Abstract
Lenalidomide is efficient in reducing red blood cell transfusion dependency and markedly lowering platelet counts in MDS/MPN‐RS‐T in the context of major platelet counts.
Keywords: lenalidomide, myelodysplastic syndrome, myelodysplastic/myeloproliferative neoplasms with ring sideroblasts and thrombosis, myeloproliferative syndrome, thrombocytosis
Lenalidomide is efficient in reducing red blood cell transfusion dependency and markedly lowering platelet counts in MDS/MPN‐RS‐T in the context of major platelet counts.

1. INTRODUCTION
Myelodysplastic/myeloproliferative neoplasms (MDS/MPN) with ring sideroblasts and thrombosis (MDS/MPN‐RS‐T), previously known as refractory anemia with ring sideroblasts and thrombocytosis (RARS‐T), are rare overlapping syndromes associating the dysplastic features of myelodysplastic syndromes with ring sideroblasts (MDS‐RS, previously known as refractory anemia with ring sideroblasts) and the myeloproliferative features of essential thrombocythemia (ET). 1 MDS/MPN‐RS‐T present with clinical, biological, and prognostic features that differ from those of MDS‐RS and ET. 2 Moreover, MDS/MPN‐RS‐T are characterized by a particular mutational pattern associating: (a) genomic abnormalities responsible for the myeloproliferative part, such as JAK2V617F mutations (40%‐50% of cases) or less frequently mutations in exon 10 of MPL (myeloproliferative leukemia) or in exon 9 of CALR (calreticulin) and (b) a high rate of splicing factor 3B subunit 1 (SF3B1) mutations, responsible for the myelodysplastic component of the disease. 2 , 3 , 4 , 5 , 6 Thus, MDS/MPN‐RS‐T is now considered as an independent entity. 1
The risk of thrombosis is higher in MDS/MPN‐RS‐T than in MDS‐RS patients without a high platelet count and this thrombotic risk often leads clinicians to use cytoreductive agents known to reduce the platelet count in almost 33% of cases. 2 However, the use of cytoreductive agents is frequently interrupted, due to the worsening of cytopenia, especially anemia. The management and treatment of this disease are currently based on thrombosis risk stratification. When platelet count is <1000 × 109/L, Patnaik and Tefferi 7 recommend stratifying patients according to two thrombosis risk factors: age > 60 years and prior arterial or venous thrombosis. Patients with no risk factors should be treated with low‐dose aspirin or observation alone in JAK2V617F‐negative diseases with absence of thrombotic risk factors. Patients with 1 or 2 risk factors should be treated with low‐dose aspirin. Cytoreductive therapy with hydroxyurea is only recommended in case of high thrombotic risk. Lenalidomide is an immunomodulatory agent frequently used in low‐risk myelodysplastic syndromes. 8 In this context, it is considered as third‐line treatment, in case of hydroxyurea failure associated with anemia. In case of a high platelet count >1000 × 109/L, aspirin may exacerbate bleeding, cytoreductive therapy often worsens anemia and lenalidomide use is not suggested. Lenalidomide has been tested in published MDS/MPN‐RS‐T cases, with conflicting results. 9 , 10 , 11 , 12 , 13 , 14 , 15 Here, we report our experience using lenalidomide on two patients with JAK2 V617F‐negative MDS/MPN‐RS‐T, one of them presenting with a major thrombocytosis.
2. CASES HISTORY
The first patient was a 78‐year‐old woman (Patient 1, Table 1), with a complete blood count (CBC) showing hemoglobin level at 85 g/L, persistent thrombocytosis (platelet count: 743 × 109/L) and a leukocyte count at 6.4 × 109/L. The bone marrow aspirates revealed erythroid hyperplasia with myelodysplastic features and 64% ring sideroblasts, no blast cells, associated with atypical megakaryocytes, leading to the diagnosis of MDS/MPN‐RS‐T, according to the revised 2016 world health organization classification's criteria. 1 Bone marrow cytogenetics showed a normal 46XX karyotype. A Lys700Glu SF3B1 mutation was noted, without JAK2 V617F, MPL exon 10 or CALR mutations. Blood transfusions were performed for one year but, in order to avoid relying on red blood cell transfusions, a treatment with lenalidomide (5 mg daily, 21/28 days) was started resulting in the decrease of platelet count from 686 × 109/L (start of treatment) to 150 × 109/L, associated with an improvement in hemoglobin levels from 80 to 100 g/L over the first 28 weeks of lenalidomide treatment. Along these 28 weeks after the beginning of lenalidomide, only 6 red blood cells (RBC) units were transfused (Figure 1A), and no transfusion was required in the subsequent 47 weeks since the hemoglobin level was above 90 g/L. However, due to the subsequent decrease in the hemoglobin level, RBC transfusions were later reinitiated, but with a lower frequency (2 units of RBC every 2 months), in association with lenalidomide. In other words, over the 20 months of treatment with lenalidomide, the RBC requirements were drastically reduced. However, grade IV neutropenia was observed (granulocytes: 0.5 × 109/L), without any infectious disease, but leading to treatment stop.
Table 1.
Biological characteristics at diagnosis of the two MDS/MPN‐RS‐T patients treated with lenalidomide
| Patient 1 | Patient 2 | |
|---|---|---|
| Age (y) | 78 | 58 |
| Sex | F | F |
| Hb level (g/L) | 85 | 114 |
| MCV (fL) | 92 | 97 |
| Platelet count (109/L) | 743 | 710 |
| Leukocytes (109/L) | 6.4 | 7.4 |
| Ring sideroblasts (%) | 64 | 24 |
| Erythroid dysplasia | Yes | Yes |
| Megakaryocytic dysplasia | Marked | Marked |
| Excess of blasts | No | No |
| Karyotype | Normal | Normal |
| SF3B1 | Mutated | N/A |
| JAK2V617F | No | No |
| MPLW515K/L | No | N/A |
| CALR | Unmutated | N/A |
| First treatment | RBC transfusions | Watch and wait |
| Evolution after 1st treatment | Increased transfusion dependency | Marked increase in the platelet count: 2000 × 109/L |
| Second treatment | Lenalidomide 5 mg daily 21 d/28 | Hydroxyurea 500 then 1000 mg/d |
| Evolution after 2nd treatment |
Normal platelet count Hb increase to 100 g/L Transfusion frequency reduced |
Adverse effects on hemoglobin levels. Irregular elevated platelet counts ranging from 1700 × 109/L to 3622 × 109/L. The decision to stop hydroxyurea and to start lenalidomide was made after a new increase in platelet count at 3106 × 109/L. EPO was maintained once a week. |
| Third treatment | Not applicable | Lenalidomide 5 then 10 mg daily 21 d/28 |
| Evolution after 3rd treatment | Not applicable |
Major decrease in platelet count Subnormal Hb levels: 118 g/L EPO every 2 wk |
Abbreviations: EPO, erythropoietin; F, female; Hb, hemoglobin; M, male; MCV, mean corpuscular volume; N, normal; N/A, nonavailable; RBC, red blood cells; y, years.
Figure 1.
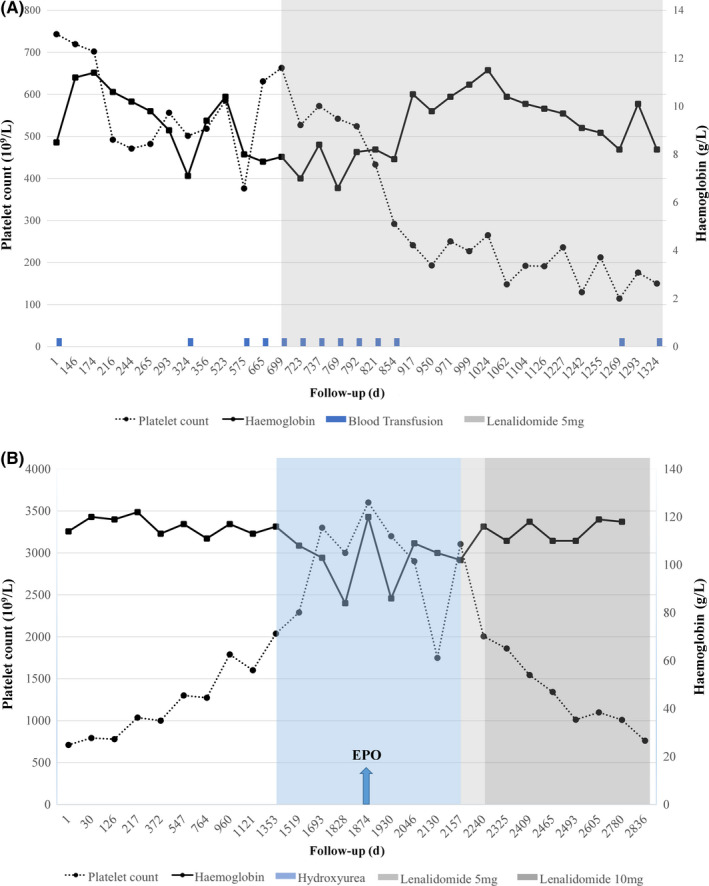
Course of blood counts for the two patients treated with lenalidomide: A, Patient 1; B, Patient 2. The left y‐axis represents the platelet counts (109/L), and the right y‐axis represents hemoglobin level (g/L). The x‐axis represents the follow‐up (in days)
The second patient was a 58‐year‐old woman (Patient 2, Table 1) whose initial CBC showed hemoglobin 114 g/L, platelet count 710 × 109/L, leukocytes 7.4 × 109/L. Bone marrow aspirate showed erythroid hyperplasia with myelodysplastic features, no excess of blasts and 24% ring sideroblasts associated with atypical megakaryocytes, leading to the diagnosis of MDS/MPN‐RS‐T. 1 Bone marrow cytogenetics showed a normal karyotype. A watch‐and‐wait strategy was initiated, but due to a marked increase in the platelet count > 2000 × 109/L, hydroxyurea (500 mg/d and then 1000 mg/d) was started. It worsened the anemia to 108 g/L and then to 84 g/L, leading to a weekly use of erythropoietin. After 18 months, hydroxyurea was stopped due to (a) its adverse effects on hemoglobin level and (b) the observation of irregular elevated platelet counts ranging from 1700 × 109/L to 3622 × 109/L. After a drop of platelet count at 1700 × 109/L, a new increase was observed at 3106 × 109/L leading to the start of a lenalidomide treatment (5 mg daily, 21/28 days), secondarily increased to 10 mg daily (21/28 days) since very well tolerated. A marked decrease in the platelet count from 3106 to 760 × 109/L was noted, while hemoglobin level raised up from 102 to 118 g/L (Figure 1B). However, for this patient, erythropoietin treatment was maintained every 2 weeks in combination with lenalidomide. Four years after the beginning of lenalidomide therapy, platelet count remains stable around 750 × 109/L. Neither adverse effects, nor thrombosis or bleeding occurred.
Informed consent for publication was obtained from both patients.
3. DISCUSSION
In conclusion, this two‐case experiment with more than 3‐year follow‐up shows the efficacy of lenalidomide in normalizing (Patient 1) or markedly reducing (Patient 2) the platelet count and allowing independency from RBC transfusion in MDS/MPN‐RS‐T. To our best knowledge, ten MDS/MPN‐RS‐T cases have been published so far (Table 2). 9 , 10 , 11 , 12 , 13 , 14 , 15 The efficacy of lenalidomide was constant in early stages, except for one case of advanced disease with rapid evolution in myelofibrosis and bone marrow failure. 10 Efficacy of lenalidomide in reducing platelet count has been observed in 3 published cases (Table 2, patients 1, 7, and 10), 9 , 14 , 15 in the context of moderately elevated platelet counts.
Table 2.
Summary of data of the literature regarding MDS/MPN‐RS‐T case reports
| Patient | Age (y) | Gender | Hb (g/L) | MCV (fL) | Platelet count (109/L) | Leukocyte count (109/L) | Ring sideroblasts (%) | Erythroid dysplasia | Megakaryocytic dysplasia | Karyotype | JAK2V617F | MPLW515K/L | CALR | SF3B1 | First treatment | Evolution after first treatment | Second treatment | Evolution after second treatment | Third treatment | Evolution after third treatment | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 81 | F | 79 | 108 | 1677 | 10 | 86 | Yes | Atypical megakaryocytes | Normal |
Yes (qPCR) 19% load |
N/A | N/A | N/A | EPO |
EPO only temporally successful After 2 y: chronic pulmonary embolism |
Hydroxyurea 500 mg 3×/d 2 wk |
Reduced platelet count Transfusion dependency |
Lenalidomide 5 mg daily |
Platelets: 100 × 109/L Transfusion independent Hb almost normalized JAK2V617F burden 0.8% |
9 |
| 2 | 60 | M | 69 | 88 | 1592 | 12.7 | 98 | Yes | Marked Hyperlobulated nuclei | Normal |
Yes (qPCR) 32,3% load |
N/A | N/A | N/A |
Pyridoxine Anabolic steroids |
Transfusion need |
Lenalidomide 10 mg daily |
3 RBC in 6 mo Plt: 680 × 109/L JAK2V617F burden unchanged |
N/A | N/A | 9 |
| 3 | 47 | F | 112 | N/A | 700 | N/A | 25‐45 | Yes | Normal | Yes | N/A | N/A | N/A | Hydroxyurea |
Minor reduction of spleen size Worsening of anemia, RBC transfusion |
Lenalidomide 10 mg daily 21 d/28 |
Pancytopenia, Increased transfusion requirement Grade 3 BM fibrosis |
Allogeneic SCT |
Graft lost Transfusion dependency Clonal evolution Death due to sepsis |
10 | |
| 4 | 84 | F | 77 | N | 1515 | N/A | 90 | Yes |
Numerous atypical megakaryocytes Hypolobulated nuclei |
5q‐ (1 mitosis) Not confirmed by FISH |
Yes 22% load |
N/A | N/A | Unmutated |
Transfusion Lenalidomide 10 mg daily |
Platelets: 281 × 109/L Transfusion independency BM normalization JAK2V617F burden <2% |
N/A | N/A | N/A | N/A | 11 |
| 5 | 39 | F | 82 | 122 | 1024 | 5.66 | 44 | Yes | Highly atypical megakaryocytes Hyperlobulated nuclei | t(2;3)(p23;q29) |
No (RT‐qPCR) |
No | N/A | N/A |
Hydroxyurea 1000 mg/d α‐interferon 3 M units 2×/wk |
Reduced platelet count but worse anemia Transfusion dependence (4 units RBC/4 wk) |
Hydroxyurea 500 mg 1×/d Pyridoxine Steroids EPO |
Failure |
Lenalidomide 5 mg daily 7 mo |
Platelet drop 363 × 109/L Hemoglobin: 90 g/L Transfusion independent Normal BM |
12 |
| 6 | 58 | M | 98 | N/A | 1163 | N/A | 30 | Yes | Large hyperlobulated nuclei |
Normal FISH neg. |
Yes (AS‐PCR) |
N/A | N/A | N/A |
Hydroxyurea 500 mg daily |
Mild decrease of hemoglobin 83 g/L without efficacy |
+ steroids EPO 40 000 units weekly |
Inefficacy Transfusion dependency |
Lenalidomide 10 mg daily 21 d/28 |
Transfusion independent; Hb > 9 g/dL Plt < 600 × 109/L |
13 |
| 7 | 68 | F | 61 | N/A | 1257 | N/A | Positive | Yes | Megakaryocytic hyperplasia | Normal | No | No | Mutated | Mutated |
Transfusion Iron supplementation |
Symptoms improvement Thrombocytosis persistence Transfusion dependency |
Lenalidomide 10 mg daily |
Platelets: 497 × 109/L Lenalidomide stopped because of severe nausea and anorexia. Platelets: 856 × 109/L |
Lenalidomide 5 mg daily |
No toxicity Platelets: 351 × 109/L Hemoglobin: 133 g/L No adverse events |
14 |
| 8 | 49 | M | 107 | 93 | 935 | 9.2 | 65 | Yes | Yes | Normal | Yes, allele burden 74% | No | No | Yes, allele burden 46% |
Lenalidomide 10 mg daily |
Decrease of platelet count to 585 × 109/L. Stop lenalidomide after 8 mo due to loss of response |
N/A | N/A | N/A | N/A | 15 |
| 9 | 73 | M | 67 | 93.8 | 669 | 7.7 | 25 | Yes | Yes | Normal | Yes, allele burden 40% | No | No | Yes, allele burden 25% | EPO | Transfusion dependency |
Lenalidomide 10 mg daily |
Transfusion independency Platelet count lowered to 470 × 109/L Stop after 17 mo due to loss of response |
N/A | N/A | 15 |
| 10 | 85 | F | 68 | 88 | 1203 | 3 | 45 | N/A | N/A | Normal | No | No | No | Yes, allele burden 44% | EPO, hydroxyurea, anagrelide |
Transfusion dependency Suboptimal response |
Lenalidomide 5 mg daily |
Platelet count decrease: 558 × 109/L Transfusion dependence |
N/A | N/A | 15 |
| 11 | 78 | F | 85 | 92 | 743 | 6.4 | 64 | Yes | Marked | Normal | No | No | Unmutated | Mutated | Transfusion 1 y | Increased transfusion dependency |
Lenalidomide 5 mg daily 21 d/28 |
Platelets: 150 × 109/L Hb:80‐100 g/L – RBC requirement drastically reduced |
N/A | N/A | Current work |
| 12 | 58 | F | 114 | 97 | 710 | 7.4 | 24 | Yes | Marked | Normal | No | N/A | N/A | N/A | Watch and wait | Marked increase in the platelet count: 2000 × 109/L |
Hydroxyurea 500 mg/d |
Platelet count 3106 × 109/L Hemoglobin: 84 g/L EPO 1×/wk Stop hydroxyurea |
Lenalidomide 5 then 10 mg daily 21 d/28 |
Platelets: 760 × 109/L Hemoglobin: 118 g/L EPO maintained every 2 wk |
Current work |
Abbreviations: AS‐PCR, allele‐specific polymerase chain reaction; BM, bone marrow; EPO, erythropoietin; F, female; Hb, hemoglobin; M, male; MCV, mean corpuscular volume; N, normal; N/A, non‐available; Plt, platelets; RBC, red blood cells; SCT, stem cell transplantation; y, years.
Our second case is striking, since it shows that lenalidomide induced a significant decrease in platelet count, even starting from very high counts (up to 3106 × 109/L), along with an increase in hemoglobin level. So far, the efficacy of lenalidomide has never been shown in MDS/MPN‐RS‐T with such a high platelet count. It highlights the interest of lenalidomide as an alternative treatment for MDS/MPN‐RS‐T, including when they present with a major platelet count. Prospective trials are still needed to confirm those encouraging results, but because of rarity of the disease, such trials are very difficult to perform.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
DM: collected data on clinical and biological parameters, analyzed data, produced the figure, and wrote the manuscript. PA: collected data on clinical and biological parameters. SM: collected data on clinical and biological parameters. VL: collected data on clinical and biological parameters. FP: collected data on clinical and biological parameters. G‐BA: collected data on clinical and biological parameters. GF: analyzed data, collected data on clinical and biological parameters, and wrote the manuscript. BJ: analyzed data, collected data on clinical and biological parameters, and wrote the manuscript.
ACKNOWLEDGMENTS
The authors thank the association “Tulipes contre le Cancer Lions Club Chalon” for the support. Published with written consent of the patient.
Divoux M, Plocque A, Sevin M, et al. Efficacy of lenalidomide in myelodysplastic/myeloproliferative neoplasms with ring sideroblasts and an extreme platelet count. Clin Case Rep. 2020;8:1774–1780. 10.1002/ccr3.3026
Francois Girodon and Julien Broséus participated equally to this work.
Contributor Information
Francois Girodon, Email: francois.girodon@chu-dijon.fr.
Julien Broséus, Email: Julien.broseus@univ-lorraine.fr.
REFERENCES
- 1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391‐2405. [DOI] [PubMed] [Google Scholar]
- 2. Broseus J, Florensa L, Zipperer E, et al. Clinical features and course of refractory anemia with ring sideroblasts associated with marked thrombocytosis. Haematologica. 2012;97(7):1036‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeromin S, Haferlach T, Weissmann S, et al. Refractory anemia with ring sideroblasts and marked thrombocytosis cases harbor mutations in SF3B1 or other spliceosome genes accompanied by JAK2V617F and ASXL1 mutations. Haematologica. 2015;100(4):125‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broséus J, Lippert E, Harutyunyan AS, et al. Low rate of calreticulin mutations in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2014;28(6):1374‐1376. [DOI] [PubMed] [Google Scholar]
- 5. Patnaik MM, Lasho TL, Finke CM, et al. Predictors of survival in refractory anemia with ring sideroblasts and thrombocytosis (RARS‐T) and the role of next‐generation sequencing. Am J Hematol. 2016;91(5):492‐498. [DOI] [PubMed] [Google Scholar]
- 6. Broséus J, Alpermann T, Wulfert M, et al. MPN and MPNr‐EuroNet (COST Action BM0902). Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2013;27(9):1826‐1831. [DOI] [PubMed] [Google Scholar]
- 7. Patnaik MM, Tefferi A. Refractory anemia with ring sideroblasts (RARS) and RARS with thrombocytosis (RARS‐T): 2017 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2017;92(3):297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. List A, Kurtin S, Roe DJ, et al. Efficacy of Lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549‐557. [DOI] [PubMed] [Google Scholar]
- 9. Huls G, Mulder AB, Rosati S, et al. Efficacy of single‐agent Lenalidomide in patients with JAK2 (V617F) mutated refractory anemia with ring sideroblasts and thrombocytosis. Blood. 2010;116(2):180‐182. [DOI] [PubMed] [Google Scholar]
- 10. Taylor G, Culligan D, Vickers MA. Refractory anemia with ring sideroblasts associated with marked thrombocytosis complicated by massive splenomegaly treated with Lenalidomide resulting in resolution of splenomegaly but severe and prolonged pancytopenia. Case Rep Hematol. 2013;2013:718480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caers J, Hafraoui K, Keutgens A, et al. Haematological and molecular responses in refractory anaemia with ring sideroblasts and thrombocytosis treated with Lenalidomide. Eur J Haematol. 2014;92(2):179‐180. [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, You Y, Li X, et al. Response to Lenalidomide of a patient with t(2;3)(p23;q29) and JAK2 non‐mutated refractory anemia with ring sideroblasts and thrombocytosis. Leuk Lymphoma. 2013l;54(7):1544‐1546. [DOI] [PubMed] [Google Scholar]
- 13. Nichele I, Ruggeri M, Rodeghiero F. Effectiveness of Lenalidomide in a patient with refractory anemia with ring sideroblasts and thrombocytosis with JAK2 (V617F) mutation. Am J Hematol. 2015;90(8):E148‐E149. [DOI] [PubMed] [Google Scholar]
- 14. Keen R, Pantin J, Savage N, et al. Treatment of refractory anemia with ring sideroblasts associated with marked thrombocytosis with lenalidomide in a patient testing negative for 5q deletion and JAK2 V617F and MPL W515K/L mutations. Hematol Rep. 2016;8(4):6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicolosi M, Mudireddy M, Vallapureddy R, et al. Lenalidomide therapy in patients with myelodysplastic syndrome/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN‐RS‐T). Am J Hematol. 2018;93(1):E27‐E30. [DOI] [PubMed] [Google Scholar]


