Abstract
Data from 1174 infants enrolled in a previous rotavirus vaccine study were analyzed to determine the effect of antibiotic exposure (from 14 days before to 7 days after vaccination) on rotavirus serum immunoglobulin A (IgA) responses. Serum IgA responses 1 month after the completion of vaccination were similar among antibiotic-exposed and nonexposed infants.
Keywords: antibiotic, microbiome, pediatric, rotavirus, serology
Standard-of-care antibiotics that were administered in a window of 14 days before to 7 days after rotavirus vaccination did not affect the serologic response observed after completion of rotavirus vaccination.
The introduction of 2 live attenuated oral rotavirus vaccines in the United States, RotaTeq (RV5 [Merck, Kenilworth, New Jersey]) and Rotarix (RV1 [GlaxoSmithKline, Philadelphia, Pennsylvania]), led to a significant decline in rotavirus disease in the United States across all ages [1, 2]. The immune response to and effectiveness of the vaccine in low- to middle-income countries have been less robust, and a proposed potential factor is the variability in the enteric microbiome, which can be altered by the frequent use of antibiotics [3]. Earlier studies in animal models and limited human data suggest that antibiotic treatment can affect fecal shedding of rotavirus and the immune response to rotavirus vaccine [4–6]. Overall, the effect of antibiotics on the immunologic response to rotavirus vaccination remains unclear, so we retrospectively analyzed serologic data from a large phase 4 rotavirus vaccine study in which a subset of infants received antibiotics and evaluated whether the receipt of antibiotics affected their serologic responses [4].
METHODS
We analyzed data from a randomized multicenter rotavirus vaccine study that examined the safety and noninferiority of mixed schedules of 2 licensed rotavirus vaccines (RV5 and RV1) compared with those of each vaccine administered as recommended [4]. Healthy infants between 6 and <15 weeks of age at first vaccination were enrolled and randomly assigned to 1 of the following 5 groups: group 1 (RV5-RV5-RV5), group 2 (RV5-RV1-RV1), group 3 (RV5-RV5-RV1), group 4 (RV1-RV1), or group 5 (RV1-RV5-RV5). Concomitant medication administration (including antibiotic use) was documented for each subject. Infants were defined as having had antibiotic exposure if the reported start or stop date of the antibiotic was within 14 days before to 7 days after each rotavirus vaccine dose. If an infant did not receive antibiotics within the defined window, he or she was classified as having had no antibiotic exposure. Serum samples were obtained approximately 1 month after completion of the rotavirus vaccination series. Participants who received all scheduled study vaccine doses and from whom a serum sample was obtained within the protocol-specified window were analyzed.
The primary outcome was immunoglobulin A (IgA) seroresponse (IgA concentration, ≥20 U/mL) according to an enzyme-linked immunoassay performed 1 month after the last vaccination against rotavirus strains WC3 and 89-12 [4]. The secondary outcome was neutralization titers against common rotavirus strains [Wa(G1P[8]), DS-1(G2P[4]), P(G3P[8]), ST3(G4P[6]), VA70(G4P[8]), and CCHMC-G9P6(G9P[6])].
The study population was characterized by assessing the baseline characteristics and outcomes of participants with and those without antibiotic exposure. Data were assessed for homogeneity across the groups and stratified according to antibiotic exposure to determine whether data could be pooled across groups. Differences between the vaccine schedule groups were found; thus, all outcomes were stratified according to vaccine schedule group. We examined differences in seroresponse rates and adjusted for treatment group, sex, race, ethnicity, and study site using logistic regression models. General linear models were used to assess associations between the covariates and the log titers against strains WC3 and 89-12. Additional prespecified secondary analyses included assessing the effect of antibiotic exposure on neutralizing antibody response rates and geometric mean titers (GMTs) of the most common rotavirus G and P types, the effect of antibiotic exposure timing (eg, receipt of antibiotic before or after rotavirus vaccination, antibiotic receipt in association with rotavirus vaccine dose 1, 2, or 3), and the effect of antibiotic class (eg, amoxicillin, cephalosporins) on participant seroresponses and GMTs.
RESULTS
Overall, 1393 participants were enrolled and randomly assigned to a group in the primary rotavirus study between March 2011 and September 2013 [4]. After excluding infants who did not meet the primary study eligibility criteria (n = 2), had missing serologic results (n = 86), did not receive all scheduled doses (n = 111), or received an incorrect or non–study vaccine (n = 20), the total population for this study included 1174 (84%) infants. Of these 1174 infants, 114 (10%) received antibiotics within 14 days before to 7 days after receipt of rotavirus vaccine. No differences with respect to sex, ethnicity, race, or age at enrollment were observed in those with and in those without antibiotic exposure. Infants who received the 2-dose rotavirus vaccine schedule (group 4 [RV1-RV1]) tended to have less antibiotic exposure than those in the other 4 groups (5.2% vs 9.7%–12.1%, respectively; P = .050), who received 3 doses.
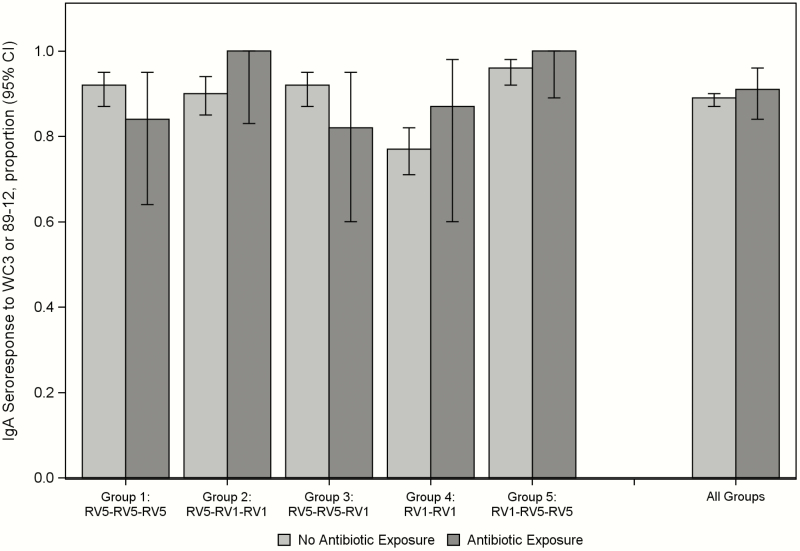
The rotavirus IgA seroresponse rates and GMTs against at least 1 vaccine antigen (WC3 and/or 89-12) 1 month after the last vaccination were similar between antibiotic-exposed and antibiotic-unexposed participants in all study groups (Table 1, Figure 1). In a multivariable logistic regression model, we found no differences in sex, race, ethnicity, study site, or antibiotic exposure (P ≥ .3 for each, model results are not shown). The only difference found was lower IgA responses in group 4, the group that received only 2 vaccine doses, which is similar to findings in the primary study (P < .0001) [4].
Table 1.
IgA GMTs Stratified According to Exposure Status
| GMT (95% CI) (U/mL) | ||||
|---|---|---|---|---|
| Treatment Arm | Antibiotic Exposureb | Na | Strain WC3 | Strain 89-12 |
| Group 1 (RV5-RV5-RV5) | Yes | 25 | 256.9 (116.6–566.4) | 45.1 (22.5–90.4) |
| No | 181 | 299.6 (232.8–385.5) | 63.5 (50.9–79.2) | |
| Group 2 (RV5-RV1-RV1) | Yes | 20 | 214.0 (114.2–401.0) | 84.0 (49.2–143.2) |
| No | 187 | 216.0 (165.2–282.5) | 119.8 (96.4–148.8) | |
| Group 3 (RV5-RV5-RV1) | Yes | 22 | 211.7 (90.2–496.8) | 77.7 (36.9–163.5) |
| No | 172 | 320.6 (247.1–416.1) | 108.0 (85.1–137.1) | |
| Group 4 (RV1-RV1) | Yes | 15 | 62.8 (30.7–128.7) | 195.1 (83.2–457.7) |
| No | 272 | 37.0 (30.7–44.7) | 96.6 (77.1–121.0) | |
| Group 5 (RV1-RV5-RV5) | Yes | 32 | 180.5 (105.9–307.7) | 205.6 (109.9–384.4) |
| No | 248 | 268.9 (221.8–326) | 213.4 (172.4–264.3) | |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; IgA, immunoglobulin A; RV1, Rotarix; RV5, RotaTeq.
aIgA assay data were missing for 2 subjects, 1 each in the WC3 and 89-12 groups.
bAntibiotic exposure was defined as receipt of an antibiotic within 14 days before to 7 days after receipt of rotavirus vaccine. No antibiotic exposure was defined as not meeting the criteria for antibiotic exposure.
Figure 1.
IgA Seroresponse to WC3 or 89-12, Stratified by Exposure Status. Abbreviations: CI, confidence interval; IgA, immunoglobulin A; RV1, Rotarix; RV5, RotaTeq.
In additional prespecified secondary analyses, we found that antibiotic exposure did not affect neutralizing antibody responses or GMTs against the most common rotavirus types (Supplemental Tables 1a and b). Furthermore, the IgA responses and GMTs did not differ according to the vaccine dose around which the antibiotic was administered (although most instances occurred around the third dose [Supplemental Table 2]), the timing of antibiotic exposure, or the antibiotic class administered, although the numbers of participants included in these subanalyses were small (Supplemental Tables 3 and 4).
DISCUSSION
The receipt of oral antibiotics in the 14 days before to 7 days after rotavirus vaccination did not affect vaccine immunogenicity in this study of 1174 infants who received RV1 and/or RV5. In the primary analysis, rotavirus-specific IgA seroresponses (IgA ≥ 20 U/mL) and GMTs against both WC3 and 89-12 were similar among infants who received and those who did not receive antibiotics (Figure 1, Table 1). Most groups achieved an IgA GMT of ≥90 U/mL (particularly against the vaccine backbone strain, WC3 or 89-12) which has correlated with rotavirus vaccine efficacy (Table 1) [5]. Similarly, antibiotic exposure did not affect serum neutralizing antibodies against common rotavirus types (Wa, DS-1, P, ST3, VA70, or CCHMC-G9P6) (Supplementary Table 1a and b). Our results did not seem to be affected by differences in infants who did and those who did not receive antibiotics, because the infants did not differ according to sex, ethnicity, race, or median age at enrollment. The number of subjects who received antibiotics in group 4 tended to be less than that in the other groups, but this result was expected because group 4 (RV1-RV1) received only 2 vaccine doses, compared with the 3 doses administered to those in the other groups. In analyses planned a priori, we observed no statistically significant differences when we analyzed the data on the basis of the vaccine dose around which the antibiotic was administered, the timing of the exposure, or the antibiotic class administered, although the numbers of participants were small for these subanalyses. Thus, we could not identify any effect of antibiotic administration around the time of rotavirus vaccination on the participants’ rotavirus IgA serologic response.
Despite the potential pathophysiological rationale for antibiotics affecting the gut microbiome, we did not observe alterations in our participants’ IgA response. Because the diversity and composition of the gut microbiome varies between children in low-income countries and those in high-income countries [6], this factor has been suggested as a potential explanation for differences observed in rotavirus effectiveness in low-income and high-income countries. A recent study of children in India receiving concomitant oral polio vaccine and azithromycin did not identify any effect of the antibiotic on serologic response to poliovirus [7]. Although it was noted in 1 study that responses to influenza and inactivated polio vaccination can be impaired by a lack of interaction between gut microbiota after antibiotic administration and Toll-like receptor 5 [8], antibiotics did not affect serologic responses in participants who received one of several alum-adjuvanted vaccines or the live-attenuated yellow fever vaccine. The increase in titers observed by 1 week after rotavirus vaccination in adults [9], the effect on the durability of serologic responses in a previous mouse study [10], and fecal rotavirus vaccine shedding could not be assessed in this study. Although we cannot address the issues raised by these previous studies, our data on the immunogenicity of rotavirus vaccine in humans exposed to antibiotics around the time of rotavirus vaccination are reassuring.
Important limitations exist in this analysis, including potential residual unmeasured confounders. Data were captured on antibiotic use 14 days before through 7 days after each vaccine dose, so antibiotic use outside this window or the selection of a narrower exposure window could have affected the results. It is possible that antibiotics blunted the immune response at an earlier time point, but no difference was observed when immunologic responses were measured after the last dose of vaccine. Furthermore, since the age of children contracting severe rotavirus infections has increased in the postvaccine era [11], early differences in seroresponses are likely to be less relevant. The immunologic responses we measured were also limited to humoral IgA and neutralizing antibody responses in serum [11]. In addition, we did not have serologic data on responses to rotavirus G12, which emerged in the United States in 2012 and is not included in either vaccine. Although this was a large study and almost 10% of the infants received antibiotics, small differences in serologic responses could have been missed. Last, serologic responses correlate with but do not completely predict the prevention of rotavirus.
Despite these important limitations, it is reassuring that we did not identify any effect of antibiotic administration on serologic responses observed after the completion of a rotavirus vaccination schedule. These data should reassure parents and healthcare providers that if antibiotics are needed for the treatment of a bacterial infection around the time of rotavirus vaccination, serologic responses will not be affected adversely. However, these findings might not generalize to infants in lower-income countries where vaccine response is worse, so studies in such a setting are needed.
Supplementary Material
These data were presented in part at IDWeek 2018, October 3–7, 2018, in San Francisco, California.
Notes
Acknowledgments. We acknowledge the valuable contributions of the 08-0017 study team that enrolled these subjects and the team at Cincinnati Children’s Hospital for conducting serologic assays.
Financial support. This work was funded by the vaccine and treatment evaluation unit (VTEU) at Vanderbilt University (grants HHSN 272200800007C and HHSN272201300023I [principal investigator, B. C.]) and Emory University (grants HHSN272200800005C and HHSN272201300018I [principal investigator, N. R.]). The network of VTEUs is supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of Interest. E. J. A. has received personal fees from AbbVie and Pfizer for consulting, and his institution receives funds to conduct clinical research from MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Novavax, Sanofi Pasteur, and Micron. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gastañaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013; 310:851–3. [DOI] [PubMed] [Google Scholar]
- 2. Anderson EJ, Shippee DB, Weinrobe MH, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis 2013; 56:755–60. [DOI] [PubMed] [Google Scholar]
- 3. Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol 2014; 35:526–37. [DOI] [PubMed] [Google Scholar]
- 4. Libster R, McNeal M, Walter EB, et al. ; VTEU Rotavirus Vaccine Study Work Group Safety and immunogenicity of sequential rotavirus vaccine schedules. Pediatrics 2016; 137:e20152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel M, Glass RI, Jiang B, et al. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284–94. [DOI] [PubMed] [Google Scholar]
- 6. Lin A, Bik EM, Costello EK, et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One 2013; 8:e53838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grassly NC, Praharaj I, Babji S, et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect Dis 2016; 16:905–14. [DOI] [PubMed] [Google Scholar]
- 8. Oh JZ, Ravindran R, Chassaing B, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014; 41:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris VC, Haak BW, Handley SA, et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host Microbe 2018; 24:197–207.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis 2014; 210:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sederdahl BK, Yi J, Jerris RC, et al. Trends in rotavirus from 2001 to 2015 in two paediatric hospitals in Atlanta, Georgia. Epidemiol Infect 2018; 146:465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.