Abstract
Background: Vast increases in life expectancy over the last century have led to shifts in population demographics and the emergence of a largely aged population, globally. This has led to a need to understand neurobiological changes associated with healthy aging. Studies on age-related changes in functional connectivity networks have largely been cross-sectional and focused on the default mode network (DMN). The current study investigated longitudinal changes in functional connectivity in multiple resting-state networks over 4 years of aging in cognitively normal older adults.
Methods: Resting-state functional magnetic resonance imaging scans from older adults (n = 16) who maintained “cognitive normal” status over 4 years were retrieved at baseline and follow-up from the Alzheimer's Disease Neuroimaging Initiative database. A seed-based approach was executed in Functional MRI of the Brain Software Library (FSL) to examine significant changes in functional connectivity within the DMN, frontoparietal network (FPN), and salience network (SN) within subjects over time.
Results: Results indicated significantly (p < 0.05, corrected) reduced functional connectivity in the FPN and SN, but not in the DMN at year 4 compared with baseline in older adults who were cognitively stable.
Conclusions: The current study highlights the importance of a longitudinal approach for understanding changes in functional connectivity. The findings also underscore the need to examine multiple networks within the same participants, given that changes were apparent in the FPN and SN but not in the DMN. Future studies should also examine changes in internetwork connectivity as well as shifts in structural connectivity over time.
Impact statement
Investigations of age-related changes in functional connectivity have largely been cross-sectional and focused on the default mode network (DMN). The current study examined the DMN as well as the frontoparietal network (FN) and salience network (SN), in a group of healthy aging adults over four years. The results revealed decreased functional connectivity over time, in the FN and SN, but not the DMN. These findings provide insights about the healthy aging brain. They also underscore the need to broaden the scope of functional connectivity analyses beyond the DMN and highlight the use of longitudinal methods.
Keywords: fMRI, functional connectivity, healthy aging, longitudinal
Introduction
Over the last century, global population demographics have shifted and an aging population has emerged. One reason for this shift is increased life expectancy, which has risen by 5.5 years between 2000 and 2016.† Given that our relative age distribution is shifting and that the majority of neuroscience research has focused on young adults, there is currently a crucial need to understand the changes that occur as individuals age. Among the most well-known changes associated with aging relate to cognitive ability (including declines in memory, executive function, and information processing speed) (Murman, 2015). To date, a wide body of literature has examined the relationships between cognitive decline and alterations in brain structure and function, many using magnetic resonance imaging (MRI) approaches.
As a noninvasive and an easily repeatable way to peer into the human brain, magnetic resonance imaging (MRI) represents an ideal method to study how the brain changes over time. In particular, developments in functional MRI (fMRI) analyses have allowed for the use of resting-state scans to better understand correlations between fluctuations in the BOLD (blood oxygen level dependent) signal between different neural regions, or functional connectivity networks.
Previous research on functional connectivity and aging has largely been cross-sectional and focused on the default mode network (DMN) in older relative to younger adults. The DMN captures regions related to mind-wandering and self-monitoring such as posterior cingulate cortex, precuneus, medial temporal lobes (MTLs), and medial prefrontal areas (Raichle, 2015). Notably, a variety of analysis approaches have been taken to examine functional connectivity networks such as the DMN, including, seed-based methods, independent component analyses, and application of graph theoretical analyses (Sala-Llonch et al., 2015). Most consistently across techniques, older adults have shown decreased functional connectivity in the DMN relative to younger adults (Damoiseaux, 2017; Dennis and Thompson, 2014; Ferreira and Busatto, 2013).
Although the DMN is the most commonly studied network, recently, several other functional connectivity networks, including the frontoparietal network (FPN) and salience network (SN) have been also been investigated in older adults. The FPN system is associated with attention shift control, cognitive control, and decision-making (Marek and Dosenbach, 2018; Vincent et al., 2008). It includes the inferior parietal cortex, ventral visual cortex, supramarginal gyrus, superior lateral occipital cortex, insula, and supplementary motor area (Dosenbach et al., 2006; Fox et al., 2005). Similarly to findings in the DMN, several studies to date have detected reduced functional connectivity in the FPN in older compared with younger adults (Andrews-Hanna et al., 2007; Huang et al., 2015; Marstaller et al., 2015; Voss et al., 2010).
The SN is anchored in the anterior insula and the dorsal anterior cingulate cortex (ACC) and contributes to a variety of complex brain functions, including communication, social behavior, and self-awareness through the integration of sensory, emotional, and cognitive information (Menon, 2015). The SN is thought to facilitate the detection of important environmental stimuli (Menon and Uddin, 2010; Seeley et al., 2007). Applying an independent component analysis approach, He et al. (2014) found decreased functional connectivity in the SN in healthy older adults compared with their younger counterparts. Likewise, a negative correlation with age of the connectivity of the bilateral insula and ACC was reported by Onoda et al. (2012). Their study has also investigated the connectivity of FPN, but no differences between groups were detected.
Few studies have examined healthy aging in multiple resting-state networks within the same set of participants over time. In a powerful approach that examined multiple networks across studies, Li et al. (2015) completed a meta-analysis of 114 task-based fMRI studies on healthy aging and examined multiple networks. They compared young and older adults and found that older adults had increased connectivity in the FPN and DMN, with the FPN showing a relationship with cognitive performance.
Primary studies that have examined more than one network within the same set of participants often included wide age ranges, also including middle-aged persons. For instance, in a large population-based study, Zonneveld et al. (2019) examined resting-state networks in 2878 persons between 50 and 95 years. The analysis revealed decreased functional connectivity in brain networks, including the anterior DMN that was most pronounced after the age of 65 years. Varangis et al. (2019) also took an important approach by examining multiple resting-state connectivity analysis methods in individuals aged 20–80 years. Their results revealed both whole-brain and network-level changes indicative of age-related decline, some of which bore a relationship with cognitive performance.
To date, changes in functional connectivity during healthy aging have mainly been inferred from cross-sectional comparisons of older versus younger adults and most studies have not compared multiple networks within the same individuals. Although such investigations often allow for larger sample sizes, a major limitation relates to the possible confounding of results owing to group differences. Longitudinal studies are needed to fully characterize and capture age-related changes in functional connectivity.
The aim of the current study was to investigate changes in functional connectivity during healthy aging, using a longitudinal approach to examine changes in multiple networks, including the DMN, FPN, and SN. To our knowledge, this is the first study to examine longitudinal changes in multiple functional connectivity networks in healthy older adults over 4 years. We hypothesized that functional connectivity would be significantly lower in each of the networks (DMN, FPN, and SN) at the 4-year follow-up compared with baseline.
Materials and Methods
Alzheimer's Disease Neuroimaging Initiative database
The data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative 2 (ADNI-2) database.‡ The ADNI was launched in 2003 as a public/private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment and early Alzheimer's disease (AD). For up-to-date information, please see the ADNI procedures manual.§
Participant selection
Selection criteria included the availability of resting-state fMRI scans as well as the corresponding structural MRI images at two time points that were 4 years apart. The current study focused entirely on healthy aging and therefore excluded individuals with subjective or mild cognitive decline and dementia. Every participant within the “cognitively normal” cohort of ADNI with resting-state fMRI (rs-fMRI) data available at an initial time point with a 4-year follow-up was included. Due to poor rs-fMRI data quality (upon individual inspection of scans for artifacts), four participants who otherwise met the criteria were excluded. The final study cohort included 16 healthy older adults with good-quality fMRI data at baseline and 4-year follow-up.
As per ADNI criteria, all participants were free of memory complaints and deemed cognitively normal based on clinical assessments by the site physician, showing an absence of significant impairment in cognitive functioning and performance of daily activities. Normal memory function was also exhibited on the Logical Memory II subscale from the revised Wechsler Memory Scale (the maximum score is 25, ≥9 for 16 years of education and above, ≥5 for 8–15 years of education, ≥3 for 0–7 years of education), a Mini-Mental State Examination score between 24 and 30 (inclusive), and a Clinical Dementia Rating of 0. For more information on group classifications, including all additional eligibility criteria, please consult the ADNI-2 procedures manual.**
All ADNI participants or their authorized representatives provided written informed consent approved by the institutional review board at each acquisition site. For the purpose of the current study, secondary use of the data was approved by the Human Research Ethics Board at the University of Victoria (Victoria, BC, Canada).
Participant demographics and behavioral analyses
Descriptive statistics were applied to calculate the mean and standard deviation of the age and education level of participants, as well as the proportion of males and females in the current study. Within-subjects t-tests were applied to investigate changes in the Mini-Mental State Examination (MMSE) scores and composite scores for executive functioning (ADNI-EF) and memory (ADNI-MEM). Composite scores were derived using data from the ADNI neuropsychological battery using item response theory methods. ADNI-EF and ADNI-MEM have been validated in published articles (Crane et al., 2012; Gibbons et al., 2012).
Image acquisition
MRI data were downloaded with permission from the ADNI. All images were acquired on 3.0 Tesla Philips MRI scanners. Whole-brain anatomical MRI scans were acquired sagittally, with a T1-weighted MPRAGE sequence, with the following parameters: a repetition time of 7 ms, an echo time of 3 ms, voxel size of 1 × 1 × 1.2 mm, and a flip angle of 9°. Functional MRI scans were obtained during an ∼7-min-long resting-state scan (with eyes open). Resting-state fMRI scans were obtained with a T2*-weighted echo-planar imaging sequence with the following parameters: a repetition time of 3000 ms, an echo time of 30 ms, 140 volumes, 48 slices, voxel size of 3.3 × 3.3 × 3.3 mm, and a flip angle of 80°.
Image preprocessing
All data obtained from the ADNI database were in DICOM format. Both the structural and functional images were converted to NIFTI format using dcm2niix in the MRIcroGL application (Li et al., 2016a). All analysis steps were performed using tools within the Functional MRI of the Brain Software Library (FSL) version 6.0.1 (Jenkinson et al., 2012).
The FEAT function was used to preprocess the data (Woolrich et al., 2001). Nonbrain tissue in the raw T1 images was removed using the automated Brain Extraction Tool (Smith, 2002), followed by visual inspection and optimization for each subject. Rigid body transformations were applied to the functional images by the use of MCFLIRT motion correction (Jenkinson et al., 2002). No spatial smoothing was applied to avoid effacing small areas of significance (Alakörkkö et al., 2017). Low-frequency artifacts were removed by high-pass temporal filtering.
Each participant's functional image was registered to his or her high-resolution structural image using the boundary-based registration algorithm (Greve and Fischl, 2009). Subsequently, registration of the structural image to standard stereotaxic space was carried out with FMRIB's linear image registration tool (Jenkinson and Smith, 2001; Jenkinson et al., 2002) using a linear 12° of freedom transformation and then further refined by applying FMRIB's non-linear image registration tool nonlinear registration of the structural image to MNI-space (Andersson et al., 2007a, 2007b).
Seed-based resting-state fMRI functional connectivity analyses
Each of three functional connectivity networks, the DMN, FPN, and SN, was examined longitudinally (baseline compared with year 4) using a seed-based approach based on anatomical hubs known from previous literature, as described below.
Selected as regions of interest were the left posterior cingulate cortex for the DMN (De Luca et al., 2006), the right inferior parietal sulcus for the FPN (Voss et al., 2010), and the right dorsal anterior cingulate for the SN (He et al., 2014). Those regions have been commonly used in the literature and have been applied to similar populations of interest. In each case, a 10 voxel (10 mm) diameter spherical region of interest (ROI) was created centered on the relevant MNI coordinates from the literature (Table 2) using the MNI 1 mm brain as a template.
Table 2.
MNI Coordinates, Peak Z Scores, and Cluster Size for the Peak Voxels That Correlated with Each Seed Region in the FPN and SN, Where Baseline Functional Connectivity Was Significantly Different at Baseline than 4-Year Follow-Up (with Reduced Connectivity Over Time)
| Brain region | MNI coordinates (x, y, z) | Z score | Cluster size (voxels) |
|---|---|---|---|
| Default mode | |||
| Left posterior cingulate cortex | −2, −51, 27 | Initial seed | |
| Frontoparietal | |||
| Right inferior parietal sulcus | 25, −62, 53 | Initial seed | |
| Left occipital pole | −12, −94, 16 | 4.29 | 915 |
| Left insular cortex | −38, 2, 6 | 4.12 | 479 |
| Right occipital pole | 14, −96, −4 | 3.99 | 407 |
| Right precentral gyrus | 56, 6, 26 | 3.75 | 347 |
| Left superior temporal gyrus | −64, −30, 6 | 3.24 | 117 |
| Left inferior frontal gyrus, pars triangularis | −44, 30, 4 | 3.68 | 109 |
| Right middle temporal gyrus, temporo-occipital part | 50, −48, 8 | 3.92 | 94 |
| Left paracingulate gyrus | −2, 24, 36 | 3.74 | 91 |
| Left frontal pole | −38, 64, 6 | 3.65 | 84 |
| Left cerebellum VI | −10, −70, −18 | 3.65 | 79 |
| Salience | |||
| Right dorsal anterior cingulate | 2, 35, 33 | Initial seed | |
| Left postcentral gyrus | −52, −32, 56 | 5.07 | 183 |
| Left angular gyrus | −44, −60, 26 | 3.45 | 118 |
| Left lateral occipital cortex, superior division | −48, −64, 34 | 4.79 | 99 |
| Right cerebellum I–IV | 4, −48, −12 | 3.55 | 81 |
The coordinates used as the center of each spherical seed are listed for each network that was examined: DMN, FPN, and SN.
DMN, default mode network; FPN, frontoparietal network; MNI, Montreal Neurological Institute; SN, salience network.
The seeds were individually registered to subjects' space using first-level FEAT. Specifically, the mean blood oxygen-level-dependent signal time series were extracted from the appropriate seed region and used as the model response function in a general linear model analysis. This allowed for examination of functional connectivity in each of the aforementioned networks through the detection of voxels with time series that correlate with that measured in the seed. In each case, the mean time series from the lateral ventricle were used as a nuisance regressor to eliminate noise (given that no meaningful signal would be expected in the ventricles). The time series statistical analyses were carried out using FILM with local autocorrelation correction (Woolrich et al., 2001).
Finally, a higher level within-group analysis was conducted to compare resting-state functional connectivity in each of the networks separately (DMN, FPN, and SN) between baseline and 4-year follow up (baseline >4 years and 4 years > baseline). The higher level analysis was carried out using a mixed-effects model, in FMRIB's local analysis of mixed effects (Beckmann et al., 2003; Woolrich et al., 2004). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = 0.05 (Worsley, 2001).
Results
Participant demographics and behavioral findings
The demographic information and behavioral findings for the participants can be viewed in Table 1. As expected, based on their placement in the cognitively normal group within the ADNI, no significant differences were present in cognitive scores (MMSE, ADNI-MEM, ADNI-EF) between baseline and follow-up.
Table 1.
Participant Demographics
| Year 1 | Year 4 | p | |
|---|---|---|---|
| n | 16 | 16 | |
| Age (years) | 74.38 ± 4.52 | 78.43 ± 4.24 | |
| Female | 9 (56.25%) | 9 (56.25%) | 0.4864 |
| Education (years) | 16.81 ± 1.91 | 16.81 ± 1.91 | |
| ADNI-MEM | 1.14 ± 0.39 | 1.14 ± 0.71 | 0.8948 |
| ADNI-EF | 0.94 ± 0.56 | 0.84 ± 0.84 | 0.6362 |
| MMSE | 28.63 ± 1.67 | 28.19 ± 2.37 | 0.6238 |
ADNI, Alzheimer's Disease Neuroimaging Initiative; ADNI-EF, ADNI executive function composite; ADNI-MEM, ADNI memory composite; MMSE, Mini-Mental State Examination.
Seed-based resting-state fMRI functional connectivity findings
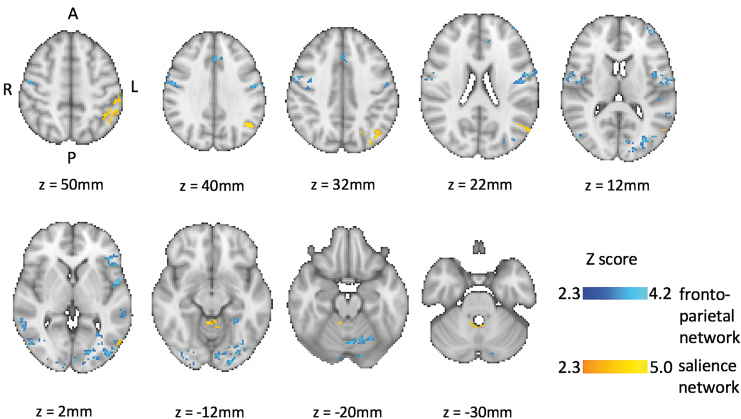
The analyses revealed significant functional connectivity differences between the baseline and the follow-up 4 years later. Significantly reduced connectivity was detected within the FPN and SN for year 4 compared with baseline. No statistically significant results were detected for either contrast within the DMN. Figure 1 depicts the regions with significantly reduced connectivity in the FNP and SN at the 4-year follow-up, and Table 2 reports the MNI coordinates for the peak voxel clusters that correlated with each seed region. For the definition of regional anatomy, the Harvard-Oxford Cortical Structural Atlas was used.
FIG. 1.
Resting-state connectivity networks baseline > year 4 (default mode network, no significant findings, frontoparietal network results in blue, salience network results in yellow; corrected for multiple comparisons, p < 0.05). Findings are displayed in axial orientation, radiological view. Color images are available online.
Discussion
The aim of the current study was to determine if there are longitudinal changes in functional connectivity, examining multiple networks, over a period of 4 years of healthy aging. Based on previous findings from cross-sectional studies, we hypothesized a decline in the functional connectivity in three different networks, including the DMN, FPN, and SN.
The results indicated significant declines in functional connectivity over 4 years within both the FPN and SN. Conversely, functional connectivity within the DMN remained stable, with no significant changes over time.
The present study was the first to investigate longitudinal changes in functional connectivity in multiple resting-state brain networks by following one group of healthy subjects over a time period of 4 years. Longitudinal findings in the FPN and SN are consistent with previously mentioned studies that took cross-sectional approaches and found decreased connectivity in the FPN (Andrews-Hanna et al., 2007; Huang et al., 2015; Marstaller et al., 2015; Voss et al., 2010) and SN (He et al., 2014) in healthy older adults. For example, our results correspond with findings from Marstaller et al. (2015) who found reduced functional connectivity in the FPN and SN and to a lesser degree in the DMN in older compared with younger adults; they demonstrated that while young adults engage the DMN as well as regions associated with the FPN and the SN, resting-state functional connectivity is only observed in the DMN and not in the FPN and SN in older adults.
In terms of longitudinal studies, our results compare with those of Chong et al. (2019), who examined a healthy elderly cohort over 2 years, using a graph theoretical approach. In this study, healthy older adults showed global decreases in integration and segregation compared with young adults, but showed only longitudinal declines in distinctiveness of three higher order cognitive modules: FPN, SN, and DMN. Therefore, we have common findings of reduced functional connectivity over time within the FPN and SN during healthy aging, although our findings in the DMN differ.
Ousdal et al. (2019) have also taken a longitudinal approach and have assessed the stability of the brain(’s) functional connectome in healthy aging. Their study showed that the connectome of the whole brain and nine subnetworks generally remains stable over a 2–3-year period in middle and older age. Interestingly, the analyses revealed a significant negative association between DMN stability and change in episodic memory performance, indicating a larger episodic memory decline between the two time points in individuals with higher DMN stability.
Interestingly, a longitudinal study by Van Hooren et al. (2018) also utilized the ADNI2 database to examine individuals along the continuum of AD. Results from the healthy control cohort revealed that the relationship between internetwork functional connectivity (in the DMN and SN, as well as in the dorsal attention network) and memory decline was moderated by Aβ. Future work should further explore the role of variables such as AB, tau, and APOE status on functional connectivity in healthy aging.
In contrast to Chong et al. (2019) and numerous cross-sectional studies of the DMN in aging (e.g., Damoiseaux, 2017), we did not observe significant decreases in the DMN over time. One potential reason that our results may differ from others is that the participants in the present study were cognitively stable over a 4-year period of time, while there is evidence that reduced connectivity in large-scale brain networks such as the DMN is linked to cognitive decline, particularly in memory and executive function (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Onoda et al., 2012). Notably, our findings are highly consistent with a longitudinal study by Persson et al. (2014), who assessed changes in the DMN in healthy adults aged 49–79 years and found stability in functional connectivity in the DMN over time.
It is possible that functional network changes first occur in the FPN and SN along with underlying structural changes, and that changes in the DMN occur later on when normal cognitive declines are more likely to be detected (perhaps longer longitudinal designs are necessary to detect this effect). Consistent with this idea, a study by Zhang et al. (2014) also suggested that the FPN may be the first target of neuronal vulnerability. It is possible that the FPN and SN are particularly sensitive to changes with age given their connection to frontal regions, which are most likely to show structural changes in both white and gray matter with age (Cabeza and Dennis; 2012).
Conversely, it is also possible that changes in the DMN occur first and then stabilize and that we were not able to detect this with our longitudinal approach, which began tracking participants in their mid-70s; the functional connectivity of the DMN is shown to be notably higher in people aged about 20 years compared with older people (Tomasi and Volkow, 2011). In this case, the implementation of a longitudinal study beginning at an earlier age/time point could elucidate the progression of changes in functional connectivity. Interestingly, Staffaroni et al. (2018) examined subnetworks of the DMN longitudinally in a large sample of healthy older adults and found age-dependent changes in functional connectivity, such that individuals aged 50–66 years showed increases in DMN connectivity, while individuals older than the age of 74 years showed declines. It is possible that the current study may have detected reduced connectivity in the DMN if participants were followed over a longer time period (past the mid-70s age range).
Another potential reason that the current study did not detect changes in the DMN may relate to the use of a posterior seed region. Recently, some studies have suggested that the DMN can be split into the posterior cingulate cortex region utilized in the current study as well as a dissociable anterior subcomponent of the network, anchored in the ventral medial prefrontal cortex (Andrews-Hanna et al., 2010; Uddin et al., 2009). Damoiseaux et al. (2008) examined both components and found that the anterior but not the posterior aspects of the DMN correlated with age.
The idea that the influence of advancing age is not homogenous within the DMN is supported by a 5-year longitudinal study by Salami et al. (2016), in which the MTL region of the DMN is investigated. Their findings revealed that the degree of functional connectivity within the anterior MTL declined after age 60, whereas elevated functional connectivity was found within the posterior MTL.
Limitations of the current study and directions for future research
There are several limitations to the current study, which may help to guide directions for future research. First, as in the case in many neuroimaging studies, there was limited statistical power in this study, due to a small sample size. Future studies should strive to include a greater number of participants. Future studies should also incorporate more than two time points and include participants who only have baseline data, to control for dropout and sampling effects. Such aims may become more achievable as large databases such as the ADNI grow over time.
Second, the current study used a seed-based approach for the identification of resting-state networks, which required a priori selection of ROIs. The inconsistent use of seed coordinates in different articles limits the comparability and replicability between studies. An additional difficulty in understanding the literature on functional connectivity changes during healthy aging is the multitude of analysis approaches that have been taken, including not only seed-based analysis but also independent component analyses and graph theoretical analyses. Moving forward, a positive step in this area of research would be to include the use of commonly accepted network seeds. Large reviews on functional connectivity changes in aging that integrate findings from the various possible approaches would also be helpful in detailing the similarities and differences between findings.
Third, the current study focused on changes in functional connectivity related to specific networks using a seed-based approach, but did not aim to examine between network connectivity. Since the SN serves to switch between the DMN and task-related networks such as the FPN (He et al., 2014; Menon and Uddin, 2010; Sridharan et al., 2008), it would be interesting to investigate the longitudinal changes on internetwork connectivity on these networks. Relatedly, future work should also examine changes in between network connectivity in the same individuals over time (e.g., as done by Grady et al., 2016). Furthermore, future research could benefit from examining changes in structural and functional connectivity within the same participants longitudinally. Currently, the ADNI does not collect both DTI and resting-state fMRI data on the same set of individuals, but this would be a relevant goal for future studies.
Conclusions
There is a fundamental need to better understand the neurobiological changes associated with healthy aging, given the globally aging population. The current study aimed to investigate changes in multiple functional connectivity networks (DMN, FPN, and SN) within the same participants over a time frame of 4 years, with hypothesized reductions in connectivity in each network over time. This approach was particularly relevant given that the majority of studies to date have focused on the DMN in cross-sectional comparisons of older versus younger adults.
The current study revealed significant changes in functional connectivity within the FPN and SN, such that connectivity was reduced over 4 years of healthy aging; no significant changes were detected within the DMN. These findings underscore the need for further longitudinal neuroimaging studies on healthy aging. Future work should aim to examine changes in multiple networks over time, in terms of both intra- and internetwork shifts in connectivity and to include multimodal imaging methods that include measurements of structural connectivity, which could provide a more comprehensive picture of the neurobiological changes in healthy aging.
Contributor Information
Collaborators: for the Alzheimer's Disease Neuroimaging Initiative
Authors' Contributions
J.R.G. conceptualized the study. M.O. was responsible for implementation of data analyses. Both J.R.G. and M.O. were involved in interpreting findings, drafting, and revising the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funding for this project was made possible by the Natural Sciences and Engineering Research Council of Canada. This project was completed with support from the neuroscience internship program at the University of Cologne. Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2- 0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- Alakörkkö T, Saarimäki H, Glerean E, Saramäki J, Korhonen O. 2017. Effects of spatial smoothing on functional brain networks. Eur J Neurosci 46:2471–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. 2007a. Non-linear Optimisation. FMRIB Technical Report TR07JA1; pp. 1–16. https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja1/tr07ja1.pdf (Last accessed November20, 2019)
- Andersson JLR, Jenkinson M, Smith S. 2007b. Non-linear Registration, Aka Spatial Normalisation. FMRIB Technical Report TR07JA2; pp. 1–21. https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf (Last accessed November20, 2019)
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain's default network. Neuron 65:550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. 2007. Disruption of large-scale brain systems in advanced aging. Neuron 56:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multilevel linear modeling for group analysis in FMRI. NeuroImage 20:1052–1063 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. 2012. Frontal lobes and aging: Deterioration and compensation. In: Stuss DT, Knight RT (eds.) Principles of Frontal Lobe Function, 2nd ed. New York: Oxford University Press [Google Scholar]
- Chong JSX, Ng KK, Tandi J, Wang C, Poh J-H, Lo JC, et al. 2019. Longitudinal changes in the cerebral cortex functional organization of healthy elderly. J Neurosci 39:5534–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, et al. ; for the Alzheimer's Disease Neuroimaging Initiative. 2012. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6:502–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS. 2017. Effects of aging on functional and structural brain connectivity. NeuroImage 160:32–40 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens Ph, Stam CJ, et al. 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864 [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. 2006. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage 29:1359–1367 [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. 2014. Functional brain connectivity using fMRI in aging and Alzheimer's disease. Neuropsychol Rev 24:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. 2006. A core system for the implementation of task sets. Neuron 50:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. 2013. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev 37:384–400 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 6:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, et al. ; for the Alzheimer's Disease Neuroimaging Initiative. 2012. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, Campbell K. 2016. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging 41:159–172 [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, et al. 2014. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease: abnormal salience network in normal aging and AD. Hum Brain Mapp 35:3446–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Hsieh W-J, Lee P-L, Peng L-N, Liu L-K, Lee W-J, et al. 2015. Age-related changes in resting-state networks of a large sample size of healthy elderly. CNS Neurosci Ther 21:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. NeuroImage 62:782–790 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156 [DOI] [PubMed] [Google Scholar]
- Li HJ, Hou XH, Liu HH, Yue CL, Lu GM, Zuo XN. 2015. Putting task activation into large-scale brain networks: a meta-analysis of 114 fMRI studies on healthy aging. Neurosci Biobehav Rev 57:156–174 [DOI] [PubMed] [Google Scholar]
- Li X, Morgan PS, Ashburner J, Smith J, Rorden C. 2016. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods 264:47–56 [DOI] [PubMed] [Google Scholar]
- Marek S, Dosenbach NUF. 2018. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci 20:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller L, Williams M, Rich A, Savage G, Burianová H. 2015. Aging and large-scale functional networks: white matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience 290:369–378 [DOI] [PubMed] [Google Scholar]
- Menon V. 2015. Salience network. Brain Mapp 597–611 [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murman DL. 2015. The impact of age on cognition. Semin Hear 36:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. 2012. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci 24:2186–2198 [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Kaufmann T, Kolskår K, Vik A, Wehling E, Lundervold AJ, et al. 2019. Longitudinal stability of the brain functional connectome is associated with episodic memory performance in aging. Hum Brain Mapp 41:697–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson LG, Nyberg L. 2014. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging 35:2107–2117 [DOI] [PubMed] [Google Scholar]
- Raichle ME. 2015. The brain's default mode network. Annu Rev Neurosci 38:433–447 [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, Junqué C. 2015. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol 6:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Wåhlin A, Kaboodvand N, Lundquist A, Nyberg L. 2016. Longitudinal evidence for dissociation of anterior and posterior MTL resting-state connectivity in aging: Links to perfusion and memory. Cereb Cortex 26:3953–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp 17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, et al. 2018. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J Neurosci 38:2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2011. Functional connectivity hubs in the human brain. NeuroImage 57:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Aging and functional brain networks. Mol Psychiatry 17:549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. 2009. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 30:625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooren RWE, Riphagen JM, Jacobs HIL; for the Alzheimer's Disease Neuroimaging Initiave. 2018. Internetwork connectivity and amyloid-beta linked to cognitive decline in preclinical Alzheimer's disease: a longitudinal cohort study. Alzheimers Res Ther 10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E, Habeck CG, Razlighi QR, Stern Y. 2019. The effect of aging on resting state connectivity of predefined networks in the brain. Front Aging Neurosci 11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, et al. 2010. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21:1732–1747 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 14:1370–1386 [DOI] [PubMed] [Google Scholar]
- Worsley KJ. 2001. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM (eds.) Functional MRI: An Introduction to Methods. Oxford: Oxford University Press, pp. 251–270 [Google Scholar]
- Zhang H-Y, Chen W-X, Jiao Y, Xu Y, Zhang X-R, Wu J-T. 2014. Selective vulnerability related to aging in large-scale resting brain networks. PLoS One 9:e108807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld HI, Pruim RHR, Bos D, Vrooman HA, Metzel RI, Hofman A, et al. 2019. Patterns of functional connectivity in an aging population: The Rotterdam Study. NeuroImage 189:432–444 [DOI] [PubMed] [Google Scholar]