional units induce the directing factors to quantize SIQP III. SIQP II seems to delocalize electronic charge throughout the molecular plan and appeared as a threadlike structure. SIQP I has an unsubstituted and electronically optimized spherical shape. Haphazard patterns of SAED depict that SIQP I does not have any set pattern of electron distribution similar to metallic nanoparticles. The constituents of SIQPs are asymmetric as the spiro center atom (SCA) adjoined the basic units of SIQPs (substituted phenyl ring, pyrrolizidine ring, NIQ). The effects of the constituents with different electronic configurations are expressed in SAED. HRTEM images are very similar to optical fibers.
Figure 5.
HRTEM ((i) inset image shows the associated electron diffraction pattern) for SIQP I (a), SIQP II (b), and SIQP III (c).
Figure 6.
(a–c) EDX images of SIQPs I, II, and III (i) (inset elemental %).
2.6. TGA Analysis
Onset temperatures in TGA for weight loss are the lowest for SIQP III and the highest for SIQP II, which point to the role of ERG and EWG due to the comparatively stronger interactions of −OCH3 and weaker interactions of −C≡N, respectively (glass-transition temperatures (Tg) for SIQPs I, II, and III are at 110.55, 204.84, and 92.88 °C, respectively) (Figures S5g, S6g, and S7g). It predicts a smaller stability of SIQP II than SIQP III due to hydrophobic–hydrophobic interactions, which is supported by the photocatalyst properties. The lowest weight loss of SIQP III at 92.88 °C shows the stability of its pyrrolizidine ring and SIQP I at 110.55 °C, while SIQP II at 204.84 °C has the highest weight loss at the first onset temperature due to its transitory crystal structure induced by an additional −C≡N group as it had developed an extended delocalization. It further opens a window for advanced research by introducing ERGs and EWGs. Nonuniform rodlike surface structures are observed for SIQPs I and III, whereas a randomly oriented larger platelike surface morphology is observed for SIQP II as they reorient and align in a 1D geometry due to the −NO2 of NIQ and the −C≡N and −OCH3 at the terminal of the phenyl ring. Hence, an extended delocalization induced by −C≡N regulates the overall geometry, which shows their ability to develop a film by maintaining the substrate temperature. Since we explored the −NO2 position contrary to other studies, it was essential and fundamental to determine the thermal stability and degradation pattern for the study of the photocatalyst activity. TGA analysis was required so that applications could be explored based on temperature.
2.7. Activation Energy (Ea) Calculation
The Arrhenius equation, eq 7, where A is the frequency or pre-exponential factor to determine the activation energy as the UV–vis photons interact to activate SIQP molecules to be adsorbed, is as follows:
| 7 |
where R = 8.314 J/(mol K) is the gas constant, T here is RT, and C is the SIQP concentration that adsorbed the photons. abs is a function of C, so plots of (1/C) vs abs produced slope values that are equal to Ea/2.303R. Putting the R value, Ea is calculated with eqs 7 and 8.
| 8 |
The ΔH, ΔG, and ΔS are calculated from Ea values (Table 4) with eqs 9 and 10.
| 9 |
| 10 |
The 25.99 J/mol Ea for SIQP I is higher than SIQPs II and III, which shows its lesser activity with a lower surface area due to centralized delocalization of phenyl–phenyl packing (Figure 7a–c). The −C≡N and −OCH3 units of SIQPs II and III, respectively, make them acoustic and vibrant to distinguish the presence of the functional group as the authentic sensor. These are supported by similar trends of exothermic ΔH data (−5.68941 > −5.704 > −5.70874 kJ/mol order). Thus, Ea and ΔH both show the least activation for EWG (extended delocalization) with a higher surface area than ERG, whereas the unsubstituted SIQP I has the maximum activation. SIQPs can distinguish EWG and ERG substituted and unsubstituted spiroheterocyclics. Table 4 lists the minimum energies (MEs) noted as the chemical potential (μ, J/mol) in the order SIQP III > I > II, which were calculated computationally with Chem 3D and MM2 along with the dipole moment in the order II > I > III. The thermodynamic basis of μ, J/mol, was derived from the following equation
| 11 |
Both calculations and experiments were made at constant temperature and atmosphere, respectively, where dT = 0 and dP = 0. Putting these values in eq 11, we get
| 12 |
Both μ and ∂G are in the order
III > I > II, despite generating them computationally and experimentally
with a UV–vis spectrophotometer, respectively. These are supported
by the reverse trends (II > I > III) of ΔS values
as per thermodynamic activities (eq 12). These retrieve their intrinsic nature and validate
a choice of our adopted research methodologies computationally and
experimentally both (Table 4). Therefore, considering the electronic activities of C6H5– (phenyl),
−C≡N (cyano), and 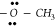 (methoxy) functional constituents
of SIQPs
supported by thermodynamics, computational, and spectroscopic parameters,
we designed the PC MB reduction experiments in various solvents. The
nature of solvents affects photocatalysis (Scheme 1), e.g., aq EtOH, aq ACN, and ACN solvents
reduced MB in the order aq EtOH > aq ACN > ACN, whereas aq EtOH
reduced
it in a shorter time (Table 5). The −NO2, −C≡N, and N constituents
develop hydrogen bonding with water, which further photocatalyzed
π → π* and n → π* transitions to produce
the h+ and e– holes. The holes overcome
the HOMO–LUMO energy gap to enhance the PC activities. The
solvent surrounds the MB to enhance the permittivity of holes to =S+– and Cl– to reduce the MB to LMB.
The photons of sunlight strike the LPE of the SIQPs that generate
the h+ and e– holes. The e– holes approach the −S+(Cl)= chemical constituent
of the MB as e– + −S+(Cl)=
→ −S– + Cl– (reduction step),
and the h+ holes approach the Cl– ion
as 2Cl– + 2h+ → Cl2 (oxidation step). The reduction of −S+(Cl)=
produced the colorless MB. From reduction and oxidation steps, turning
blue to colorless MB indicated the scavenging or reduction of Cl– into Cl2 gas. The Cl2 gas generated
a pressure in a lid-fitted reaction vessel, resulting in opening of
the lid due to Cl2 generation. This experiment was repeated
several times to ensure lid opening by Cl2 generation.
The Cl2 production was also chemically analyzed by bringing
a moistened NH4OH glass rod near the open mouth of the
reaction vessel. It produced white dense fumes and a white precipitate
on a glass rod as the NH4OH + Cl2 → NH4Cl↓ + HCl↑. The operating string is materialized
in situ where the SIQPs were highly monodispersed along with the monodispersion
of MB. The SIQPs released the h+ and e– holes vis-à-vis MB. The e– holes reduced
−S+(Cl)= into colorless −S–
MB. Therefore, it was the Cl– ion that acted as
a reducing radical for the reduction reaction in sunlight. Also, HIn
did not produce the PCR because it has no Cl– ion
in its molecular structure. Therefore, the generation of the Cl– radical reduced the MB, but no color change was noticed
with HIn. It constitutes an advanced greener and nanoreduction model.24 Protic solvents like CH3OH, n-butanol, and nitromethane could exponentially catalyze
photocatalysis.
(methoxy) functional constituents
of SIQPs
supported by thermodynamics, computational, and spectroscopic parameters,
we designed the PC MB reduction experiments in various solvents. The
nature of solvents affects photocatalysis (Scheme 1), e.g., aq EtOH, aq ACN, and ACN solvents
reduced MB in the order aq EtOH > aq ACN > ACN, whereas aq EtOH
reduced
it in a shorter time (Table 5). The −NO2, −C≡N, and N constituents
develop hydrogen bonding with water, which further photocatalyzed
π → π* and n → π* transitions to produce
the h+ and e– holes. The holes overcome
the HOMO–LUMO energy gap to enhance the PC activities. The
solvent surrounds the MB to enhance the permittivity of holes to =S+– and Cl– to reduce the MB to LMB.
The photons of sunlight strike the LPE of the SIQPs that generate
the h+ and e– holes. The e– holes approach the −S+(Cl)= chemical constituent
of the MB as e– + −S+(Cl)=
→ −S– + Cl– (reduction step),
and the h+ holes approach the Cl– ion
as 2Cl– + 2h+ → Cl2 (oxidation step). The reduction of −S+(Cl)=
produced the colorless MB. From reduction and oxidation steps, turning
blue to colorless MB indicated the scavenging or reduction of Cl– into Cl2 gas. The Cl2 gas generated
a pressure in a lid-fitted reaction vessel, resulting in opening of
the lid due to Cl2 generation. This experiment was repeated
several times to ensure lid opening by Cl2 generation.
The Cl2 production was also chemically analyzed by bringing
a moistened NH4OH glass rod near the open mouth of the
reaction vessel. It produced white dense fumes and a white precipitate
on a glass rod as the NH4OH + Cl2 → NH4Cl↓ + HCl↑. The operating string is materialized
in situ where the SIQPs were highly monodispersed along with the monodispersion
of MB. The SIQPs released the h+ and e– holes vis-à-vis MB. The e– holes reduced
−S+(Cl)= into colorless −S–
MB. Therefore, it was the Cl– ion that acted as
a reducing radical for the reduction reaction in sunlight. Also, HIn
did not produce the PCR because it has no Cl– ion
in its molecular structure. Therefore, the generation of the Cl– radical reduced the MB, but no color change was noticed
with HIn. It constitutes an advanced greener and nanoreduction model.24 Protic solvents like CH3OH, n-butanol, and nitromethane could exponentially catalyze
photocatalysis.
Table 4. Calculated Electronic Activation Energies Ea, Enthalpies ΔH, Gibbs Free Energies ΔG, and Entropy ΔS of SIQPs (C = 1.5 mmol) in J/mol Relative to Corresponding Reagents.
| slope | A | Ea | ΔG | ΔH | ΔS | MEaa | dipole (D)a | |
|---|---|---|---|---|---|---|---|---|
| SIPQ I | –1.3576 | 0.26 | 25.99 | –1530.58 | –5689.41 | –33.08 | 70.26 | 15.51 |
| SIPQ II | –0.3483 | 2.05 | 6.66 | –1783.78 | –5708.74 | –32.23 | 68.78 | 16.02 |
| SIQP III | –0.5443 | 1.08 | 10.42 | –205.75 | –5704.98 | –37.51 | 78.04 | 15.48 |
Minimum energy (ME) (kcal/mol) and dipole moment (D) were calculated by MM2.
Scheme 1. Plausible Reaction Mechanism for Photocatalysis of MB to LMB by SIQPs with Colorless Gas (Cl2) Evolution.
Table 5. MB (18 ppm) Photocatalytic Reduction Rate by SIQPs (1.5 ppm) in Different Solvents in Comparison with CdS-GO (10–3 M).
| color
change time (∼min.) |
rate
of catalysis (%) |
|||||
|---|---|---|---|---|---|---|
| sample | ACN | aq ACN | aq EtOH | ACN | aq ACN (∼g/min) | aq EtOH (∼g/min) |
| SIQP I | 120 | 95 | 70 | 41.3 | 3.0 × 10–3 | 4.2 × 10–3 |
| SIQP II | 45 | 43 | 30 | 85.5 | 7.0 × 10–3 | 1.0 × 10–2 |
| SIQP III | 70 | 54 | 45 | 80.9 | 5.5 × 10–3 | 6.7 × 10–3 |
| CdS-GO | 160 | 3.5%/min | ||||
2.8. Mechanism of Photocatalysis
With increasing concentrations, the SIQPs linearly increased the absorption without blue or red shifts, which confirms the nondegradation of SIQP in sunlight (Figure S5e). The increase in abs is perfectly fitted in the reaction abs = εcl. First, we studied the SIQPs PC effect in the absence of a dye with ACN, aq ACN, MeOH, and aq MeOH solvents, which resulted in no change. It can be explained by the availability of LPE in the solvent itself, which did not induce adequate transitions. Thus, no h+ and e– hole formation occurred and no reduction occurred with these solvents. Hence, there was no change in PCR in the absence of MB. Since the SIQPs (1.5 ppm) and MB (18 ppm) were taken at fixed concentration for PCR study. The PCR experiments were conducted in sunlight, where the photons were exposed to SIQP molecules, since with time, the molecules acquired Ea and explored their PC activity. Therefore, with time, the molecules of SIQPs get activated, which generates the h+ and e– holes to reduce the MB. Therefore, as per the law of mass action, the concentration did not increase with time. Thus, the molecules of SIQPs that were considered are fully activated with time and reduced the MB. The PC kinetics with MB reduction was studied. The concentrations of both SIQPs and MB were fixed so that each molecule becomes activated and participated in the redox cycle as a = ao e–Ea/kBT, where “a” is concentration at time t, “a0” is the initial concentration, kB is the Boltzmann distribution constant, T is the reaction temperature, and Ea is the activation energy. Had their concentration been increased, then there would have been the lowest activation of these molecules in fractions. For PCR kinetics, the 1.5 ppm SIQPs and 18 ppm MB were separately prepared in ACN. Next, 0.01 mL of MB was added to each sample solution of SIQPs at RT. The UV–vis absorption (λmax) and images were separately recorded at initial and final PC reductions. In sunlight, initially, the intense blue color of MB with SIQP II was reduced to a pale yellow within ∼10 min, and then it became colorless, whereas the disappearance of the blue color with SIQPs I and III took a longer time. SIQPs on absorbing sunlight generated h+ and e– holes, which interacted with −S+= and Cl– species of MB. LPE of the N-atom of −NO2 in NIQ had further repelled out the sp2 electrons to expeditiously develop the e– holes on electron–electron repulsion (EER) induced by the LPE of the N-atom as per the Born–Oppenheimer approximation discussed below. The PC rate of SIQP II for MB reduction is greater than that of SIQP III (Table 5) as no such EWG mechanism exists with SIQPs I and III, so its sp2 electrons vis-à-vis the redox cycle may not promote the valance-band (VB)-to-conduction-band (CB) shift expeditiously. SIQPs further quantize the sp2 electrons to reach the e– holes due to the LPE of the N-atom of NO2. Had there not been LPE, the sp2 electron would have faced resistance (shear stress η) to develop the ζVB–CB noted as the following equation13
| 13 |
where ζVB-CB is the ζ potential, ε(e–)×(h+) is the dielectric constant, εmedium is the medium permittivity, ηSIQP–MB is the shear stress, and μ(e–)×(h+) is the electron mobility. Equation 13 reflects the dynamics of h+ and e– holes vis-à-vis their generation, mobility, solvent contribution, alignment, and interactions with =S+– and Cl– of MB as oxidizing agents. The solvents play a critical role in establishing a higher medium permittivity to shift the respective holes from SIQPs to MB polar species. The LPE of 2N atoms of NIQ inhibits the delocalization of sp2 electrons so that they move to initiate the sp2 → sp3 (h+ to e–) holes and then orient toward the −S+= and Cl–, respectively, as a medium has moderate shear stress. Rotational, vibrational, and transitional motions of the pyrrolizidine due to SCA seem to orient the N≡C–Ph– and N≡C– toward quinoxaline and indeno units, which further catalyze the sp2 → sp3 (h+ to e–) holes. The ζVB–CB promotes the electrokinetics of h+ and e− holes despite the Fermi energy. The shear in GO for h+ and e− holes is higher that lowers the value of μ(e−)×(h+) from sp2 → sp3. Thus, the frequencies of the redox cycle, i.e., sp2 → sp3 for h+ to e– holes and vice versa for SIQP II, are more (Scheme 1) due to the higher μ(e–)×(h+) from sp2 → sp3 electron clouds. The MB photocatalytic initiation and efficiency depend on a mutual shift of quantized electron clouds. Thus, −NO2 further creates an efficient sp2 → sp3 transfer mechanism to quickly reorient the holes vis-à-vis the sp2 → sp3 shift of NIQ. The −NO2 promotes photocatalysis. sp2 → sp3 and sp → sp2 populations are generated, which greatly support the VB → CB shifts for expeditious MB reduction, which are missing in GO. The SIQPs supersede the use of GO by acting as a green photocatalyst for fluorescent dye reduction.25 SIQP I with the phenyl ring reduced the MB by 60%, as compared to 90% by SIQP II with 90–60 = 30% more MB PC reduction. SIQP II with sp2 → sp3 and sp → sp2 electronic shifts generated exponential holes due to a terminal C≡N. The MB photocatalysis with SIQP III is 70%, which a lower than the reduction with SIQP II. Thus, compared to ERG, EWG increased photocatalysis (Table 5) as EER of ERG inhibits the expeditious shift from sp2 → sp3, which lowers the photocatalysis by 90–70 = 20%. These shifts are prompt but in GO are generated by exfoliating its sheets through sonication, which weaken its intersheet van der Waals forces. The functional defects or edges that generate sp2 → sp3 are mechanically induced in GO in contrast to being naturally available in SIQP structures. Functional edges of GO are induced by exfoliating GO sheets on sonication using suitable solvents. However, SIQP II does not require sonication to generate such functional edges (Figure 7b). These edges already exist in SIQP structures and act as superactive sites with their intrinsic intramolecular entropy noted as tentropy. The moment photons strike these superactive electronic sites, sp2 → sp3 and sp → sp2 populations are generated to photo-reduce the MB (Scheme 1). The cyclic sp → sp2 and sp2 → sp3 electronic transitions of −C≡N and double bonds of SIQPs complete a redox cycle (Scheme 1) contrary to GO with sp2 → sp3. SIQP III with ERG −OCH3 in ACN splits in electron transitions as electrons of −CH3 tend to move toward the O-atom. The electronically saturated −O– atom may not favorably accommodate an electron cloud of ERG to induce EER. The SIQPs have distinguished the electronic configurations with their electron potential energies, and they are not synchronized and express their photonic response at different λmax due to a contrast in absorption activities of ERG and −O– atoms. Since the −O– atom is EWG, it attracts the sp2 electron of the phenyl ring, but two LPEs, which are in a transition state due to the electron cloud of ERG to be pumped toward the −O– atom, may not favorably accommodate the sp2 electrons. There is no space to synchronize the ERG-O-atom and the sp2 electron of the phenyl ring and utilize their energy. Stretching was performed with separate energies as per Hook’s law (eq 14), which on Fourier transform on the time scale develop intensified energy peaks.
| 14 |
The νi optimizes at different λmax with 320 nm, which after 30 min splits within 298–305 nm (Figure S8a–c) with the SIQPs in the close wavelength range being expressed. These involve their electrons for photocatalysis along with the ν values of each constituent that may generate the most vibrant web space, which could neutralize the active structures of mosaic-type moieties like coronavirus. Thus, closely packed stretching vibrations with ERG depict the active electronic transitions of LPE and delocalization interrupted by −S+= and Cl– ionic states that synchronize with extraordinarily active electronic transitions and clouds of SIQPs. Thus, MB-UV electronic transitions induce collisions derived from EER, and the negative–positive charge attraction interactions are as reported. These sharper peaks within 200–246 nm absorb maximum UV–vis light, while that at 291 nm absorbs the lowest. Electronic transitions of SIQPs vis-à-vis MB with ACN induce favorable electronic transitions within a single MB molecule in a combinatorial manner. The −S+= and Cl– PC active ionic constituents of MB differently respond to the UV–vis light vis-à-vis h+ and e– holes. Similar to GO, the SIQPs upon absorbing photons from solar radiation produced h+ and e– holes, which were transferred from their conduction band (sp2) to valence bands (sp3) (Scheme 1). As per Le Chatelier’s law, the h+ and e– holes interact with −S+= and Cl– species, respectively, to counterbalance their charges within the Lennard-Jones potential that reduce the MB. Patterns of UV–MB interactions with SIQP II effectively reduced the MB in ACN as ACN brings the SIQP II and MB together. The UV–vis absorption is reduced to a negative value as the electronic transition of SIQP II is diverted to interact with electronic transitions of MB. Thus, before reaching LUMO states, the electrons are captured by MB so the MB undergoes a reduction as ACN electronically exists as CH2+–CN–. The MB synchronizes and reorients within the SIQP semiconductor mechanism, which differs from GO as it has a hexagonal structural sheet bound together with van der Waals forces. The GO expresses the symmetric h+ and e– hole alignments to develop a double layer with positive and negative charges with the ζ potential, but SIQPs do not have symmetric sheet arrangements, so the h+ and e– hole alignment may not be symmetric. Thus, the electron–electron cyclic attraction could be disrupted and SIQPs of electrons move toward MB to reduce the S+, which is counterbalanced by h+ as they move toward Cl– to reduce MB (Scheme 1, step 3). SIQPs have manifold nucleophilic domains, which result from the electrophilic domain of MB. These domains also support the lowest absorption as these electrophilic and nucleophilic domains of MB and SIQPs, respectively, bring them together through Coulombic interactions reported in the following equation15
| 15 |
The Coulombic mechanism results in a closer distance, where h+ and e– reached S+ and Cl–, respectively. ACN with 3.92 D dipole moment acted as a dispersion medium (Figure S8i,ii). The solvent engages the H+, which was produced due to absorption of the photon via an electron-release mechanism. The solvents created extra spontaneity to engage the H+. The H+-release mechanism is supported by SIQPs. Since PCR was conducted in sunlight in November 2019 from 11 am to 1 pm, the samples were kept in an open box with a rigid body so that air fluctuations were minimized and solar radiations in almost equal amounts with time were absorbed by the sample that tuned the intensity. PCR under similar experimental conditions with similar stoichiometries of SIQP and MB was conducted at the same time in sunlight. Each experiment generated h+ and e– holes, which reduced a similar amount of MB with ±0.1% variation. Hence, the reproducibility of PCR was determined by conducting authentic experiments that reproduced the same results.
2.9. Adsorption Activity of SIQPs with MB
The MB reduction rate by SIQPs is determined with eq 16,25 and the highest rate with SIQP II was found (Table 5) as
| 16 |
The % MB photocatalytic reduction by SIQP I in 120 min in ACN is calculated with the following equation
| 17 |
where Co is the initial MB concentration at time t = 0, and Ct is the reduced MB concentration at time t min.
The % MB reduction by SIQP II in sunlight for 30 min in ACN is
| 18 |
The % MB reduction by SIQP III in sunlight for 70 min in ACN is
| 19 |
MB reduction to transform it into LMB has the order SIQP II (85.5) > SIQP III (80.9) > SIQP I (41.3%); their rates are calculated in terms of percentage (%) (Table 5). The higher % of MB reduction of SIQP II compared to SIQPs I and III in a shorter duration made it more effective. It can be explained by the illustrated mechanism. The photons interact with the sp2 electron orbitals of SIQPs forming h+ and e– holes, i.e., from valance (VB) and conduction (CB) bands, respectively. These holes tend toward MB to induce its sp2 electrons, which reduce the MB to LMB. Therefore, other molecules of MB dyes could easily be reduced to their leuco state. The process was conducted in ACN, aq ACN, and aq EtOH (variable dipole moment) solutions with different reduction powers (Chart 1), where H2, O2, and Cl2 gases were formed along with MB reduction to LMB (colorless). High polarity of the solvent medium hindered the PCR due to the resistance faced by strong dipolar interactions of h+ and e– holes. Thus, the polarity and PCR are inversely proportional. SIQP II is better than SIQPs I and III for the PC studies based on the rate of reaction for photocatalysis (% MB reduction). Since the MB has quaternary N, it was extraordinarily active and highly sensitive toward light. However, SIQPs I, II, and III produced electrons (e–), which reduced the MB. So these activities tend to stabilize the MB via reduction. It is observed that the solution is monodispersed and is not agglomerated in the solution, and it does not consume oxygen in the solution. It is merely participating in the interacting modes with the chemical process in the reaction medium. If there is an adequate amount of MB to consume the released e– holes, then there is no effect of the presence of oxygen because oxygen transfers into the oxide ion with e– holes, which consumes more Ea compared to MB. However, the situation is different when the MB is in less amount and oxygen is present; then, excess e– holes may react as O2 + 4e– → 2O2–; 2O2– → O2 + 4e–. This depends on the concentration of MB. Since h+ and e– holes are active and the oxygen gas molecules also respond to the freely available e– holes, when the MB is completely reduced, then e– approaches the oxygen gas. To balance the h+ and e– holes, the O2 → O2– and O2– → O2 redox cycles keep going on. Hence, SIQP is fully balanced with h+ and e– holes. There is the possibility that ACN generates CH2=C=N– and H+ and develops the neutral H2O molecule as 2H+ + O2– → H2O. However, it responds to pH and the chemical potential. Each reacting molecule individually is dispersed in the solvent without undergoing coagulation or coalescence vis-à-vis the solvent or medium. Thereby, a reduction prominently occurs rather than a coagulation so the SIQPs remained monodispersed, which facilitates kinetic orientation to align the reacting species favoring a reaction. Therefore, any salt of monovalent, divalent, trivalent, or transition metals if added could induce robust PC activities for MB reduction. After conducting valid experiments for PCR of MB, a similar PCR experiment setup was extended for HIn. However, it was not reduced as it does not have any quaternary atom in its structure, which could accept the h+ and e– charges individually. In fact, with time, HIn developed two liquid phases and it remained dispersed in the bottom layer. Thus, the usability of SIQP in sunlight is for those dyes that have quaternary atoms to furnish the dipoles to be neutralized by charges of holes generated by SIQP (Scheme 2). Therefore, in terms of PC activities and efficiency, SIQP favorably generated h+ and e– holes; these holes by SIQPs with photons photocatalyzed the MB to LMB through the redox cycle. These activities electronically transform the MB to colorless LMB. Thus, the reduction of MB using the generated h+ and e– holes is called PC efficiency, i.e., the quantum yield for reducing the % of MB to LMB. Thus, the PC activity leads to PC efficiency.
Chart 1. (i) Dipole Moment for the Solvent Used in Photocatalysis. (ii) Reduction Power.
Scheme 2. Respective Data Comparison for Photocatalytic Reduction Property and pH.
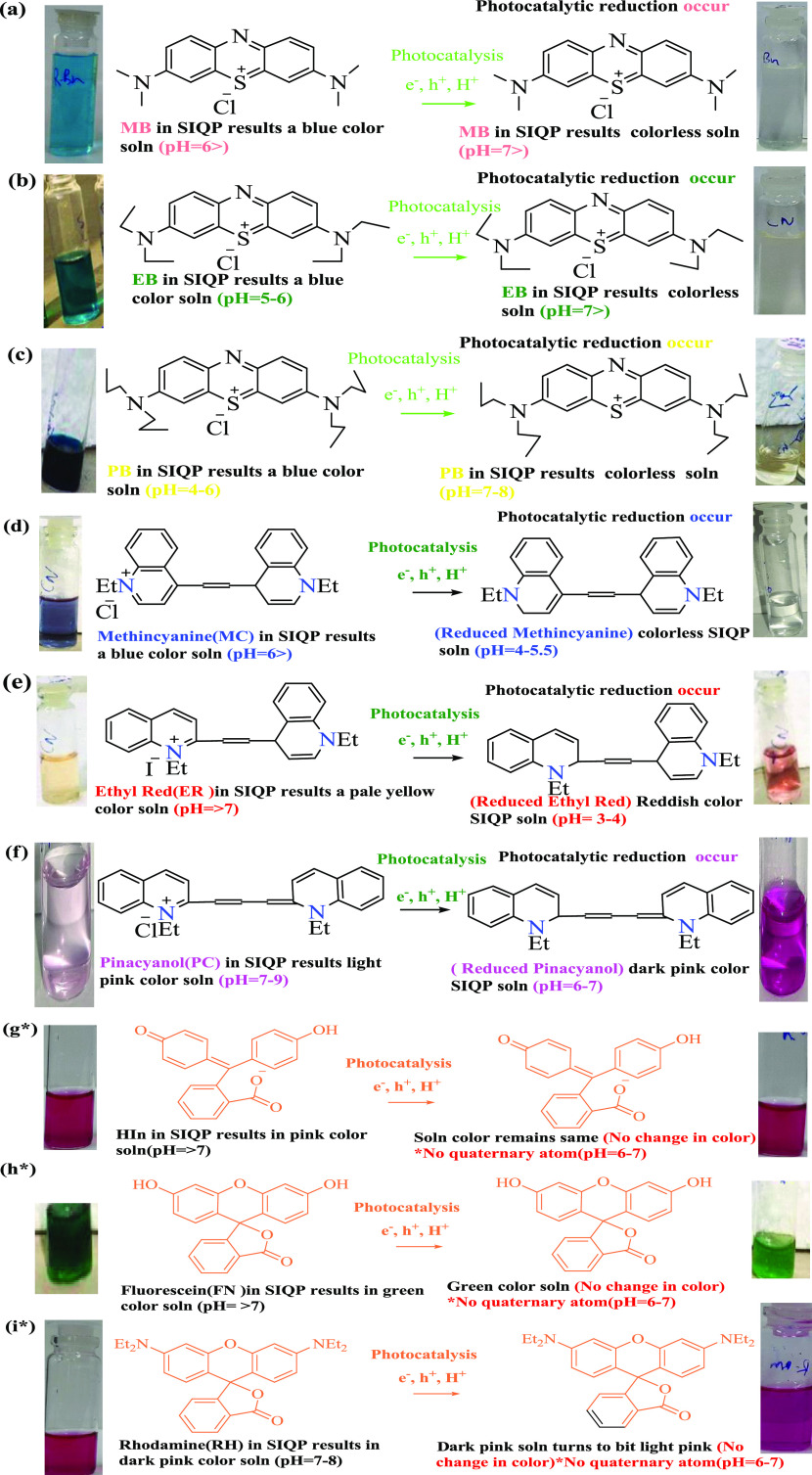
For (a) MB, (b) ethylene blue (EB), (c) propylene blue (PB), a series of quinoline dyes like (d) methincyanine (MC), (e) ethyl red (ER), and (f) pinacyanol (PC), and phthalein series like (g*) HIn, (h*) fluorescein (FN), and (i*) rhodamine (RH) under sunlight (*SIQPs do not show PCR, as used dyes have no quaternary atoms).
2.10. Raman Analysis
Vibrational frequencies (ν) and intensity of Raman spectra elucidate the molecular structure (Figure 8). Raman frequencies for SIQP I are 1129 cm–1 (ν(C=C aromatic ring stretching)) and 1508 (ν(NO)) as well as intensity shifts for D and G bands are 81 127 au, 1506 cm–1 and 78 191 au, 1514 cm–1, respectively. Raman frequencies for SIQP II are 1487 (ν(C=C aromatic ring stretching)), 1654 (ν(NO)), 1958 (ν(CN)), and 2052 cm–1 (ν(CN)) as well as intensity shifts for D and G bands are 285 523 au, 1654 cm–1 and 279 899 au, 1658 cm–1, respectively. Raman frequencies for SIQP III are 1181 (ν(C=C aromatic ring structure)), 1314 (ν(C–O)), 1564 (ν(NO)), and 2222 cm–1 (ν(CN)) as well as intensity shifts for D and G bands are 44 955 au, 1555 cm–1 and 44 028 au, 1566 cm–1, respectively (Figure 8). SIQP II has the highest Raman intensity due to para-substituted EWG like −CN compared to SIQP III with ERG like −OMe, and hence it is a Raman-active compound. Thus, the −C≡N group acts as a sensor to depict a population of phonon generations contrary to phenyl and −OMe. Thus, SIQP II may be categorized as an upconversion nanoparticle (UCNP). The Raman intensities for SIQPs I and III are comparable due to their hydrophobic nature (Chart 2). The functional-group-dependent Raman intensity differentiates the nature of SIQPs to act as sensors. The D and G bands like GO showed disordered and synergistic structures, respectively. The intensities of the D and G bands have ratios 1.8:6.3:1 and 1.7:6.5:1 (Table 6), showing that the phenyl ring alone and the −C≡N at the para position (SIQP II) induced the maximum electronic disorder compared to the para-OMe in SIQP III. Comparatively, the lower electronic disruption of SIQP III depicts the EER due to −OCH3 as ERG and the −O-atom that holds the two LPEs. The noticeable impacts of phenyl, −C≡N, and −OCH3 at the para position have almost a similar ratio of intensities. The G band frequencies are comparatively higher than the D ones as the disruption of the electronic structure inhibits the phonon generation and its frequency. Since the G band depicts the reordering, its electronic transition optimizes the frequency and hence a higher frequency indicates the same. Raman studies confirm the impact of −C≡N at the para position of phenyl of pyrrolizidine and −NO2 of NIQ because their EWG SIQP II substantially catalyzed the semiconductor h+ and e– hole-generating abilities. Thus, h+ and e– hole generation with EWG at terminal positions of NIQ might have affected the Fermi energy (EF) level (Figure S9) with a shorter HOMO–LUMO gap (band gap) in the case of specially SIQP II. The Fermi–Dirac distribution (eq 20)
| 20 |
where E is the energy, T is the temperature, kB is the Boltzmann distribution constant, and νRaman is the Raman frequency. Using the equation in Table 6, the HOMO–LUMO gaps for SIQPs I, II, and III are calculated at Raman frequencies (νRaman) 1506, 1654, and 1555 cm–1, respectively, as
 |
20a |
 |
20b |
 |
20c |
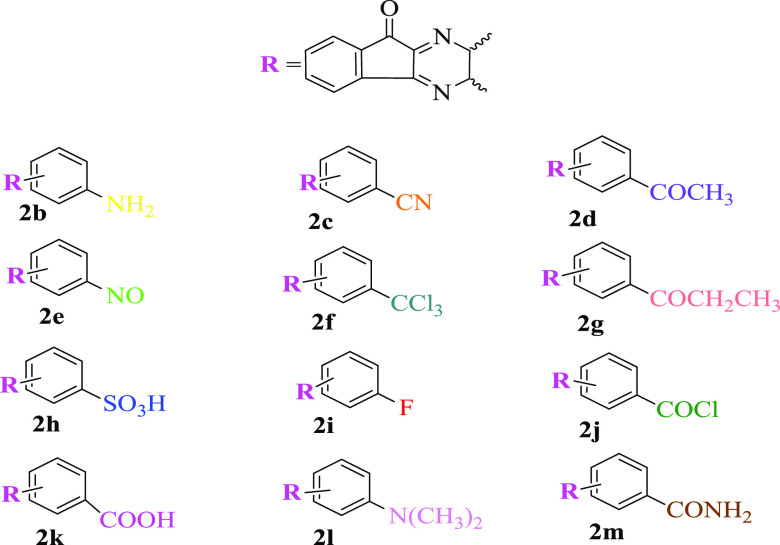
The E – EF value is negligible (eqs 20 and 20a–c; Table 6) for SIQPs, and hence the semiconductor mechanism effectively works for PCR conjugated and quaternary atom holding dye but not for dyes that lack a quaternary atom in structures like HIn and so on (Scheme 2). The mechanistic path followed by SIQPs for acting as reliable sensors to distinguish and separate conjugated dyes with quaternary atoms in their structures, e.g., MB, by photocatalytic reduction was studied (Scheme 3a and Table 7). These SIQPs also separated dyes like HIn, which do not have a quaternary atom in their structures (Scheme 3b and Chart 3). The SIQPs may minimize the challenges of global warming, resulting from a mixture of fluorescent dyes present in industrial effluents, whereas the reduced dyes do not cause global warming. The scope of SIQP was further extended by engineering the probable structure of NIQ as depicted in Table 8 with the derivatives of 1,2-diaminebenzaldehyde for the probable products. The IQ unit could be modified by substituting EWG groups for synthesizing the products in desired yields (2a) (Table 8). A series of functional groups including primary and tertiary amines (2b and 2l), cyano (2c), alkoxyl (2d and 2g), nitroso (2e), trichloro (2f), sulfo (2h), fluoro (2i), acid chloride (2j), carboxylic acid (2k), and amide (2m) groups could be explored through 2a, having −NO2, via HRTEM, Raman, and EDX analyses. Since in spiroheterocyclics all of the constituents have special electronic configurations and energy distributions based on the Born–Oppenheimer approximation, the molecule has a critical special geometry, which may affect the photocatalyst too. Thus, HRTEM, Raman, and EDX are essential and remarkable to study the internal morphology, energy distribution, and different energy levels of the SIQP molecules in different modes.
Figure 8.

Raman shifts for SIQPs I, II, and III.
Chart 2. D and G Bands in Raman Shifts for SIQPs.

Table 6. Raman D and G Shift Ratios with Their Frequencies (ν) for SIQPs.
| sample | E – EF | D shift | G shift | D freq. (Dν) | G freq. (Gν) |
|---|---|---|---|---|---|
| SIQP I | –2.7580 × 10–24 | 81 127 | 78 191 | 1506 | 1514 |
| SIQP II | –2.4887 × 10–24 | 285 523 | 279 899 | 1654 | 1658 |
| SIQP III | –2.6472 × 10–24 | 44 955 | 44 028 | 1555 | 1566 |
Scheme 3. HOMO–LUMO Gap (i.e., Band Gap) for MB (Allowed) and HIn (Forbidden) Reduction.
Table 7. PC Property Comparison for Different Natures of Dyes in the pH Range 4–7.
| nature of dyes used | minimum energy (kcal/mol) | dipole moment (D) | no. of π e– | quaternary atom (present/absent) | SIQPs PCR activity26 |
|---|---|---|---|---|---|
| (a) MB | –41.8298 | 0.0231 | 7 | yes | yes |
| (b) EB | –36.5970 | 0.0205 | 7 | yes | yes |
| (c) PB | –35.0474 | 0.0201 | 7 | yes | yes |
| (d) MC | –36.3374 | –0.2330 | 10 | yes | yes |
| (e) ER | –28.8062 | –0.2326 | 10 | yes | yes |
| (f) PC | –33.4505 | 0.0000 | 11 | yes | yes |
| (g*) HIn | 35.6056 | 0.0209 | 11 | no | phase out |
| (h*) FN | 20.8949 | 7.2629 | 10 | no | no change |
| (i*) RH | 48.4709 | 7.0520 | 10 | no | no change |
Chart 3. Minimization Energies of MB, EB, PB, MC, ER, PC, HIn, FN, and RH.
Table 8. Reaction Scope (Scheme 7)a.
3. Conclusions
Novel photocatalyzing chiral SIQPs have been synthesized via a one-pot three-reacting species with 87–98% yields. 1H NMR, 13C NMR, NOESY, FT-IR, UV–vis, fluorescence, Raman spectroscopy, liquid chromatography-mass spectrometry (LC-MS), TGA, HRTEM, and energy-dispersive analysis with X-ray spectroscopy (EDX) processes have confirmed the structures and relation with photocatalytic activity. Ea, ΔH, ΔS, and ΔG thermodynamic parameters calculated from UV–vis spectrophotometric absorption established their optical and interacting activities in various solvents. SIQPs photocatalytically reduced the fluorescent MB dye but salted out the HIn to a newly developed liquid phase. Our PC methodology is being extended to several other fluorescent dyes like malachite green, mitosensor green, octadecyl rhodamine B chloride, and rhodamine 123. SIQPs act as an adsorbent for several toxic heavy metals in industrial waste effluents and various techniques.27 In place of the phenyl ring, several other superactive molecules could be used to explore competent constitutional units and in vitro activities for biological applications28,29,26 in the future.
4. Experimental Section
4.1. Materials
Materials used in this study were thin-layer chromatography (TLC) plates, analytical-grade hexane (Sigma-Aldrich, ≥99%), ethyl acetate (Sigma-Aldrich, ≥99%), dichloromethane (DCM) (Sigma-Aldrich, ≥99.8%), dimethylformamide (DMF) (Sigma-Aldrich, ≥99.8%), acetone (Sigma-Aldrich, ≥99.9%), MeOH (Sigma-Aldrich, ≥99.9%), and ACN (Sigma-Aldrich, ≥99.8%). Solvents were redistilled and used. Benzaldehyde, para-cyanobenzaldehyde, para-methoxybenzaldehyde, ninhydrin, malononitrile, lithium bromide (LiBr), acetic acid (CH3COOH), methanol (CH3OH), l-proline, magnetic beads, and Whatman filter paper were used as received.
4.2. Characterization Methods
Structures were analyzed with 1H NMR, 13C NMR, and NOESY (mixing time 0.5 s) (500 MHz, Bruker Avance spectrometer) in CDCl3 and dimethyl sulfoxide (DMSO)-d6 at 500 and 125 MHz using tetramethylsilane (TMS) as the internal standard; FT-IR spectra from 200 to 800 cm–1 with KBr pellets on the PerkinElmer TL8000 TG-IR interface; mass spectra on an Agilent Technology 6545 Q-TOF LC-MS mass spectrometer operating at 70 eV; UV–vis spectra from 190 to 1100 nm with UV-1800 SHIMADZU (UV spectrophotometer) in the electrospray ionization (ESI) mode; high-resolution transmission electron microscopy (HRTEM) with a JEOL JEM-2100 electron microscope at 200 kV operating voltage; thermal gravimetric analysis (TGA) with an intercooler PerkinElmer TGA-6000 thermometer for ∼25–500 °C; fluorescent spectra with Edinburgh Instruments Mark McCallum (λmax = 200–800 nm, counts 0–107); and Raman spectroscopy and EDX with EDX QUANTA FEG 250 SEM.
4.3. Central Theme for the Interacting Mechanism and Activities
SCA interconnects NIQ and pyrrolizidine together through covalent bonds for SIQP synthesis. Pyrrolizidine (2-phenylhexahydro-1H-pyrrolizine-1,1-dicarbonitrile) was prepared by mixing 2-benzylidene malononitrile and l-proline with NIQ. 4-Cyano and 4-methoxy 2-benzylidene malononitriles separately replaced 2-benzylidene malononitrile for SIQPs II and III. SIQPs with a unique electronic configuration and favorable HOMO–LUMO gap along with biocompatible −NO2 represent a novel science area. −NO2 enabled SCA to symmetrize, synergize, and optimize electronic clouds expressed with thermodynamic functions. SCA enables rotational, vibrational, translational, and electronic motions responsible for acoustic and physicochemical sensing activities. EDX and HRTEM depict the activities of SIQPs as optical fibers as a novelty.
4.4. Synthesis of the Starting Material
Benzaldehyde (100 mmol, 1.0 equiv, 10.6 mL) (Scheme 4) (Table S1), 4-cyano-benzaldehyde (100 mmol, 1.0 equiv, 13.1 g) (Scheme 5) (Table S2), 4-methoxy benzaldehyde (100 mmol, 1.0 equiv, 13.6 mL) (Scheme 6), and malononitrile (100 mmol, 1.0 equiv, 6.6 g) (Table S3) with LiBr (catalytic amount) in DMF solvent were separately taken in RB and stirred for 1 h at RT. This synthesis did not work with NaBr but worked with the LiBr catalyst due to its smaller cationic size. Starting materials remained unreacted with NaBr (studied from TLC). The main reaction was monitored with TLC, and the product was filtered and dried under vacuum. Structures were analyzed with 1H NMR.
Scheme 4. Synthesis of 2-Benzylidene Malononitrile (Figure S1).
Scheme 5. Synthesis of 2-(4-Cyanobenzylidene) Malononitrile (Figure S2).
Scheme 6. Synthesis of 2-(4-Methoxybenzylidene) Malononitrile (Figure S3).
4.5. Synthesis of 7-Nitro-11H-indeno[1,2-b]quinoxalin-11-one (NIQ)
4-Nitrophenylene-1, 2-diamine (11 mmol, 1.1 equiv, 1.7 g) (Scheme 7) and ninhydrin (10 mmol, 1.0 equiv, 1.8 g) were stirred in a CH3COOH (10 mL) and CH3OH (30 mL) mixture for 30 min at RT (Table S4). The reaction was monitored by TLC. A pale-yellow product was obtained, which was filtered and washed with CH3OH (unreacted CH3COOH was washed out with chilled water) and dried under vacuum. Structures were analyzed with 1H NMR.
Scheme 7. Synthesis of 7-Nitro-11H-indeno[1,2-b]quinoxalin-11-one (NIQ) (Figure S4).

Note: The solvent system in a 1:3 ratio of CH3COOH (10 mL) and CH3OH (30 mL) was used to maintain the pH to maximize the yield. Here, CH3COOH acted as a source of H+ to remove water.
4.6. Mechanism of SIQPs
Scheme 8 shows how derivatives of benzylidene malononitrile (d) from benzaldehyde (R = H, −CN, −OMe) (a) and malononitrile (b) in DMF (c) with LiBr were synthesized. LiBr catalyzed the reaction by rearranging a dispersion phase of benzaldehyde (R = H, −CN, −OMe) with malononitrile in DMF to induce favorable collisions.
Scheme 8. Proposed Mechanism for the Formation of SIQPs and Explaining the Role of LiBr and l-Proline.
4.7. Synthesis of SIQPs
2-Benzylidene malononitrile (1.0 mmol, 1.0 equiv, 154.5 g) (Scheme 9) (Table S5), 2-(4-cyanobenzylidene) malononitrile (1.0 mmol, 1.0 equiv, 131.1 g) (Scheme 10) (Table S6), and 2-(4-methoxybenzylidene) (Scheme 11) (Table S7) were separately added to NIQ (1.0 mmol, 1.0 equiv, 277 g) and l-proline (1.0 mmol, 1.0 equiv, 126.5 g) in ACN (10 mL) solvent. Reacting mixtures were stirred and refluxed for 3 h. The reaction was monitored by TLC on aluminum plates coated with silica gel-G F254 (ACME) with 0.25 mm thickness and visualized with UV–vis light and iodine. Excess solvent was removed by the rota evaporator. The crude product was washed with DCM and lime water (∼3 times) for purification and isolated by column chromatography (ACME, 60–120 mesh) with 70% petroleum ether + 30% ethyl acetate eluting mixture and then dried under a vacuum. Products SIQPs I, II, and III were synthesized. The condensation reactions depicted in Schemes 9–11 proceeded linearly with time, and since no undesired product was developed, they were first-order reactions. The PC undergoes the semiconductor mechanism. The MB reduction was directly proportional because h+ and e– holes are produced continuously with time until SIQPs saturate vis-à-vis the photon-receiving ability.
Scheme 9. Synthesis of 7-Nitro-2′-phenyl-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP I) (Figures S5a–g, 5a, and 6a).
Scheme 10. Synthesis of 2′-(4-Cyanophenyl)-7-nitro-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP II) (Figures S6a–g, 5b, and 6b).
Scheme 11. Synthesis of 2′-(4-Methoxyphenyl)-7-nitro-5′,6′,7′,7a′-tetrahydrospiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-1′,1′(2′H)-dicarbonitrile (SIQP III) (Figures S7a–g, 5c, and 6c).
Acknowledgments
The authors are thankful to the Central University of Gujarat, India, for infrastructural support; CSIR Bhavnagar for EDX, fluorescent, and mass spectroscopy; SNU for Raman spectroscopy; and specially thankful to Nakul Kumar, a research scholar, SCS, Central University of Gujarat.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02976.
Spectral data of 1H NMR, 13C NMR, NOESY, FT-IR, LC-MS, UV–vis, and TGA of all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Huisgen R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem., Int. Ed. 1963, 2, 565–598. 10.1002/anie.196305651. [DOI] [Google Scholar]
- Saraswat P.; Jeyabalan G.; Hassan M. Z.; Rahman M. U.; Nyola N. K. Review of Synthesis and Various Biological Activities of Spiro Heterocyclic Compounds Comprising Oxindole and Pyrrolidine Moities. Synth. Commun. 2016, 46, 1643–1664. 10.1080/00397911.2016.1211704. [DOI] [Google Scholar]
- Yang J.-M.; Hu Y.; Li Q.; Yu F.; Cao J.; Fang D.; Huang Z.-B.; Shi D.-Q. Efficient and Regioselective Synthesis of Novel Functionalized Dispiropyrrolidines and Their Cytotoxic Activities. ACS Comb. Sci. 2014, 16, 139–145. 10.1021/co400096c. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Huang Y.-X.; Sun J.; Yan C.-G. A [3+2] Cycloaddition Reaction for the Synthesis of Spiro[Indoline-3,3′-Pyrrolidines] and Evaluation of Cytotoxicity towards Cancer Cells. New J. Chem. 2019, 43, 8903–8910. 10.1039/C9NJ00994A. [DOI] [Google Scholar]
- Shahrestani N.; Salahi F.; Tavakoli N.; Jadidi K.; Hamzehloueian M.; Notash B. Asymmetric Synthesis Approach of Enantiomerically Pure Spiro-Indenoquinoxaline Pyrrolidines and Spiro-Indenoquinoxaline Pyrrolizidines. Tetrahedron: Asymmetry 2015, 26, 1117–1129. 10.1016/j.tetasy.2015.08.013. [DOI] [Google Scholar]
- Pattanaik P.; Nayak S.; Ranjan Mishra D.; Panda P.; Prasad Raiguru B.; Priyadarsini Mishra N.; Mohapatra S.; Arjunreddy Mallampudi N.; Purohit C. S. One Pot, Three Component 1,3 Dipolar Cycloaddition: Regio and Diastereoselective Synthesis of Spiropyrrolidinyl Indenoquinoxaline Derivatives. Tetrahedron Lett. 2018, 59, 2688–2694. 10.1016/j.tetlet.2018.05.087. [DOI] [Google Scholar]
- Shaabanzadeh M.; Khabari F. One-Pot Diastereoselective Synthesis of New Spiro Indenoquinoxaline Derivatives Containing Cyclopropane Ring. Arkivoc 2009, 2009, 307. 10.3998/ark.5550190.0010.b28. [DOI] [Google Scholar]
- Saragi T. P. I.; Spehr T.; Siebert A.; Fuhrmann-Lieker T.; Salbeck J. Spiro Compounds for Organic Optoelectronics. Chem. Rev. 2007, 107, 1011–1065. 10.1021/cr0501341. [DOI] [PubMed] [Google Scholar]
- Mahns B.; Roth F.; Grobosch M.; Lindner S.; Knupfer M.; Saragi T.; Reichert T.; Salbeck J.; Hahn T. Electronic Properties of Spiro Compounds for Organic Electronics. J. Chem. Phys. 2012, 136, 124702 10.1063/1.3698280. [DOI] [PubMed] [Google Scholar]
- Balewski Ł.; Sączewski F.; Gdaniec M.; Kornicka A.; Cicha K.; Jalińska A. Synthesis and Fluorescent Properties of Novel Isoquinoline Derivatives. Molecules 2019, 24, 4070 10.3390/molecules24224070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Fu Q. Y.; Gao G.; He H. Y.; Zhang Y.; Wu Y. S.; Zhang Z. H. Catalyst-Free, Visible-Light Promoted One-Pot Synthesis of Spirooxindole-Pyran Derivatives in Aqueous Ethyl Lactate. ACS Sustainable Chem. Eng. 2017, 5, 6175–6182. 10.1021/acssuschemeng.7b01102. [DOI] [Google Scholar]
- Kong D.-l.; Lu G.-p.; Wu M.-s.; Shi Z.-f.; Lin Q. One-Pot, Catalyst-Free Synthesis of Spiro[Dihydroquinoline-Naphthofuranone] Compounds from Isatins in Water Triggered by Hydrogen Bonding Effects. ACS Sustainable Chem. Eng. 2017, 5, 3465–3470. 10.1021/acssuschemeng.7b00145. [DOI] [Google Scholar]
- Xie L. Y.; Duan Y.; Lu L. H.; Li Y. J.; Peng S.; Wu C.; Liu K. J.; Wang Z.; He W. M. Fast, Base-Free and Aqueous Synthesis of Quinolin-2(1H)-Ones under Ambient Conditions. ACS Sustainable Chem. Eng. 2017, 5, 10407–10412. 10.1021/acssuschemeng.7b02442. [DOI] [Google Scholar]
- Suresh A.; Baiju T. V.; Kumar T.; Namboothiri I. N. N. Synthesis of Spiro- and Fused Heterocycles via (4+4) Annulation of Sulfonylphthalide with o-Hydroxystyrenyl Derivatives. J. Org. Chem. 2019, 84, 3158–3168. 10.1021/acs.joc.8b03039. [DOI] [PubMed] [Google Scholar]
- Barkov A. Y.; Zimnitskiy N. S.; Korotaev V. Y.; Kutyashev I. B.; Moshkin V. S.; Sosnovskikh V. Y. Regio- and Stereoselective 1,3-Dipolar Cycloaddition of Indenoquinoxalinone Azomethine Ylides to β-Nitrostyrenes: Synthesis of Spiro[Indeno[1,2-b]Quinoxaline-11,3′-Pyrrolizidines] and Spiro[Indeno[1,2-b]Quinoxaline-11,2′-Pyrrolidines]. Chem. Heterocycl. Compd. 2017, 53, 451–459. 10.1007/s10593-017-2074-0. [DOI] [Google Scholar]
- Zheng Y.; Tice C. M.; Singh S. B. The Use of Spirocyclic Scaffolds in Drug Discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- Pardasani R. T.; Pardasani P.; Chaturvedi V.; Yadav S. K.; Saxena A.; Sharma I. Theoretical and Synthetic Approach to Novel Spiroheterocycles Derived from Isatin Derivatives and L-Proline via 1,3-Dipolar Cycloaddition. Heteroat. Chem. 2003, 14, 36. 10.1002/hc.10063. [DOI] [Google Scholar]
- Galliford C. V.; Scheidt K. A. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]
- Han Y.; Wu Q.; Sun J.; Yan C. G. Synthesis of the Functionalized Spiro[Indoline-3,5′-Pyrroline]-2, 2′-Diones via Three-Component Reactions of Arylamines, Acetylenedicarboxylates, and Isatins. Tetrahedron 2012, 68, 8539–8544. 10.1016/j.tet.2012.08.030. [DOI] [Google Scholar]
- Shahrestani N.; Salahi F.; Tavakoli N.; Jadidi K.; Hamzehloueian M.; Notash B. Asymmetric Synthesis Approach of Enantiomerically Pure Spiro-Indenoquinoxaline Pyrrolidines and Spiro-Indenoquinoxaline Pyrrolizidines. Tetrahedron: Asymmetry 2015, 26, 1117–1129. 10.1016/j.tetasy.2015.08.013. [DOI] [Google Scholar]
- Gavaskar D.; Suresh Babu A. R.; Raghunathan R.; Dharani M.; Balasubramanian S. An Expedient Sequential One-Pot Four Component Synthesis of Novel Steroidal Spiro-Pyrrolidine Heterocycles in Ionic Liquid. Steroids 2016, 109, 1–6. 10.1016/j.steroids.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Inwati G. K.; Rao Y.; Singh M. In Situ Growth of Low-Dimensional Silver Nanoclusters with Their Tunable Plasmonic and Thermodynamic Behavior. ACS Omega 2017, 2, 5748–5758. 10.1021/acsomega.7b00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. D.; Dang V. S.; Nguyen V. H.; Nguyen T. M. T.; Dang C. H. Synthesis and Photophysical Characterization of Several 2,3-Quinoxaline Derivatives: An Application of Pd(0)/PEG Nanoparticle Catalyst for Sonogashira Coupling. Polycyclic Aromat. Compd. 2018, 38, 42–50. 10.1080/10406638.2016.1143848. [DOI] [Google Scholar]
- Singh M. Extra Elements Detection in Organic Compounds by Nonbreakable Sodium Ignition Apparatus (NOSIA). Green Chem. Lett. Rev. 2015, 8, 1–7. 10.1080/17518253.2014.969330. [DOI] [Google Scholar]
- Dev S.; Singh M. Metallic Sulfide Nanoparticles Anchored Graphene Oxide: Synthesis, Characterization and Reduction of Methylene Blue to Leuco Methylene Blue in Aqueous Mixtures. J. Phys. Chem. Solids 2020, 139, 109335 10.1016/j.jpcs.2020.109335. [DOI] [Google Scholar]
- Naikwade A. G.; Jagadale M. B.; Kale D. P.; Gophane A. D.; Garadkar K. M.; Rashinkar G. S. Photocatalytic Degradation of Methyl Orange by Magnetically Retrievable Supported Ionic Liquid Phase Photocatalyst. ACS Omega 2020, 5, 131–144. 10.1021/acsomega.9b02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saju J.; Balasundaram O. N. Optimization and Characterization of NiO Thin Films Prepared via NSP Technique and Its P-N Junction Diode Application. Mater. Sci. 2019, 37, 338–346. 10.2478/msp-2019-0049. [DOI] [Google Scholar]
- Bryan M. C.; Dunn P. J.; Entwistle D.; Gallou F.; Koenig S. G.; Hayler J. D.; Hickey M. R.; Hughes S.; Kopach M. E.; Moine G.; et al. Key Green Chemistry Research Areas from a Pharmaceutical Manufacturers’ Perspective Revisited. Green Chem. 2018, 20, 5082–5103. 10.1039/C8GC01276H. [DOI] [Google Scholar]
- Jin L.; Liang F. A Facile One-Pot Construction of Succinimide-Fused Spiro[Pyrrolidine-2,3′-Oxindoles] via 1,3-Dipolar Cycloaddition Involving 3-Amino Oxindoles and Maleimides. Molecules 2018, 23, 582 10.3390/molecules23030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.