Abstract
MicroRNAs (miRNAs) was confirmed to play an active role in the pathogenesis of prostate cancer (PCa). The expression and biological function for miR-92a in PCa remains unknown. In this study, we demonstrated that miR-92a expression was decreased in PCa tissues and cells lines. Overexpression miR-92a inhibited the cell viability, migration and invasion of PC-3 while inhibition of miR-92a led to opposite alteration of cell viability and metastasis of DU-145 cells. Mechanically, we confirmed that miR-92a interacted with 3’-UTR of SOX4 through the complementary sequences by luciferase reporter assay. qRT-PCR and western blot confirmed that miR-92a inhibited the expression of SOX4 in PCa cells. Moreover, overexpression of SOX4 reversed the inhibitory effects of miR-92a overexpression on PC-3 cell viability, migration and invasion, while knockdown of SOX4 suppressed the promoting effects of miR-92a knockdown on these biological functions of DU-145 cells. Therefore, our study indicates that miR-92a inhibits the growth and metastasis of prostate cancer by targeting SOX4, and can potentially serve as a biomarker and treatment target for PCa patients.
Keywords: miR-92a, prostate cancer, SOX4, viability, metastasis
Introduction
Prostate cancer (PCa) ranks as the second leading cause of cancer-associated mortality in males, particularly in Western countries.1 Recent study in 2016 estimated that around 180,000 patients in the United States were newly diagnosed with PCa and around 26,000 cases succumbed to PCa.2 The incidence and mortality of PCa is increasing in China in recent years.3 Although remarkable advancements have been achieved in cancer treatment, PCa remains as an intractable malignancy due to uncontrolled proliferation, enhanced metastatic ability and frequent recurrence of PCa. Currently, the mechanism underlying the growth and metastasis of PCa cells remains largely uncovered. Thus, it is of great value to explore the critical molecules and exact mechanisms mediating the progression of PCa.
MicroRNAs (miRNAs) are a group of endogenous non-coding RNAs which regulate the expression of target genes post-transcriptionally by interacting with the 3’-untranslated region (UTR) of mRNAs.4 Previous studies have demonstrated that miRNAs play critical roles in various cellular processes including cell proliferation, differentiation and movement.5 Increasing evidences showed that miRNAs were aberrantly expressed in cancer tissues and acted as either oncogenes or tumor suppressors.6 They were proposed as the promising biomarkers and therapeutic targets of human cancers including PCa.7,8 Recent studies reported that miR-92a played critical roles in the progression of colorectal cancer,9,10 hepatocellular carcinoma,11 pancreatic cancer,12 cervical cancer13 and osteosarcoma.14 It was found to promote the proliferation, metastasis, and metabolism of cancer cells and its expression levels in cancer tissues was correlated with the clinical prognosis of patients. Previous study demonstrated a suppressive function of miR-92a in Pca.15 However, the exact role of miR-92a in PCa and the underlying mechanisms remain unknown.
In this study, we found that miR-92a expression was decreased in PCa tissues and cell lines. MiR-92a inhibited the viability, migration and invasion of PCa cells. Mechanically, we found that SOX4 was the downstream target of miR-92a. MiR-92a exerted inhibitory effect on the viability and metastasis of PCa cells by inhibiting the expression of SOX4.
Material and Methods
Clinical Specimens
40 pairs of PCa tissues and adjacent normal prostate tissues were collected from PCa patients who underwent resection operation in Seventh Affiliated Hospital of Sun Yat-Sen University. Written informed consents were obtained from all enrolled patients, and the protocol of study was approved by Ethics Committee of Seventh Affiliated Hospital of Sun Yat-Sen University (Ethics approval number: 18-029).
Cell Lines and Culture
Normal prostate epithelial cells (RWPE-1) and PCa cell lines including LNCaP, PC-3, 22RV-1 and DU-145 were obtained from American Type Culture Collection (Manassas, VA, USA). RWPE-1 cells were cultured in keratinocyte serum-free media supplemented with recombinant epidermal growth factor (5 ng/ml), bovine pituitary extract (0.05 mg/ml), streptomycin (100 mg/ml), and penicillin (100 U/ml). All PCa cell lines were grown in RPMI-1640 supplemented with fetal bovine serum (FBS, 10%), streptomycin (100 mg/ml) and penicillin (100 U/ml). All cells cultures were maintained in a humidified cell incubator with 5% CO2 at temperature of 37˚C.
Cell Transfection
The miR-92a mimic, control mimic, antisense oligonucleotides of miR-92a (miR-92a inhibitor), and negative control oligonucleotides (NC) were obtained from GenePharma (Shanghai, China). SOX4 siRNA and negative control siRNA, and, SOX4 overexpression vector and empty control plasmid, were purchased from Obio Technology Co., Ltd. (Shanghai, China). For cell transfection, PCa cells were seeded into 6-well plates with 2.5 x 104 cells/well and transfected with miR-92a mimic (2.5ug) or inhibitor (2ug) using Lipofectamine 2000 (Life Technologies) based on the manufacturer’s instructions. SOX4 siRNA (2ug) or SOX4 overexpression vector (4ug) was transfected into PCa cells using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientifc, Inc.). The media was replaced with fresh culture media 24 h after transfection. After incubated for 48 h, the efficacy of cell transfection was evaluated by real-time quantitative polymerase chain reaction (qRT-PCR) analysis.
RNA Extraction and qRT-PCR
The RNA was extracted from clinical specimens and cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientifc, Inc.). miRNA and total RNA was reversed transcribed into cDNA using MiR-X™ miRNA First-Strand Synthesis (Takara Biotechnology Co., Ltd., Kusatsu, Japan) and PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.), respectively. qRT-PCR was further performed using FastStart Universal SYBR Green Master (Roche Diagnostics, Indianapolis, IN, USA). Thermal cycling of qRT-PCR was set as: 95˚C for 5 min, 95˚C for 10 sec and 60˚C for 20 sec, repeated for 40 cycles. U6 and GAPDH was used as the internal control for miR-92a and SOX4, respectively. The primers for miR-92a were designed by and purchased from Tiangen Biotech Company (Beijing, China). The primer sequences for miR-92a, SOX4, U6 and GAPDH were listed as below: miR-92a forward: 5’-GCTGAGTATTGCACTTGTCCCG-3’ and reverse: 5’ –GTGTCGTGGAGTCGGCAA-3’; SOX4 forward: 5’- GGCCTCGAGCTGGGAATCGC-3’ and reverse: 5’- GCCCACTCGGGGTCTTGCAC-3’; U6 forward: 5’-CTCGCTTCGGCAGCACATATACT-3’ and reverse: 5’-ACGCTTCACGAATTTGCGTGTC-3’; GAPDH forward: 5’-GTCTCCTCTGACTTCAACAGCG-3’ and reverse: 5’-ACCACCCTGTTGCTGTAGCCAA-3’. The 2-ΔΔCt method was used to analyze the relative expression of miR-92a and SOX4.16
Western Blot
RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China) was used for the extraction of cellular protein. Protein concentration was measured using the BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA). Protein lysates (10-30ug) were separated by 10% SDS-PAGE gel (Thermo Fisher Scientifc, Inc.) and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked with nonfat milk (5%) and incubated with SOX4 antibody (1:2,000, ab85051; Abcam, Cambridge, UK), Vimentin antibody (1:1000, #5741, Cell Signaling Technology, CA, USA), AKT antibody (1:1000, #4691, Cell Signaling Technology), p-AKT antibody (1:1000, #4060, Cell Signaling Technology) or GAPDH antibody (1:4,000, 5632 -1; Epitomics, Burlingame, CA, USA) at 4°C overnight. Then membranes were washed with Tris buffered saline Tween (TBST) solution and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. ECL reagent was used for the visualization and detection of protein signals.
Cell Viability Assay
The PCa cells seeded into 96-well plates with 4,000 cells/well 24 h following the cell transfection. The viability of the cells was detected using MTT solution (5 mg/ml; Sigma, St. Louis, MO, USA). Relative numbers of viable cells were evaluated by the absorbance at the wavelength of 490 nm at 24, 48 and 72 h after cell seeding. Before subjected to absorbance measurement, the medium was discarded, and the cells were incubated with MTT solution for 2 hours.
Cell Migration and Invasion Assay
The migration and invasion of PCa cells were analyzed in 24-well Transwell devices with 8 µm pore size polycarbonate membranes (Corning Inc., Corning, NY, USA). A total of 4 x 104 PCa cells suspended in 200uL serum-free medium were added into the upper chamber while the lower chamber was added with medium containing 10% FBS as chemo-attractant. In the invasion assays, the membranes of upper chambers were covered with diluted Matrigel (70uL with 1:6 dilution, BD Biosciences, San Diego, CA, USA) before cell seeding. 24 h after cell seeding, cells were wiped from the upper surface of membranes with a cotton swab while cells on the lower surface of the membrane were fixed in methanol and stained with crystal violet. Cell number was counted under light microscope.
Dual-Luciferase Reporter Assay
The wild type 3’-UTR of SOX4 (wt SOX4 3’-UTR) contained the complementary sequences with miR-92a were cloned into pGL luciferase vector (Promega, Madison, WI, USA). The 3’-UTR of SOX4 containing the mutation of miR-92a recognition sites (mutated SOX4 3’-UTR) was constructed using a Site-Directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA) and cloned into the pGL luciferase vector (Promega). Luciferase assay was performed using a dual-Glo luciferase assay system (Promega).
Statistical Analysis
All numerical data were expressed as mean ± SD from at least 3 independent replicates. SPSS software, 16.0 (SPSS, Inc, Chicago, IL, USA) was used for statistical analysis, and a 2-tailed Student t-test or ANOVA analysis were employed to analyze the differences between 2 groups or multiple groups, respectively. Differences were considered as statistically significant at P < 0.05.
Results
MiR 92a Expression Is Downregulated in PCa Tissues and Cell Lines
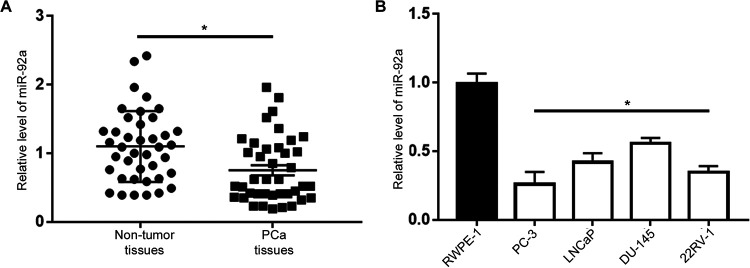
The expression levels of miR-92a in PCa tissues and adjacent non-tumor tissues were examined by qRT-PCR. The results demonstrated that compared with adjacent non-tumor tissues, miR-92a expression was significantly decreased in PCa tissues (P < 0.05, Figure 1A). Similarly, qRT-PCR was performed to determine miR-92a expression levels in 4 PCa cell lines (LNCaP, PC-3, 22RV-1 and DU-145) and normal prostate epithelial cells (RWPE-1). As depicted in Figure 1B, compared with in RWPE-1 cells, the expression level of miR-92a was significantly decreased in 4 PCa cell lines (P < 0.05). The level of miR-92a was lowest in PC-3 cells and highest in DU-145 cells (Figure 1B). These results indicated that miR-92a may function as a tumor suppressor in the progression of PCa.
Figure 1.
The expression status of miR-92a in PCa. (A) qRT-PCR results for the expression of miR-92a in PCa tissues (n = 40) and matched adjacent normal tissues (n = 40). (B) Relative expression level of miR-92a in PCa cell lines (PC-3, LNCaP, 22RV-1 and DU-145) and the RWPE-1 cells. *P < 0.05.
MiR-92a Overexpression Inhibits the Viability, Migration and Invasion of PCa Cells
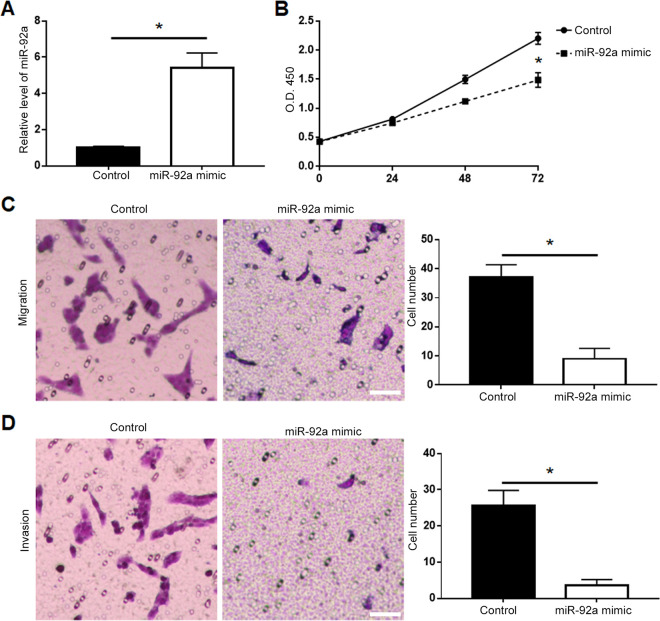
To the functional influence of miR-92a on PCa cells, we performed gain-of-function experiments by transfection of miR-92a mimic into PC-3 cells. qRT-PCR showed that transfection of miR-92a mimic resulted in significantly increased level of miR-92a in PC-3 cells (P < 0.05, Figure 2A). MTT assay showed that forced expression of miR-92a led to decreased cell viability of PC-3 cells (P < 0.05, Figure 2B). Overexpression of miR-92a led to decreased migration (P < 0.05, Figure 2C) and invasion (P < 0.05, Figure 2D) of DU-145 cells. Additionally, western blot demonstrated that miR-92a overexpression led to decreased expression of p-AKT and vimentin (P < 0.05, Supplementary Figure 1A).
Figure 2.
Overexpression of miR-92a inhibited the viability, migration and invasion of PC-3 cells. (A) Transfection of miR-92a mimic significantly increased the expression of miR-92a in PC-3 cells, as determined by qRT-PCR assay. (B) The viability of PC-3 cells was significantly decreased after overexpression of miR-92a, as determined by MTT assay. (C) The migration and (D) invasion of PC-3 cells was significantly suppressed after overexpression of miR-92a, as determined by Transwell assay. Scale bar: 100um, *P < 0.05.
MiR-92a Knockdown Enhances the Viability, Migration and Invasion of PCa Cells
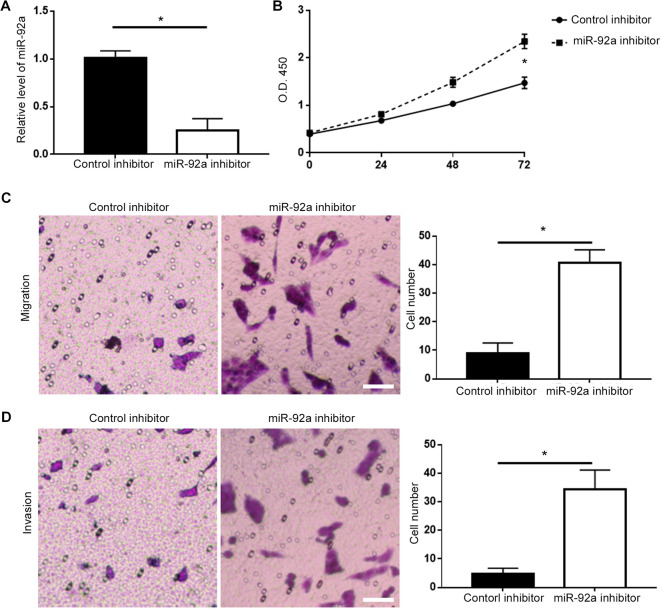
On the other hand, we performed loss-of-function experiments by transfection of miR-92a inhibitor into DU-145 cells. Transfection of miR-92a inhibitor effectively reduced the level of miR-92a in DU-145 cells (P < 0.05, Figure 3A), and subsequently resulted in increased cell viability of DU-145 cells (P < 0.05, Figure 3B). Knockdown of miR-92a led to increased migration (P < 0.05, Figure 3C) and invasion (P < 0.05, Figure 3D) of DU-145 cells. Additionally, western blot demonstrated that miR-92a knockdown led to increased expression of p-AKT and vimentin (P < 0.05, Supplementary Figure 1B).
Figure 3.
Knockdown of miR-92a promoted the viability, migration and invasion of DU-145 cells. (A) Transfection of miR-92a inhibitor significantly decreased the expression of miR-92a in DU-145 cells, as determined by qRT-PCR assay. (B) The viability of DU-145 cells was significantly increased after knockdown of miR-92a, as determined by MTT assay. (C) The migration and (D) invasion of DU-145 cells was significantly enhanced after knockdown of miR-92a, as determined by Transwell assay. Scale bar: 100um, *P < 0.05.
MiR-92a Directly Targets SOX4 in PCa Cells
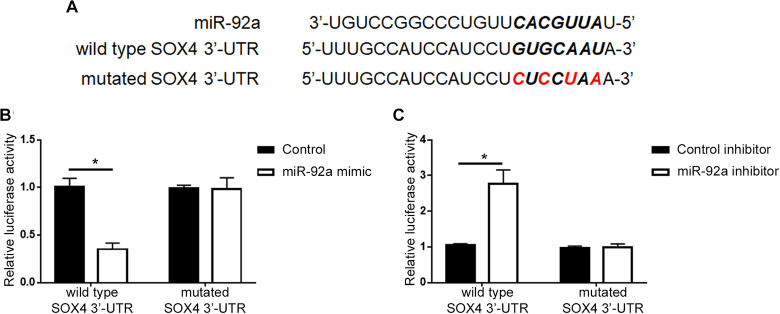
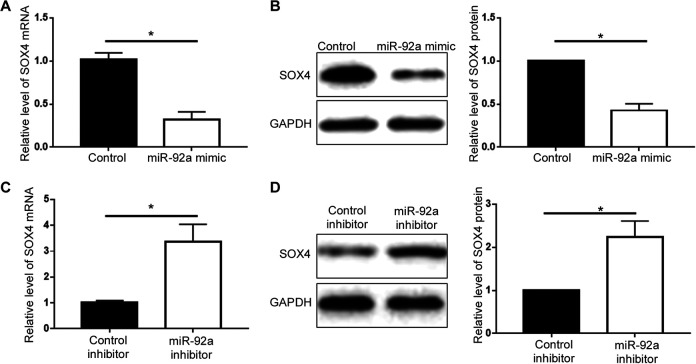
To understand the underlying mechanisms by which miR-92a exerted its promoting effects on cell viability and metastasis, we explored the website of Targetscan (http://www.targetscan.org/vert_71/) to identify the potential targets of miR-92a, and found that SOX4 was predicted to be the downstream target of miR-92a (Figure 4A). To validate the interaction between miR-92a and the 3’-UTR of SOX4, we performed luciferase reporter assay in 293 T cells. Co-transfection of wt SOX4 3’-UTR construct with miR-92a mimic into 293 T cells resulted in a significant decrease of cellular luciferase activity compared with cells transfected with control mimic (P < 0.05, Figure 4B). Overexpression of miR-92a did not affect the luciferase activity of mutant SOX4 3’-UTR (Figure 4B). In contrary, transfection of miR-92a inhibitor led to increased luciferase activity of wt SOX4 3’-UTR construct in 293 T cells (P < 0.05, Figure 4C) while had no effect on that of mutant SOX4 3’-UTR (Figure 4C). These results indicate that miR-92a directly targets the 3’-UTR of SOX4 through the complementary sequences. The expression level of SOX4 in PCa cell lines was demonstrated in Supplementary Figure 2. qRT-PCR and western blot were further performed to assess miR-92a could regulate the expression of SOX4 in PCa cells. As shown in Figure 5A and 5B, overexpression of miR-92a decreased the mRNA and protein level of SOX4 in PC-3 cells (P < 0.05). In contrary, knockdown of miR-92a in DU-145 cells increased the expression of SOX4 mRNA and protein (Figure 5C and 5D, P < 0.05).
Figure 4.
MiR-92a interacted with SOX4 3’-UTR through the complementary sequences. (A) Wild type 3’-UTR of SOX4 contained the sequences for the interaction with miR-92a. Mutated 3’-UTR of SOX4 was constructed by the site-directed mutagenesis. (B) miR-92a overexpression in 293 T cells suppressed the luciferase activity of wild-type 3’-UTR of SOX4, with no obvious effect on that of mutated 3’-UTR of SOX4. (C) miR-92a knockdown in 293 T cells increased the luciferase activity of wild-type 3’-UTR of SOX4, with no obvious effect on that of mutated 3’-UTR of SOX4. *P < 0.05.
Figure 5.
MiR-92a inhibited SOX4 expression in PCa cells. (A) miR-92a overexpression significantly reduced the mRNA level of SOX4 in PC-3 cells. (B) miR-92a overexpression reduced the protein level of SOX4 in PC-3 cells. (C) miR-92a knockdown increased SOX4 mRNA level in DU-145 cells. (D) miR-92a knockdown increased SOX4 protein level in DU-145 cells. *P < 0.05.
MiR-92a Exerts Its Tumor Suppressive Functions in PCa Cells by Inhibiting SOX4 Expression
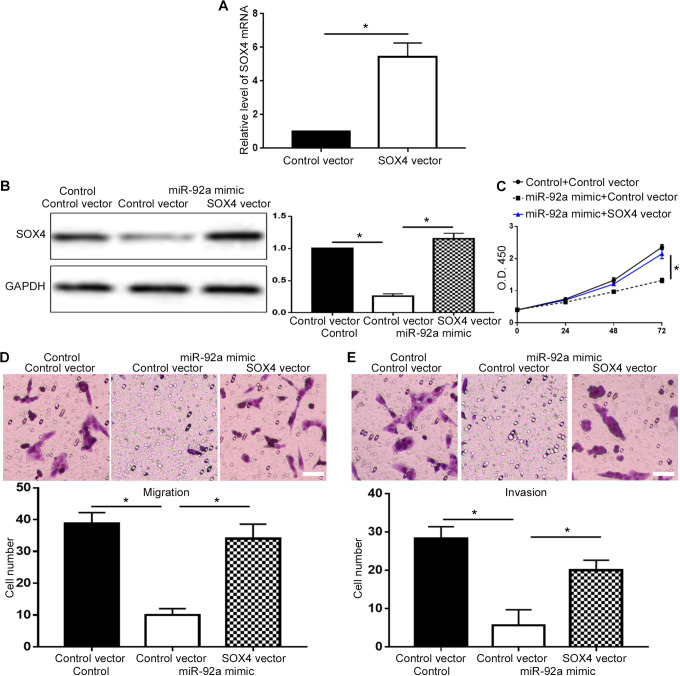
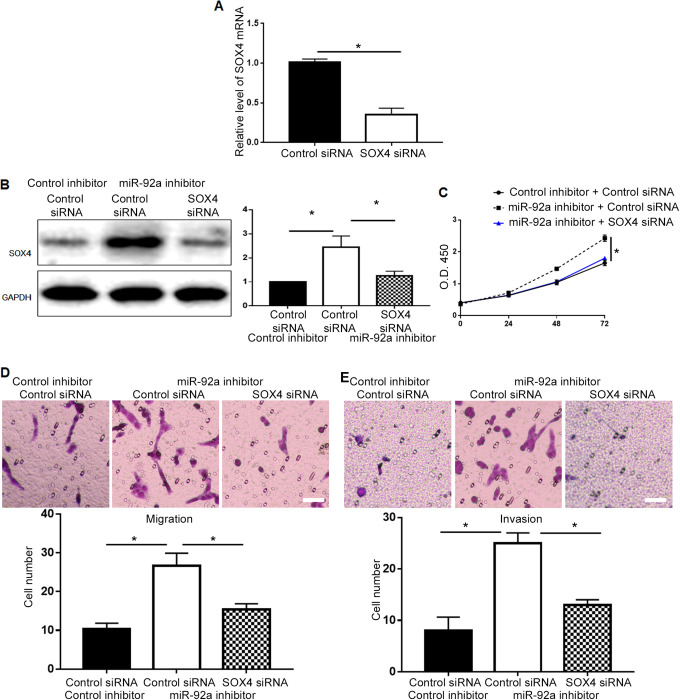
To verify whether miR-92a exerted its biological functions via SOX4 in PCa, we investigated whether restoration of SOX4 could abrogate the inhibitory effect of miR-92a overexpression on cell viability and metastasis. Transfection of SOX4 plasmid effectively increased the level of SOX4 mRNA in PC-3 cells (P < 0.05, Figure 6A), and restored SOX4 protein level in PC-3 cells overexpressing miR-92a (P < 0.05, Figure 6B). Restoring SOX4 expression abrogated the decrease of cell viability induced by miR-92a overexpression (P < 0.05, Figure 6C). The decrease of cell migration and invasion induced by miR-92a overexpression was inhibited by SOX4 restoration (P < 0.05, Figure 6D and 6E). On the other hand, transfection of SOX4 siRNA effectively reduced SOX4 mRNA level in DU-145 cells (P < 0.05, Figure 7A), and decreased SOX4 protein in DU-145 cells with miR-92a knockdown (P < 0.05, Figure 7B) and suppressed the increase of cell viability (P < 0.05, Figure 7C), migration (P < 0.05, Figure 7D) and invasion (P < 0.05, Figure 7E) induced by miR-92a knockdown.
Figure 6.
Overexpression of SOX4 suppressed the inhibitory effects of miR-92a mimic on cell viability, migration and invasion. (A) SOX4 vector increased the level of SOX4 mRNA in PC-3 cells. (B) SOX4 overexpression vector significantly increased the expression of SOX4 protein in PC-3 cells overexpressing miR-92a. Forced expression of SOX4 reversed the inhibitory effects of miR-92a overexpression on (C) cell viability, (D) migration, (E) invasion. Scale bar: 100um, *P < 0.05.
Figure 7.
Inhibition of SOX4 abrogated the promoting effects of miR-92a inhibitor on cell viability, migration and invasion. (A) SOX4 siRNA decreased the level of SOX4 mRNA in DU-145 cells. (B) SOX4 siRNA significantly decreased SOX4 protein in DU-145 cells with miR-92 knockdown. Knockdown of SOX4 abrogated the promoting effects of miR-92a knockdown on (C) cell viability, (D) migration, (E) invasion. Scale bar: 100um, *P < 0.05.
Discussion
PCa is one of most common types of malignancy in men, which shows an increasing morbidity and mortality rate.2 Over past decades, the molecular mechanisms underlying the initiation and progression of PCa have been intensively investigated. miRNAs are found to play important roles in regulating the viability, metastasis and other malignant behaviors of PCa cells and proposed as promising biomarkers and therapeutic targets of PCa.17
In the present study, we investigated the expression and biological functions of miR-92a in PCa, and showed that miR-92 expression was significantly decreased in clinical tissues collected from PCa patients, as well as in PCa cell lines, implying a tumor suppressive function of miR-92a in PCas. Functionally, overexpression of miR-92a decreased the viability, migration and invasion of PCa cells while miR-92a knockdown resulted in opposite effects on cell viability and metastasis. These indicate miR-92a exerts its suppressive functions in PCa by inhibiting cell viability and metastasis. Further experiments demonstrated that SOX4 was confirmed as a downstream target of miR-92a in PCa cells, through which miR-92a exerted its tumor suppressive functions in PCa cells. This is the first attempt, to the best of our knowledge, to illuminate the expression and functions of miR-92a in PCa. However, due to limited number of included samples, the correlation between miR-92a level and the clinical features of PCa patients remains unknown, which is worth to be investigated in the future.
Enhanced ability of cell proliferation is a fundamental characteristic of human cancers, and occurrence of systemic metastasis is the major cause of cancer mortality.18 Therefore, identifying critical and novel molecules regulating proliferation and metastasis of cancer cells may contribute to the advancement of cancer treatment. MiR-92a has been shown to affect numerous cellular processes including cell viability, apoptosis, metastasis and drug resistance, suggests that it is important for cancer progression. In the studies of colorectal cancer, hepatocellular carcinoma, pancreatic cancer, cervical cancer and osteosarcoma, miR-92a was reported to exert oncogenic influence on cancer progression.9-14 However, our data revealed a tumor suppressive role of miR-92a in PCa using both gain- and loss- of function assays. These indicate that the functional influence of miR-92a in human cancers differs in different types of human cancers.
SOX4, a classical transcriptional factor, was found to promote the progression of PCa.19,20 Elevated expression of SOX4 was correlated with poor prognosis of PCa patients. Previous studies demonstrated that SOX4 was critical for the tumorigenesis, metastasis and epithelial-mesenchymal transition of PCa cells.21-23 In this study, we revealed that miR-92a could directly interact with 3’-UTR of SOX4 and inhibited the expression of SOX4 in PCa cells. More importantly, rescue experiments proved that miR-92a exerted its inhibitory effect on viability and metastasis of PCa cells by inhibiting SOX4 expression. In studies of other cancer types, molecules including Fbxw7, KLF4, GSK3β and DKK3 were found to be under the regulation of miR-92a in different types of cancers.10,13 In cervical cancer, miR-92a promoted the proliferation and invasion of cancer cells by targeting Fbxw7.13 In colorectal cancer, miR-92a upregulates the Wnt/beta-catenin signaling activity via directly targeting KLF4, GSK3beta and DKK3.10 These demonstrate that miR-92a can target different genes in different cancer cells, and exerted its biological functions in human cancers through various molecular mechanisms. The functional influence of miR-92a in each type of human cancers is largely dependent on its downstream targets in the cancer cells.
In conclusion, the present study suggested that downregulation of miR-92a is a frequent event in PCa and inhibited the viability and metastasis of PCa. SOX4 was identified to be the downstream target of miR-92a in PCas. MiR-92a exerted its tumor suppressive effect on cell viability and metastasis by targeting SOX4. Therefore, miR-92 may be a potential biomarker and therapeutic target for PCa.
Supplemental Material
Supplemental Material, S1 for MicroRNA-92a Inhibits the Cell Viability and Metastasis of Prostate Cancer by Targeting SOX4 by Guolong Liao, Haiyun Xiong, Jiani Tang, Yamei Li and Ying Liu in Technology in Cancer Research & Treatment
Supplemental Material, S2 for MicroRNA-92a Inhibits the Cell Viability and Metastasis of Prostate Cancer by Targeting SOX4 by Guolong Liao, Haiyun Xiong, Jiani Tang, Yamei Li and Ying Liu in Technology in Cancer Research & Treatment
Footnotes
Author Contribution: Guolong Liao and Haiyun Xiong are authors contributed equally to this study.
Availability of data and materials: All data regarding this manuscript is available for the readers upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Guolong Liao  https://orcid.org/0000-0002-6421-3449
https://orcid.org/0000-0002-6421-3449
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Yamamoto S, Kawakami S, Yonese J, et al. Long-term oncological outcome and risk stratification in men with high-risk prostate cancer treated with radical prostatectomy. Jpn J Clin Oncol. 2012;42(6):541–547. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Peyromaure EM, Mao K, Sun Y, et al. A comparative study of prostate cancer detection and management in China and in France. Can J Urol. 2009;16(1):4472–4477. [PubMed] [Google Scholar]
- 4. Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 6. Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayub SG, Kaul D, Ayub T. Microdissecting the role of microRNAs in the pathogenesis of prostate cancer. Cancer Genet 2015;208(6):289–302. [DOI] [PubMed] [Google Scholar]
- 8. Wang YL, Wu S, Jiang B, Yin FF, Zheng SS, Hou SC. Role of micrornas in prostate cancer pathogenesis. Clin Genitourin Cancer. 2015;13(4):261–270. [DOI] [PubMed] [Google Scholar]
- 9. Lao IW, Cui F, Zhu H. Quantitation of microRNA-92a in colorectal adenocarcinoma and its precancerous lesions: co-utilization of in situ hybridization and spectral imaging. Oncol Lett. 2015;9(3):1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang GJ, Li LF, Yang GD, et al. MiR-92a promotes stem cell-like properties by activating Wnt/beta-catenin signaling in colorectal cancer. Oncotarget. 2017;8(60):101760–101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Wu J, Xie C. miR-92a promotes hepatocellular carcinoma cells proliferation and invasion by FOXA2 targeting. Iran J Basic Med Sci. 2017;20(7):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He G, Zhang L, Li Q, Yang L. miR-92a/DUSP10/JNK signalling axis promotes human pancreatic cancer cells proliferation. Biomed Pharmacother. 2014;68(1):25–30. [DOI] [PubMed] [Google Scholar]
- 13. Zhou C, Shen L, Mao L, Wang B, Li Y, Yu H. miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem Biophys Res Commun. 2015;458(1):63–69. [DOI] [PubMed] [Google Scholar]
- 14. Jiang X, Li X, Wu F, et al. Overexpression of miR-92a promotes the tumor growth of osteosarcoma by suppressing F-box and WD repeat-containing protein 7. Gene. 2017;606:10–16. [DOI] [PubMed] [Google Scholar]
- 15. Ottman R, Levy J, Grizzle WE, Chakrabarti R. The other face of miR-17-92a cluster, exhibiting tumor suppressor effects in prostate cancer. Oncotarget. 2016;7(45):73739–73753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 17. Moustafa AA, Kim H, Albeltagy RS, El-Habit OH, Abdel-Mageed AB. MicroRNAs in prostate cancer: from function to biomarker discovery. Exp Biol Med (Maywood). 2018;243(10):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 19. Chen J, Ju HL, Yuan XY, Wang TJ, Lai BQ. SOX4 is a potential prognostic factor in human cancers: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18(1):65–72. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Zeng S, Jiang X, Lai D, Su Z. SOX4 induces tumor invasion by targeting EMT-related pathway in prostate cancer. Tumour Biol. 2017;39(5):1010428317694539. [DOI] [PubMed] [Google Scholar]
- 21. Bilir B, Osunkoya AO, Wiles WG, et al. SOX4 is essential for prostate tumorigenesis initiated by PTEN ablation. Cancer Res. 2016;76(5):1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu W, Tao T, Qi M, et al. MicroRNA-132/212 upregulation inhibits TGF-beta-mediated epithelial-mesenchymal transition of prostate cancer cells by targeting SOX4. Prostate. 2016;76(16):1560–1570. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Li Y, Yang X, et al. ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells. Prostate. 2014;74(6):647–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, S1 for MicroRNA-92a Inhibits the Cell Viability and Metastasis of Prostate Cancer by Targeting SOX4 by Guolong Liao, Haiyun Xiong, Jiani Tang, Yamei Li and Ying Liu in Technology in Cancer Research & Treatment
Supplemental Material, S2 for MicroRNA-92a Inhibits the Cell Viability and Metastasis of Prostate Cancer by Targeting SOX4 by Guolong Liao, Haiyun Xiong, Jiani Tang, Yamei Li and Ying Liu in Technology in Cancer Research & Treatment