Abstract
Endothelial dysfunction caused by high glucose is recognized as an important event in the pathogenesis of diabetes-related vascular complications. Ropivacaine is considered to have the best safety profile among the commonly used amide local anesthetics, but the extent of its actions remains incompletely understood. Here, we used human umbilical vein endothelial cells exposed to high glucose to explore the effects of ropivacaine on oxidative stress and markers of inflammation. Ropivacaine treatment exerted significant beneficial effects by rescuing oxidative stress and downregulating interleukin (IL)-1β and IL-18. We also found that ropivacaine could inhibit the secretion of the high-mobility group box 1 protein and improve cell viability. Importantly, sirtuin-1 (SIRT1) knockdown experiments show that the inhibitory effects of ropivacaine against NLRP3 inflammasome activation are dependent on SIRT1. Taken together, these results demonstrate the potential of ropivacaine as a promising therapy against diabetic endothelial dysfunction.
1. Introduction
Cardiovascular disease is considered the most common complication associated with diabetes.1,2 The etiopathogenesis of diabetes-associated cardiovascular disease is complicated, but endothelial inflammation and dysfunction are recognized as playing major roles in disease development and progression. In particular, the chronic hyperglycemic state resulting from diabetes-associated reduced insulin production leads to oxidative stress and a sustained inflammatory response in endothelial cells, thereby causing a loss of function and increased apoptosis.3,4 Endothelial dysfunction alters the ability of the endothelium to regulate vasodilation and increases inflammation. It has been reported that among other things, high glucose-induced endothelial dysfunction impairs signal transduction, upregulates the expression of endothelial constricting factors, and downregulates the release of endothelium-derived relaxing factors. Meanwhile, the degradation of endothelial-derived relaxing factors is increased, thereby further promoting vascular constriction.5 Oxidative stress has also been implicated in endothelial dysfunction. High glucose increases cellular and mitochondrial reactive oxygen species (ROS) and nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4). NOX4 regulates the production of ROS, and suppression of NOX4 has been shown to ameliorate diabetes-associated endothelial dysfunction.6 Thioredoxin-interacting protein (TxNIP) is a crucial inhibitor that is upregulated in response to high glucose and works to inhibit the antioxidant thioredoxin, thereby promoting further imbalance of the oxidant/antioxidant ratio. TxNIP is also a mechanism for activating the NLRP3 inflammasome.7,8
The term inflammasome is used to describe a set of multi-protein systems that are activated after infection, inflammation, and the autoimmune response.9 The NLRP3 inflammasome, an important complex involved in high glucose-induced endothelial inflammation, is composed of NLRP3, apoptosis-associated specklike protein containing a C-terminal caspase-recruitment domain (ASC), and caspase-1. This inflammasome plays a vital role in promoting inflammation by guiding the maturation of interleukin (IL)-1β and IL-18.10,11 Inhibition of the NLRP3 inflammasome is considered as a promising therapeutic target against the development of type I and type II diabetes9,12 as well as several diabetes-associated complications, including atherosclerosis,13,14 diabetic cardiomyopathy,15 and diabetic nephropathy.16 However, the cellular pathways involved in NLRP3 inflammasome activation are poorly understood.
Ropivacaine is one of the most widely used amide-based local anesthetics in both clinical and dental settings. Local anesthetics work by blocking neural signal transduction through inhibition of sodium-ion uptake in a limited area of the body and for a limited amount of time.17 While research has suggested potential cytotoxic and apoptotic effects of amino amide local anesthetics including bupivacaine, ropivacaine, levobupivacaine, and mepivacaine, a recent literature review reported that among them, ropivacaine had the best safety profile in terms of cytotoxicity.18 Recent research has also demonstrated a potential anti-inflammatory effect of ropivacaine through inhibition of proinflammatory cytokines.19,20 Interestingly, it was recently shown that ropivacaine could prevent apoptosis, inhibit the expression of IL-1β, IL-6, and TNF-α, and suppress the activation of nuclear factor kappa B (NF-κB) inflammatory signaling pathways, thereby significantly suppressing inflammation.20 Sirtuin-1 (SIRT1) is involved in regulating innate immunity, cell senescence, apoptosis, metabolism, and the cell cycle. SIRT1 has been demonstrated to exert a protective effect against endothelial dysfunction by inhibiting premature cell senescence and downregulating the expression of plasminogen activator inhibitor 1 and endothelial nitric oxide.21 Importantly, SIRT1 negatively regulates the activity of the NLRP3 inflammasome.22 Here, we investigated the possible role of ropivacaine in high glucose-induced NLRP3 inflammasome activation in human umbilical vein endothelial cells (HUVECs).
2. Results
2.1. Ropivacaine Reduced the Production of Mitochondrial ROS
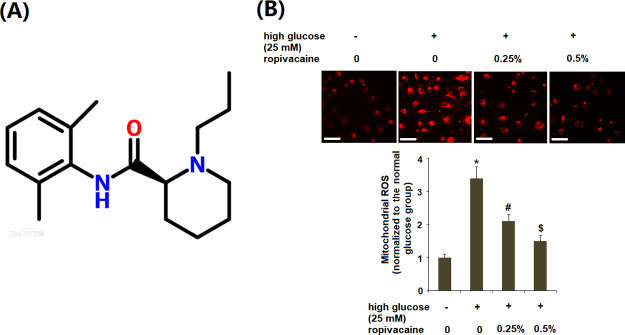
Concentrations of 0.25 and 0.5% ropivacaine are used clinically for regional anesthesia.23,24 The molecular structure of ropivacaine is shown in Figure 1A. Therefore, these two doses of ropivacaine were used. The effects of ropivacaine treatment on high glucose-induced oxidative stress were determined by measuring the generation of ROS and NOX4. As shown in Figure 1B, the results of MitoSOX staining reveal that exposure to high glucose resulted in a greater than 3-fold increase in production of ROS, which was decreased to only a 1.5-fold baseline by ropivacaine (0.25, 0.5%).
Figure 1.
Ropivacaine ameliorated the production of mitochondrial ROS in HUVECs. (A) Molecular structure of ropivacaine and (B) mitochondrial ROS was measured with MitoSOX. Scale bars, 100 μm [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
2.2. Ropivacaine Reduced High Glucose-Induced NOX4
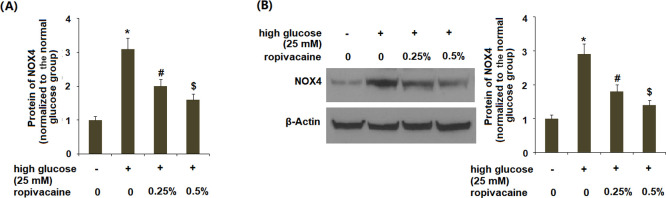
Next, we determined the roles of ropivacaine on NOX4. The results in Figure 2A,B indicate that high glucose elevated NOX4 at both the gene and protein levels, which was ameliorated by ropivacaine.
Figure 2.
Ropivacaine reduced the expression of NOX4. (A) mRNA of NOX4 and (B) protein of NOX4 [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
2.3. Ropivacaine Suppressed the High Glucose-Induced Release of Lactate Dehydrogenase
We assessed cell viability upon exposure to high glucose as well as the two doses of ropivacaine (0.25 and 0.5%) by determining the release of lactate dehydrogenase (LDH). Exposure to high glucose significantly increased the rate of cell death, which was attenuated by ropivacaine (Figure 3).
Figure 3.
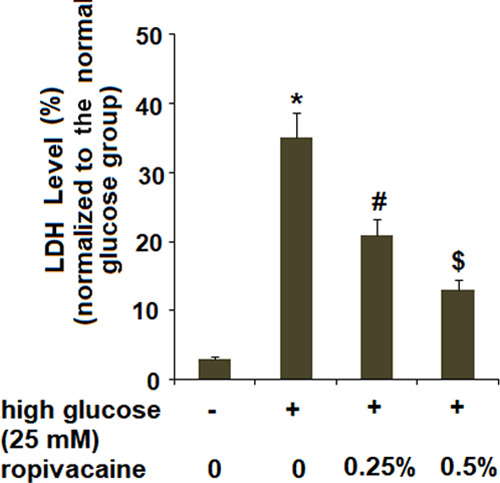
Ropivacaine suppressed the release of LDH. The release of LDH was measured using a kit [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 6].
2.4. Ropivacaine Inhibited High Glucose-Induced Secretions of High-Mobility Group Box 1
Next, we determined the effects of ropivacaine on the release of high-mobility group box 1 (HMGB1). The results obtained using a commercial HMGB1 kit revealed that high glucose significantly increased the secretion of HMGB1, which was suppressed by ropivacaine (Figure 4).
Figure 4.
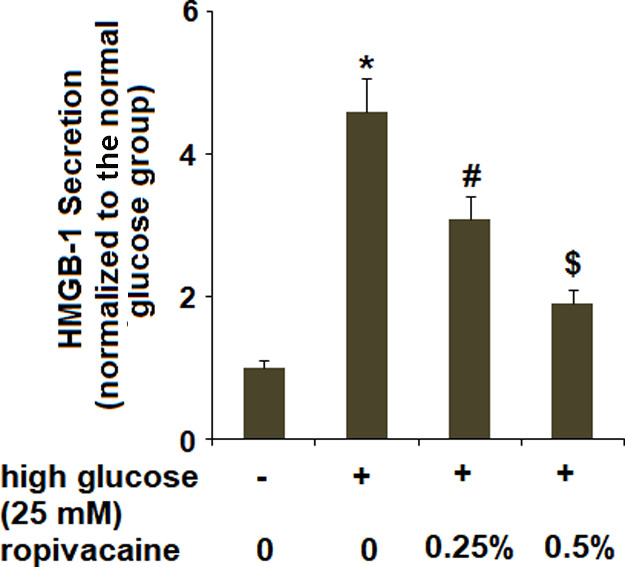
Ropivacaine inhibited secretions of HMGB-1. The secretions of HMGB-1 were determined using a commercial kit [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 6].
2.5. Ropivacaine Reduced TxNIP
We then determined whether ropivacaine treatment impacted TxNIP expression. As shown in Figure 5A,B, the two doses of ropivacaine could prevent the increase in TxNIP caused by high glucose.
Figure 5.
Ropivacaine reduced TxNIP. (A) messenger RNA (mRNA) of TxNIP and (B) protein of TxNIP [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
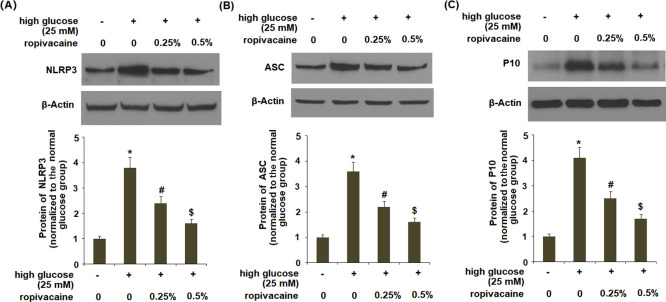
2.6. Ropivacaine Inhibited the Activation of the NLRP3 Inflammasome in HUVECs
The results in Figure 6A–C indicate that ropivacaine significantly reduced the levels of the NLRP3 protein, ASC, and P10 induced by high glucose.
Figure 6.
Ropivacaine inhibits the activation of the NLRP3 inflammasome in HUVECs. (A) NLRP3, (B) ASC, and (C) P10 [*, P < 0.01 vs normal group; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
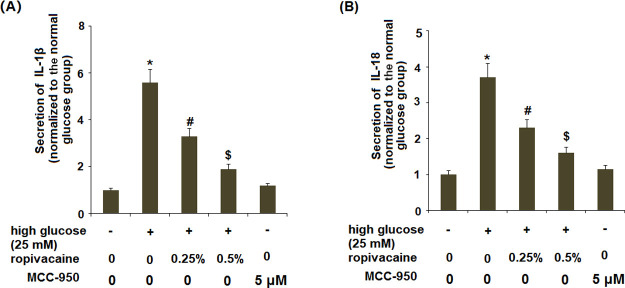
2.7. Ropivacaine Inhibited the Expression of IL-1β and IL-18
To confirm that the ropivacaine-mediated reduced TxNIP expression and the NLRP3 inflammasome could inhibit the production of IL-1β, we determined the effects of ropivacaine on high glucose-induced IL-1β and IL-18. As shown in Figure 7, the two doses of ropivacaine significantly ameliorated both high glucose-induced increased secreted levels (Figure 7A,B) and intracellular levels (Figure S1) of IL-1β and IL-18. MCC950, a selective small-molecule inhibitor specific to NLRP3, was used as a positive control. The presence of MCC950 significantly inhibited the high glucose-induced expression of IL-1β and IL-18 (Figure S1A,B).
Figure 7.
Ropivacaine inhibits the expression of IL-1β and IL-18. HUVECs were stimulated with high glucose (25 mM) with or without ropivacaine (0.25, 0.5%) or MCC950 (5 μM) for 24 h. (A) Secretion of IL-1β and (B) secretion of IL-18 [*, P < 0.01 vs normal glucose; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
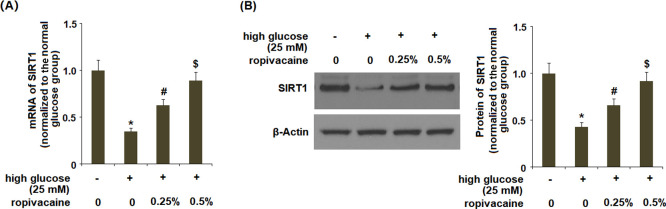
2.8. Ropivacaine Inhibited the Reduction of SIRT1
We assessed the role of SIRT1 in NLRP3 inflammasome activation. The results in Figure 8A,B showed that ropivacaine treatment could reverse the reduction in SIRT1 induced by high glucose in a dose-dependent manner.
Figure 8.
Ropivacaine inhibits the reduction of SIRT1. (A) mRNA expression of SIRT1 and (B) protein of SIRT1 [*, P < 0.01 vs normal glucose; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.25%), N = 5–6].
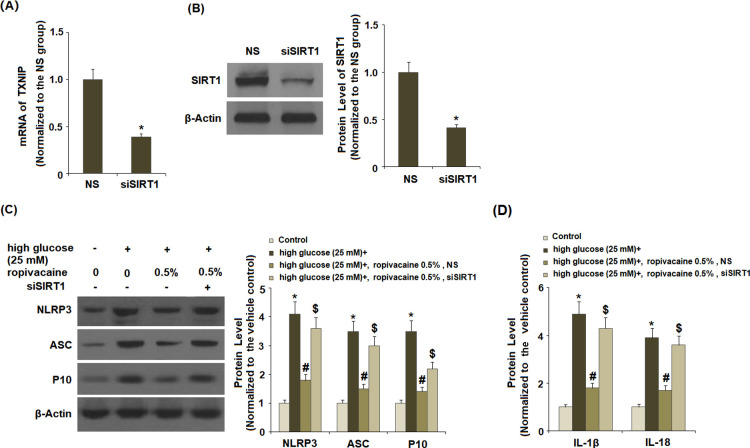
2.9. Knockdown of SIRT1 Broke the Effects of Ropivacaine on the NLRP3 Inflammasome
Next, we determined whether ropivacaine-mediated inhibition of NLRP3 is mediated through SIRT1. Successful knockdown of SIRT1 was verified (Figure 9A,B). Silencing of SIRT1 broke the negative regulatory effects of ropivacaine on the expression of NLRP3 (Figure 9C), ASC, and P10. Additionally, inhibition of SIRT1 ameliorated the roles of ropivacaine on both secreted levels (Figure 9D) and intracellular levels (Figure S2) of IL-1β and IL-18.
Figure 9.
Knockdown of SIRT1 abolished the effects of ropivacaine on activation of NLRP3. NS, nonspecific group; siSIRT1, SIRT1 small interfering RNA. (A,B) Successful silencing of SIRT1 was verified (*, P < 0.01 vs NS group), (C) levels of NLRP3, ASC, and P10, and (D) IL-1β and IL-18 production [*, P < 0.01 vs normal glucose; #, P < 0.01 high glucose only; $, P < 0.01 vs high glucose + ropivacaine (0.5%) + NS group, N = 5–6].
3. Discussion
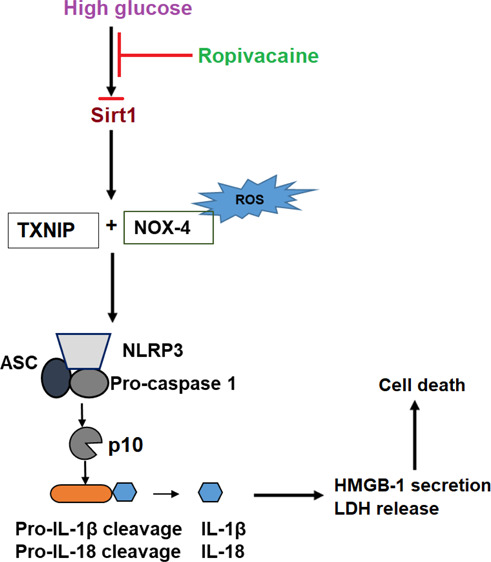
Endothelial inflammation and dysfunction play a leading role in diabetes-associated cardiovascular disease by altering the vasoconstrictive function of endothelial cells and creating a proinflammatory environment. Among the factors contributing to high glucose-induced endothelial dysfunction are oxidative stress, increased cell apoptosis, and exacerbation of inflammation. ROS-mediated activation of TxNIP and the subsequent NLRP3 inflammasome activation are critical events in the pathological development of atherosclerosis and cardiovascular risks.23,24 However, the exact mechanisms driving NLRP3 inflammasome activation and the inflammatory response in diabetic endothelial dysfunction remain unclear. Here, we studied the effects of the widely used amide local anesthetic ropivacaine in NLRP3 inflammasome activation and inflammation using HUVECs. We found that exposure to high glucose induces endothelial dysfunction through ROS- and TxNIP-mediated activation of the NLRP3 inflammasome. However, treatment with ropivacaine significantly inhibits endothelial dysfunction by suppressing IL-1β, IL-18, and HMGB1. Furthermore, these protective effects of ropivacaine appear to be mediated through the SIRT1 pathway.
Endothelial dysfunction, characterized by dysregulation of vascular constriction and relaxation, is an important event in diabetic vascular disease.25 Hyperglycemia is well documented to induce endothelial dysfunction by disrupting the oxidant/antioxidant balance and promoting the expression of IL-1β, IL-18, and HMGB1.26−30 The findings in Figures 1 and 2 indicate that ropivacaine could suppress the production of ROS and NOX4, thereby exerting a protective antioxidative stress capacity in HUVECs. Previous studies investigating the effects of ropivacaine and other amide local anesthetics on endothelial cell viability have yielded conflicting results.30,31 Our findings demonstrate that ropivacaine significantly improved diminished endothelial cell viability induced by high glucose.
HMGB1 is a cellular stress response signaling protein released by immune cells upon cell death or injury and contributes to diabetic endothelial dysfunction.32,33 A recent study provided evidence that downregulation of HMGB1 may serve as a potential approach for preventing endothelial dysfunction by inhibiting inflammation and apoptosis.33 Our findings demonstrate that ropivacaine treatment can significantly inhibit the high glucose-induced expression of HMGB1. TxNIP is an antioxidant-inhibitory protein that activates the NLRP3 inflammasome. Importantly, TxNIP/NLRP3 inflammasome activation is important for the development of diabetic vascular disease and endothelial dysfunction.32,34 Our results indicate that ropivacaine obviously suppressed the activation of the NLRP3 inflammasome complex by suppressing the activation of TxNIP. Generation of IL-1β and IL-18, as well as their precursors, is orchestrated by the NLRP3 inflammasome. The endothelial release of IL-1β induced by high glucose has been suggested as the origin of upregulated IL-1β associated with diabetic retinopathy and is well recognized as a major proinflammatory mediator of diabetic vascular disease.34
SIRT1 is a protective factor that has been shown to inhibit the inflammatory response in endothelial cells by suppressing the NLRP3 inflammasome. SIRT1 was also shown to be reduced in response to lipopolysaccharide and adenosine triphosphate exposure.35 Concordantly, our findings indicate that high glucose reduces SIRT1 and that ropivacaine treatment can ameliorate this effect. Importantly, these results indicate that SIRT1 signaling plays a key role in the beneficial effects of ropivacaine treatment demonstrated by the other experiments performed in this study.
There are several limitations to the current study. First, high glucose will induce a change in the osmotic strength of the cell culture medium. Altered osmotic strength will exert various impacts on gene action. Therefore, future investigations should include the influence of osmotic strength. Second, we only examined the effects of ropivacaine in an in vitro primary endothelial cell culture model. Further in vivo investigations will help to verify the pharmacological function of ropivacaine in endothelial inflammation.
4. Conclusions
Our results demonstrate the potential of ropivacaine as a possible therapy against diabetic endothelial dysfunction.
5. Materials and Methods
5.1. Cell Culture and Treatment
HUVECs were maintained in an EGM-2 (Lonza, Switzerland) supplemented with fetal bovine serum (10%) (Gibco, USA) and P/S (1%) (Sigma-Aldrich, USA) in T75 cell culture flasks. The cells were divided into four groups and incubated in 12-well cell culture plates: (1) normal group (5.5 mM glucose), (2) high glucose treatment group (25 mM glucose),7,13 and (3) high glucose + ropivacaine groups (25 mM glucose + 0.25%, 0.5% ropivacaine) (Cat# 1605497, Sigma-Aldrich).
5.2. Real-Time Polymerase Chain Reaction Analysis
Upon completion of the necessary treatment, RNA was extracted using TRIzol. The concentration and purity of the RNA samples from the different groups were determined using a NanoDrop 2000. Purified RNA was then used to produce complementary DNA (cDNA) using a kit. The generated cDNA was then used for real-time polymerase chain reaction (PCR) to measure the target genes on an ABI 7500 fast real-time PCR system. The expression was calculated by normalizing to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. The primers used are as follows: SIRT1 [forward (for): 5′-TCACCACCAGATTCTTCAGTG-3′; reverse (rev): 5′-CCTCTTGATCATCTCCATCAGTC-3′]; TxNIP (for: 5′-TGGATCTGGTGGATGTCAATAC-3′; rev: 5′-TCTGAGTCAGCACCTTGGTCTG-3′); NOX-4 (for: 5′-CTTTTGGAAGTCCATTTGAG-3′; rev: 5′-CGGGAGGGTGGGTATCTAA-3′); and GAPDH (for: 5′-ACTGGCGTCTTCACCACCAT-3′; rev: 5′-AAGGCC ATGCCAGTGAGCTT-3′).
5.3. Western Blot
Upon completion of the necessary treatment, HUVECs were washed. Proteins were isolated from HUVECs with the radioimmunoprecipitation assay buffer in the presence of protease cocktail inhibitors (Sigma-Aldrich, USA). Protein samples (20 μg) from each experimental group were loaded onto the sodium dodecyl sulfate–polyacrylamide (10%) gel, followed by electrophoresis. Separated protein samples were transferred to a polyvinylidene difluoride membrane (Millipore, USA). Membranes were then blocked with non-fat milk (5%) on a shaker and incubated with primary antibodies. After three washes with Tris-buffered saline–Tween 20, membranes were probed with the horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h. The immunoblot bands were detected using an ECL Detection Kit. Western blot data were analyzed using the ImageJ software (NIH, USA). Briefly, the images were converted to grayscale. The background was then subtracted. The integrated density of each band was determined.
5.4. Enzyme-Linked Immunosorbent Assay Analysis
Upon completion of the necessary treatment, the supernatant pipetted into 96-well plates to measure the secretion of IL-1β and IL-18 using ELISA kits (Cat# SLB50, R&D system; Cat# QK318, R&D system). The optical density values were measured at 450 nM.
5.5. Determination of Mitochondrial ROS
Upon completion of the necessary treatment, HUVECs were stained with the indicator MitoSOX Red to assess the levels of mitochondrial superoxide. Briefly, the cells were washed three times and loaded with MitoSOX Red (5 μM) for 30 min. Fluorescence signals were detected using a LSM710 laser confocal microscope (Zeiss, Germany) with a ×20 objective lens under 543 nm excitation light.
5.6. LDH Release
The amount of released LDH was determined using a LDH kit. After stimulation, the supernatants were used to determine the LDH release activity.
5.7. Determination of HMGB1 Secretion
The amount of secreted HMGB1 was tested using an HMGB1 commercial kit (Shino-Test Corporation, Kanagawa, Japan). After stimulation, the supernatants were used to determine the levels of secreted HMGB1.
5.8. Statistical Analysis
The experimental data in the current study are shown as means ± standard error of the mean. The significance of differences was assessed using ANOVA, followed by Bonferroni’s test. P < 0.05 was considered statistically significant.
Acknowledgments
This study was supported by the Ningbo Scientific Support Project (no. 172102610022).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03143.
Ropivacaine inhibiting high glucose-induced expression of intracellular IL-1β and IL-18 and silencing of SIRT1 abolishing the inhibitory effects of ropivacaine on intracellular IL-1β and IL-18 production (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Abdul-Ghani M.; DeFronzo R. A.; Del Prato S.; Chilton R.; Singh R.; Ryder R. E. J. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived?. Diabetes Care 2017, 40, 813–820. 10.2337/dc16-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altabas V. Diabetes, endothelial dysfunction, and vascular repair: what should a diabetologist keep his eye on?. Int. J. Endocrinol. 2015, 2015, 848272. 10.1155/2015/848272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingueti C. P.; Dusse L. M. S. A.; das Graças Carvalho M.; de Sousa L. P.; Gomes K. B.; Fernandes A. P. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Chen J.; Brodsky S. V.; Goligorsky D. M.; Hampel D. J.; Li H.; Gross S. S.; Goligorsky M. S. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ. Res. 2002, 90, 1290–1298. 10.1161/01.res.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- De Vriese A. S.; Verbeuren T. J.; Van de Voorde J.; Lameire N. H.; Vanhoutte P. M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Li X.; Zhang Y.; Huang Y.; Zhang Y.; Ma Q. Oxymatrine ameliorates diabetesinduced aortic endothelial dysfunction via the regulation of eNOS and NOX4. Journal of cellular biochemistry. J. Cell. Biochem. 2019, 120, 7323. 10.1002/jcb.28006. [DOI] [PubMed] [Google Scholar]
- Wang J. S.; Huang Y.; Zhang S.; Yin H. J.; Zhang L.; Zhang Y. H.; Song Y. W.; Li D.-D. A Protective Role of Paeoniflorin in Fluctuant Hyperglycemia-Induced Vascular Endothelial Injuries through Antioxidative and Anti-Inflammatory Effects and Reduction of PKCβ1. Oxid. Med. Cell. Longevity 2019, 2019, 5647219. 10.1155/2019/5647219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.; Xia L.; Goldberg H.; Lee K. W.; Quaggin S. E.; Fantus I. G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. 10.1074/jbc.m112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abderrazak A.; El Hadri K.; Bosc E.; Blondeau B.; Slimane M.-N.; Buchele B.; Simmet T.; Couchie D.; Rouis M. Inhibition of the inflammasome NLRP3 by arglabin attenuates inflammation, protects pancreatic β-cells from apoptosis, and prevents type 2 diabetes mellitus development in ApoE2Ki mice on a chronic high-fat diet. J. Pharmacol. Exp. Ther. 2016, 357, 487–494. 10.1124/jpet.116.232934. [DOI] [PubMed] [Google Scholar]
- Lebreton F.; Berishvili E.; Parnaud G.; Rouget C.; Bosco D.; Berney T.; Lavallard V. NLRP3 inflammasome is expressed and regulated in human islets. Cell Death Discovery 2018, 9, 726. 10.1038/s41419-018-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F. L.; Netea M. G.; Dinarello C. A.; Joosten L. A. B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Grishman E. K.; White P. C.; Savani R. C. Toll-like receptors, the NLRP3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr. Res. 2012, 71, 626. 10.1038/pr.2012.24. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wu Z.; He Y.; Zhang H.; Tian L.; Zheng C.; Shang T.; Zhu Q.; Li D.; He Y. Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2. Mol. Immunol. 2018, 101, 245–250. 10.1016/j.molimm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Han Z.; Fan Y.; Zhang J.; Chen K.; Gao L.; Zeng H.; Cao J.; Wang C. MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell. Physiol. Biochem. 2017, 41, 1555–1571. 10.1159/000470822. [DOI] [PubMed] [Google Scholar]
- Luo B.; Li B.; Wang W.; Liu X.; Liu X.; Xia Y.; Zhang C.; Zhang Y.; Zhang M.; An F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc. Drugs Ther. 2014, 28, 33–43. 10.1007/s10557-013-6498-1. [DOI] [PubMed] [Google Scholar]
- Yang S.-M.; Ka S.-M.; Wu H.-L.; Yeh Y.-C.; Kuo C.-H.; Hua K.-F.; Shi G.-Y.; Hung Y.-J.; Hsiao F.-C.; Yang S.-S.; et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-κB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 2014, 57, 424–434. 10.1007/s00125-013-3115-6. [DOI] [PubMed] [Google Scholar]
- Becker D. E.; Reed K. L. Local Anesthetics: Review of Pharmacological Considerations. Anesth Prog. 2012, 59, 90–102. 10.2344/0003-3006-59.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram P.; Kennedy D. J.; Yeh P.; Dragoo J. Chondrotoxic Effects of Local Anesthetics on Human Knee Articular Cartilage-A Systematic Review. PM&R 2019, 11, 379. 10.1002/pmrj.12007. [DOI] [PubMed] [Google Scholar]
- Behnaz F.; Soltanpoor P.; Teymourian H.; Tadayon N.; Reza M. G.; Ghasemi M. Sympatholytic and Anti-Inflammatory Effects of Ropivacaine and Bupivacaine After Infraclavicular Block in Arterio Venous Fistula Surgery. Reg. Anesth. Pain Med. 2019, 9, e85704 10.5812/aapm.85704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Li L.; Wang F.; Wu X.; Zhao X.; Xue N. Anti-Inflammatory Effect of Local Anaesthetic Ropivacaine in Lipopolysaccharide-Stimulated RAW264. 7 Macrophages. Pharmacology 2019, 103, 228–235. 10.1159/000496425. [DOI] [PubMed] [Google Scholar]
- Ota H.; Akishita M.; Eto M.; Iijima K.; Kaneki M.; Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yang X.; He Y.; Wang W.; Zhang J.; Zhang W.; Jing T.; Wang B.; Lin R. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology 2017, 222, 552–561. 10.1016/j.imbio.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Sun X.; Jiao X.; Ma Y.; Liu Y.; Zhang L.; He Y.; Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Parikh G. P.; Shah V. R.; Vora K. S.; Parikh B. K.; Modi M. P.; Panchal A. Ultrasound guided peritubal infiltration of 0.25% ropivacaine for postoperative pain relief in percutaneous nephrolithotomy. Middle East J Anaesthesiol 2014, 58, 293. 10.4103/0019-5049.135040. [DOI] [PubMed] [Google Scholar]
- Younis W. H.; Al-Rawi N. H.; Mohamed M. A.-H.; Yaseen N. Y. Molecular events on tooth socket healing in diabetic rabbits. Br. J. Oral. Maxillofac. Surg. 2013, 51, 932–936. 10.1016/j.bjoms.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Cosentino F.; Lüscher T. F. Endothelial dysfunction in diabetes mellitus. J. Cardiovasc. Pharmacol. 1998, 32, S54–S61. [PubMed] [Google Scholar]
- Suchanek H.; Mysliwska J.; Siebert J.; Wieckiewicz J.; Hak Ł.; Szyndler K.; Kartanowicz D. High serum interleukin-18 concentrations in patients with coronary artery disease and type 2 diabetes mellitus. Eur. Cytokine Network 2005, 16, 177–185. [PubMed] [Google Scholar]
- Liu Y.; Costa M. B.; Gerhardinger C. IL-1β is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1β autostimulation. PLoS One 2012, 7, e36949 10.1371/journal.pone.0036949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.-t.; Wang F.; Shao M.; Wang Y.; Zhu H.-q. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vasc. Pharmacol. 2017, 88, 48–55. 10.1016/j.vph.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Wohlrab P.; Kaun C.; Saleh L.; et al. Ropivacaine Causes Inflammation and Apoptosis in Human Umbilical Vein Endothelial Cells and Human Placental Trophoblasts. Anaesth., Pain Intensive Care 2018, 53, 18. 10.1055/s-0038-1675498. [DOI] [Google Scholar]
- Piegeler T.; Votta-Velis E. G.; Bakhshi F. R.; Mao M.; Carnegie G.; Bonini M. G.; Schwartz D. E.; Borgeat A.; Beck-Schimmer B.; Minshall R. D. Endothelial Barrier Protection by Local AnestheticsRopivacaine and Lidocaine Block Tumor Necrosis Factor-α–induced Endothelial Cell Src Activation. Int J Anesthetic Anesthesiol 2014, 120, 1414–1428. 10.1097/aln.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Tang D.; Kang R. Oxidative stress-mediated HMGB1 biology. Front. Plant Physiol. 2015, 6, 93. 10.3389/fphys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone L.; Devi T. S.; Hosoya K.-i.; Terasaki T.; Singh L. P. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J. Cell. Physiol. 2009, 221, 262–272. 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- Liu R.; Luo Q.; You W.; Jin M. MicroRNA-106 attenuates hyperglycemia-induced vascular endothelial cell dysfunction by targeting HMGB1. Gene 2018, 677, 142–148. 10.1016/j.gene.2018.07.063. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yang J.; Chen M.-H.; Wang Q.; Qin M.-J.; Zhang T.; Chen X.-Q.; Liu B.-L.; Wen X.-D. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015, 99, 101–115. 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.