The coronavirus disease 2019 (COVID-19) pandemic confronts the medical and public health professions with the difficult task of controlling the first and maybe the second waves of infection with limited antiviral medications and without vaccines. So, we must rely on nonpharmaceutical interventions as a mainstay of prevention. In this issue of The Journal of Pediatric Infectious Diseases, Cherry addresses the need for eye protection as one of these interventions. Fortunately, advances in infectious disease, exposure and aerosol sciences, aerobiology, and industrial hygiene put us in a far better position to understand modes of transmission and to design and implement effective controls than was possible in the past. Unfortunately, scientists in these several fields too often don’t speak the same language. In particular, the vocabulary used in exposure science and industrial hygiene to describe aerosols and their respiratory tract deposition does not correspond well to the way the terms “respiratory droplets” and “aerosols” are frequently used by the medical infectious disease community. The resulting communication difficulty hampers effective and timely collaborative efforts—at a time when we urgently need to bring together the best science from many disciplines. My purpose is to help translate so that we can come to a better and ultimately transdisciplinary understanding of the modes of respiratory infection transmission.
By definition, an aerosol is a suspension of particles in a gas. The particles can be solid, liquid, or a combination. By suspended, we usually mean that the particles have a residence time in air for more than a few seconds and are carried along with air currents. Meteorologists distinguish droplets and cloud particles (<200 µm) that remain suspended in air or evaporate before reaching the ground from drops (liquid precipitation with diameters >200 µm) and raindrops (>500 µm) [1]. Fog droplets can be as large as 20 µm or more, although most are ≤10 µm [2].
The settling velocities of particles in the range of 10 µm in still air suggest that they are suspended in the air for 5 minutes after being released from a cough at 1 meter above the floor [3]. But indoor air is not still. The upward velocity of air in the thermal plume from a human body is greater than the settling velocity of a 50-µm droplet [4]. Indoor air velocities are generally sufficient to keep particles of at least 10 µm suspended and wafted along with air currents, and particles of up to 20–30 µm can travel far from their site of release. Because particles much larger than 5 µm (whether liquid droplets or the dried residual material of a respiratory droplet) can be suspended in air and wafted on air currents, such particles are true aerosols. So, the common medical use of the term “aerosols” to mean only particles ≤5 µm is out of sync with what we know from modern aerosol physics.
In the context of a reported COVID-19 restaurant outbreak in Guangzhou [5], the apparently high air velocity due to ceiling-mounted air conditioning units likely maintained droplets and their residual solid particles with diameters well in excess of 10 µm suspended in the breathing zone of the diners. But what is the significance of aerosols composed of such relatively large particles floating through the breathing zone of unsuspecting restaurant patrons?
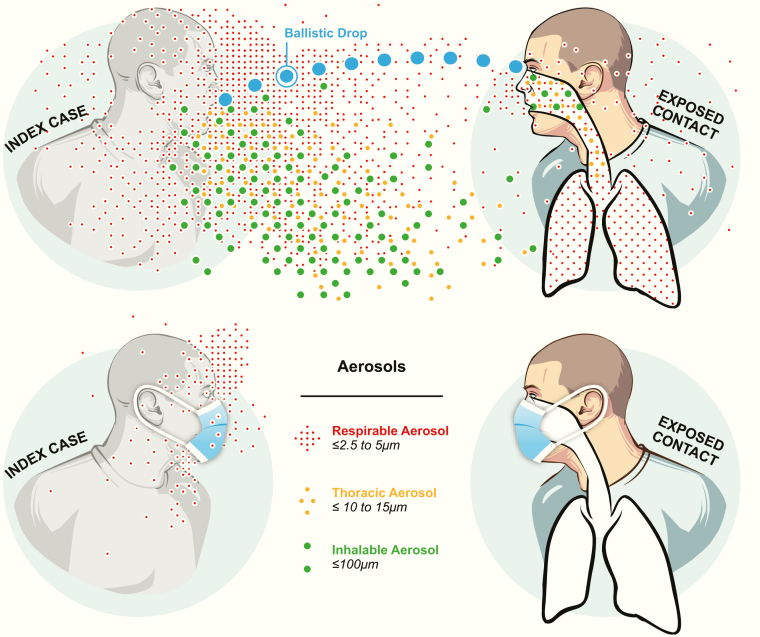
Exposure scientists and industrial hygienists classify aerosols based on where they deposit in the respiratory tract as respirable aerosols or particulate matter <2.5 μm (PM2.5); thoracic aerosols or particulate matter <10 μm (PM10); and inhalable aerosols or total suspended particulates (TSP) [6]. Respirable aerosols are defined as those particles small enough to reach the respiratory bronchioles and alveoli and include particles ≤5 µm. In the context of ambient air pollution measurement, PM2.5 is the standard metric. Thoracic aerosols are those larger particles (up to 10–15 µm) able to penetrate into the trachea and large intrathoracic airways. For ambient air pollution, the standard metric is PM10. Inhalable aerosols are the largest particles, up to about 100–200 µm, that can be aspirated into the nose. For ambient air pollution, the roughly corresponding metric is TSP.
Which aerosol fraction—respirable, thoracic, or inhalable—is important from a health perspective depends on the agent and its target tissue. In the case of Mycobacterium tuberculosis, because the target resides in the alveoli, only the respirable fraction is relevant [7]. For a virus that uses a receptor present on the surface of cells throughout the length of the respiratory tract [8], all of these aerosol fractions are likely to be important (Figure 1). In addition, because the conjunctiva are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, a spray of ballistic drops hitting the eyes or the anterior nares also poses a risk, in addition to the well-accepted risk of self-inoculation of these sites with fingers contaminated after touching surfaces.
Figure 1.
Short-range transmission potential of ballistic drops and droplet aerosols in the inhalable, thoracic, and respiratory aerosol size ranges and the impact of face masks as source control.
When considering recommendations for protection of healthcare workers who must get close to their patients in potentially highly contaminated environments, eye as well as respiratory protection is important. For the general population, physical distance will limit exposure to splash and spray and contaminated surfaces, but will be less effective at blocking aerosols of even 20- to 30-µm particles that can travel considerable distances. Face masks that block shedding of inhalable and thoracic aerosols and reduce shedding of respirable aerosols [9] can be expected to make an important contribution as source control. There is evidence that physical distance, face masks, and eye protection all contribute to reducing the spread of betacoronavirus infections [10].
As evidence accumulates for SARS-CoV-2 transmission via aerosols, engineering controls—especially ventilation and air disinfection—will be an important component of the path ahead [11, 12]. As a result, bridging the language barrier between the medical and the exposure science and engineering communities is an important task. The medical terms, established more than a century ago [13], that artificially dichotomize droplet and aerosol transmission served the profession well in banishing the idea of miasmas. Now, it is time to move on to more precise and nuanced terminology to facilitate the communication and transdisciplinary collaboration necessary to limit the damage from COVID-19 and get everyone safely back to school and work, together, again.
Notes
Acknowledgments. The author acknowledges Professors Catherine Noakes, William W. Nazaroff, Lidia Morawska, and Russell R. Dickerson for helpful discussions about aerosols, terminology, and fact checking.
Potential conflicts of interest. The author: No potential conflicts. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The author has received research support from the National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Surveillance (CEIRS) definitive contract HHSN272201400008C and the Defense Advanced Research Projects Agency (DARPA) BTO under the auspices of Col. Matthew Hepburn through agreement N66001-17-2-4023 and N66001-18-2-4015. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the funding agency and no official endorsement should be inferred.
References
- 1. American Meteorological Society. Droplet. Meteorology glossary.2012. Available at: http://glossary.ametsoc.org/wiki/Droplet. Accessed June 17, 2020.
- 2. Podzimek J. Droplet concentration and size distribution in haze and fog. Stud Geophys Geod 1997; 41:277–96. [Google Scholar]
- 3. Hinds WC. Aerosol technology: properties, behavior, and measurement of airborne particles. 2nd ed. New York: Wiley; 1999. [Google Scholar]
- 4. Gena AW, Voelker C, Settles GS. Qualitative and quantitative schlieren optical measurement of the human thermal plume. Indoor Air 2020; 30:757–66. [DOI] [PubMed] [Google Scholar]
- 5. Lu J, Gu J, Li K, et al. COVID-19 Outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis 2020; 26:1628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volkwein JC, Maynard AD, Harper M. Workplace aerosol measurement. In: Kulkarni P, Baron PA, Willeke K, eds. Aerosol measurement. Hoboken, NJ: John Wiley & Sons, Inc, 2011:571–90. [Google Scholar]
- 7. Wells WF. Airborne contagion and air hygiene: an ecological study of droplet infection. Cambridge, MA: Harvard University Press, 1955. [Google Scholar]
- 8. Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract [manuscript published online ahead of print 27 May 2020]. Cell 2020. doi:10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu DK, Akl EA, Duda S, et al. ; COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395:1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 2020; 142:105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nardell EA, Nathavitharana RR. Airborne spread of SARS-CoV-2 and a potential role for air disinfection [Published online June 1, 2020]. JAMA 2020. doi: 10.1001/jama.2020.7603 [DOI] [PubMed] [Google Scholar]
- 13. Chapin CV. The sources and modes of infection. 1st ed. New York: J. Wiley & Sons, 1910. Available at: https://lccn.loc.gov/12021189. Accessed May 25, 2020. [Google Scholar]