Supplemental Digital Content is Available in the Text.
Key Words: HIV, lost to follow-up, mother-to-child transmission, South Africa, patient outcome assessment, retention
Background:
Undetermined attrition prohibits full understanding of the coverage and effectiveness of HIV programs. Outcomes following loss to follow-up (LTFU) among antiretroviral therapy (ART) patients may differ according to their reasons for ART initiation.
Setting:
We compare the true outcomes of adult patients previously identified as LTFU by reason for ART initiation in 8 health facilities in north eastern South Africa.
Methods:
Adult HIV patient records were linked to health and demographic surveillance system (HDSS) data from 2014 to 2017. Outcomes of adults categorized as LTFU (>90 days late for the last scheduled clinic visit) were determined through clinic and routine tracing record reviews, consultation of HDSS data, and supplementary tracing. We calculated the proportion of patients per outcome category and performed competing risk survival analysis to estimate the cumulative incidence of death, transfer, migration, ART interruption, and re-engagement following LTFU.
Results:
Of 895/1017 patients LTFU with an outcome ascertained, 120 (13.4%) had died, 225 (25.1%) re-engaged, 50 (5.6%) migrated out of the HDSS, 75 (8.4%) were alive and not on treatment, and 315 (35.1%) transferred their treatment. These outcomes varied by sex and pregnancy status at ART initiation. Mortality was less likely among pregnant women, patients with higher baseline CD4, and more likely among older patients.
Conclusions:
Patient survival and transfers to other facilities are considerably higher than those suggested in earlier studies. Outcomes differ for women who were pregnant or postpartum when initiating ART, with this population less likely to have died and more likely to have migrated.
INTRODUCTION
As HIV programs in sub-Saharan Africa have expanded, emphasis has been put on initiating patients on antiretroviral therapy (ART) as early as possible in the course of HIV infection.1,2 Eligibility for ART has changed since the adoption of Option B+ which made all pregnant and postpartum women eligible for ART as soon as they tested HIV positive and “Treat all” which extended this eligibility to all people living with HIV.3 Although ART initiation rates among people diagnosed with HIV have increased,4–6 many programs have experienced high attrition rates, especially among women who initiate ART for prevention of mother-to-child transmission of HIV (PMTCT).7 Many of these patients are classified as lost to follow-up (LTFU), a general term for unknown outcomes of patients who have not returned for a scheduled clinic visit. LTFU is often an amalgamation of “silent” (undocumented) clinic transfers, treatment interruptions or stoppages, and deaths,8–12 which are challenging to accurately document using routine reporting mechanism.13–15
Misclassification of patients as being LTFU can lead to as much as a five-fold underestimation of retention and deaths.16 Understanding true outcomes among patients who are reported as LTFU is important to accurately monitor and report on indicators for national ART programs and better target tracing efforts.10 Accurate mortality estimates are also important for parameterizing epidemic projections in software programs such as the UNAIDS Spectrum package.17
A systematic review of HIV patient tracing studies conducted in sub-Saharan Africa from 2001 to 2012 reported that 39% of patients documented as LTFU in clinic records had died, 18.6% had self-transferred to other HIV clinics, and 28.6% had stopped ART but were still alive.12 An earlier review covering studies in sub-Saharan Africa undertaken between 2004 and 2008 reported that 42% of patients documented as LTFU in HIV clinics had died.18
These 2 reviews were conducted in the earlier stages of sub-Saharan African ART programs when ART patient profiles included a higher proportion with severe immunosuppression at treatment initiation and before universal ART for HIV-positive pregnant women (Option B+) had been introduced.19 In addition, decentralization of ART programs means ART can be provided closer to patients' homes,12 which may have increased the number of “silent” transfers taking place within these programs. Furthermore, the proportion of pregnant and postpartum women in ART programs has increased since the adoption of Option B+. This population differs from the general adult population on ART in several ways that are likely to affect the true outcomes among those LTFU, yet few studies have traced women LTFU from PMTCT programs.20 First, ART initiation eligibility criteria for pregnant women have included higher CD4 counts in many settings over the past decade, such that on average they are more likely to initiate treatment while still asymptomatic.21 In addition, childbirth is a risk factor for default from treatment programs22,23 for reasons including postpartum depression or out-referral from PMTCT programs after delivery.24–26
With recent randomized control trials of universal test and treat showing modest and mixed results regarding reducing HIV incidence,27–29 it is imperative that we understand outcomes among nonadherent patients including those LTFU. This will help to develop and direct innovative ways to identify and reach those who have truly disengaged from care. In this context, we conducted a tracing study in Agincourt in rural north-eastern South Africa to ascertain the true outcomes of patients who were LTFU, disaggregated by whether they were pregnant or postpartum when initiating ART (PMTCT) or not, to better understand the outcomes of this group and compare them to the adult ART population who met other criteria for ART initiation.
METHODS
Setting
The Agincourt Health and Demographic Surveillance System (HDSS) is located in Mpumalanga province in rural north-eastern South Africa. Established in 1992, the site is approximately 475 square kilometers and has conducted annual demographic surveys within the HDSS population to capture births, deaths, and migrations since 1999.30,31 In 2013, HIV prevalence in the HDSS population aged 15 years or older was estimated at 19.4%32
The HDSS also collects verbal autopsy (VA) data to ascertain probable causes of death.33 In brief, a structured interview was conducted with people who were closely related to or cared for the deceased during the final illness and could report on symptoms and signs they observed during this period. The interview was conducted using a locally validated tool, in the local language. Until 2010, 2 medical doctors independently reviewed the data to assign a cause of death based on international classification of diseases (ICD-10) conventions,34 with a third doctor used in the event of a lack of consensus. The cause was coded “undetermined” if this failed to yield any agreement.30,35 Since 2011, causes of death are assigned using the InterVA-4 probabilistic model.36
There are 5 primary health facilities and 3 secondary community health centers located within the Agincourt HDSS, all of which offer HIV services including testing and treatment. All health facilities routinely trace patients that are late for a scheduled appointment, with some clinics receiving tracing support from 2 nonprofit organizations, Right-to-Care (RtC) (6 facilities) and Home-Based Carers (HBC) (7 facilities). Routine tracing is described in detail elsewhere.37 Briefly, tracing procedures are triggered once a patient is more than 5 working days late for a scheduled visit and usually involves 2 steps, 3 phone calls, and a home visit if the phone calls do not yield a satisfactory outcome. Patients are considered LTFU if they have not returned to the clinic 90 days after their scheduled visit.
In 2014, an initiative was started to identify registered HDSS residents when they visited local health facilities. The point-of-contact interactive record linkage (PIRL) matches chronic care (HIV, diabetic and hypertensive) patient information at the health facility to their HDSS record. This is done in the presence of the patient to resolve any indecision about their identity in the event of multiple resident matches.38
Record Review and Tracing Study
Using the PIRL database, we identified patients who were more than 90 days late for a scheduled HIV clinic appointment on August 15, 2017 at any of the 8 health facilities located in the Agincourt HDSS. Patients were included in the cohort if they were 18 years or older, had ever declared residency in the HDSS, and had enrolled in HIV treatment after PIRL was established at the health facilities.
Patients who had not yet initiated ART were excluded from our analyses because they did not have a next scheduled visit and as such it was impossible to determine whether they were LTFU or just visited the clinics less frequently. Furthermore, this population would not be comparable to patients who had potentially accrued some benefits from taking ART.
Patients were followed up to ascertain whether they were still alive and still on treatment. Trained fieldworkers conducted a thorough record review, on a case-by-case basis, to resolve each patient outcome by comparing the list of patients LTFU against (1) TIER.Net (the electronic medical records database for health facilities in South Africa)39 (2) paper-based patient clinic files, and (3) logbooks kept by RtC and HBC. The PIRL database was also reviewed for duplicate patients who were then considered silent transfers. Residency and vital status were also checked in the HDSS demographic surveillance database.
Home-Based Carers conducted a further home visit for all patients without an outcome resolution (ie, no definitive outcome after the record review and for whom routine tracing had not previously been done). For all patients remaining LTFU, searches were undertaken in TIER.Net databases of clinics in close proximity to their residence to capture any further silent transfers (see Supplementary Figure 1, Supplemental Digital Content, http://links.lww.com/QAI/B486).
Definitions
A patient was considered to have died if they were reported as deceased in their patient file or in TIER.Net or if they were reported to have died through HDSS surveillance data.
A patient was considered to have re-engaged in care if they were found to still be in care at the same clinic where they initiated treatment but were >90 days late for their last appointment.
A patient was defined as having transferred if they had either reported taking treatment at another clinic, if the clinic at which they initiated ART had communicated with and ascertained their transfer to another clinic, or if there was a record of them collecting treatment from another clinic within the Agincourt HDSS.
Patients were defined as having migrated if they were recorded as such (movement outside the study area) through the HDSS, the migration event happened after their last clinic visit and there was no evidence of them taking treatment at another clinic.
A patient had stopped ART if they had been found and reported that they stopped ART, denied their HIV status or refused to return to the clinic.
A patient was alive with ART status unknown if additional tracing yielded no definitive outcome, but they were found to still be alive through the most recent demographic surveillance round, with a surveillance date after their last clinic visit.
A data error was a situation where a patient was <90 days late for their next scheduled appointment but was erroneously classified as LTFU.
Statistical Analyses
Counts and proportions were calculated for socio-demographic, baseline clinical characteristics, patient tracing outcomes, and VA causes of death. A Pearson's χ2 test was used to compare categorical variables.
Competing risk survival analysis methods were used to estimate the cumulative incidence of death, transfer, migration, ART stoppage, and re-engagement following loss to follow-up (LTFU). Follow-up time began on the date of each patient's last recorded clinic visit as we suspected that some outcomes especially deaths would occur closely following a last visit and before patients would have been categorized as LTFU. Using these cumulative probabilities, status plots were produced stratified by sex, pregnancy status at ART initiation, and baseline CD4.
A Cox regression model was used to determine the factors associated with death, with all other outcomes considered to be right-censored. Bivariate analyses were conducted with a priori selected variables that had been shown to be associated with death in previous studies.18,40–42 All variables with P < 0.1 were included in the multivariable Cox regression model. A parsimonious model was achieved using Wald tests. All analyses were conducted using Stata 15.43 All models accounted for clustering at the clinic level and used robust standard errors.
Ethics
Ethical approval was obtained from the London School of Hygiene and Tropical Medicine, the University of Witwatersrand, and the Mpumalanga Department of Health.
RESULTS
Population Characteristics
Over the study period, 4089 patients were added to the PIRL database and met the inclusion criteria. Of these 4089, 1325 (32.4%) met the LTFU criteria and were eligible for inclusion in the record review and tracing study. Of these 1325 patients, 166 (12.5%) did not have an ART initiation date. Further investigation of these 166 patients found 46 (27.7%) had initiated ART after record linkage, 59 (35.5%) had not yet initiated ART, and 61 (36.7%) had initiated ART before record linkage began. These 61 patients and the 59 non-ART patients were excluded from further analyses. Of the remaining 1205 patients, 188 (15.6%) were misclassified as LTFU due to data errors (missed clinic visits in the PIRL database) and were excluded from further analysis (see Supplementary Figure 2, Supplemental Digital Content, http://links.lww.com/QAI/B486). Analyses of these 188 patients to evaluate the utility of routine tracing are presented in supplementary information (see Supplementary information 1, Supplemental Digital Content, http://links.lww.com/QAI/B486). The remaining 1017 patients were 91–1188 days late (see Supplementary Figure 3, Supplemental Digital Content, http://links.lww.com/QAI/B486).
Of the 1017 remaining patients, 280 (27.5%) initiated ART for PMTCT, 767 (75.4%) were women and 849 (83.5%) linked to an HDSS record (Table 1). Pregnant women were younger with a median age of 29 years (IQR: 25, 33) compared with nonpregnant women, 33 years (IQR: 28, 42) and men, 41 years (IQR: 34, 48). Of 280 patients who initiated ART for PMTCT, 52 (18.6%) had a baseline CD4 <200 cells/µL compared with 193 of 487 (39.6%) nonpregnant women and 146 of 250 (58.4%) men. None of the patients who initiated ART for PMTCT with baseline CD4 <200 cells/µL were categorized as WHO stage III/IV compared with 53 of 193 (27.5%) nonpregnant women and 45 of 146 (30.8%) men. Furthermore, 5.0% of women who initiated treatment for PMTCT had a CD4 less than 100 cells/µL compared with 21.8% of nonpregnant women and 34.4% of men. The main reason for ART initiation for nonpregnant patients was CD4 count criteria (74.5%) (Table 1).
TABLE 1.
Patient Demographic and Clinical Characteristics, and Final Outcomes Disaggregated by Pregnancy Status at ART Initiation
| LTFU | Pregnant Women | Nonpregnant Women | Men | |
| 1017 | 280 | 487 | 250 | |
| N (%) | N (%) | N (%) | N (%) | |
| Age | ||||
| 18–29 | 333 (32.7) | 150 (53.6) | 157 (32.2) | 26 (10.4) |
| 30–44 | 484 (47.6) | 124 (44.3) | 226 (46.4) | 134 (53.6) |
| 45–59 | 141 (13.9) | 6 (2.1) | 70 (14.4) | 65 (26.0) |
| 60+ | 58 (5.7) | 0 (0) | 33 (6.8) | 25 (10.0) |
| Missing | 1 (0.1) | 0 (0) | 1 (0.2) | 0 (0) |
| ART reason | ||||
| CD4 | 549 (54.0) | 0 (0) | 376 (77.2) | 173 (69.2) |
| PMTCT | 280 (27.5) | 280 (100.0) | 0 (0) | 0 (0) |
| WHO Stage | 77 (7.6) | 0 (0) | 45 (9.2) | 32 (12.8) |
| Test and treat | 43 (4.2) | 0 (0) | 23 (4.7) | 20 (8.0) |
| TB | 39 (3.8) | 0 (0) | 17 (3.5) | 22 (8.8) |
| Missing | 29 (2.9) | 0 (0) | 26 (5.3) | 3 (1.2) |
| ART start yr | ||||
| 2014 | 211 (20.8) | 58 (20.7) | 101 (20.7) | 52 (20.8) |
| 2015 | 414 (40.7) | 105 (37.5) | 212 (43.5) | 97 (38.8) |
| 2016 | 350 (34.4) | 107 (38.2) | 157 (32.2) | 86 (34.4) |
| 2017 | 42 (4.1) | 10 (3.6) | 17 (3.5) | 15 (6.0) |
| Time on ART | ||||
| ≤3 mo | 325 (32.0) | 89 (31.8) | 136 (27.9) | 100 (40.0) |
| 3–6 mo | 190 (18.7) | 70 (25.0) | 88 (18.1) | 32 (12.8) |
| 6–12 mo | 228 (22.4) | 70 (25.0) | 114 (23.4) | 44 (17.6) |
| 12–24 mo | 219 (21.5) | 39 (13.9) | 120 (24.6) | 60 (24.0) |
| >24 mo | 55 (5.4) | 12 (4.3) | 29 (6.0) | 14 (5.6) |
| Baseline CD4 | ||||
| <100 | 206 (20.2) | 14 (5.0) | 106 (21.8) | 86 (34.4) |
| 100–199 | 185 (18.2) | 38 (13.6) | 87 (17.9) | 60 (24.0) |
| 200–349 | 261 (25.7) | 71 (25.4) | 129 (26.5) | 61 (24.4) |
| 350–499 | 193 (19.0) | 74 (26.4) | 95 (19.5) | 24 (9.6) |
| 500+ | 145 (14.3) | 64 (22.9) | 64 (13.1) | 17 (6.8) |
| Missing | 27 (2.6) | 19 (6.8) | 6 (1.2) | 2 (0.8) |
| Baseline WHO stage | ||||
| I | 722 (71.9) | 261 (93.2) | 329 (67.6) | 132 (52.8) |
| II | 143 (14.1) | 17 (6.1) | 73 (15.0) | 53 (21.2) |
| III | 129 (12.7) | 2 (0.7) | 70 (14.4) | 57 (22.8) |
| IV | 10 (1.0) | 0 (0) | 6 (1.2) | 4 (1.6) |
| Missing | 13 (1.3) | 0 (0) | 9 (1.8) | 4 (1.6) |
| Refill schedule | ||||
| 1 mo | 672 (66.1) | 188 (67.1) | 322 (66.1) | 162 (64.8) |
| 2 mo | 233 (22.9) | 68 (24.3) | 102 (20.9) | 63 (25.2) |
| 3 mo | 79 (7.8) | 20 (7.1) | 44 (9.0) | 15 (6.0) |
| >3 mo | 33 (3.2) | 4 (1.4) | 19 (3.9) | 10 (4.0) |
| Health facility | ||||
| Agincourt | 272 (26.7) | 74 (26.4) | 141 (28.9) | 57 (22.8) |
| Belfast | 186 (18.3) | 64 (22.9) | 80 (16.4) | 42 (16.8) |
| Cunningmore | 58 (5.7) | 16 (5.7) | 32 (6.6) | 10 (4.0) |
| Justicia | 120 (11.8) | 42 (15.0) | 42 (8.6) | 36 (14.4) |
| Kildare | 117 (11.5) | 25 (8.9) | 62 (12.7) | 30 (12.0) |
| Lillydale | 166 (16.3) | 32 (11.4) | 81 (16.6) | 53 (21.2) |
| Thulamahashe | 25 (2.5) | 9 (3.2) | 12 (2.5) | 4 (1.6) |
| Xanthia | 73 (7.2) | 18 (6.4) | 32 (7.6) | 18 (7.2) |
| Time since last appointment | ||||
| ≤1 yr | 526 (51.7) | 130 (46.4) | 255 (52.4) | 141 (56.4) |
| 1–2 yrs | 369 (36.3) | 117 (41.8) | 176 (36.1) | 76 (30.4) |
| >2 yrs | 122 (12.0) | 33 (11.8) | 56 (11.5) | 33 (13.2) |
| AHDSS outcome | ||||
| Still in HDSS | 505 (49.7) | 142 (50.7) | 237 (48.7) | 126 (50.4) |
| Deceased | 74 (7.3) | 6 (2.1) | 42 (8.6) | 26 (10.4) |
| Migrated | 270 (26.5) | 99 (35.4) | 125 (25.7) | 46 (18.4) |
| Not linked | 168 (16.5) | 33 (11.8) | 83 (17.0) | 52 (20.8) |
| Final outcome | ||||
| Deceased | 120 (11.8) | 10 (3.6) | 60 (12.3) | 50 (20.0) |
| Transferred out | 315 (31.0) | 82 (29.3) | 176 (36.1) | 57 (22.8) |
| Stopped ART | 75 (7.4) | 28 (10.0) | 20 (4.1) | 27 (10.8) |
| Migrated | 49 (4.8) | 21 (7.5) | 22 (4.5) | 6 (2.4) |
| Reengaged | 225 (22.1) | 54 (19.3) | 110 (22.6) | 61 (24.4) |
| Alive: ART unknown | 111 (10.9) | 45 (16.1) | 45 (9.2) | 21 (8.4) |
| LTFU | 122 (12.0) | 40 (14.3) | 54 (11.1) | 28 (11.2) |
Sources of Resolution
Of the 1017 patients LTFU, 895 (88.0%) were resolved, with 536 (59.9%) of these occurring through record review, 155 (17.3%) through demographic surveillance data (23 migrations, 21 deaths, 111 alive), 72 (8.0%) through subsequent visit data in the PIRL database, 53 (5.9%) through supplementary tracing, 57 (6.4%) identified as duplicates in the PIRL database (one person matching to multiple clinic records), and 22 (2.5%) through a search of patient records in clinics in close proximity to the patient's residence.
Patient Outcomes
Of 1017 patients LTFU, 120 [11.8%, 95% confidence interval (CI): 9.9 to 13.9] had died, 315 (31.0%, CI: 28.1 to 33.9) had transferred to another facility, 75 (7.4%, CI: 5.8 to 9.1) had stopped ART, 49 (4.8%, CI: 3.6 to 6.3) had migrated, 225 (22.1%, CI: 19.6 to 24.8) re-engaged in care, 111 (10.9%, CI: 9.1 to 13.0) were alive with an unknown treatment status, and 122 (12.0%) remained LTFU. These outcomes differed (all P < 0.001) by sex, age, baseline CD4 count, time on ART, clinic visit schedule, health facility, time since a missed appointment, and ART initiation reason. Women who initiated treatment while pregnant or postpartum were less likely to have died [3.6% (CI: 1.7 to 6.5) compared with 14.9% (CI: 12.4 to 17.7)] and more likely to have migrated [7.5% (CI: 4.7 to 11.2) compared with 3.8% (CI: 2.5 to 5.4)], to be alive with their ART status unknown [16.1% (CI: 12.0 to 20.9) compared with 8.9% (CI: 7.0 to 11.2)] or stopped ART [10.0% (CI: 6.7 to 14.1) compared with 6.4% (CI: 4.7 to 8.4)] (Table 2).
TABLE 2.
Patient Outcomes Disaggregated by Patient Demographic and Clinical Characteristics
| Outcome | Total | |||||||
| Deceased | Transferred out | Stopped ART | Migrated | Reengaged | Alive: ART unknown | Still LTFU | All LTFU | |
| 120 | 315 | 75 | 49 | 225 | 111 | 122 | 1017 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Sex (P < 0.001) | ||||||||
| Female | 70 (9.1) | 258 (33.6) | 48 (6.3) | 43 (5.6) | 164 (21.4) | 90 (11.7) | 94 (12.2) | 767 (75.4) |
| Male | 50 (20.0) | 57 (22.8) | 27 (10.8) | 6 (2.4) | 61 (24.4) | 21 (8.4) | 28 (11.2) | 250 (24.6) |
| Age (P < 0.001) | ||||||||
| 18–29 | 17 (5.1) | 117 (35.1) | 24 (7.2) | 25 (7.5) | 61 (18.3) | 46 (13.8) | 43 (12.9) | 333 (32.7) |
| 30–44 | 55 (11.4) | 147 (30.4) | 37 (7.6) | 21 (4.3) | 116 (24.0) | 50 (10.3) | 58 (12.0) | 484 (47.6) |
| 45–59 | 27 (19.1) | 38 (26.9) | 11 (7.8) | 2 (1.4) | 35 (24.8) | 13 (9.2) | 15 (10.6) | 141 (13.9) |
| 60+ | 21 (36.2) | 13 (22.4) | 3 (5.2) | 1 (1.7) | 12 (20.7) | 2 (3.4) | 6 (10.3) | 58 (5.7) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (0.1) |
| ART reason (P < 0.001) | ||||||||
| Non-PMTCT | 110 (14.9) | 233 (31.6) | 47 (6.4) | 28 (3.8) | 171 (23.2) | 66 (8.9) | 82 (11.1) | 737 (72.5) |
| PMTCT | 10 (3.6) | 82 (29.3) | 28 (10.0) | 21 (7.5) | 54 (19.3) | 45 (16.1) | 40 (14.3) | 280 (27.5) |
| ART start yr (P = 0.251) | ||||||||
| 2014 | 28 (13.3) | 58 (27.5) | 14 (6.6) | 18 (8.5) | 50 (23.7) | 19 (9.0) | 24 (11.4) | 211 (20.7) |
| 2015 | 41 (9.9) | 149 (36.0) | 33 (8.0) | 16 (3.9) | 82 (19.8) | 44 (10.6) | 49 (11.8) | 414 (40.7) |
| 2016 | 46 (13.1) | 100 (28.6) | 24 (6.9) | 14 (4.0) | 82 (23.4) | 41 (11.7) | 43 (12.3) | 350 (34.4) |
| 2017 | 5 (11.9) | 8 (19.0) | 4 (9.5) | 1 (2.4) | 11 (26.2) | 7 (16.7) | 6 (14.3) | 42 (4.1) |
| Time on ART (P < 0.001) | ||||||||
| ≤3 mo | 54 (16.6) | 89 (27.3) | 29 (8.9) | 13 (4.0) | 47 (14.5) | 41 (12.6) | 52 (16.0) | 325 (32.0) |
| 3–6 mo | 18 (9.5) | 62 (32.6) | 13 (6.8) | 8 (4.2) | 31 (16.3) | 30 (15.8) | 28 (14.7) | 190 (18.7) |
| 6–12 mo | 25 (11.0) | 79 (34.6) | 12 (5.3) | 17 (7.5) | 42 (18.4) | 25 (11.0) | 28 (12.3) | 228 (22.4) |
| 12–24 mo | 16 (7.3) | 76 (34.7) | 17 (7.8) | 9 (4.1) | 75 (34.2) | 13 (5.9) | 13 (5.9) | 219 (21.5) |
| >24 mo | 7 (12.7) | 9 (16.4) | 4 (7.3) | 2 (3.6) | 30 (54.5) | 2 (3.6) | 1 (1.8) | 55 (5.4) |
| Baseline CD4 (P < 0.001) | ||||||||
| <100 | 50 (24.3) | 64 (31.1) | 8 (3.9) | 4 (1.9) | 38 (18.4) | 13 (6.3) | 29 (14.1) | 206 (20.2) |
| 100–199 | 32 (17.3) | 46 (24.9) | 16 (8.6) | 8 (4.3) | 41 (22.2) | 19 (10.3) | 23 (12.4) | 185 (18.2) |
| 200–349 | 19 (7.3) | 69 (26.4) | 23 (8.8) | 12 (4.6) | 63 (24.1) | 43 (16.5) | 32 (12.3) | 261 (25.7) |
| 350–499 | 11 (5.7) | 72 (37.3) | 16 (8.3) | 14 (7.3) | 36 (18.6) | 20 (10.4) | 24 (12.4) | 193 (19.0) |
| 500+ | 8 (5.5) | 53 (36.5) | 11 (7.6) | 10 (6.9) | 41 (28.3) | 12 (8.3) | 10 (6.9) | 145 (14.3) |
| Missing | 0 (0) | 11 (40.7) | 1 (3.7) | 1 (3.7) | 6 (22.2) | 4 (14.8) | 4 (14.8) | 27 (2.6) |
| Baseline WHO stage (P = 0.017) | ||||||||
| I | 65 (9.0) | 230 (31.8) | 55 (7.6) | 38 (5.3) | 159 (22.0) | 88 (12.2) | 87 (12.0) | 722 (71.0) |
| II | 21 (14.7) | 42 (29.4) | 12 (8.4) | 6 (4.2) | 34 (23.8) | 11 (7.7) | 17 (11.9) | 143 (14.1) |
| III | 26 (20.1) | 39 (30.2) | 7 (5.4) | 4 (3.1) | 28 (21.7) | 9 (7.0) | 16 (12.4) | 129 (12.7) |
| IV | 5 (50.0) | 1 (10.0) | 1 (10.0) | 0 (0) | 2 (20.0) | 0 (0) | 1 (10.0) | 10 (1.0) |
| Missing | 3 (23.1) | 3 (23.1) | 0 (0) | 1 (7.7) | 2 (15.4) | 3 (23.1) | 1 (7.7) | 13 (1.3) |
| Refill schedule (P < 0.001) | ||||||||
| 1 mo | 84 (12.5) | 210 (31.2) | 48 (7.1) | 30 (4.5) | 143 (21.3) | 77 (11.4) | 80 (11.9) | 672 (66.1) |
| 2 mo | 24 (10.3) | 71 (30.5) | 21 (9.0) | 14 (6.0) | 43 (18.4) | 24 (10.3) | 36 (15.5) | 233 (22.9) |
| 3 mo | 9 (11.4) | 30 (38.0) | 3 (3.8) | 5 (6.3) | 18 (22.8) | 9 (11.4) | 5 (6.3) | 79 (7.8) |
| >3 mo | 3 (9.1) | 4 (12.1) | 3 (9.1) | 0 (0) | 21 (63.6) | 1 (3.0) | 1 (3.0) | 33 (3.2) |
| Health facility (P < 0.001) | ||||||||
| Agincourt | 27 (9.9) | 66 (24.3) | 15 (5.5) | 11 (4.0) | 110 (37.1) | 21 (7.7) | 22 (8.1) | 272 (26.7) |
| Belfast | 16 (8.6) | 52 (28.0) | 13 (7.0) | 12 (6.4) | 32 (17.2) | 29 (15.6) | 32 (17.2) | 186 (18.3) |
| Cunningmore | 11 (19.0) | 21 (36.2) | 8 (13.8) | 1 (1.7) | 7 (12.1) | 5 (8.6) | 5 (8.6) | 58 (5.7) |
| Justicia | 20 (16.7) | 30 (25.0) | 13 (10.8) | 7 (5.8) | 14 (11.7) | 11 (9.2) | 25 (20.8) | 120 (11.8) |
| Kildare | 16 (13.7) | 50 (42.7) | 10 (8.5) | 8 (6.8) | 14 (12.0) | 9 (7.7) | 10 (8.5) | 117 (11.5) |
| Lillydale | 19 (11.4) | 51 (30.7) | 9 (5.4) | 7 (4.2) | 37 (22.3) | 24 (14.5) | 19 (11.4) | 166 (16.3) |
| Thulamahashe | 3 (12.0) | 4 (16.0) | 1 (4.0) | 0 (0) | 7 (28.0) | 6 (24.0) | 4 (16.0) | 25 (2.4) |
| Xanthia | 9 (12.2) | 41 (55.4) | 6 (8.1) | 3 (4.0) | 4 (5.4) | 6 (8.1) | 5 (6.8) | 74 (7.3) |
| Time since last appointment (P < 0.001) | ||||||||
| ≤1 yr | 48 (9.1) | 146 (27.8) | 40 (7.6) | 16 (3.0) | 171 (32.5) | 51 (9.7) | 54 (10.3) | 526 (51.7) |
| 1–2 yrs | 53 (14.4) | 134 (36.3) | 26 (7.0) | 19 (5.1) | 46 (12.5) | 44 (11.9) | 47 (12.7) | 369 (36.3) |
| >2 yrs | 19 (15.6) | 35 (28.7) | 9 (7.4) | 14 (11.5) | 8 (6.6) | 16 (13.1) | 21 (17.2) | 122 (12.0) |
| AHDSS outcome (P < 0.001) | ||||||||
| Still in HDSS | 17 (3.4) | 177 (35.0) | 52 (10.3) | 7 (1.4) | 141 (27.9) | 111 (22.0) | 0 (0) | 505 (49.7) |
| Deceased | 70 (94.6) | 4 (5.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 74 (7.3) |
| Migrated | 22 (8.1) | 86 (31.8) | 19 (7.0) | 34 (12.6) | 58 (21.5) | 0 (0) | 51 (18.9) | 270 (26.5) |
| Not linked | 11 (6.5) | 48 (28.6) | 4 (2.4) | 8 (4.8) | 26 (15.5) | 0 (0) | 71 (42.3) | 168 (16.5) |
Most deaths occurred in the groups where baseline CD4 <200 cells/µL (Figure 1). Men were at highest risk of mortality, and pregnant women were at the lowest risk (Figure 2). Men and pregnant women also had higher risks of being alive and not in care compared with nonpregnant women (Figure 2). The mortality risk appeared to be similar for all CD4 categories for pregnant women unlike for nonpregnant women (see Supplementary Figure 4 and 5, Supplemental Digital Content, http://links.lww.com/QAI/B486). We also report on probable causes of death ascertained using VA data (see Supplementary information 2, Supplemental Digital Content, http://links.lww.com/QAI/B486).
FIGURE 1.
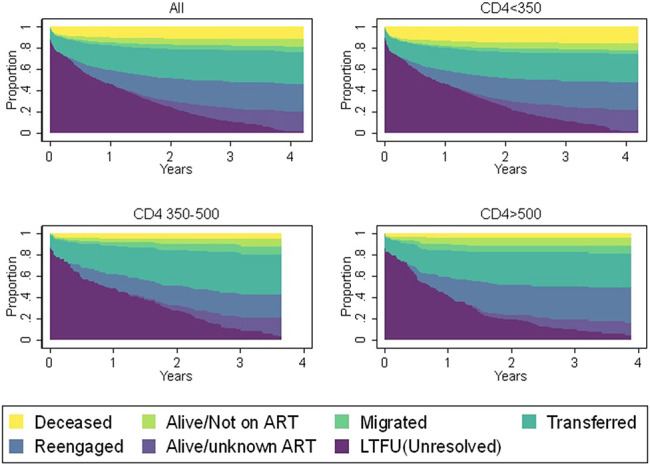
Status of patients by baseline CD4 and years since their last clinic visit.
FIGURE 2.
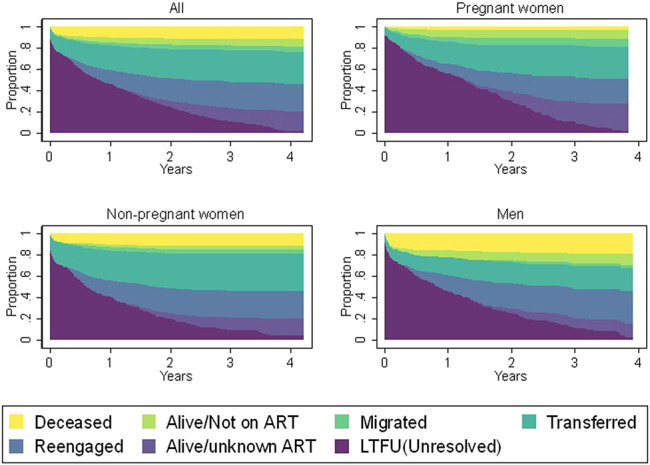
Status of patients by sex, pregnancy status at ART initiation, and years since their last clinic visit.
Factors Associated With Death
Of 120 deaths, 48 (37.2%) occurred before the patient's next visit date, 42 (32.6%) occurred after the patient's next scheduled visit date but before they would have met the criteria for LTFU, 30 (23.3%) occurred after the patient had met the criteria for LTFU, and 9 (7.0%) had a missing date of death.
In multivariable competing risk regression, being pregnant at ART initiation (aHR: 0.36, 95% CI: 0.15 to 0.87), and longer time on ART (12–24 months aHR: 0.44, 0.23 to 0.85) were associated with lower hazard of death following LTFU. Older age (≥60 years aHR: 8.86, 3.90–20.14) and lower CD4 at ART initiation (<100 cells/µL aHR: 3.77, 2.31–6.15; 100–199 cells/µL aHR: 2.35, 1.49–3.69) were associated with a higher hazard of death (Table 3).
TABLE 3.
Factors Associated With Death
| HR (95% CI) | P | aHR (95% CI) n = 932 | P | |
| Sex | ||||
| Female | Reference | __ | ||
| Male | 2.10 (1.57 to 2.81) | <0.001 | ||
| Age | ||||
| 18–29 | Reference | __ | Reference | __ |
| 30–44 | 2.68 (1.30 to 5.51) | 0.007 | 2.37 (0.98 to 5.75) | 0.056 |
| 45–59 | 4.73 (3.13 to 7.15) | <0.001 | 2.96 (1.44 to 6.08) | 0.003 |
| 60+ | 11.31 (5.32 to 24.06) | <0.001 | 8.86 (3.90 to 20.14) | <0.001 |
| ART reason | ||||
| Non-PMTCT | Reference | __ | Reference | __ |
| PMTCT | 0.17 (0.07 to 0.43) | <0.001 | 0.36 (0.15 to 0.87) | 0.022 |
| ART start yr | ||||
| 2014 | 1.29 (0.82 to 2.04) | 0.268 | ||
| 2015 | Reference | __ | ||
| 2016 | 1.20 (0.67 to 2.14) | 0.536 | ||
| 2017 | 1.28 (0.83 to 1.97) | 0.258 | ||
| Time on ART | ||||
| ≤3 mo | Reference | __ | Reference | __ |
| 3–6 mo | 0.56 (0.31 to 0.99) | 0.048 | 0.76 (0.46 to 1.25) | 0.276 |
| 6–12 mo | 0.74 (0.49 to 1.13) | 0.167 | 0.82 (0.56 to 1.20) | 0.307 |
| 12–24 mo | 0.53 (0.31 to 0.91) | 0.023 | 0.44 (0.23 to 0.85) | 0.015 |
| >24 mo | 0.91 (0.41 to 2.05) | 0.828 | 0.60 (0.23 to 1.56) | 0.297 |
| Baseline CD4 | ||||
| <100 | 4.26 (3.11 to 5.82) | <0.001 | 3.77 (2.31 to 6.15) | <0.001 |
| 100–199 | 2.57 (1.60 to 4.12) | <0.001 | 2.35 (1.49 to 3.69) | <0.001 |
| 200–349 | Reference | __ | Reference | __ |
| 350–499 | 0.78 (0.39 to 1.55) | 0.483 | 1.11 (0.53 to 2.36) | 0.776 |
| 500+ | 0.82 (0.24 to 2.79) | 0.756 | 1.13 (0.35 to 3.67) | 0.840 |
| Baseline WHO stage | ||||
| I | Reference | __ | Reference | __ |
| II | 1.71 (0.98 to 3.00) | 0.061 | 0.86 (0.40 to 1.86) | 0.706 |
| III | 2.70 (1.77 to 4.14) | <0.001 | 1.36 (0.94 to 1.96) | 0.102 |
| IV | 6.64 (3.08 to 14.32) | <0.001 | 3.14 (1.14 to 8.59) | 0.026 |
| Refill schedule | ||||
| 1 mo | Reference | __ | ||
| 2 mo | 0.83 (0.37 to 1.86) | 0.647 | ||
| 3 mo | 0.93 (0.49 to 1.75) | 0.824 | ||
| >3 mo | 0.74 (0.22 to 2.42) | 0.615 | ||
| Health facility | ||||
| Agincourt | Reference | __ | Reference | __ |
| Belfast | 1.03 (0.97 to 1.09) | 0.345 | 0.80 (0.61 to 1.05) | 0.108 |
| Cunningmore | 3.14 (2.98 to 3.31) | <0.001 | 3.39 (2.92 to 3.94) | <0.001 |
| Justicia | 2.10 (1.98 to 2.24) | <0.001 | 1.70 (1.55 to 1.86) | <0.001 |
| Kildare | 1.90 (1.84 to 1.95) | <0.001 | 1.08 (0.78 to 1.50) | 0.639 |
| Bhubezi | 1.26 (1.19 to 1.34) | <0.001 | 0.96 (0.73 to 1.28) | 0.810 |
| Thulamahashe | 0.93 (0.91 to 0.95) | <0.001 | 1.59 (1.15 to 2.22) | 0.005 |
| Xanthia | 1.75 (0.70 to 1.80) | <0.001 | 1.98 (1.64 to 2.38) | <0.001 |
| Time since last appointment | ||||
| ≤1 yr | Reference | __ | Reference | __ |
| 1–2 yrs | 1.57 (1.03 to 2.39) | 0.037 | 1.75 (1.10 to 2.78) | 0.018 |
| >2 yrs | 1.65 (0.73 to 3.75) | 0.228 | 0.81 (0.39 to 1.67) | 0.564 |
All CD4 data was retrieved from clinic records (files and TIER.Net). All other clinical data was retrieved from the PIRL database (sex and age were crosschecked in clinic and HDSS records).
DISCUSSION
We describe the treatment outcomes of HIV patients enrolled in care between April 2014 and August 2017 who had become LTFU in a rural South African setting as determined through a comprehensive record review and tracing study. Using multiple data sources and methods, we managed to ascertain the outcomes of 88% of the patients LTFU, a figure that is higher than most studies included in a recent systematic review of tracing studies in sub-Saharan Africa.12 We found that 31% of patients LTFU had transferred to another facility, 22% had re-engaged in care, and 12% of patients had died. These percentages varied by sex, reason for ART initiation, and baseline CD4 cell count. The differences for pregnant and postpartum women are particularly pertinent given that they represent the first iterations of treatment as prevention and could provide an indication for what to expect with the move to test and treat for all people living with HIV.
The proportion of patients reported as LTFU who had died in our study was substantially lower than the 42% and 39% reported in earlier systematic reviews of tracing studies from sub-Saharan Africa.12,18 Even if all the patients remaining LTFU after record review and tracing had died, mortality in our study would only rise to 24%. This lower percentage of deaths compared with the previous reviews is likely to be because of a healthier cohort of patients initiating treatment. We found that pregnant women were less likely to have died, an encouraging trend if it does translate to the general ART treatment population as less immunocompromised people begin to initiate ART. Mortality following LTFU may decrease further as universal test and treat policies result in growing proportions of asymptomatic patients initiating ART.
In competing risk survival analysis, being pregnant at ART initiation, higher baseline CD4, and longer time on ART were protective against death, whereas older age was found to be associated with a higher hazard of death following LTFU. Our findings suggest baseline CD4 cell count, WHO stage, and older age remain accurate measures for determining which patients are at highest risk for death,42,44,45 and these characteristics could be used to help prioritize tracing interventions. Whereas mortality risk appeared to wane with increasing CD4 at baseline for nonpregnant women and men, mortality appeared to be similar for all CD4 categories for women who initiated treatment for PMTCT. This may reflect the fact that their mortality risk was more influenced by other factors such as pregnancy related complications than by HIV.46,47 This could also be because of the fact that pregnant women were healthier in WHO staging compared with nonpregnant women and men, given the same CD4 at baseline, also reflected by the lower proportion of pregnant patients with a baseline CD4 <100 cells/µL. This discrepancy could also be related to temporary declines in CD4 count during pregnancy.48
Patients lost early on in treatment were at higher risk of death and this remained statistically significant even when controlling for baseline CD4, indicating that a longer duration on ART before attrition may reduce the risk of death. This protective effect appeared to be strongest for those who had been on ART 12–24 months before they became LTFU. This suggests that in settings with limited resources, tracing should be considered most urgent for newly ART-initiated patients who drop out of care. On the other hand, it may also indicate that some patients are still initiating treatment too late. In this study, 11% of nonpregnant patients had a CD4 cell count >500 cells/µL (compared with 23% of pregnant women), reflecting the fact that universal test and treat was not adopted in South Africa until September 2016.49,50 Men were disproportionately over-represented in the <200 cells/µL baseline CD4 category despite South African guidelines for ART initiation with CD4 <500 cells/µL having been in effect since January 2015.51 Men especially appear to be harder to reach and come into care later, similar to findings from other studies,52–55 and emphasizes the need to reach men earlier with ART.56–58
However, as the proportion of LTFU attributable to mortality dwindles, other outcomes are likely to become more prevalent. In our study, transfer to another facility accounted for 31% of patients who were reported as LTFU, which is higher than a previous systematic review.12 Other studies have suggested transfers become more common as programs expand and offer ART closer to patients' homes.12,59,60 Women were more likely to have transferred their care to another clinic. For pregnant women, this could reflect the higher mobility common during pregnancy and childbirth.13,61,62 Furthermore, given that most of these transfers were not reported to the sending facility similar to previous studies,12,15 these types of transfers could potentially lead to the spread of drug resistance in situations where ART experienced patients are offered regimens that have lost any therapeutic value due to drug resistance.63 Silent transfers may also lead to over-estimates of the number of people newly initiating ART and the number of people who have ever initiated ART. The current system of transferring patients could be improved by better referral systems, patient education, regular information exchange between clinics, and provider training.64
We found that 7.4% of patients had stopped treatment, with this being more common for women who initiated ART while pregnant, which adds to findings from previous studies that suggest that feeling healthy contributes to attrition for pregnant women.65,66 This figure is lower than the 28.6% of treatment interruptions reported in a recent systematic review.12 This may suggest that interventions to reduce interruptions, including routine tracing, are working well in this setting, further supported by the number of re-engagements in care that were observed in our study.
Our data showed that pregnant women and the general treatment cohort still differ significantly especially regarding immune system markers such as CD4. However, with the advent of test and treat, these groups may increasingly become similar in this regard and hence outcomes for pregnant women living with HIV could represent what treatment programs may expect to see in the future regarding patients that become LTFU especially those of a similar age. With ART programs in sub-Saharan Africa maturing, and with less immunologically compromised patients initiating ART, patients that become LTFU will be less likely to have died, whereas ART cessation or interruption and re-engagement in care are likely to become more common. Treatment programs will increasingly need to reallocate resources to deal with improving the clinic transfer process and invest in tracing and psychosocial support to get patients back in care or else risk having high community viral load which may increase the probability of onward transmission. We showed that 6% of patients who were late for a scheduled appointment returned before they officially became categorized as LTFU. These patients in theory would have received the routine tracing intervention offering further evidence of its utility, in line with a previous study that has highlighted how early active tracing of patients LTFU may improve patient outcomes and retention in care.8
Furthermore, given that most resolutions came through record review of tracing logbooks and clinic records, this study demonstrates that routine patient tracing still has utility for improving the completeness and accuracy of patient records. The availability of these data within the clinics suggests that routinely-collected data, especially those from the 2 organizations that assist in patient tracing needs to be better collated, integrated and recorded to ensure that patient outcomes are reflected in their clinic files and on TIER.Net. This study also demonstrates the utility of other data sources such as HDSS data. Given the push to integrate national ID numbers in patient profiles, clinics operating within similar health and demographic surveillance sites should consider liaising with these sites to improve the capture of deaths and migrations. Policy makers should also consider using South Africa's national death registry within clinics as this has been shown to be useful in other studies.67,68
This study had several limitations. First, the record review was cross-sectional; we only consulted clinic records at one point in time, whereas, some of these records may have subsequently been updated. Furthermore, we only consulted HBC and RtC logbooks that were afforded to us and it is possible that we might have missed some with information on patients we were trying to find. The observational nature of the study limited our ability to assess predictive factors and causality. We failed to ascertain the outcomes for 12% of our cohort and this may introduce some downward bias to our estimates. Finally, as we only resolved cause of death in 48.3% of patients found to have died, this data should be interpreted with caution. As we attempted to trace all adult patients LTFU, rather than a sample, these results are likely to be generalizable to other rural sub-Saharan settings. A strength of this study is the use of multiple data sources.
In conclusion, our study offers evidence for the growing utility for routine patient tracing. The different distribution of outcomes among Option B+ women suggests that different program mortality and attrition correction factors will be needed as universal test and treat becomes more established. Higher mortality among men emphasizes the importance of programmatic efforts to reach men earlier and treatment programs need to improve transfer procedures to make it more conducive for patients to move between clinics.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all the participants in the study.
Footnotes
Supported by the Economic and Social Research Council (ES/JS00021/1), the Bill and Melinda Gates Foundation for the MeSH Consortium (OPP1120138), the Bill and Melinda Gates Foundation ALPHA grant (OPP1164897), and the MRC SHAPE UTT grant (MR/P014313/1).
The authors have no conflicts of interest to disclose.
The study was conceived by D.E., A.W. and G.R. Fieldwork was planned and executed by D.E., F.X.G.O. and C.W.K. Data collection was supervised by D.E. Analyses were conducted by D.E. with input from all authors. All authors contributed to the interpretation of the findings. The manuscript was drafted by D.E. with input from J.R., B.R. and all the authors. All authors read and approved the final manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The dataset used in these analyses are not yet publicly available as they currently being utilised for the first author's PhD research. They will be made available on request at the end of his PhD in 2021. Data from the PIRL database are available by making a data request to the Agincourt HDSS data manager.
REFERENCES
- 1.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. [DOI] [PubMed] [Google Scholar]
- 2.Group TISS. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Guideline on when to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 4.Tymejczyk O, Brazier E, Yiannoutsos CT, et al. Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: regression discontinuity analysis. PLoS Med. 2019;16:e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS Lond Engl. 2010;24:563–572. [DOI] [PubMed] [Google Scholar]
- 6.Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy: a systematic review and meta-analysis. AIDS Lond Engl. 2018;32:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knettel BA, Cichowitz C, Ngocho JS, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ era: a systematic review and meta-analysis of studies in Africa. JAIDS J Acquir Immune Defic Syndr. 2017;77:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the “Back-to-Care” project in Lilongwe, Malawi. Trop Med Int Health. 2010;15(suppl 1):82–89. [DOI] [PubMed] [Google Scholar]
- 9.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011;16:1297–1313. [DOI] [PubMed] [Google Scholar]
- 10.McMahon JH, Elliott JH, Hong SY, et al. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One. 2013;8:e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Retention in HIV Programmes: Defining the Challenges and Identifying Solutions: Meeting Report, 13–15 September 2011. World Health Organization; 2012. Available at: https://apps.who.int/iris/handle/10665/44878. Accessed January 21, 2020. [Google Scholar]
- 12.Wilkinson LS, Skordis-Worrall J, Ajose O, et al. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20:365–379. [DOI] [PubMed] [Google Scholar]
- 13.Clouse K, Vermund SH, Maskew M, et al. Mobility and clinic switching among postpartum women considered lost to HIV care in South Africa. J Acquir Immune Defic Syndr. 2017;74:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tweya H, Feldacker C, Estill J, et al. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PLoS One. 2013;8:e75761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zürcher K, Mooser A, Anderegg N, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health 2017;22:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover J, Johnson P, Hallett T, et al. The Spectrum projection package: improvements in estimating incidence by age and sex, mother-to-child transmission, HIV progression in children and double orphans. Sex Transm Infect. 2010;86(suppl 2):ii16–ii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman S, Wu Y, Lahuerta M, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS Lond Engl. 2014;28:2429–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19:1360–1366. [DOI] [PubMed] [Google Scholar]
- 21.Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 Years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis. 2018;66(suppl_2):S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS Lond Engl. 2012;26:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–107. [DOI] [PubMed] [Google Scholar]
- 24.Dow A, Dube Q, Pence BW, et al. Postpartum depression and HIV infection among women in Malawi. J Acquir Immune Defic Syndr. 2014;65:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lettow M, Bedell R, Mayuni I, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc. 2014;17:18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clouse K, Schwartz S, Van Rie A, et al. What they wanted was to give birth; nothing else: barriers to retention in option B+ HIV care among postpartum women in South Africa. J Acquir Immune Defic Syndr. 2014;67:e12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes R, Donnell D, Floyd S, et al. PopART Study Team. Impact of Universal Testing and Treatment in Zambia and South Africa: HPTN071 (PopART). Seattle, WA; 2019. Available at: http://www.croiconference.org/sites/default/files/uploads/92LB.pdf. Accessed May 16, 2019. [Google Scholar]
- 28.Dabis F; TasP study group. The Impact of Universal Test and Treat on HIV Incidence in a Rural South African Population: ANRS 12249 TasP Trial, 2012–2016. Durban, South Africa; 2016. Available at: http://programme.aids2016.org/Abstract/Abstract/10537. Accessed May 16, 2019. [Google Scholar]
- 29.Havlir D; SEARCH collaboration. SEARCH Community Cluster Randomized Study of HIV “Test and Treat” Using Multi- Disease Approach and Streamlined Care in Rural Uganda and Kenya. Amsterdam, Netherlands; 2018. Available at: https://programme.aids2018.org/Abstract/Abstract/13469. Accessed May 16, 2019. [Google Scholar]
- 30.Kahn K, Collinson MA, Gómez-Olivé FX, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol. 2012;41:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tollman SM, Herbst K, Garenne M, et al. The Agincourt demographic and health study—site description, baseline findings and implications. S Afr Med J. 1999;89:858–864. [PubMed] [Google Scholar]
- 32.Gómez-Olivé FX, Angotti N, Houle B, et al. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care. 2013;25:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byass P, Hussain-Alkhateeb L, D'Ambruoso L, et al. An integrated approach to processing WHO-2016 verbal autopsy data: the InterVA-5 model. BMC Med. 2019;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO|ICD-10 Online Versions. WHO; Available at: http://www.who.int/classifications/icd/icdonlineversions/en/. Accessed April 20, 2020. [Google Scholar]
- 35.Kahn K, Tollman SM, Garenne M, et al. Validation and application of verbal autopsies in a rural area of South Africa. Trop Med Int Health. 2000;5:824–831. [DOI] [PubMed] [Google Scholar]
- 36.Byass P, Calvert C, Miiro-Nakiyingi J, et al. InterVA-4 as a public health tool for measuring HIV/AIDS mortality: a validation study from five African countries. Glob Health Action. 2013;6:22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.South Africa: National Department of Health. Adherence Guidelines for HIV, TB and NCDs: Policy and Service Delivery Guidelines for Linkage to Care, Adherence to Treatment and Retention in Care. 2016. Available at: http://www.differentiatedservicedelivery.org/Portals/0/adam/Content/3QvfVVZSK0G1PlKvq1vTVw/File/15%202%2016%20AGL%20policy%20and%20service%20delivery%20guidelines.pdf. Accessed March 8, 2018. [Google Scholar]
- 38.Rentsch CT, Kabudula CW, Catlett J, et al. Point-of-contact Interactive Record Linkage (PIRL): a software tool to prospectively link demographic surveillance and health facility data. Gates Open Res. 2018;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17:18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacPherson P, Moshabela M, Martinson N, et al. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103:588–593. [DOI] [PubMed] [Google Scholar]
- 41.Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. 2014;67:e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.StataCorp. Stata Statistical Software. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 44.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. [DOI] [PubMed] [Google Scholar]
- 45.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garenne M, Kahn K, Collinson MA, et al. Maternal mortality in rural South Africa: the impact of case definition on levels and trends. Int J Womens Health. 2013;5:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garenne M, Kahn K, Collinson M, et al. Protective effect of pregnancy in rural South Africa: questioning the concept of indirect cause of maternal death. PLoS One. 2013;8:e64414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heffron R, Donnell D, Kiarie J, et al. A prospective study of the effect of pregnancy on CD4 counts and plasma HIV-1 RNA concentrations of antiretroviral-naive HIV-1 infected women. J Acquir Immune Defic Syndr. 2014;65:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer-Rath G, Johnson LF, Pillay Y, et al. Changing the South African national antiretroviral therapy guidelines: the role of cost modelling. PLoS One. 2017;12:e0186557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.South Africa: National Department of Health. Implementation of the Universal Test and Treat Strategy for HIV Positive Patients and Differentiated Care for Stable Patients. 2016. Available at: https://sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Decongestion%20CCMT%20Directorate.pdf. Accessed July 24, 2019. [Google Scholar]
- 51.South Africa: National Department of Health. National Consolidated Guidelines for the Prevention of Mother-To-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. 2015. Available at: https://www.scribd.com/doc/268965647/National-Consolidated-Guidelines-for-PMTCT-and-the-Management-of-HIV-in-Children-Adolescents-and-Adults. Accessed July 24, 2019. [Google Scholar]
- 52.Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9:e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braitstein P, Boulle A, Nash D, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health. 2008;17:47–55. [DOI] [PubMed] [Google Scholar]
- 54.Stringer JSA, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. [DOI] [PubMed] [Google Scholar]
- 55.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS Lond Engl. 2010;24:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills EJ, Beyrer C, Birungi J, et al. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9:e1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katirayi L, Chadambuka A, Muchedzi A, et al. Echoes of old HIV paradigms: reassessing the problem of engaging men in HIV testing and treatment through women's perspectives. Reprod Health 2017;14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulerwitz J, Michaelis A, Verma R, et al. Addressing gender dynamics and engaging men in HIV programs: lessons learned from horizons research. Public Health Rep. 2010;125:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nglazi MD, Kaplan R, Orrell C, et al. Increasing transfers-out from an antiretroviral treatment service in South Africa: patient characteristics and rates of virological non-suppression. PLoS One. 2013;8:e57907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B, Losina E, Stark R, et al. Loss to follow-up in a community clinic in South Africa—roles of gender, pregnancy and CD4 count. S Afr Med J. 2011;101:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson L, Lewis J, Grant AD, et al. Patient attrition between diagnosis with HIV in pregnancy-related services and long-term HIV care and treatment services in Kenya: a retrospective study. J Acquir Immune Defic Syndr. 2012;60:e90–97. [DOI] [PubMed] [Google Scholar]
- 63.Castro H, Pillay D, Sabin C, et al. UK Collaborative Group on HIV Drug Resistance. Effect of misclassification of antiretroviral treatment status on the prevalence of transmitted HIV-1 drug resistance. BMC Med Res Methodol. 2012;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katirayi L, Chouraya C, Kudiabor K, et al. Lessons learned from the PMTCT program in Swaziland: challenges with accepting lifelong ART for pregnant and lactating women—a qualitative study. BMC Public Health 2016;16:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim MH, Zhou A, Mazenga A, et al. Why did I stop? Barriers and facilitators to uptake and adherence to ART in option B+ HIV care in lilongwe, Malawi. PLoS One. 2016;11:e0149527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox MP, Brennan A, Maskew M, et al. Using vital registration data to update mortality among patients lost to follow-up from ART programs: evidence from the themba lethu clinic, South Africa. Trop Med Int Health. 2010;15:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Cutsem G, Ford N, Hildebrand K, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6:e14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


