Abstract
Purpose of Review
We aim to summarize recent insights and provide an up-to-date overview on the role of intra-aortic balloon pump (IABP) counterpulsation in cardiogenic shock (CS).
Recent Findings
In the largest randomized controlled trial (RCT) of patients with CS after acute myocardial infarction (AMICS), IABP did not lower mortality. However, recent data suggest a role for IABP in patients who have persistent ischemia after revascularization. Moreover, in the growing population of CS not caused by acute coronary syndrome (ACS), multiple retrospective studies and one small RCT report on significant hemodynamic improvement following (early) initiation of IABP support, which allowed bridging of most patients to recovery or definitive therapies like heart transplant or a left ventricular assist device (LVAD).
Summary
Routine use of IABP in patients with AMICS is not recommended, but many patients with CS either from ischemic or non-ischemic cause may benefit from IABP at least for hemodynamic improvement in the short term. There is a need for a larger RCT regarding the role of IABP in selected patients with ACS, as well as in patients with non-ACS CS.
Keywords: Intra-aortic balloon counterpulsation, Mechanical circulatory support, Cardiogenic shock, Heart failure
Introduction
Although the use of (percutaneous and non-percutaneous) mechanical circulatory support devices (MCSDs) such as veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has increased considerably last years, intra-aortic balloon pump (IABP) counterpulsation globally remains the most used first-line support in patients with cardiogenic shock (CS) [1, 2]. In this article, we aim to summarize recent insights and provide an up-to-date overview of the use of IABP in patients with CS.
Technique
IABP is a mechanical support device that consists of a flexible 30–50-cc helium-filled balloon catheter attached to a console that times periodic inflation and deflation according to the cardiac cycle. The distal tip of the balloon should be placed in the descending aorta, approximately 1 cm distal to the origin of the left subclavian artery. The IABP was first placed by surgical cut-down of the femoral artery by Dr. Adrian Kantrowitz in the 1960s. Currently, implantation is usually done by a percutaneous (Seldinger) technique via the femoral approach, although surgical insertion in the subclavian artery [3–5, 6•] or percutaneous introduction via the axillary artery [7•] is also possible.
Hemodynamics
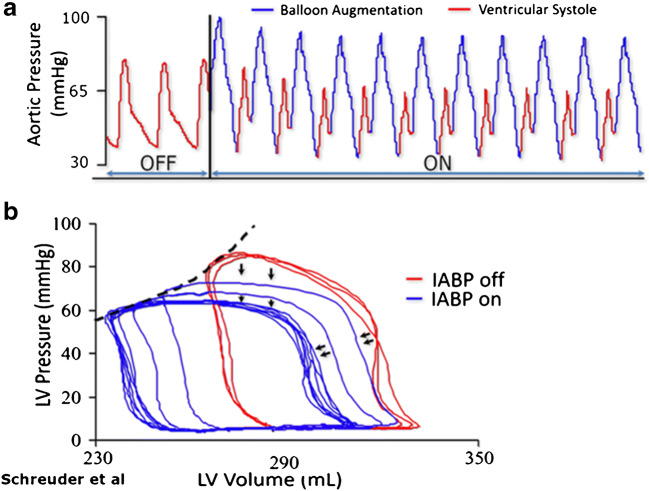
Its physiological effect is dual. By inflating the balloon immediately after aortic valve closure, diastolic and mean arterial pressures rise and coronary perfusion improves. On the other hand, a vacuum effect—caused by rapid deflation of the balloon just before aortic valve opening—provides a reduction in left ventricle afterload and thereby passively augments cardiac output (CO) [8]. The hemodynamic effect will vary based on the clinical setting and the overall stroke volume. In vivo left pressure-volume loops, measured invasively with a conductance catheter, show an acute decrease in left ventricular end-systolic volume by 6%, a decrease in left ventricular end-systolic pressure by 18%, and an increase in stroke volume by 14% (see Fig. 1b) [9]. Left ventricle stroke work is reduced [10]. The primary objectives of the IABP are an increase in myocardial oxygen supply, a decrease in oxygen demand, and optimization of end-organ perfusion [10]. The bedside effects on aortic pressure curves are generally characterized by a decrease in systolic blood pressure, an increase in diastolic blood pressure, and an increase in mean arterial pressure (Fig. 1a) [8]. A reduction in pulmonary capillary wedge pressure (PCWP) and an increase in stroke volume can be measured with right heart catheterization or estimated with echocardiography [8].
Fig. 1.
Hemodynamic effects of an IABP in patients with reduced ejection fraction. a Immediate effect on aortic pressure curve after initiation of IABP in a patient with 14% ejection fraction. b Corresponding pressure-volume loops showing left shift with reduction in systolic pressure, and increased stroke volume. Copied with permission from Bastos et al. [8] and Schreuder et al. [9]
Indications
IABP has been applied in a wide spectrum of indications.
Acute Myocardial Infarction Without Shock
* Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP-AMI) was a multicenter randomized controlled trial (RCT) that showed no reduction in infarct size or mortality by a strategy of percutaneous coronary intervention (PCI) with prophylactic IABP support versus PCI alone in 337 patients with anterior ST-elevation myocardial infarction (STEMI) without CS [11]. Nine percent of the patients in the PCI group crossed over to rescue IABP therapy. However, there was a significant difference in the exploratory composite end point of time to death, shock, or new or worsening heart failure (HF) (P = 0.03), which was solely driven by the development of shock in patients after PCI.
* In 2015, a meta-analysis to assess IABP efficacy in AMI included 12 RCTs containing a total of 2123 patients [12]. The authors concluded that IABP did not have any statistically significant effect on mortality.
* Recently, Van Nunen and colleagues evaluated the effect of the IABP in 100 patients with large STEMI complicated by persistent ischemia (defined by < 50% of ST-elevation resolution after PCI) [13]. Placement of IABP in this selected group resulted in more frequent ST-elevation resolution (73 ± 17%) compared with the control group (56 ± 26%; P < 0.01), after a mean of 3 h. The composite end point of death, necessity of left ventricular assist device (LVAD) implantation, or re-admission for HF within 6 months was numerically lower in the IABP group compared with the control group. The authors found no significant difference in infarct size.
High-Risk Percutaneous Coronary Intervention
* In BCIS-1, a multicenter trial, 301 elective patients with severe coronary artery disease and left ventricular ejection fraction (LVEF) of < 30% were randomized to receive PCI with or without IABP support [14]. Twelve percent of the no-IABP group required bailout IABP therapy. This study was primarily designed to address in-hospital MACCE (a composite end point of death, AMI, further revascularization, and cerebrovascular events) at 28 days, and no difference between the groups was seen. However, all-cause mortality at a median follow-up of 51 months was significantly lower in the planned IABP group vs the PCI alone group (HR 0.66; 95% CI 0.44–0.98; P = 0.039).
* In the PROTECT II study, 452 symptomatic patients with complex 3-vessel or unprotected left main or last patent coronary artery disease with a LVEF of ≤ 35% were randomized to hemodynamic support by IABP or Impella 2.5 during non-emergent high-risk PCI [15]. Impella provided better hemodynamic support, which was the secondary outcome measure. There was no significant difference in the primary composite end point of MACCE and device-related adverse events after 30 days. However, there was a significantly better outcome of this composite end point in the Impella group after 90 days in the per-protocol analysis (51% in IABP vs 40% in Impella; P = 0.02).
* In a recent meta-analysis of 16 RCTs, prophylactic use of IABP during high-risk PCI was not associated with a decrease in 30-day or 6-month all-cause mortality, re-infarction, stroke/transient ischemic attack (TIA), HF, repeat revascularization, embolization, or arrhythmia [16]. Percutaneous ventricular assist devices (pVADs) were more likely to reduce repeat revascularization but showed an increased risk of bleeding events compared with IABP.
* A retrospective analysis of 21,848 patients who underwent non-emergent PCI requiring mechanical circulatory support showed that patients supported with a pVAD had lower in-hospital mortality compared with IABP, despite the observation that patients in this group had more comorbidities [17]. Patients with pVAD also had lower cardiac, vascular, and respiratory complications and their duration of hospital stay was shorter. After applying propensity score matching, these findings remained significant.
Prior to High-Risk Coronary Artery Bypass Graft Surgery
Although some meta-analyses suggest a benefit in mortality and MACCE, the prophylactic pre-operative insertion of IABP in patients undergoing high-risk coronary artery bypass grafting (CABG) remains controversial [18–21].
As a Left Ventricular Vent During VA-ECMO Support
In patients with CS requiring VA-ECMO, the concomitant use of IABP is associated with significantly lower mortality, although direct unloading by the concomitant use of a (more expensive) Impella device might be even more effective [22, 23]. However, Impella requires larger vascular access and may be associated with more adverse effects (bleeding, hemolysis, limb ischemia).
Mechanical Complications of AMI
A final indication includes mechanical complications of AMI (i.e., ventricular septal rupture, mitral regurgitation, or free wall rupture) as a bridge to surgical repair which is still a class IIa/C recommendation for IABP placement in European guidelines [24, 25].
Adverse Events
Compared with other MCSDs like micro-axial pVADs (Impella, Abiomed, Danvers, MA; USA) and Tandem Heart (CardiacAssist Inc., Pittsburgh, PA, USA), extracorporeal centrifugal-flow LVAD, and VA-ECMO, complication rates of IABP are low. The reported incidence of adverse events in femoral IABP implantation ranges between 0.9 and 31.1% [26•, 27, 28•, 29, 30••], but these rates also include minor adverse events (e.g., access site hematoma, transient loss of pulsations, or need for blood transfusion). The most frequent device-related complication is (most often reversible) limb ischemia with a roughly estimated incidence of 5% (range from 0.9 to 26.7%) [27, 29, 31]. However, we have to consider that complications may be the result of the CS itself, since the complication rate in IABP supported patients was equal compared with controls in IABP-SHOCK [31]. When the IABP is implanted by an axillary or subclavian approach, the following complications have been reported: malfunction due to kinking, rupture, or migration requiring removal or reposition (15–37%), stroke (0–3%), upper limb ischemia (0–4%), transient brachial plexus injury (0–2%), mesenteric ischemia (0–3%), local vascular complications (0–7%), bacteremia requiring antibiotics (0–9%), and bleeding needing transfusion (0–16%) [4, 5, 7•, 32].
Recent Insights Regarding the Use of IABP in CS
Cardiogenic Shock After Acute Myocardial Infarction
While cardiogenic shock following acute myocardial infarction (AMICS) was the main indication for an IABP for many years, the results of the IABP-SHOCK II trial in 2012, the largest IABP trial so far, caused a severe decline in its routine use [2, 33, 34]. In this RCT, 600 patients with AMICS were randomized to IABP or conservative therapy, both including routine revascularization [31]. No difference in all-cause mortality after 30 days was observed. On the other hand, IABP was not associated with increased adverse events like re-infarction, stent thrombosis, bleeding, sepsis, or stroke. In 2015, a meta-analysis of 7 RCTs including 790 patients with AMICS showed similar results of no survival benefit by the routine placement of an IABP in this population [35]. As a consequence of these results, both European and American guideline recommendations were downgraded (ESC: III/B; ACC/AHA: IIb/B) [24, 36, 37]. Because only 13% of patients in the IABP group of the IABP-SHOCK II trial received the IABP before revascularization, a meta-analysis including 1348 patients with AMICS was performed in order to clarify the role of the timing of its placement [38]. However, no difference was seen with respect to short- or long-term (≥ 6 months) survival between patients supported upstream or only after primary PCI. Also, no significant outcome difference in terms of re-infarction, repeat revascularization, stroke, renal failure, and major bleeding was seen.
IABP vs Impella
It is hypothesized that the Impella device, by direct unloading, may reduce infarct size, particularly when starting pre-PCI in patients with AMICS who are revascularized [39]. Patients with CS who were treated with pVAD (Tandem Heart® or Impella®) had a significantly higher mean arterial pressure and a faster decrease in lactate levels compared with patients treated with IABP [40]. However, in the same meta-analysis including 148 patients, no significant difference in 30-d mortality was seen, whereas bleeding occurred more frequently in patients with pVAD (RR 2.50; P < 0.001) [40]. Of note, sample sizes of the 4 RCTs included in this meta-analysis were small. Critics also emphasize that 92% of patients in the latest IMPRESS (IMPella versus IABP REduces mortality in STEMI patients treated with primary PCI in Severe cardiogenic SHOCK) study had been resuscitated from cardiac arrest, resulting in a 46% death rate due to anoxic brain damage [41].
Two important observational studies were recently published. First, Schrage and colleagues retrospectively matched 237 patients with AMICS treated with Impella to an equal number of patients from the IABP-SHOCK II trial treated with medical therapy or IABP [42]. The authors found no significant difference in 30-d all-cause mortality, while severe or life-threatening bleeding and peripheral vascular complications occurred significantly more often in the Impella group. Second, in a large US retrospective study including 1680 propensity-matched paired patients with AMICS undergoing PCI, there was a significantly higher risk of in-hospital death and major bleeding associated with the use of pVADs compared with treatment with IABP (45% vs 34% and 31% vs 16% respectively; P for both < .001) [43]. These findings were remarkable since patients with pVADs were significantly younger and less likely to have STEMI compared with patients treated with IABP.
Large-Volume IABP May Be Better
In the past decade, a larger-capacity (50-cc) IABP was introduced into clinical practice. Compared with previously used 40-cc IABPs, patients who received a 50-cc IABP showed higher-peak augmented diastolic pressure, higher magnitude of diastolic augmentation, and a greater slope and magnitude of deflation pressure from peak augmented diastolic pressure to reduced aortic end-diastolic pressure [44]. In 50-cc IABP recipients, diastolic pressure and PA occlusion pressure were reduced, and CO, cardiac index, and PA oxygen saturation were increased, while these PA catheter–derived measurements did not significantly change in patients with a 40-cc IABP. The absolute increase in CO was 1.4 ± 1.0 L/min in the 50-cc IABP group versus 0.7 ± 0.9 L/min in the 40-cc IABP group, which represented a relative increase of CO compared with baseline of 40% and 18% respectively (P = .08). Fifty cubic centimeters IABP also resulted in a greater systolic unloading and a larger reduction in pulmonary capillary occlusion pressure, compared with 40-cc IABP. The magnitude of systolic unloading correlated directly with the magnitude of diastolic augmentation and inversely with the PA occlusion pressure [44]. Also in later studies, 50-cc IABP caused significant diastolic pressure augmentation (Δ + 42 mmHg), systolic unloading (Δ − 15 mmHg), increased CO (Δ + 1.03 L/min), and decreased cardiac filling pressures in the majority of patients [45, 46].
Non-ACS Cardiogenic Shock
Although the use of IABP in patients with AMICS is now controversial, 20–70% of all CS is not caused by an ACS [2, 47–49]. This non-ACS CS group (also defined as ADHF-CS: acute decompensated HF with cardiogenic shock) includes acute decompensated chronic HF but also CS as a presentation of de novo HF. Importantly, this group seems to be a different population with regard to age, gender, ventricular function, and ventricular dimensions [2, 47, 49, 50••]. Patients with non-ACS CS also have less atherosclerotic cardiovascular risk factors and are more likely to have chronic kidney disease and pre-existing HF, compared with patients with AMICS [47, 48, 50••]. In contrast to AMICS, the etiology of non-ACS CS is diverse, reaching from temporary cardiac disturbances like arrhythmias (responsive to interventions or even self-limiting) until expressions of end-stage HF without any traceable provoking events. Although the role of IABP in this population remains insufficiently defined, several small uncontrolled studies have been performed in order to elucidate its feasibility in this subgroup. These studies are summarized in Table 1.
Table 1.
Chronologic overview of recently published studies regarding the use of IABP in non-ACS cardiogenic shock and end-stage chronic heart failure
| Author, publication year [reference] | Study design (volume of balloon) « insertion site » |
Inclusion criteria/study population | No. of pts treated with IABP# | Duration of IABP therapy (range) | Effects on hemodynamics, echocardiography and laboratory tests^ | Clinical outcomes |
|---|---|---|---|---|---|---|
| Norkiene, 2007 [51] |
Retrospective, observational (40 cc IABP) « femoral » |
Acute decompensated DCM, listed for urgent OHT or LVAD, NYHA 4, MAP < 65, CI < 2, PCWP > 20, refractory to all means of OMT | 11 | Mean 182 ± 82 h (72 to 360) | MAP ↑; LVEF ↑; CVP ↓ | 27% recovery; 27% LVAD; 18% OHT; 27% died (2 after IABP removal and 1 after LVAD) |
| Gjesdal, 2009 [52] |
Retrospective (40–50 cc IABP) « femoral » |
IABP: Terminal HF, IABP as an intended BTT due to clinical deterioration not responding to OMT Control: Pts who received OHT in a hemodynamic stable situation (without IABP) |
40 (control group: 135) |
Mean 21 ± 16 days (3 to 66) from onset IABP to OHT Mean 25 ± 21 days (1 to 49) from IABP to MCS |
Creatinine ↓; urea ↓; ASAT and ALAT ↓; bilirubin ↓; sodium ↑; potassium ↓ | 95% OHT, but 15% needed escalation to ECMO (10%) and LVAD (5%); 5% died (2.5% on IABP and 2.5% on LVAD); equal post-OHT mortality after 30 d, 1 y, and 3 y between IABP and control; post-OHT RHC and TTE variables equal after 30 d and 1 y |
| Russo, 2012 [5] |
Retrospective, observational (size NA) « subclavian » |
IABP to support severe decompensated HF while awaiting OHT | 17$ | Mean 17 ± 13 days (3 to 48) | NA | 82% OHT; 12% needed escalation to VAD (further outcome unknown); 6% still waiting for OHT; 0% died |
| Umakanthan, 2012 [32] |
Retrospective, observational (size NA) « axillary& » |
End-stage HF and failure on or intolerance to inotropes | 18 |
Mean 27 ± 18 days (5 to 63) Median 19 days |
CI ↑; mPAP ↓; sPAP ↓; CVP ↓ | 72% OHT; 28% died (6% despite escalation to LVAD); longest walking distance 5.5× ↑; 1 m survival 89%; 6 m survival 72% |
| Mizuno, 2014 [53] |
Prospective, non-randomized, observational, multicenter cohort (size NA) « femoral » |
ADHF who meet the modified Framingham criteria, > 20 y, and considered suitable by the attending physicians; IABP vs control (without IABP) | 123 (control group: 4678) | NA | NA | 71% discharged alive; 29% mortality during hospitalization; mean length of hospital stay 48 days |
| Ntalianis, 2015 [54] |
Prospective, unicenter, observational (size NA) « femoral » |
End-stage HF, NYHA IV, INTERMACS 1 or 2, despite OMT, severe LV and RV systolic dysfunction, with contra-indications for durable HRT, IABP as prolonged support in order to improve the RV function to recover or regain LVAD candidacy | 15 |
Mean 73 ± 50 days (13 to 155) Median 72 days |
RAP ↓; mPAP ↓; CI ↑; RVSWI ↑; PCWP ↓; creatinine ↓; total bilirubin↓; LVEF ↑; RVEDD ↓; Sm ↑ | 20% recovery (without MCS and all alive/NYHA1 after 6 m); 40% LVAD after a mean of 66 d (reversal of previous contra-indications by IABP); 40% died |
| Sintek, 2015 [55] |
Single-centre, retrospective (mean size 42 cc) « femoral » |
Systolic CHF who developed CS refractory to OMT and, INTERMACS 1 or 2, pts. who received LVAD after bridge with IABP | 54 | Median 2 days for decompensated pts and 3 days for stabilized pts | CI ↑; PCWP ↓; CPI ↑; UP ↑; sPAP ↓ only in subgroup of responders | 57% stabilized*; 43% decompensated (26% medication increase; 11% escalation to MCS); 17% died |
| Tanaka, 2016 [4] |
Single-centre, retrospective (size 34/40/50 cc) « subclavian& » |
Advanced DCHF (clinical diagnosis confirmed by RHC), 56% on inotropes, mean CI 1.9 ± 0.6, as a bridge to definitive HRT | 88 | Median 21 ± 22 days (4 to 135) | CVP↓; mPAP ↓; PCWP ↓; CI ↑; creatinine ↓ | 93% of patients LVAD, OHT, or recovery (3.4% with escalation to MCS); 7% died; 96% able to walk > 3×/d and received physical rehabilitation during IABP; TMST ↑ |
| Den Uil, 2017 [56] |
Single center, retrospective (50cc IABP) « femoral » |
Inotrope-dependent HF with signs of hypoperfusion and tissue hypoxia, INTERMACS 1/2 | 27 | Median 4 days (3 to 9) | MAP ↑; sVO2 ↑; RAP ↓; fb ↓; lactate ↓; sodium ↑ | 67% successful (26% recovery; 19% LVAD; 22% OHT); 7% escalation to ECMO; 26% died; 30-day survival 67%; 1 y survival 63% |
| Annamalai, 2017 [10] |
Single-centre, prospective (50 cc IABP) « femoral » |
Stage D HF, NYHA 3/4, INTERMACS 2/3, inotrope-dependent with persistently low CO, within 48 h of LVAD surgery | 10 | < 48 h | LVSW ↓; LVESP ↓; DPTI ↑; PAP ↓; myocardial oxygen supply/demand ratio ↑; PVR ↓; CPO ↑ | 100% successful LVAD |
| Hsu, 2018 [26•] |
Single-centre, retrospective, cohort study (size NA) « femoral » |
> 18 y, CS (89% systolic CHF) defined as SBP < 90 for > 30 min with evidence of poor end-organ perfusion or need for inotropic support | 74 | NA | CI ↑; SVR ↓; HR ↓; SBP ↓; DBP ↓; RAP ↓; PCWP ↓; PAP ↓; LVCPI ↑; | 20% recovery; 45% LVAD; 7% OHT; 4% urgent escalation to MCS; 24% died |
| Morici, 2018 [57] |
Bicentre, prospective, phase II study (size NA) « femoral » |
≥ 18 y, < 80 y, severe LV dysfunction, SBP < 90, or MAP < 60 after fluid challenge or with RAP > 12 or PCWP > 14 with ≥ 1 sign of ongoing organ hypoperfusion, failure of OMT (88% after failure of inotropes) | 17$ | Median 7 days (IQR 4 to 9) | NA for IABP alone group | 12% recovery; 53% LVAD; 12% OHT; 6% escalation to ECMO; 18% died |
| Fried, 2018 [28•] |
Single-centre, retrospective, cohort study (size NA) « femoral except for 1 axillary» |
≥ 18 y, ADCHF with CS (CI < 2.2 and SBP < 90 or need for vasoactive medications to maintain this level) (87% on ≥ 1 inotrope and 28% on ≥ 1 vasopressor) | 132 |
Median 96 h (IQR 48 to 144) for entire cohort Median 111 h (IQR 48 to 168) for those who received LVAD or OHT Median 84 h (IQR 44–235) for those with clinical deterioration |
CO and CI ↑; mPAP ↓ | 78% discharged after HRT or recovery; 16% recovery; 52% LVAD; 6% OHT; 8% escalation to other MCS; 18% died; 84% overall 30-d survival |
| Imamura, 2018 [6•] |
Single-centre, retrospective (size NA) « subclavian » |
Advanced HF, IABP to treat hemodynamic deterioration (69% on inotropes) | 91 | Mean 25 ± 20 days; 65% continued IABP support for ≥ 14 days | PCWP ↓; CVP ↓; CI ↑; creatinine ↓; lactate ↑ | 12% recovery; 69% LVAD or OHT; 4% escalation to other MCS; 9% died |
| Malick, 2019 [50••] |
Single-centre, retrospective, cohort study (size NA) « femoral » |
≥ 18 y, ADHF with CS (CI < 2.2 and either SBP < 90 or need for vasoactive medications to achieve this SBP) | 132$ | Median 3 days (IQR 2 to 5) | CO and CI ↑; CPO ↑; CPI ↑; CVP ↓; SVR ↓; mPAP ↓ | 16% recovery; 62% HRT; 22% died; (8% escalation to MCS of which ½ died and ½ received OHT) |
| Bhimaraj, 2020 [7•] |
Single-centre, retrospective, (size NA) « axillary » |
Advanced HF who needed maintenance of hemodynamic support until HRT (71% on inotropes), mean sVO2 54% | 195 | Median 19 days (IQR 12 to 43), max 169 days | WBC ↓; BUN ↓; bilirubin ↓ | 68% successful HRT (62% OHT and 7% LVAD); 9% escalation to MCS; 11% IABP removal due to complications; 8% died and 3% IABP removal because of lack of candidacy for HRT |
ACS acute coronary syndrome, ADCHF acute decompensated chronic heart failure, ADHF acute decompensated heart failure, ALAT alanine aminotransferase, ASAT aspartate aminotransferase, BTT bridge to transplant, BUN blood urea nitrogen, cc cubic centimetre, CHF chronic heart failure, CI cardiac index (in L/min/m2), CO cardiac output, CPO cardiac power output, CS cardiogenic shock, CVP central venous pressure, DBP diastolic blood pressure (in mmHg), DCHF decompensated chronic heart failure, DCM dilated cardiomyopathy, DPTI diastolic pressure time index, ECMO extracorporeal membrane oxygenation, fb fluid balance, HF heart failure, HR heart rate, HRT heart replacement therapy (conventional cardiac surgery, heart transplant, or LVAD implantation), IABP intra-aortic balloon pump, INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support profile, IQR interquartile range, LV left ventricle, LVAD left ventricular assist device, CPI cardiac power index, LVEF left ventricular ejection fraction, LVESP left ventricular end-systolic pressure, LVSW left ventricle stroke work, m month, MAP mean arterial pressure (in mmHg), max maximum, MCS mechanical circulatory support, mPAP mean pulmonary artery pressure (in mmHg), NA not available, No. number, NYHA New York Heart Association classification, OHT orthotopic heart transplantation, OMT optimal medical (drug) therapy including inotropic and/or vasopressive support, PAP pulmonary artery pressure (in mmHg), PCWP pulmonary capillary wedge pressure (in mmHg), Pts patients, PVR peripheral vascular resistance, RAP right atrial pressure (in mmHg), RHC right heart catheterization, RV right ventricle, RVEDD right ventricle end-diastolic diameter, RVSWI right ventricle stroke work index, SBP systolic blood pressure (in mmHg), Sm tricuspid annular systolic tissue Doppler velocity, sPAP systolic pulmonary artery pressure (in mmHg), sVO2 central venous oxygen saturation, TMST two-minute step in place test, TTE transthoracic echocardiography, UP urinary production, VAD ventricular assist device, WBC white blood count, y year(s)
#Only studies with ≥ 10 patients were included in this table
^Only significant (P < 0.05) results are listed
$The overall study population also contained patients with AMICS, other indication for IABP than CS, or control patients without IABP but these patients were excluded from this table
*Stabilization means that all the following 5 criteria were met: (1) did not need any other form of temporary mechanical support; (2) did not require an increase in dose or number of vasopressor or inotrope support; (3) did not need renal replacement therapy or mechanical ventilation; (4) did not have refractory ventricular arrhythmias; or (5) did not experience worsening metabolic acidosis
&Patients first underwent femoral IABP placement to evaluate if any hemodynamic benefit was achieved
A study of particular interest is the one by Malick and colleagues, in which the effect of IABP placement was directly compared between patients with AMICS (n = 73; 36%) and those with non-ACS CS (n = 132; 64%) [50••]. Baseline characteristics showed that patients with non-ACS CS had significantly higher PAP (mean 38 ± 9 vs 31 ± 8 mmHg), lower LVEF (18 ± 9 vs 30 ± 12%), higher left ventricular end-diastolic dimension (7 ± 1 vs 5 ± 1 cm), higher serum creatinine (1.97 ± 1.06 vs 1.59 ± 1.11 mg/dL), lower serum lactate (2.54 ± 2.50 vs 4.92 ± 4.21 mmol/L), higher PA pulsatility index (2.91 ± 3.35 vs 2.00 ± 1.69), and more vasoactive agents (1.7 ± 1.0 vs 1.4 ± 0.8). Interestingly, patients with non-ACS CS experienced a 5-fold greater CO augmentation compared with patients with AMICS (0.58 ± 0.79 L/min vs 0.12 ± 1.00 L/min; P = 0.0009). Patients with non-ACS CS experienced an increase by almost a quarter (24%) of their baseline CO, while the increase in patients with AMICS was only 10% (P = 0.02). Systemic vascular resistance decreased significantly in non-ACS CS patients but remained equal in patients with AMICS (P < 0.05).
We recently performed the first RCT regarding IABP therapy versus inotropy in the early phase of non-ACS CS [30••]. The population included both de novo and acute on chronic HF patients without signs of acute ischemia. All patients (n = 32) had a systolic blood pressure of < 100 mmHg, fluid retention, at least moderate tricuspid valve regurgitation and/or mitral valve regurgitation, a dilated inferior cava vein, high filling pressure, low CO, a neutral or positive fluid balance despite fluid restriction, and high-dose intravenous loop diuretics, together with dysfunction of at least 1 other organ. Sixteen patients were treated with a 50 cc IABP and 16 with inotropes. After 48 h, those treated with IABP had significant higher central venous oxygen saturation (+ 17 vs. + 5%), a better increase in cardiac power output (+ 0.27 vs + 0.09 W/m2), lower N-terminal pro B-type natriuretic peptide levels (− 59 vs − 16 ng/L), a more negative cumulative fluid balance (− 3.066 vs − 1.198 L), and a better decrease in dyspnea severity score (− 4 vs − 2). In addition, mean arterial pressure increased more in the IABP group, and mean PAP and PCWP decreased more in the IABP group. Fewer patients in the IABP group ended up with moderate to severe mitral valve regurgitation. Finally, patients treated with an IABP tended to have lower major adverse cardiovascular events (a combined end point of crossover or other escalation of therapy, death, HF, re-hospitalization or TIA/stroke) (38% vs 69%), and mortality at 90 days (25% vs 56%), when compared with the group of patients who were treated by inotropes only.
Discussion
Advantages of IABP Compared With Other MCSDs
Although other MSCDs like Impella, Tandem Heart, or VA-ECMO provide more hemodynamic support, (first-line) IABP has multiple advantages. First of all, it is relatively cheap [1] and IABPs are largely available and applicable, also in non-tertiary centers. Insertion of an IABP device is more straightforward and can be performed in the intensive care unit without the need for fluoroscopy. Compared with other devices, IABP placement is associated with fewer adverse events like vascular complications [58] or hemolysis [39]. Although mobilization of patients with femoral IABPs is compromised, placement in the axillary or subclavian artery allows mobilization and early physical rehabilitation [3–5, 6•, 7•]. When the IABP fails or cannot be weaned, rapid escalation is possible to percutaneous MCSDs, VA-ECMO, or advanced HF therapies like durable MCSDs (e.g. LVAD) or orthotopic heart transplant (OHT) [59]. Finally, an IABP is easily removed and the presence of an IABP does not complicate native heart excision in case of bridging to OHT.
Why Did IABP Not Provide Benefit in AMICS?
The hemodynamic effects of an IABP stand out better with larger balloon size. Several recent studies demonstrate that the use of larger 50-cc balloons resulted in a greater reduction in cardiac filling pressures and increased CO compared with the 40-cc IABPs [44–46]. Unfortunately, 50-cc IABPs were generally not used in the major landmark studies so far, since the 50-cc IABP was only introduced in 2012. Since the number of patients achieving optimal hemodynamic benefit from IABP activation may be < 50% with the older 30–40-cc IABPs, this could potentially have contributed to the failure of previous IABP studies [44].
Although the supposed additional beneficial effect of improved coronary blood flow by IABP would be expected to be extra beneficial for patients with AMICS, IABP-SHOCK II showed no benefit of survival [31]. Several limitations of the IABP-SHOCK II should be mentioned. As discussed previously, most patients were treated with conventional, small-volume IABP-catheters. Besides, 10% of patients in the control group experienced crossover to IABP. Moreover, since almost half of all patients were included after cardiopulmonary resuscitation, a substantial amount might have died due to post-anoxic damage. Finally, a large percentage of patients in this trial were already on vasopressors/inotropes (90%), and thus IABP therapy might have been initiated too late.
Besides the limitations of this study, there are also several possible pathophysiological explanations for the neutral findings of IABP in patients with AMICS. First, ACS-driven (extensive) myocardial damage triggers inflammatory and other systemic responses, which may be insufficiently counter-attacked by an IABP that only passively supports the circulation [37]. Second, the effect of improved coronary blood flow is possibly non-existent in vivo due to intact coronary autoregulation [13]. Hence, Van Nunen and colleagues postulated the hypothesis that IABP only improves coronary blood flow in case of exhausted coronary autoregulation, which was not the case in IABP-SHOCK II, since 90% of the total study population obtained successful reperfusion (i.e., final TIMI flow grade 2 or 3 in the infarct-related artery (IRA)) [13, 31]. Patients with AMI and persistent ischemia despite primary PCI were supposed to have impaired autoregulation and Van Nunen proved that the IABP resulted in more rapid ST-elevation resolution in this subgroup. Also, death, necessity of LVAD implantation, or re-admission for HF tended to occur less frequently after IABP implantation in this subgroup [13]. Hawranek retrospectively evaluated patients with AMICS from the prospective nationwide registry who had unsuccessful PCI (i.e., final TIMI flow grade 0 to 1 in the IRA) [60•]. Although conclusions are limited by its observational design, IABP in this subgroup was associated with lower short-term and 12-month mortality.
Why Is the Augmentation of Cardiac Output in Patients With Non-ACS CS More Pronounced Than in Patients With AMICS?
Due to improved survival after ACS, the incidence of end-stage HF and non-ACS CS is rising [61]. However, at this time, no large RCTs for the acute mechanical treatment of this subgroup are available [36]. The first (small) RCT showed significant improvement of central venous oxygen saturation, cardiac power output, and urine output by IABP compared with medical therapy [30••]. Baseline hemodynamic parameters were equal to those reported in previous studies on AMICS [62]. Besides, as we show in Table 1, multiple retrospective studies reported that the use of an IABP in non-ACS CS temporarily stabilized hemodynamics and end-organ perfusion and allowed a bridge to recovery of the native cardiac function, decision-making, or more durable heart replacement therapy like OHT and LVAD. The increase of the cardiac index in non-ACS CS ranged from 0.3 to 0.9 L/min/m2 [6•, 28•, 32, 50••, 54], and one may imagine that such a (limited) CO augmentation may be sufficient to stabilize patients with chronic HF and CS who are used to have a low CO under stable conditions. Previous studies of patients with AMICS demonstrated less CO augmentation by IABP [62–64], which probably explains the lack of efficacy in (tachycardic) patients suffering from an acute decrease in stroke volume as included in the IABP-SHOCK II trial [31].
Malick et al. also described that the augmentation of CO occurred to a less extent in patients with AMICS [50••]. The exact reasons for the difference in treatment response between non-ACS CS and AMICS remain unclear. One hypothesis is that IABP support depends on the intrinsic contractile reserve [50••, 65]. Although baseline stroke volume may be identical in AMICS versus non-ACS CS [50••], baseline PAP was higher in non-ACS CS. Since low output may be mainly triggered by high filling pressures in non-ACS CS, and the IABP may be more effective in lowering afterload and optimizing renal perfusion in this subgroup, the IABP may function better in a high-volume status rather than in an acutely developed low-flow contractile state. This explanation is supported by Fried’s finding that non-ACS patients with high baseline mean PAP had the greatest CO augmentation by IABP [28•]. Also in Imamura’s study, patients with higher filling pressures were most likely to benefit from IABP support [6•].
Clinical Outcomes After IABP in Non-ACS CS
The proportion of patients successfully weaned from IABP in CS is significantly lower in patients with STEMI compared with patients with NSTEMI and congestive HF (P = 0.04) [66]. In this retrospective analysis, even 97.8% of congestive HF patients were weaned from IABP support [66]. In Thiele’s IABP-SHOCK II trial, only 4% of patients who received an IABP were bridged to durable mechanical circulatory support with good long-term outcome [31], and in most other AMICS studies, the rates of successful bridging to durable heart replacement therapy were unfortunately not reported [59]. As shown in Table 1, many patients with non-ACS CS treated with IABP were successfully bridged to durable heart replacement therapy like LVAD or OHT. In our recently published RCT, non-ACS CS patients treated with IABP were significantly more often bridged to LVAD or OHT compared with patients treated with inotropes (31 vs 0% respectively; P < 0.05) [30••]. Recent literature shows that patients with ischemic or non-ischemic heart failure who needed pre-operative IABP have similar short- and long-term survival rates after LVAD implantation (88% and 78% after 3 and 12 months respectively), compared with patients who received LVAD without the need for pre-operative mechanical circulatory support (91% and 82% after 3 and 12 months respectively) [67••]. Also, after OHT, no significant difference in short- or long-term survival post-OHT between pre-OHT IABP and a control group was seen [52]. Unfortunately, most studies looking specifically at IABP in non-ACS CS (Table 1) did not report long-term survival rates.
Patient Selection
As already mentioned, CS cannot be seen as one single entity, but rather as a wide spectrum of different aetiologies, hemodynamic characteristics, degree of severity, and response to therapy. This heterogeneity is the main reason that estimating the possible effect of IABP in daily clinical practice remains challenging. Even within the non-ACS CS subgroup, part of the patients appeared to be non-responders [28•]. In 60/75 patients who underwent right heart catheterization in the before-mentioned cohort of Visveswaran, CO and cardiac index increased up to 7 L/min and 3.4 L/min/m2 respectively, while in the remaining 20% non-responders CO decreased. Remarkably, the mortality rate between responders and non-responders was equal [46]. In Hsu’s study, all patients showed an initial improvement in CO within the first 24 h, but in patients with adverse events, CO declined after 24–48 h post IABP implantation [26•]. Some authors suggest that the IABP is less effective in patients with non-ACS CS and underlying ischemic cardiomyopathy [26•, 30••]. Others showed that patients with too bad left and/or right ventricle function at baseline were less likely to show clinical stabilization after IABP insertion [10, 26•, 28•, 55, 56, 68]. Many other prognostic parameters at baseline have been proposed (e.g., left ventricular end-diastolic pressure, left ventricle end-systolic pressure, end-systolic pressure-volume relationship, dP/dTmax, right atrial pressure, PAP, right atrial pressure to PCWP ratio, PCWP, left ventricular end-diastolic dimension, heart rate, systemic vascular resistance, absence of biventricular failure, and the degree of inflammation and multi-organ dysfunction), but most study populations were small, sometimes data are conflicting, and underlying mechanisms remain insufficiently understood [6•, 7•, 10, 28•, 30••, 44]. Also, the fact that persisting arrhythmias can cause opposite disadvantageous hemodynamic effects in patients with IABP should always be taken into consideration [4, 8].
What Is the Correct Timing of IABP Placement?
Although recommended as first-line therapy of CS [36], the beneficial effect of intravenous positive inotropes and/or vasopressors is never proven and observational data even point towards increased mortality [69, 70]. Possible deleterious effects can be explained by an increased incidence of arrhythmias and aggravation of myocardial ischemia. Since primary IABP placement showed substantial and fast hemodynamic benefit as compared with inotrope therapy [30••], early IABP implantation might result in better outcomes. In Gul’s study, placement of IABP within 1 h of onset of CS showed remarkably lower mortality compared with delayed implantation (35% vs 49% respectively; P < 0.001) [27], suggesting that early IABP placement instead of waiting too long for the possible benefit of inotropes could be beneficial. This is endorsed by the finding that patients who stabilized after IABP were on fewer vasopressors or inotropes in observational studies [28•, 55]. Unfortunately, in the currently available retrospective studies regarding non-ACS CS (Table 1), the timing of IABP insertion and phase of shock is very heterogeneous and sometimes poorly defined. Also in this population, the timing of implantation seems to be a crucial factor, since the time to mechanical support is proportional to the amount of organ preservation. Finally, also the timing of IABP weaning seems to be crucial and is actually poorly defined in previous studies.
Areas to Be Discovered
Results of randomized trials like the DanGer Shock and ECLS SHOCK are expected to elucidate the effect on LVEF and mortality by respectively Impella CP and ECMO in patients with AMICS. Since IABP might still provide benefit in selected patients with AMICS and unsuccessful revascularization or patients with non-ACS CS, larger RCTs are required to evaluate its effect in those patients. We would recommend hemodynamically guided placement of IABP in those subgroups. Investigators should preferably evaluate not only outcomes like short-term mortality, but also time to reversal of shock, end-organ failure, duration of hospital stay, and long-term mortality and functionality.
Conclusion
The IABP remains a relatively cheap and easily applicable device with low complication rates that offers sufficient hemodynamic support in many patients and allows direct escalation to more powerful support devices if necessary. Although IABP is already in use for several decades, strong evidence by large RCTs is still lacking. The largest RCT of IABP in patients with AMICS reported no mortality benefit, but recent data suggest that IABP may still be useful in a selected subgroup (patients with persistent ischemia or unsuccessful revascularization). Moreover, IABP was not harmful either and more importantly this trial did not address CS complicating (chronic) HF without ACS. Available evidence suggests that the IABP has a clear beneficial effect on many hemodynamic parameters in this non-ACS CS group, allowing the clinician to, at least temporarily, stabilize the hemodynamic profile. Although further research is required, the IABP in this particular group seems promising. More studies should be performed to better define other subgroups with good IABP response, particularly in an era where alternative MSCDs or VA-ECMO are available.
Abbreviations
- 30-d
30 day
- ACC
American College of Cardiology
- ACS
Acute coronary syndrome
- AHA
American Heart Association
- AMI
Acute myocardial infarction
- AMICS
Cardiogenic shock after acute myocardial infarction
- BCIS-1
Balloon pump–assisted Coronary Intervention Study
- CABG
Coronary artery bypass grafting
- Cc
Cubic centimeter
- CO
Cardiac output
- CRISP-AMI
Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction
- CS
Cardiogenic shock
- ESC
European Society of Cardiology
- HF
Heart failure
- IABP
Intra-aortic balloon pump
- LVAD
Left ventricular assist device
- LVEF
Left ventricular ejection fraction
- MACCE
Major adverse cardiac and cerebrovascular events
- MCSD
Mechanical circulatory support device
- NSTEMI
non ST-elevation myocardial infarction
- OHT
Orthotopic heart transplantation
- PCI
Percutaneous coronary intervention
- PROTECT II
Prospective Multicenter Randomized Trial Comparing IMPELLA to IABP in High-Risk PCI II
- PA
Pulmonary artery
- PAP
Pulmonary artery pressure
- PCI
Percutaneous coronary intervention
- PCWP
Pulmonary capillary wedge pressure
- pVAD
Percutaneous ventricular assist device
- RCT
Randomized controlled trial
- STEMI
ST-elevation myocardial infarction
- US
United States
- TIA
Transient ischemic attack
- TIMI
Thrombolysis in myocardial infarction
- IRA
Infarct-related artery
- VA-ECMO
Veno-arterial extra-corporal membrane oxygenation
Compliance with Ethical Standards
Conflict of Interest
Dr. Van Mieghem reports grants and personal fees from PulseCath BV, grants and personal fees from Abbott Vascular, grants and personal fees from Medtronic, grants and personal fees from Biotronik, grants and personal fees from Boston Scientific, and personal fees from Abiomed, all outside the submitted work. All other authors declare no conflicts of interest related to the content of this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Key Points
• The routine use of IABP in patients with AMICS after successful PCI was not shown to be beneficial or harmful compared with optimal medical therapy, regardless of the timing of placement. However, in the subgroup of patients with impaired coronary autoregulation due to unsuccessful primary PCI, IABP might still be helpful.
• Although pVADs like Impella may be more appropriate to use in high-risk PCI, the use of pVADs has so far demonstrated equal or higher mortality compared with IABP in patients with AMICS.
• Main trials have focused on AMICS, and therefore, there is a need for (larger) RCTs regarding the use of IABP in non-ACS CS and advanced HF, which concerns over 50% of patients with CS in recent studies.
• Studies that reflect clinical experience or pilot experiments of IABP in non-ACS CS show good hemodynamic improvement which allowed stabilization and clinical decision-making. A high percentage of these patients can be bridged to recovery or may receive destination therapy with good long-term outcome.
This article is part of the Topical Collection on Cardiogenic Shock: Progress in Mechanical Circulatory Support
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Doshi R, Patel K, Decter D, Gupta R, Meraj P. Trends in the utilisation and in-hospital mortality associated with short-term mechanical circulatory support for heart failure with reduced ejection fraction. Heart Lung Circ. 2019;28(4):e47–e50. doi: 10.1016/j.hlc.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, Jorde UP. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 3.Raman J, Loor G, London M, Jolly N. Subclavian artery access for ambulatory balloon pump insertion. Ann Thorac Surg. 2010;90(3):1032–1034. doi: 10.1016/j.athoracsur.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka A, Tuladhar SM, Onsager D, Asfaw Z, Ota T, Juricek C, Lahart M, Lonchyna VA, Kim G, Fedson S, Sayer G, Uriel N, Jeevanandam V. The subclavian intraaortic balloon pump: a compelling bridge device for advanced heart failure. Ann Thorac Surg. 2015;100(6):2151–2157. doi: 10.1016/j.athoracsur.2015.05.087. [DOI] [PubMed] [Google Scholar]
- 5.Russo MJ, Jeevanandam V, Stepney J, Merlo A, Johnson EM, Malyala R, Raman J. Intra-aortic balloon pump inserted through the subclavian artery: a minimally invasive approach to mechanical support in the ambulatory end-stage heart failure patient. J Thorac Cardiovasc Surg. 2012;144(4):951–955. doi: 10.1016/j.jtcvs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Imamura T, Juricek C, Nguyen A, Chung B, Rodgers D, Sayer G, et al. Predictors of hemodynamic improvement and stabilization following intraaortic balloon pump implantation in patients with advanced heart failure. J Invasive Cardiol. 2018;30(2):56–61. [PMC free article] [PubMed] [Google Scholar]
- 7.Bhimaraj A, Agrawal T, Duran A, Tamimi O, Amione-Guerra J, Trachtenberg B, et al. Percutaneous left axillary artery placement of intra-aortic balloon pump in advanced heart failure patients. JACC Heart Fail. 2020;8(4):313–323. doi: 10.1016/j.jchf.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Bastos MB, Burkhoff D, Maly J, Daemen J, den Uil CA, Ameloot K, Lenzen M, Mahfoud F, Zijlstra F, Schreuder JJ, van Mieghem NM. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41(12):1286–1297. doi: 10.1093/eurheartj/ehz552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreuder JJ, Maisano F, Donelli A, Jansen JR, Hanlon P, Bovelander J, et al. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005;79(3):872–880. doi: 10.1016/j.athoracsur.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 10.Annamalai SK, Buiten L, Esposito ML, Paruchuri V, Mullin A, Breton C, Pedicini R, O’Kelly R, Morine K, Wessler B, Patel AR, Kiernan MS, Karas RH, Kapur NK. Acute hemodynamic effects of intra-aortic balloon counterpulsation pumps in advanced heart failure. J Card Fail. 2017;23(8):606–614. doi: 10.1016/j.cardfail.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Y, Sen S, Shun-Shin MJ, Ouyang J, Finegold JA, Al-Lamee RK, et al. Intra-aortic balloon pump therapy for acute myocardial infarction: a meta-analysis. JAMA Intern Med. 2015;175(6):931–939. doi: 10.1001/jamainternmed.2015.0569. [DOI] [PubMed] [Google Scholar]
- 13.van Nunen LX, van 't Veer M, Zimmermann FM, Wijnbergen I, Brueren GRG, Tonino PAL, et al. Intra-aortic balloon pump counterpulsation in extensive myocardial infarction with persistent ischemia: the SEMPER FI pilot study. Catheter Cardiovasc Interv. 2020;95(1):128–135. doi: 10.1002/ccd.28289. [DOI] [PubMed] [Google Scholar]
- 14.Perera D, Stables R, Clayton T, De Silva K, Lumley M, Clack L, et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127(2):207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 16.Shi W, Wang W, Wang K, Huang W. Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention: a meta-analysis of randomized trials. Medicine (Baltimore) 2019;98(37):e17107. doi: 10.1097/MD.0000000000017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khadra Y, Alraies MC, Darmoch F, Pacha HM, Soud M, Kaki A, et al. Outcomes of nonemergent percutaneous coronary intervention requiring mechanical circulatory support in patients without cardiogenic shock. Catheter Cardiovasc Interv. 2020;95(3):503–512. doi: 10.1002/ccd.28383. [DOI] [PubMed] [Google Scholar]
- 18.Deppe AC, Weber C, Liakopoulos OJ, Zeriouh M, Slottosch I, Scherner M, Kuhn EW, Choi YH, Wahlers T. Preoperative intra-aortic balloon pump use in high-risk patients prior to coronary artery bypass graft surgery decreases the risk for morbidity and mortality-a meta-analysis of 9,212 patients. J Card Surg. 2017;32(3):177–185. doi: 10.1111/jocs.13114. [DOI] [PubMed] [Google Scholar]
- 19.Rampersad PP, Udell JA, Zawi R, Ouzounian M, Overgaard CB, Sharma V, Rao V, Farkouh ME, Džavík V. Preoperative intraaortic balloon pump improves early outcomes following high-risk coronary artery bypass graft surgery: a meta-analysis of randomized trials and prospective study design. J Invasive Cardiol. 2018;30(1):2–9. [PubMed] [Google Scholar]
- 20.Escutia-Cuevas HH, Suarez-Cuenca JA, Espinoza-Rueda MA, Macedo-Calvillo L, Castro-Gutierrez A, Garcia-Garcia JF, et al. Preoperative use of intra-aortic balloon pump support reduced 30-day mortality in a population with LVEF >35% and high surgical risk after coronary artery bypass graft surgery. Cardiology. 2020;145(5):267–274. [DOI] [PubMed]
- 21.Ranucci M, Castelvecchio S, Biondi A, de Vincentiis C, Ballotta A, Varrica A, Frigiola A, Menicanti L. A randomized controlled trial of preoperative intra-aortic balloon pump in coronary patients with poor left ventricular function undergoing coronary artery bypass surgery*. Crit Care Med. 2013;41(11):2476–2483. doi: 10.1097/CCM.0b013e3182978dfc. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, et al. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11(9):e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930. [DOI] [PubMed] [Google Scholar]
- 23.Baldetti L, Gramegna M, Beneduce A, Melillo F, Moroni F, Calvo F, Melisurgo G, Ajello S, Fominskiy E, Pappalardo F, Scandroglio AM. Strategies of left ventricular unloading during VA-ECMO support: a network meta-analysis. Int J Cardiol. 2020;312:16–21. doi: 10.1016/j.ijcard.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 25.Kettner J, Sramko M, Holek M, Pirk J, Kautzner J. Utility of intra-aortic balloon pump support for ventricular septal rupture and acute mitral regurgitation complicating acute myocardial infarction. Am J Cardiol. 2013;112(11):1709–1713. doi: 10.1016/j.amjcard.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Hsu S, Kambhampati S, Sciortino CM, Russell SD, Schulman SP. Predictors of intra-aortic balloon pump hemodynamic failure in non-acute myocardial infarction cardiogenic shock. Am Heart J. 2018;199:181–191. doi: 10.1016/j.ahj.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gul B, Bellumkonda L. Usefulness of intra-aortic balloon pump in patients with cardiogenic shock. Am J Cardiol. 2019;123(5):750–756. doi: 10.1016/j.amjcard.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Fried JA, Nair A, Takeda K, Clerkin K, Topkara VK, Masoumi A, et al. Clinical and hemodynamic effects of intra-aortic balloon pump therapy in chronic heart failure patients with cardiogenic shock. J Heart Lung Transplant. 2018;37(11):1313–1321. doi: 10.1016/j.healun.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jong MM, Lorusso R, Al Awami F, Matteuci F, Parise O, Lozekoot P, et al. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion. 2018;33(2):96–104. doi: 10.1177/0267659117727825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.den Uil CA, Van Mieghem NM, Bastos MB, Jewbali LS, Lenzen MJ, Engstrom AE, et al. Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: a randomised trial. EuroIntervention. 2019;15(7):586–593. doi: 10.4244/EIJ-D-19-00254. [DOI] [PubMed] [Google Scholar]
- 31.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K, IABP-SHOCK II Trial Investigators Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 32.Umakanthan R, Hoff SJ, Solenkova N, Wigger MA, Keebler ME, Lenneman A, Leacche M, DiSalvo TG, Ooi H, Naftilan AJ, Byrne JG, Ahmad RM. Benefits of ambulatory axillary intra-aortic balloon pump for circulatory support as bridge to heart transplant. J Thorac Cardiovasc Surg. 2012;143(5):1193–1197. doi: 10.1016/j.jtcvs.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Helgestad OKL, Josiassen J, Hassager C, Jensen LO, Holmvang L, Udesen NLJ, Schmidt H, Berg Ravn H, Moller JE. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart. 2020;7(1):e001214. doi: 10.1136/openhrt-2019-001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallabhajosyula S, Prasad A, Sandhu GS, Bell MR, Gulati R, Eleid MF, et al. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes. EuroIntervention. 2019;EIJ-D-19-00226. [DOI] [PMC free article] [PubMed]
- 35.Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;27(3):CD007398. doi: 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 37.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–ee68. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 38.Cui K, Lyu S, Liu H, Song X, Yuan F, Xu F, Zhang M, Zhang M, Wang W, Zhang D, Tian J, Yan Y, Zhou K, Chen L. Timing of initiation of intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: a meta-analysis. Clin Cardiol. 2019;42(11):1126–1134. doi: 10.1002/clc.23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieri M, Sorrentino T, Oppizzi M, Melisurgo G, Lembo R, Colombo A, Zangrillo A, Pappalardo F. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol. 2018;31(6):717–724. doi: 10.1111/joic.12569. [DOI] [PubMed] [Google Scholar]
- 40.Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, de Waha S. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38(47):3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 41.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius-Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schäfer A, Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 43.Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020;e200254. [DOI] [PMC free article] [PubMed]
- 44.Kapur NK, Paruchuri V, Majithia A, Esposito M, Shih H, Weintraub A, Kiernan M, Pham DT, Denofrio D, Kimmelstiel C. Hemodynamic effects of standard versus larger-capacity intraaortic balloon counterpulsation pumps. J Invasive Cardiol. 2015;27(4):182–188. [PubMed] [Google Scholar]
- 45.Visveswaran GK, Cohen M, Seliem A, DiVita M, McNamara JKR, Dave A, et al. A single center tertiary care experience utilizing the large volume mega 50cc intra-aortic balloon counterpulsation in contemporary clinical practice. Catheter Cardiovasc Interv. 2017;90(4):E63–E72. doi: 10.1002/ccd.26908. [DOI] [PubMed] [Google Scholar]
- 46.Baran DA, Visveswaran GK, Seliem A, DiVita M, Wasty N, Cohen M. Differential responses to larger volume intra-aortic balloon counterpulsation: hemodynamic and clinical outcomes. Catheter Cardiovasc Interv. 2018;92(4):703–710. doi: 10.1002/ccd.27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis A, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12(3):e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrage B, Weimann J, Dabboura S, Yan I, Hilal R, Becher PM, et al. Patient characteristics, treatment and outcome in non-ischemic vs. ischemic cardiogenic shock. J Clin Med. 2020;9(4):931. [DOI] [PMC free article] [PubMed]
- 49.Harjola VP, Lassus J, Sionis A, Kober L, Tarvasmaki T, Spinar J, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 50.Malick W, Fried JA, Masoumi A, Nair A, Zuver A, Huang A, et al. Comparison of the hemodynamic response to intra-aortic balloon counterpulsation in patients with cardiogenic shock resulting from acute myocardial infarction versus acute decompensated heart failure. Am J Cardiol. 2019;124(12):1947–1953. doi: 10.1016/j.amjcard.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norkiene I, Ringaitiene D, Rucinskas K, Samalavicius R, Baublys A, Miniauskas S, Sirvydis V. Intra-aortic balloon counterpulsation in decompensated cardiomyopathy patients: bridge to transplantation or assist device. Interact Cardiovasc Thorac Surg. 2007;6(1):66–70. doi: 10.1510/icvts.2006.140160. [DOI] [PubMed] [Google Scholar]
- 52.Gjesdal O, Gude E, Arora S, Leivestad T, Andreassen AK, Gullestad L, Aaberge L, Brunvand H, Edvardsen T, Geiran OR, Simonsen S. Intra-aortic balloon counterpulsation as a bridge to heart transplantation does not impair long-term survival. Eur J Heart Fail. 2009;11(7):709–714. doi: 10.1093/eurjhf/hfp078. [DOI] [PubMed] [Google Scholar]
- 53.Mizuno M, Sato N, Kajimoto K, Sakata Y, Minami Y, Munakata R, Hagiwara N, Takano T, Acute Decompensated Heart Failure Syndromes [ATTEND] investigators Intra-aortic balloon counterpulsation for acute decompensated heart failure. Int J Cardiol. 2014;176(3):1444–1446. doi: 10.1016/j.ijcard.2014.08.154. [DOI] [PubMed] [Google Scholar]
- 54.Ntalianis A, Kapelios CJ, Kanakakis J, Repasos E, Pantsios C, Nana E, Kontogiannis C, Malliaras K, Tsamatsoulis M, Kaldara E, Charitos C, Nanas JN. Prolonged intra-aortic balloon pump support in biventricular heart failure induces right ventricular reverse remodeling. Int J Cardiol. 2015;192:3–8. doi: 10.1016/j.ijcard.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Sintek MA, Gdowski M, Lindman BR, Nassif M, Lavine KJ, Novak E, Bach RG, Silvestry SC, Mann DL, Joseph SM. Intra-aortic balloon counterpulsation in patients with chronic heart failure and cardiogenic shock: clinical response and predictors of stabilization. J Card Fail. 2015;21(11):868–876. doi: 10.1016/j.cardfail.2015.06.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.den Uil CA, Galli G, Jewbali LS, Caliskan K, Manintveld OC, Brugts JJ, et al. First-line support by intra-aortic balloon pump in non-ischaemic cardiogenic shock in the era of modern ventricular assist devices. Cardiology. 2017;138(1):1–8. doi: 10.1159/000471846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morici N, Oliva F, Ajello S, Stucchi M, Sacco A, Cipriani MG, de Bonis M, Garascia A, Gagliardone MP, Melisurgo G, Russo CF, la Vecchia C, Frigerio M, Pappalardo F. Management of cardiogenic shock in acute decompensated chronic heart failure: the ALTSHOCK phase II clinical trial. Am Heart J. 2018;204:196–201. doi: 10.1016/j.ahj.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Ouweneel DM, Henriques JP. Percutaneous cardiac support devices for cardiogenic shock: current indications and recommendations. Heart. 2012;98(16):1246–1254. doi: 10.1136/heartjnl-2012-301963. [DOI] [PubMed] [Google Scholar]
- 59.den Uil CA, Akin S, Jewbali LS, Dos Reis MD, Brugts JJ, Constantinescu AA, et al. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017;52(1):14–25. doi: 10.1093/ejcts/ezx088. [DOI] [PubMed] [Google Scholar]
- 60.Hawranek M, Gierlotka M, Pres D, Zembala M, Gasior M. Nonroutine use of intra-aortic balloon pump in cardiogenic shock complicating myocardial infarction with successful and unsuccessful primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(18):1885–1893. doi: 10.1016/j.jcin.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 62.Prondzinsky R, Unverzagt S, Russ M, Lemm H, Swyter M, Wegener N, Buerke U, Raaz U, Ebelt H, Schlitt A, Heinroth K, Haerting J, Werdan K, Buerke M. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock. 2012;37(4):378–384. doi: 10.1097/SHK.0b013e31824a67af. [DOI] [PubMed] [Google Scholar]
- 63.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 64.Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 65.Kapur NK, Hirst CS. Counterpulsation requires pulsation: IABP use in patients with heart failure without acute MI. Catheter Cardiovasc Interv. 2018;92(4):711–712. doi: 10.1002/ccd.27878. [DOI] [PubMed] [Google Scholar]
- 66.Lauten P, Rademacher W, Goebel B, Kretzschmar D, Figulla HR, Lauten A, Ferrari M. Intra-aortic counterpulsation for hemodynamic support in patients with acute ischemic versus non-ischemic heart failure. J Invasive Cardiol. 2012;24(11):583–588. [PubMed] [Google Scholar]
- 67.Ton VK, Xie R, Hernandez-Montfort JA, Meyns B, Nakatani T, Yanase M, et al. Short- and long-term adverse events in patients on temporary circulatory support before durable ventricular assist device: An IMACS registry analysis. J Heart Lung Transplant. 2020;39(4):342–352. doi: 10.1016/j.healun.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Krishnamoorthy A, DeVore AD, Sun JL, Barnett AS, Samsky MD, Shaw LK, et al. The impact of a failing right heart in patients supported by intra-aortic balloon counterpulsation. Eur Heart J Acute Cardiovasc Care. 2017;6(8):709–718. doi: 10.1177/2048872616652262. [DOI] [PubMed] [Google Scholar]
- 69.Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014;63(20):2069–2078. doi: 10.1016/j.jacc.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J, ADHERE Scientific Advisory Committee and Investigators. ADHERE Study Group In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46(1):57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]