Abstract

In this study, we developed a rapid and easy method to determine cyanide (CN) intoxication by quantification of CN and 2-aminothiazoline-4-carboxylic acid (ATCA), which is a new and reliable indicator of CN exposure, in the human blood using probe electrospray ionization tandem mass spectrometry (PESI/MS/MS) named RECiQ. For CN, we applied the previously reported one-pot derivatization method using 2,3-naphthalenedialdehyde and taurine, which can directly derivatize CN in the blood. The analytical conditions of the CN derivatization were optimized as a 10 min reaction time at room temperature. In contrast, ATCA could be directly detected in the blood by PESI/MS/MS. We developed quantitative methods for the derivatized CN and ATCA using an internal standard method and validated them using quality control samples, demonstrating that the linearities of each calibration curve were greater than 0.995, and intra- and interday precisions and accuracies were 5.1–15 and 1.1–14%, respectively. Moreover, the lower limit of detections for CN and ATCA were 42 and 43 ng/mL, respectively. Finally, we applied RECiQ to three postmortem blood specimens obtained from victims of fire incidents, which resulted in the successful quantification of CN and ATCA in all samples. As PESI/MS/MS can be completed within 0.5 min, and the sample volume requirement of RECiQ is only 2 μL of blood, these methods are useful not only for the rapid determination of CN exposure but also for the estimation of the CN intoxication levels during an autopsy.
Introduction
Cyanide (CN) is an ancient toxic compound that inhibits cellular respiration, primarily due to the inhibition of glycolysis when CN combines with Fe3+ of cytochrome oxidase.1 CN analysis is mandatory for forensic toxicologists because CN is often used to commit murder or suicide and is also detectable in victims of fire incidents. In fact, Kudo et al. reported that there were 12 poisoning cases involving cyanide (CN) in 2003–2006.2 In other words, three CN poisoning cases occurred on an average every year in Japan. Furthermore, it was necessary for forensic toxicologists to perform CN analysis for autopsy samples in Japan since there were serial murders involving CN in 2013. In particular, measuring the blood CN level is crucial for estimating CN intoxication; however, some drawbacks exist: (1) CN is unstable in the blood at room temperature3,4 and (2) its half-life in the blood is relatively short.5,6 Thus, a new indicator, 2-aminothiazoline-4-carboxylic acid (ATCA), of CN intoxication has been demonstrated.7−18 It is produced by the reaction of cystine with CN; approximately 20% of the injected CN is metabolized to ATCA8,14 and does not metabolize further.14 Additionally, ATCA is stable in the blood,16 making it a suitable analytical target to indicate CN exposure. Ruzycka et al. have reported that ATCA functions as an indicator of CN intoxication, though perhaps not at sublethal levels.16 However, an animal study by Yu et al. has demonstrated that plasma ATCA concentration levels increased significantly with sublethal CN dose levels.9 In addition, there have been several reports on ATCA in cases of CN exposure in the United States and Europe, but none from Asia. Thus, more information on ATCA in cases of CN exposure, especially using postmortem blood, is required. Moreover, it is important to develop a rapid and easy analytical method to confirm the feasibility of ATCA as an indicator of CN intoxication in postmortem blood.
As ATCA is a highly polar compound, it is tedious to extract in the blood. As such, appropriate derivatization methods are required to detect ATCA by gas chromatography–mass spectrometry (GC/MS)8,10 and a column specialized for separating polar compounds (e.g., hydrophilic interaction chromatography (HILIC) column) is essential for the analysis of ATCA by liquid chromatography–tandem mass spectrometry (LC/MS/MS).9,11,15,16 These analytical methods can be used to identify ATCA in biological specimens, although it takes a relatively long time to obtain the results. However, in an autopsy, a rapid method to determine CN exposure is strongly required, and ambient ionization mass spectrometry (AIMS) meets this demand.
AIMS is defined as an ionization technique for the analysis under open-air conditions, allowing direct analysis of the sample with little or no preparation,19 and it has been applied in the direct identification of various substances. Probe electrospray ionization (PESI), invented by Hiraoka et al.,20 is an ambient ionization that uses a thin probe needle as the sampling and ionization unit. Our group is the first to have combined PESI with tandem mass spectrometry (PESI/MS/MS) and applied it to an intact metabolite analysis of mouse tissues,21−23 achieving successful detection of the metabolites in the tissues without tedious sample preparation. Therefore, PESI/MS/MS has a high potential to directly detect CN and ATCA in biological samples, although the molecular weight of CN is extremely low, requiring an appropriate and rapid derivatization method.
Sano et al. have reported the direct derivatization of CN in the blood using 2,3-naphthalenedialdehyde (NDA) and taurine.24,25 This is a simple derivatization method because CN can be derivatized by mixing CN in the blood with NDA and taurine at room temperature for 30 min. Tracqui et al. have combined this derivatization method with liquid chromatography–mass spectrometry (LC/MS), through which CN can be detected in its derivatized form (1-cyanobenz[f]isoindole).26
In this study, we have developed a rapid and easy method for determining CN intoxication by CN and ATCA quantification in the human blood using PESI/MS/MS named RECiQ. We first applied PESI/MS/MS to analyze CN and ATCA in the blood. Here, ATCA is directly detectable, whereas CN is detected in its derivatized form using NDA and taurine. We optimize the derivatization time of CN to a 10 min reaction time at room temperature. Furthermore, we constructed calibration curves using an internal standard (IS) method, demonstrating sufficient linearity (R2 > 0.995), and further validated these quantitative methods using quality control (QC) samples. Finally, we apply RECiQ to three postmortem blood samples obtained from victims of fire incidents, proving the method’s practicality.
Results and Discussion
PESI/MS/MS of Derivatized CN
Sano et al. reported that CN easily reacts with NDA and taurine in the human blood at room temperature.25 The reaction scheme is presented in Figure S1 in the Supporting Information. Additionally, Tracqui et al. reported that this derivative can be ionized by electrospray ionization (ESI).26 Thus, there was a high potential that PESI/MS/MS would be able to detect derivatized CN directly in the blood, which we confirmed (Figure 1).
Figure 1.
Detection of derivatized CN in the blood by PESI/MS/MS. (a) Fragmentation patterns of the derivatized CN and (b) chronogram of the derivatized CN (1000 ng/mL) in the blood.
The product ion at m/z 191 corresponded to the fragment ion with a loss of sulfoethyl moiety, and the product ion at m/z 81 was a sulfonic ion (Figure 1a). As there are no reports on the optimal derivatization conditions of CN with NDA and taurine, we optimized them. In pre-experiments, we evaluated the amount of blood and taurine solution and changed the concentrations of taurine and NDA (Table S1). The optimal conditions were determined as follows: the amount of blood, 2 μL and the amount and concentration of the derivatization solvent, 38 μL and 5.0 mM for the taurine solution and 40 μL and 4.3 mM for the NDA solution, respectively. Under these conditions, we further evaluated the derivatization time (1, 5, 10, 20, and 30 min, n = 3 at each time point). As presented in Figure 2, the derivatization apparently reached a plateau at 10 min, and thus we determined this to be the optimal derivatization time.
Figure 2.
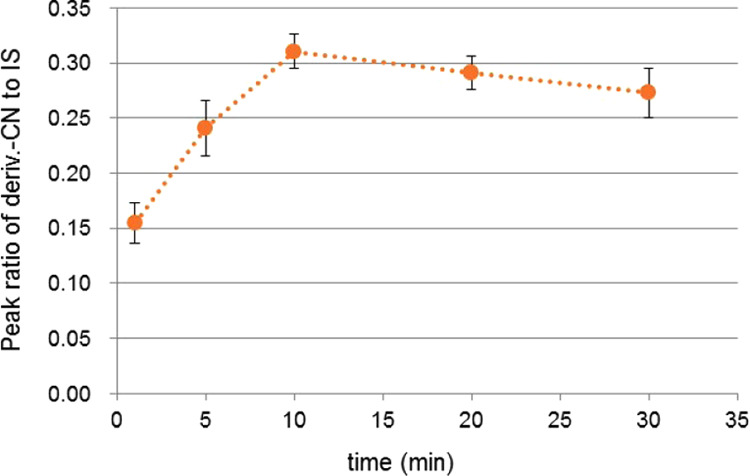
Time course of the derivatized CN (CN concentration: 200 ng/mL) in the blood (n = 3) at room temperature.
To evaluate the dilution effect, the final sample was diluted with a 50% aqueous ethanol solution (2- and 4-fold dilution), and the analytical results were compared, confirming that the peak area ratio (derivatized CN/IS) rose higher by the 4-fold dilution, as presented Table S1.
Moreover, we evaluated the possible inhibitory effect of thiocyanate, another CN metabolite, on the derivatization of CN. We tested the CN-spiked blood at 500 ng/mL with and without the coexistence of thiocyanate at 50 000 ng/mL. As presented in Figure S2, there were no inhibitory effects of thiocyanate on the CN derivatization, demonstrating that this derivatization method can selectively react with CN.
PESI/MS/MS of ATCA
To confirm whether PESI/MS/MS can detect ATCA directly in the blood, we analyzed ATCA in the spiked blood at 1000 ng/mL by PESI/MS/MS and successfully detected it (Figure 3). The product ion at m/z 101 corresponded to the decarboxyl fragment ion, and the product ion at m/z 59 was a ring cleavage ion (Figure 3a).
Figure 3.
Detection of ATCA in the blood by PESI/MS/MS. (a) Fragmentation patterns of ATCA and (b) chronogram of ATCA (1000 ng/mL) in the blood.
Quantitative Methods of CN and ATCA in the Blood
The calibration curves of CN and ATCA were constructed using an IS method. The validation results are presented in Table 1. The linearity of the calibration curves was sufficient (R2 > 0.995), and the intra- and interday precisions and accuracies were below 15%. Thus, these methods exhibited high repeatability and quantitativity. The lower limit of detection (LLOD) and lower limit of quantitation (LLOQ) were 42 and 127 ng/mL for CN and 43 and 131 ng/mL for ATCA, respectively. In comparison with several earlier reports (Table 2), the LLOD and LLOQ of the present methods were slightly higher.8,15,27−32 However, sufficient sensitivity for practical use was obtained because PESI exhibited high ionization efficiency like nano-ESI,33 even without analyte extraction. The endogenous and exogenous levels of CN and ATCA in the human blood are presented in Table 3.10,15,16,28,34−36 It became apparent that the present methods can be applied to the concentration ranges of fire incidents and CN poisoning and are satisfactory for forensic purposes. The raw data of the calibrants and QC samples are presented in Tables S2 and S3.
Table 1. Validation Results for CN and ATCA by PESI/MS/MS (n = 5).
| low
QC |
high
QC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| intraday |
interday |
intraday |
interday |
|||||||||
| compounds | range (ng/mL) | R2 | LLOD (ng/mL) | LLOQ (ng/mL) | precision (%) | accuracy (%) | precision (%) | accuracy (%) | precision (%) | accuracy (%) | precision (%) | accuracy (%) |
| CN | 50–2500 | 0.997 | 42 | 127 | 250 ng/mL | 2000 ng/mL | ||||||
| 7.8 | 1.9 | 5.6 | 1.1 | 5.1 | 5.2 | 15 | 14 | |||||
| ATCA | 50–1500 | 0.995 | 43 | 131 | 250 ng/mL | 1000 ng/mL | ||||||
| 8.3 | 4.8 | 7.0 | 5.9 | 9.7 | 3.3 | 5.4 | 3.8 | |||||
Table 2. Analytical Performance of the Published Methods for CN and ATCA.
| technique | analyte | matrix | LLOD (ng/mL) | LLOQ (ng/mL) | range (ng/mL) | R2 | runtime (min) | sample size (μL) | refs |
|---|---|---|---|---|---|---|---|---|---|
| HS-GC/NPDa | CN | blood | 11 | 50 | 50–5000 | 0.975–0.999 | 10 | 500 | (27) |
| HS-GC/MSb | CN | blood | 20 | 70 | 0–50 000 | 0.999 | 19.1 | 250 | (28) |
| GC/MSc | CN | blood | 130 | N/A | 260–26 000 | 0.999 | 23 | 200 | (29) |
| GC/MS | CN | plasma | 26 | 260 | 260–420 000 | 0.999 | 18.1 | 100 | (30) |
| LCMS/MSd | CN | blood | 10 | 26 | 25–26 000 | 0.992 | 6 | 25 | (31) |
| LC/MS/MS | ATCA | blood | 0.43 | 1.5 | 30–900 | 0.999 | 16 | 100 | (15) |
| GC/MS | ATCA | blood | 24 | 34 | 30–1000 | 0.992 | 13 | 100 | (32) |
| GC/MS | ATCA | urine | 25 | 50 | 10–2000 | N/A | 11 | 100 | (8) |
Headspace gas chromatography with a nitrogen–phosphorus detector.
Headspace gas chromatography–mass spectrometry.
Gas chromatography–mass spectrometry.
Liquid chromatography–tandem mass spectrometry.
Table 3. Blood Concentration Levels of CN and ATCA (Endogenous, Fire Incident, and CN Poisoning).
Application to Real Autopsy Samples
To demonstrate the feasibility of RECiQ, we applied it to the postmortem blood samples of three victims of fire incidents. The samples were prepared as described in the Experimental Section. The raw data of the postmortem samples are presented in Table S4. Both CN and ATCA were successfully quantitated in the three blood samples (Table 4). It was noted that ATCA could be analyzed during the CN derivatization; thus, the total analysis time was approximately 10 min. As presented in Table 3, the ATCA blood concentrations of victims of fire incidents ranged from 100 to 191 ng/mL in previous reports.16 However, our results revealed higher ATCA blood concentration levels. Although the reason for this was not discussed in this study, there may have been a difference in the periods between CN exposure and death or a racial difference in the metabolism of CN to ATCA. Therefore, in future studies, it is necessary to accumulate data on ATCA blood concentration using real postmortem blood to confirm the applicability of ATCA.
Table 4. Concentrations of CN and ATCA in the Blood of Fire Victims.
| victim no. | CN (ng/mL) | ATCA (ng/mL) |
|---|---|---|
| 1 | 214 | 536 |
| 2 | 941 | 582 |
| 3 | 746 | 991 |
Limitations and Future Perspectives
Although RECiQ demonstrated practicality for the fire cases, it still needs to be applied to acute CN poisoning cases, as we were unable to obtain such blood samples in this study. Moreover, performing forensic toxicological analysis with a small volume of biological samples is preferable. As demonstrated above, RECiQ can analyze CN and ATCA with only 2 μL of blood, which is a low sample volume in comparison with previous reports (Table 2). In the sample preparation in RECiQ, 2 μL of blood was diluted with the solutions, and final volumes were 330 μL for the CN analysis and 80 μL for ATCA analysis, respectively. Since mass spectrometric analysis in RECiQ requires 15 μL per run, triplicate analysis (i.e., 15 μL × 3) can be performed in RECiQ if necessary. In addition, the analysis time of RECiQ is 0.5 min, which is obviously shorter than that in previous reports. This method can directly detect derivatized CN and ATCA in the blood without extraction, enabling nonprofessional individuals to execute such a method without special training. However, it should be noted that a positive control sample must be checked before making a concrete decision.
As mentioned previously, ATCA is generated by the reaction of CN with cystine in the body.8 Cystine is an intermediate of methionine metabolism, and methionine plays a significant role in methionine-centered metabolism.37 The biosynthesis of ATCA can be altered by diet, which may change the body’s cystine concentration levels. Thus, the generation of ATCA in the blood may be dependent on the endogenous levels of cystine, and a study on ATCA generation in consideration of food intake is underway.
Conclusions
In this study, we developed RECiQ, a rapid and easy method for determining CN intoxication by CN and ATCA quantification in the human blood using PESI/MS/MS. Data acquisition can be completed within 0.5 min, and only 2 μL of blood is required for the analysis. To analyze CN in the blood, we applied derivatization with NDA and taurine at room temperature for 10 min, whereas ATCA in the blood could be directly analyzed. The validation results for derivatized CN and ATCA were satisfactory, demonstrating the good quantitativity of the methods. To evaluate the practicality of RECiQ, we applied it to real postmortem blood from fire incident cases and successfully detected CN and ATCA by PESI/MS/MS. The ATCA concentration levels in this study were slightly higher than those in previous reports, which may have been due to racial differences or a difference in the periods between CN exposure and death.
As RECiQ does not require tedious sample pretreatments, such as extraction and evaporation, beginners in the toxicological analysis also can quantitate CN and ATCA in the blood without special training. Thus, RECiQ is useful not only for rapidly determining CN exposure but also for estimating CN poisoning levels during an autopsy. Moreover, it may be applicable to the investigation of the relationship between ATCA blood concentration and CN-induced toxic effects in the future, especially in Asians.
Experimental Section
Materials
Potassium CN and potassium thiocyanate were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan), and NDA and taurine were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Secobarbital-d5 was purchased from Cerilliant Corporation (Texas); ATCA and 2-aminothiazole-4-carboxylic acid (ATZA) were purchased from Combi-Blocks Inc. (San Diego). The other reagents used were of HPLC grade. Pooled blood was purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan). A stock standard solution of CN was prepared at 10 mg/mL by dissolving potassium CN in a 0.1 mol/L sodium hydroxide aqueous solution. A stock standard solution of thiocyanate was prepared at 10 mg/mL by dissolving potassium thiocyanate in water. The working standard solutions of CN and thiocyanate were prepared prior to use by diluting the stock standard solutions with a 0.1 mol/L sodium hydroxide aqueous solution and water, respectively.
The NDA was dissolved in methanol, and its concentration was adjusted to 4.3 mM with ethanol. Taurine was dissolved in 50 mM potassium tetraborate/50 mM potassium hydrogen phosphate buffer (50:50, v/v), and its concentration was adjusted to 5.0 mM. Secobarbital-d5 was dissolved in a 50% aqueous ethanol solution, and its concentration was adjusted to 90 ng/mL. The stock standard solutions of ATCA and ATZA were prepared at 1 mg/mL by dissolving a 50% aqueous ethanol solution. The working standard solutions of ATCA were prepared prior to use by diluting the stock standard solutions with water. A working standard solution of ATZA was dissolved in water, and its concentration was adjusted to 500 ng/mL. These solutions were stored at −20 °C until analysis. Postmortem blood samples were obtained from three fire victims (three males, aged 66–82 years at death) upon autopsy. Their postmortem intervals were not determined correctly. All postmortem samples were stored at −30 °C until analysis.
This work was approved by the Ethics Committee of Nagoya University Graduate School of Medicine (approval number 2017-0175).
Analytical Conditions of PESI/MS/MS
An LCMS-8040 triple quadrupole tandem mass spectrometer with a probe electrospray ion source (Shimadzu Corporation, Kyoto, Japan) was used. Photographs of the instrument are shown in Figure 4.
Figure 4.
Photographs of (a) probe, (b) sample plate, (c) details of PESI ion source, and (d) exterior of PESI/MS/MS.
The PESI needle (tip diameter: 700 nm) was manufactured by Shimadzu Corporation. The resolution of the mass spectrometer was set to unit resolution, and the selected reaction monitoring (SRM) mode was applied. The SRM transitions and collision energies were optimized using the authentic standards listed in Table 5.
Table 5. SRM Transitions and Collision Energies for CN, ATCA, and the ISs.
| quantifier
ion |
qualifier
ion |
|||||
|---|---|---|---|---|---|---|
| compounds | precursor ion (m/z) | polarity | m/z | CE (V) | m/z | CE (V) |
| CN | 299 | neg | 191 | 25 | 81 | 25 |
| secobarbital-d5 (IS) | 242 | neg | 199 | 12 | ||
| ATCA | 147 | pos | 101 | –23 | 59 | –30 |
| ATZA (IS) | 145 | pos | 127 | –23 | ||
The loop time of the data acquisition of the mass spectrometer was set at 40 ms, and the frequency of the probe movement was set at 3.26 Hz. The measurement time was set at 0.5 min to obtain sufficient data points. The heat block and desolvation line temperatures were set at 50 and 300 °C, respectively. The peak areas were calculated by integrating all peaks for each compound per run using the built-in software, LabSolutions (Shimadzu, ver. 5.86). The probe needle was changed between sample analyses to prevent contamination.
Workflow for RECiQ
The workflow for RECiQ is presented in Figure 5.
Figure 5.
Workflow for RECiQ. (a) For analyzing CN, NDA and taurine solutions were added to 2 μL of blood and the solution was vortexed quickly, followed by allowing to stand for 10 min. After the addition of IS and 50% aqueous ethanol solutions, 15 μL of the solution was poured to a sample plate. (b) For analyzing ATCA, IS solution and ethanol were added to 2 μL of blood. After 50% aqueous ethanol solution was added to it, 15 μL of the solution was poured to a sample plate.
The CN standard solution was spiked to pooled blood, and the concentrations were adjusted to 50–2500 ng/mL. The workflow for the CN analysis is presented in Figure 5a. Then, 38 μL of the taurine solution (5.0 mM) and 40 μL of the NDA solution (4.3 mM) were added to 2 μL of the spiked blood sample. The mixture was vortexed quickly and allowed to stand for 10 min at room temperature. Then, 10 μL of the IS solution (secobarbital-d5, 90 ng/mL) and 240 μL of a 50% aqueous ethanol solution were added to the mixture. Finally, 15 μL of the solution was poured onto a sample plate (Shimadzu Corporation), and PESI/MS/MS was performed.
The ATCA standard solution was spiked to pooled blood, and the concentrations were adjusted to 50–1500 ng/mL. The workflow of the ATCA analysis is presented in Figure 5b.
To 2 μL of the spiked blood, 18 μL of the IS solution (ATZA, 500 ng/mL) and 20 μL of ethanol were added. This solution was diluted with 40 μL of a 50% aqueous ethanol solution. Finally, 15 μL of the solution was poured onto a sample plate (Shimadzu), and PESI/MS/MS was performed.
Method Validation
The calibration curves for the derivatized CN and ATCA were constructed by analyzing their spiked blood (calibrants); the ranges of the calibration curves are presented in Table 1. The methods were validated using QC samples, and their intra- and interday precisions and accuracies were calculated. The lower limit of detection (LLOD) and lower limit of quantitation (LLOQ) were calculated from the calibration curves using the following formulas: LLOD = 3.3 σblank/S and LLOQ = 10 σblank/S, where σblank is the standard division of the blank and S is the slope of each calibration curve.38
Acknowledgments
The authors are grateful for the financial support from the JSPS KAKENHI Grant Numbers 17H00689, 18H00506, and 18K10122. This work has been performed under the approval of the Ethics Committee of Nagoya University Graduate School of Medicine (Approval Number 2017-0175). I am very grateful to Dr. Issey Takahashi for making a great graphical abstract. Last but not least, we would like to thank the anonymous reviewer who strongly improved this manuscript with their feedback and comments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03229.
Derivatization scheme of CN; effect of thiocyanate’s interference; conditions for the sample preparation of derivatized CN in the blood; raw data of calibrants and QC samples for CN and ATCA; and raw data of postmortem samples (PDF)
Author Contributions
K.Z. conceived the idea and designed and supervised the experiments. K.H. and K.Z. wrote the manuscript. K.H. performed the entire experiment and executed data analysis. K.Z., K.H., T.M., and Y.H. discussed the results. K.O., H.T., M.H., and A.I. commented on the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Cooper C. E.; Brown G. C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- Kudo K.; Ishida T.; Hikiji W.; Usumoto Y.; Umehara T.; Nagamatsu K.; Tsuji A.; Ikeda N. Pattern of poisoning in Japan: selection of drugs and poisons for systematic toxicological analysis. Forensic Toxicol. 2010, 28, 25–32. 10.1007/s11419-009-0088-8. [DOI] [Google Scholar]
- McAllister J. L.; Roby R. J.; Levine B.; Purser D. Stability of Cyanide in Cadavers and in Postmortem Stored Tissue Specimens: A Review. J. Anal. Toxicol. 2008, 32, 612–620. 10.1093/jat/32.8.612. [DOI] [PubMed] [Google Scholar]
- Lundquist P.; Rosling H.; Sörbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clin. Chem. 1985, 31, 591–595. 10.1093/clinchem/31.4.591. [DOI] [PubMed] [Google Scholar]
- Kirk M. A.; Gerace R.; Kulig K. W. Cyanide and methemoglobin kinetics in smoke inhalation victims treated with the cyanide antidote kit. Ann. Emerg. Med. 1993, 22, 1413–1418. 10.1016/S0196-0644(05)81988-2. [DOI] [PubMed] [Google Scholar]
- Felscher D.; Wulfmeyer M. A New Specific Method to Detect Cyanide in Body Fluids, Especially Whole Blood, by Fluorimetry. J. Anal. Toxicol. 1998, 22, 363–366. 10.1093/jat/22.5.363. [DOI] [PubMed] [Google Scholar]
- Baskin S. I.; Petrikovics I.; Platoff G. E.; Rockwood G. A.; Logue B. A. Spectrophotometric Analysis of the Cyanide Metabolite 2-Aminothiazoline-4-Carboxylic Acid (ATCA). Toxicol. Mech. Methods 2006, 16, 339–345. 10.1080/15376520600616933. [DOI] [PubMed] [Google Scholar]
- Logue B. A.; Kirschten N. P.; Petrikovics I.; Moser M. A.; Rockwood G. A.; Baskin S. I. Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005, 819, 237–244. 10.1016/j.jchromb.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Yu J. C.; Martin S.; Nasr J.; Stafford K.; Thompson D.; Petrikovics I. LC-MS/MS analysis of 2-aminothiazoline-4-carboxylic acid as a forensic biomarker for cyanide poisoning. World J. Methodol. 2012, 2, 33–41. 10.5662/wjm.v2.i5.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue B. A.; Maserek W. K.; Rockwood G. A.; Keebaugh M. W.; Baskin S. I. The analysis of 2-amino-2-thiazoline-4-carboxylic acid in the plasma of smokers and non-smokers. Toxicol. Mech. Methods 2009, 19, 202–208. 10.1080/15376510802488165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikovics I.; Yu J. C.; Thompson D. E.; Jayanna P.; Logue B. A.; Nasr J.; Bhandari R. K.; Baskin S. I.; Rockwood G. Plasma persistence of 2-aminothiazoline-4-carboxylic acid in rat system determined by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2012, 891–892, 81–84. 10.1016/j.jchromb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Vinnakota C. V.; Peetha N. S.; Perrizo M. G.; Ferris D. G.; Oda R. P.; Rockwood G. A.; Logue B. A. Comparison of cyanide exposure markers in the biofluids of smokers and non-smokers. Biomarkers 2012, 17, 625–633. 10.3109/1354750X.2012.709880. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R.; Singh P.; Palit M.; Waghmare C.; Singh A. K.; Gopalan N.; Kumar D. Time-dependent comparative evaluation of some important biomarkers of acute cyanide poisoning in rats: an aid in diagnosis. Biomarkers 2014, 19, 241–251. 10.3109/1354750X.2014.902996. [DOI] [PubMed] [Google Scholar]
- Bhandari R. K.; Oda R. P.; Petrikovics I.; Thompson D. E.; Brenner M.; Mahon S. B.; Bebarta V. S.; Rockwood G. A.; Logue B. A. Cyanide toxicokinetics: the behavior of cyanide, thiocyanate and 2-amino-2-thiazoline-4-carboxylic acid in multiple animal models. J. Anal. Toxicol. 2014, 38, 218–225. 10.1093/jat/bku020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebułtowicz J.; Ruzycka M.; Fudalej M.; Krajewski P.; Wroczynski P. LC-MS/MS method development and validation for quantitative analyses of 2-aminothiazoline-4-carboxylic acid--a new cyanide exposure marker in post mortem blood. Talanta 2016, 150, 586–592. 10.1016/j.talanta.2015.12.076. [DOI] [PubMed] [Google Scholar]
- Rużycka M.; Giebultowicz J.; Fudalej M.; Krajewski P.; Wroczynski P. Application of 2-Aminothiazoline-4-carboxylic Acid as a Forensic Marker of Cyanide Exposure. Chem. Res. Toxicol. 2017, 30, 516–523. 10.1021/acs.chemrestox.6b00219. [DOI] [PubMed] [Google Scholar]
- Giebułtowicz J.; Sobiech M.; Rużycka M.; Luliński P. Theoretical and experimental approach to hydrophilic interaction dispersive solid-phase extraction of 2-aminothiazoline-4-carboxylic acid from human post-mortem blood. J. Chromatogr. A 2019, 1587, 61–72. 10.1016/j.chroma.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Li S. Y.; Petrikovics I.; Yu J. The Potential Use of 2-Aminothiazoline-4-carboxylic Acid (ATCA) as a Forensic Marker for Cyanide Exposure in Medicolegal Death Investigation: A Review. Forensic Sci. Rev. 2019, 31, 45–58. [PubMed] [Google Scholar]
- Zaitsu K.Chapter 1 - Introduction to ambient ionization mass spectrometry. In Ambient Ionization Mass Spectrometry in Life Sciences, Zaitsu K., Ed.; Elsevier, 2019; pp 1–32. [Google Scholar]
- Hiraoka K.; Nishidate K.; Mori K.; Asakawa D.; Suzuki S. Development of probe electrospray using a solid needle. Rapid Commun. Mass Spectrom. 2007, 21, 3139–3144. 10.1002/rcm.3201. [DOI] [PubMed] [Google Scholar]
- Zaitsu K.; Hayashi Y.; Murata T.; Ohara T.; Nakagiri K.; Kusano M.; Nakajima H.; Nakajima T.; Ishikawa T.; Tsuchihashi H.; Ishii A. Intact Endogenous Metabolite Analysis of Mice Liver by Probe Electrospray Ionization/Triple Quadrupole Tandem Mass Spectrometry and Its Preliminary Application to in Vivo Real-Time Analysis. Anal. Chem. 2016, 88, 3556–3561. 10.1021/acs.analchem.5b04046. [DOI] [PubMed] [Google Scholar]
- Hayashi Y.; Zaitsu K.; Murata T.; Ohara T.; Moreau S.; Kusano M.; Tanihata H.; Tsuchihashi H.; Ishii A.; Ishikawa T. Intact metabolite profiling of mouse brain by probe electrospray ionization/triple quadrupole tandem mass spectrometry (PESI/MS/MS) and its potential use for local distribution analysis of the brain. Anal. Chim. Acta 2017, 983, 160–165. 10.1016/j.aca.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Zaitsu K.; Hayashi Y.; Murata T.; Yokota K.; Ohara T.; Kusano M.; Tsuchihashi H.; Ishikawa T.; Ishii A.; Ogata K.; Tanihata H. In Vivo Real-Time Monitoring System Using Probe Electrospray Ionization/Tandem Mass Spectrometry for Metabolites in Mouse Brain. Anal. Chem. 2018, 90, 4695–4701. 10.1021/acs.analchem.7b05291. [DOI] [PubMed] [Google Scholar]
- Sano A.; Takezawa M.; Takitani S. Fluorometric determination of cyanide with 2,3-naphthalenedialdehyde and taurine. Talanta 1987, 34, 743–744. 10.1016/0039-9140(87)80233-3. [DOI] [PubMed] [Google Scholar]
- Sano A.; Takimoto N.; Takitani S. High-performance liquid chromatographic determination of cyanide in human red blood cells by pre-column fluorescence derivatization. J. Chromatogr. B: Biomed. Sci. Appl. 1992, 582, 131–135. 10.1016/0378-4347(92)80311-D. [DOI] [PubMed] [Google Scholar]
- Tracqui A.; Raul J. S.; Géraut A.; Berthelon L.; Ludes B. Determination of Blood Cyanide by HPLC-MS. J. Anal. Toxicol. 2002, 26, 144–148. 10.1093/jat/26.3.144. [DOI] [PubMed] [Google Scholar]
- Gambaro V.; Arnoldi S.; Casagni E.; Dell’Acqua L.; Pecoraro C.; Froldi R. Blood Cyanide Determination in Two Cases of Fatal Intoxication: Comparison Between Headspace Gas Chromatography and a Spectrophotometric Method*. J. Forensic Sci. 2007, 52, 1401–1404. 10.1111/j.1556-4029.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- Desharnais B.; Huppé G.; Lamarche M.; Mireault P.; Skinner C. D. Cyanide quantification in post-mortem biological matrices by headspace GC–MS. Forensic Sci. Int. 2012, 222, 346–351. 10.1016/j.forsciint.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Kudo K.; Usumoto Y.; Sameshima N.; Okumura M.; Tsuji A.; Ikeda N. Reliable determination of cyanide, thiocyanate and azide in human whole blood by GC–MS, and its application in NAGINATA–GC–MS screening. Forensic Toxicol. 2018, 36, 160–169. 10.1007/s11419-017-0397-2. [DOI] [Google Scholar]
- Bhandari R. K.; Oda R. P.; Youso S. L.; Petrikovics I.; Bebarta V. S.; Rockwood G. A.; Logue B. A. Simultaneous determination of cyanide and thiocyanate in plasma by chemical ionization gas chromatography mass-spectrometry (CI-GC-MS). Anal. Bioanal. Chem. 2012, 404, 2287–2294. 10.1007/s00216-012-6360-5. [DOI] [PubMed] [Google Scholar]
- Lacroix C.; Saussereau E.; Boulanger F.; Goullé J. P. Online Liquid Chromatography-Tandem Mass Spectrometry Cyanide Determination in Blood. J. Anal. Toxicol. 2011, 35, 143–147. 10.1093/anatox/35.3.143. [DOI] [PubMed] [Google Scholar]
- Li S. Y.; Petrikovics I.; Yu J. Development of magnetic carbon nanotubes for dispersive micro solid phase extraction of the cyanide metabolite, 2-aminothiazoline-4-carboxylic acid, in biological samples. J. Chromatogr. B 2019, 1109, 67–75. 10.1016/j.jchromb.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.; Zhao Y.; Cai S.; Fu S.; Yang C.; Zhang S.; Zhang X. Single Cell Analysis with Probe ESI-Mass Spectrometry: Detection of Metabolites at Cellular and Subcellular Levels. Anal. Chem. 2014, 86, 3809–3816. 10.1021/ac500882e. [DOI] [PubMed] [Google Scholar]
- Baselt R. C.Disposition of Toxic Drugs and Chemicals in Man, 11th ed.; Biomedical Publications: Seal Beach, CA, 2017; pp 554–556. [Google Scholar]
- Stamyr K.; Thelander G.; Ernstgård L.; Ahlner J.; Johanson G. Swedish forensic data 1992–2009 suggest hydrogen cyanide as an important cause of death in fire victims. Inhalation Toxicol. 2012, 24, 194–199. 10.3109/08958378.2012.660285. [DOI] [PubMed] [Google Scholar]
- Rhee J.; Jung J.; Yeom H.; Lee H.; Lee S.; Park Y.; Chung H. Distribution of cyanide in heart blood, peripheral blood and gastric contents in 21 cyanide related fatalities. Forensic Sci. Int. 2011, 210, e12–e15. 10.1016/j.forsciint.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Gao X.; Sanderson S. M.; Dai Z.; Reid M. A.; Cooper D. E.; Lu M.; Richie J. P.; Ciccarella A.; Calcagnotto A.; Mikhael P. G.; Mentch S. J.; Liu J.; Ables G.; Kirsch D. G.; Hsu D. S.; Nichenametla S. N.; Locasale J. W. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology; ICH; U.S. Department of Health and Human Services: CDER; CBER; Rockville, 1996.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






