Abstract
Background
Patients with coronary disease may have unknown diabetes or prediabetes. We evaluated 3‐year outcomes after percutaneous coronary intervention (PCI) with contemporary drug‐eluting stents (DES) in patients with silent diabetes, prediabetes, and normoglycemia.
Methods
All BIO‐RESORT trial (NCT01674803) participants without known diabetes, enrolled at our center, were invited for oral glucose tolerance testing (OGTT) and measurements of fasting plasma glucose and glycated hemoglobin (HbA1c).
Results
OGTT detected silent diabetes in 68 (6.9%), prediabetes in 132 (13.4%), and normoglycemia in 788 (79.8%) of all 988 study participants. Follow‐up was available in 986 (99.8%) patients. The main endpoint target vessel failure (TVF: cardiac death, target vessel‐related myocardial infarction [MI], or target vessel revascularization) differed between groups (14.8, 9.9, and 5.6%; p = .002), driven by MI during the first 48 hr and by cardiac death (p < .001; p = .026). Between 48 hr and 3‐years, there was no significant between‐group difference in TVF, target vessel MI, and target vessel revascularization. Multivariable analysis demonstrated that silent diabetes was independently associated with TVF (adjusted HR: 2.52, 95%‐CI: 1.26–5.03). An alternative diagnostic approach—HbA1c and fasting plasma glucose—detected silent diabetes and prediabetes in 33 (3.3%) and 217 (22.0%) patients, and normoglycemia in 738 (74.7%); TVF rates were 12.1, 7.9, and 6.0% (p = .23).
Conclusion
In patients without known diabetes, abnormal glucose metabolism by OGTT was independently associated with higher 3‐year TVF rates after PCI with contemporary DES. This difference was driven by periprocedural MI and cardiac death. After the first 48 hr, the rates of TVF, target vessel MI, and target vessel revascularization were low and did not differ significantly between metabolic groups.
Keywords: diabetes mellitus, glycated hemoglobin, oral glucose tolerance testing, percutaneous coronary intervention, prediabetes, second‐generation drug‐eluting stents
1. INTRODUCTION
An abnormal glucose metabolism has been found in up to 50% of patients with coronary artery disease and no history of diabetes when oral glucose tolerance testing (OGTT) was used as the diagnostic tool.1, 2 These patients with “silent” diabetes or prediabetes are known to have more extensive and complex atherosclerotic coronary disease.3, 4 Diabetes associations suggest that OGTT, fasting plasma glucose (FPG), and measuring glycated hemoglobin (HbA1c) are equally appropriate for diagnosing diabetes. Nevertheless, OGTT is able to identify more individuals with diabetes, while HbA1c testing is convenient but more susceptible for interference by hemoglobinopathies or anemia.5
During the first 12 months after treatment with contemporary drug‐eluting stents (DES), patients with diabetes develop more adverse cardiovascular events than normoglycemic patients.6, 7, 8 Nevertheless, there is a lack of long‐term outcome data after percutaneous coronary intervention (PCI) with new‐generation DES in patients with abnormal glucose metabolism, newly detected by OGTT.
The randomized BIO‐RESORT trial,9 which investigates the outcome of all‐comer patients treated with three new‐generation DES, has shown low and similar clinical event rates for all treatment arms up to the 3‐year follow‐up.9 At Thoraxcentrum Twente, we prospectively assessed OGTT and HbA1c with FPG to evaluate the prevalence of silent diabetes and prediabetes among 988 trial participants without known diabetes.10 In one out of three of these “nondiabetic” patients, abnormal glucose tolerance was detected and independently associated with an up to four‐fold higher 1‐year risk of adverse cardiovascular events.10 In the current analysis, we assessed the 3‐year outcomes of the participants in the BIO‐RESORT Silent Diabetes study, comparing patients with abnormal glucose metabolism with true normoglycemic patients.
2. METHODS
2.1. Study design and participants
BIO‐RESORT Silent Diabetes is a prespecified, prospective study, performed under the umbrella of the randomized BIO‐RESORT trial. Details of the main trial (ClinicalTrials.gov NCT01674803) have been previously reported.9 In brief, this investigator‐initiated, patient‐ and assessor‐blinded, randomized trial was performed between December 2012 and August 2015. A total of 3,514 PCI all‐comers were enrolled at four study sites. All coronary syndromes and lesion types were permitted, and there was no limit for lesion length, reference vessel size, and number of vessels to be treated. Patients were treated with the ultrathin strut biodegradable polymer Orsiro sirolimus‐eluting stent (Biotronik, Bülach, Switzerland), the very thin strut biodegradable polymer Synergy everolimus‐eluting stent (Boston Scientific, Marlborough, MA), or the thin strut durable polymer Resolute Integrity zotarolimus‐eluting stent (Medtronic, Santa Rosa, CA). No statistically significant differences in 3‐year clinical outcome were found.11
In the BIO‐RESORT Silent Diabetes study, 1,899 patients who were treated at Thoraxcentrum Twente and had no known history of diabetes were invited to participate.10 A total of 988 agreed to participate and underwent OGTT measured by a central laboratory 4–6 weeks after PCI (Hexokinase, Roche Diagnostics, Almere, the Netherlands).10 HbA1c levels were measured with a Tina‐quant third generation assay on Cobas 6,000 analyzer (Roche Diagnostics). Patients and general practitioners received a letter with laboratory results and advice on current guidelines. Patients who were not enrolled in the BIO‐RESORT Silent Diabetes study underwent routine clinical assessment of their cardiovascular risk factors, which generally included testing of their glucose metabolism by means of HbA1c or FPG. As all diagnostic measurements (OGTT, HbA1c, and FPG) were required to classify patients, data of nonparticipants were not used in this prospective study.
The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee Twente and the institutional review boards of the participating center. All patients provided written informed consent.
2.2. Procedures, follow‐up, and monitoring
Coronary interventions, the choice of concomitant medication, and type and duration of antiplatelet therapy were based on standard techniques, routine clinical practice, current international guidelines, and the operator's judgment. Electrocardiographs and cardiac biomarkers were systematically assessed after PCI, with subsequent serial measurements in case of suspected ischemia. Trial and data management was done by Cardiovascular Research and Education Enschede (Enschede, the Netherlands). Clinical follow‐up was obtained at visits to outpatient clinics, by telephone follow‐up, or by questionnaire. An independent clinical research organization (Diagram, Zwolle, the Netherlands) performed data monitoring and independent clinical event adjudication.
2.3. Definitions and clinical endpoints
Definitions of glycemic state were based on the World Health Organization 1999 criteria for OGTT and the International Expert Committee 2009 criteria for HbA1c with FPG.5, 12 When using OGTT, a patient was considered to have normal glucose metabolism if FPG was <6.1 mmoL/L and 2‐h glucose was <7.8 mmoL/L, prediabetes if FPG was <7.0 mmoL/L and 2‐h glucose was 7.8–11.0 mmoL/L, and silent diabetes if FPG was ≥7.0 mmoL/L or 2‐h glucose was ≥11.1 mmoL/L. When using FPG and HbA1c, normal glucose metabolism was defined as FPG <6.1 mmoL/L and HbA1c ≤ 41 mmoL/moL, pre‐diabetes as FPG of 6.1–6.9 mmoL/L and HbA1c of 42‐47 mmoL/moL, and silent diabetes as FPG ≥7.0 mmoL/L or HbA1c ≥48 mmoL/moL.5, 12
The prespecified endpoints are based on suggestions of the Academic Research Consortium,13, 14 as previously described.9 The main clinical endpoint target vessel failure (TVF) is a composite of cardiac death, target vessel‐related myocardial infarction (MI), or repeat target vessel revascularization. Death was considered cardiac, unless unequivocal noncardiac cause could be established. MI was defined by any creatine kinase concentration of more than double the upper limit of normal with elevated confirmatory cardiac biomarkers. Periprocedural MI was defined as an MI that occurred within 48 hr of the PCI procedure. We also assessed the more global composite clinical endpoint of major adverse cardiac events (MACE), defined as all‐cause death, any MI, emergent coronary artery bypass grafting, or clinically indicated target lesion revascularization.
2.4. Statistical analysis
The Pearson chi‐square test was used to compare categorical variables, and the Student t test to compare continuous variables. The time to primary endpoint and components thereof were assessed according to Kaplan–Meier methods, and log‐rank testing was applied for between‐group comparisons. We performed Cox proportional hazards regression analyses to investigate the effect of abnormal glucose metabolism on 3‐year clinical outcome. The following variables, associated with the main composite endpoint (TVF), were included in multivariable models: gender, age, body mass index, hypercholesterolemia, hypertension, previous coronary artery bypass grafting, renal insufficiency, total stent length, and previous MI. Using stepwise backward selection, variables with a nonsignificant association (p > .15) with the outcome were excluded from the multivariable model. Although body mass index, age, and gender were likely confounders based on the literature, these variables did not change the effect estimate and were therefore omitted from this model. The final multivariable model included previous MI and total stent length. All statistical tests were 2‐tailed and p‐values <.05 were considered significant. Statistical analyses were performed with SPSS version 24 (IBM Corporation, Armonk, NY).
3. RESULTS
3.1. Glycemic state and baseline characteristics
Of all 988 study participants, OGTT classified 68 (6.9%) as having silent diabetes and 132 (13.4%) as having prediabetes; HbA1c and FPG detected silent diabetes in 33 (3.3%) and prediabetes in 217 (22.0%) patients. Combining both diagnostic approaches (i.e., OGTT or FPG/HbA1c were positive), 330 patients (33.4%) were classified as having an abnormal glucose metabolism while 658 (66.6%) patients had a normal glucose metabolism (i.e., both approaches negative). Table 1 presents details of patients and PCI procedures. Three‐year follow‐up was available in 986 (99.8%) of all 988 patients. The Tables S1 and S2 present the glycemic state and the use of oral anti‐diabetic medication during follow‐up in patients with silent diabetes at baseline.
Table 1.
Baseline characteristics of study population
| OGTT‐based metabolic states | p‐value | HbA1c‐ and FPG‐based metabolic states | p‐value | |||||
|---|---|---|---|---|---|---|---|---|
| Abnormal glucose metabolism | Normal glucose metabolism | Abnormal glucose metabolism | Normal glucose metabolism | |||||
| Silent DM | Pre‐DM | Silent DM | Pre‐DM | |||||
| n = 68 | n = 132 | n = 788 | n = 33 | n = 217 | n = 738 | |||
| Age (years) | 63.9 ± 9.2 | 62.5 ± 9.8 | 61.3 ± 10.2 | .08 | 62.1 ± 8.1 | 63.1 ± 10.3 | 61.2 ± 10.1 | .07 |
| Mean | 53 (77.9) | 98 (74.2) | 623 (79.1) | .46 | 24 (72.7) | 179 (82.5) | 571 (77.4) | .20 |
| BMI (kg/m2) | 28.5 ± 4.5 | 28.5 ± 3.8 | 27.0 ± 3.9 | <.001 | 28.5 ± 4.6 | 28.3 ± 4.1 | 27.0 ± 3.9 | <.001 |
| Hypertension | 29 (42.6) | 64 (48.5) | 301 (38.2) | .07 | 16 (48.5) | 89 (41.0) | 289 (39.2) | .52 |
| Systolic BP (mmHg) | 136.2 ± 24.5 | 139.8 ± 24.8 | 133.6 ± 23.8 | .02 | 134.3 ± 21.8 | 134.8 ± 24.0 | 134.6 ± 24.2 | .99 |
| Hyper‐cholesterolemia | 36 (52.9) | 52 (39.4) | 331 (42.0) | .16 | 15 (45.5) | 96 (44.2) | 308 (41.7) | .76 |
| Current smoker | 23 (35.4) | 39 (30.5) | 230 (29.7) | .63 | 23 (35.4) | 68 (32.4) | 213 (29.3) | .57 |
| Family history of CAD | 31 (49.2) | 70 (54.7) | 398 (52.4) | .77 | 17 (56.7) | 109 (52.4) | 373 (52.3) | .90 |
| Previous MI | 18 (26.5) | 18 (13.6) | 127 (16.1) | .06 | 11 (33.3) | 47 (21.7) | 105 (14.2) | .001 |
| Previous PCI | 12 (17.6) | 24 (18.2) | 111 (14.1) | .38 | 7 (21.2) | 42 (19.4) | 98 (13.3) | .05 |
| Previous CABG | 8 (11.8) | 6 (4.5) | 46 (5.8) | .11 | 3 (9.1) | 17 (7.8) | 40 (5.4) | .32 |
| LVEF <30% | 0 | 0 | 6 (0.8) | .47 | 0 | 1 (0.5) | 5 (0.7) | .84 |
| Renal insufficiencya | 4 (5.9) | 4 (3.0) | 13 (1.6) | .05 | 1 (3.0) | 7 (3.2) | 13 (1.8) | .39 |
| Hb (g/dL) | 14.8 ± 1.5 | 14.6 ± 1.7 | 14.5 ± 1.3 | .39 | 14.7 ± 1.5 | 14.5 ± 1.5 | 14.6 ± 1.4 | .47 |
| MI with systolic BP <90 mmHg | 0 | 1 (0.8) | 14 (1.8) | .39 | 0 | 2 (0.9) | 13 (1.8) | .52 |
| Clinical presentation | .66 | .97 | ||||||
| STEMI | 15 (22.1) | 42 (31.8) | 249 (31.6) | 11 (33.3) | 65 (30.0) | 230 (31.2) | ||
| Non‐STEMI | 12 (17.6) | 28 (21.2) | 147 (18.7) | 6 (18.2) | 40 (18.4) | 141 (19.1) | ||
| Unstable angina | 17 (25.0) | 26 (19.7) | 157 (19.9) | 8 (24.2) | 42 (19.4) | 150 (20.3) | ||
| Stable angina | 24 (35.3) | 36 (27.3) | 235 (29.8) | 8 (24.2) | 70 (32.3) | 217 (29.4) | ||
| Multivessel treatment | 16 (23.5) | 29 (22.0) | 139 (17.6) | .23 | 5 (15.2) | 45 (20.7) | 134 (18.2) | .60 |
| LAD | 30 (44.1) | 71 (53.8) | 404 (51.3) | .26 | 16 (48.5) | 100 (46.1) | 389 (52.7) | .22 |
| Total stent length/patient (mm) | 51.8 ± 34.2 | 43.8 ± 27.2 | 43.7 ± 30.0 | .04 | 48.6 ± 30.0 | 43.9 ± 30.0 | 44.2 ± 30.0 | .69 |
| Reference vessel diameter (mm)b | 2.5 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.6 | .32 | 2.5 ± 0.4 | 2.6 ± 0.6 | 2.6 ± 0.6 | .81 |
Note: Data are n(%) or mean ± SD.
Abbreviations: BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; FPG, fasting plasma glucose; Hb, hemoglobin; HbA1c, glycated hemoglobin; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NG, normal glucose metabolism; OGTT, oral glucose tolerance testing; PCI, percutaneous coronary intervention; Pre‐DM, pre‐diabetes; Silent DM, silent diabetes mellitus; STEMI, ST‐segment‐elevation myocardial infarction.
Renal insufficiency defined as an estimated glomerular filtration rate of less than 30 mL per minute per 1.73 m2 of body‐surface area or the need for dialysis.
Smallest angiographic reference vessel diameter per patient.
3.2. Abnormal glucose metabolism, based on OGTT
When using OGTT to diagnose abnormal glucose metabolism, there was a significant difference in the rates of the main clinical endpoint TVF between patients with silent diabetes, prediabetes, and normoglycemia (14.8, 9.9, and 5.6%; plog‐rank = 0.002). Patients with silent diabetes (based on OGTT) had higher rates of TVF, MACE, and various individual clinical endpoints, including cardiac death and target vessel MI, versus patients with normoglycemia. In patients with prediabetes, TVF and any revascularization showed rates that were higher than in patients with normoglycemia (Table 2). Kaplan–Meier curves of TVF and its components are presented in Figure 1. Landmark analysis of TVF and its components showed that from 48 hr to 3‐year follow‐up, there was no significant difference in most clinical endpoints between patients with silent diabetes, prediabetes, and normoglycemia (Table 2). The rates of definite or probable stent thrombosis were low (0.0, 0.8, and 0.9%; plog‐rank = 0.74).
Table 2.
Clinical event rates at 3‐year follow‐up and between 48 hr and 3 years
| Based on OGTT | p‐value | Based on HbA1c and FPG | p‐value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abnormal glucose metabolism | Normal glucose metabolism | Overall P log‐rank | Silent DM versus NG | Pre‐DM versus NG | Abnormal glucose metabolism | Normal glucose metabolism | Overall P log‐rank | Silent DM versus NG | Pre‐DM versus NG | |||
| Silent DM | Pre‐DM | Silent DM | Pre‐DM | |||||||||
| Total n = 988 | n = 68 | n = 132 | n = 788 | n = 33 | n = 217 | n = 738 | ||||||
| Target vessel failurea | 10 (14.8) | 13 (9.9) | 42 (5.6) | 0.002 | 0.002 | 0.046 | 4 (12.1) | 17 (7.9) | 44 (6.0) | 0.23 | 0.14 | 0.31 |
| Death | 4 (5.9) | 2 (1.5) | 13 (1.7) | 0.046 | 0.024 | 0.91 | 1 (3.0) | 5 (1.8) | 13 (1.8) | 0.79 | 0.60 | 0.60 |
| Cardiac death | 2 (3.0) | 1 (0.8) | 2 (0.3) | 0.010 | 0.014 | 0.37 | 0 | 3 (1.4) | 2 (0.3) | 0.12 | 0.99 | 0.07 |
| Any MI | 7 (10.3) | 7 (5.3) | 28 (3.7) | 0.020 | 0.008 | 0.33 | 4 (12.1) | 11 (5.1) | 27 (3.7) | 0.042 | 0.020 | 0.35 |
| Target vessel MI | 7 (10.3) | 6 (4.5) | 22 (2.8) | 0.004 | 0.002 | 0.29 | 4 (12.1) | 10 (4.6) | 21 (2.9) | 0.010 | 0.006 | 0.20 |
| Periprocedural MI | 7 (10.3) | 5 (3.8) | 12 (1.5) | <0.001 | <0.001 | 0.08 | 4 (12.1) | 8 (3.7) | 12 (1.6) | <0.001 | <0.001 | 0.06 |
| Revascularization, any | 5 (7.4) | 16 (12.1) | 55 (7.0) | 0.13 | 0.88 | 0.046 | 3 (9.1) | 17 (7.9) | 56 (7.6) | 0.95 | 0.78 | 0.89 |
| TVR | 3 (4.4) | 7 (5.3) | 28 (3.6) | 0.61 | 0.69 | 0.34 | 0 | 8 (3.7) | 30 (4.1) | 0.50 | 0.97 | 0.81 |
| MACE | 12 (17.6) | 11 (8.3) | 53 (6.7) | 0.003 | 0.001 | 0.49 | 5 (15.2) | 19 (8.8) | 52 (7.1) | 0.16 | 0.08 | 0.38 |
| Landmark analysis between 48 hr and 3 years | ||||||||||||
| Target vessel failurea | 3 (4.9) | 8 (6.3) | 30 (3.9) | 0.44 | 0.67 | 0.21 | 0 | 9 (4.3) | 32 (4.4) | 0.52 | 0.97 | 0.95 |
| Cardiac death | 2 (3.0) | 1 (0.8) | 2 (0.3) | 0.010 | 0.014 | 0.37 | 0 | 3 (1.4) | 2 (0.3) | 0.12 | 0.99 | 0.07 |
| Target vessel MI | 0 | 1 (0.8) | 10 (1.3) | 0.61 | 0.98 | 0.63 | 0 | 2 (1.0) | 9 (1.3) | 0.79 | 0.99 | 0.74 |
| TVR | 3 (4.4) | 7 (5.3) | 27 (3.5) | 0.55 | 0.65 | 0.30 | 0 | 8 (3.7) | 29 (4.0) | 0.51 | 0.88 | 0.97 |
Note: Data are n(%). Patients who were censored during the first 48 hr of follow‐up were excluded from the landmark analysis.
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MACE, major adverse cardiac events; MI, myocardial infarction; NG, normal glucose metabolism; OGTT, oral glucose tolerance testing; Pre‐DM, pre‐diabetes; Silent DM, silent diabetes mellitus; TVR, target vessel revascularization.
The main study endpoint target vessel failure consists of cardiac death, target vessel myocardial infarction, or target vessel revascularization. MACE consists of any death, any MI, emergent CABG or clinically indicated target lesion revascularization.
Figure 1.
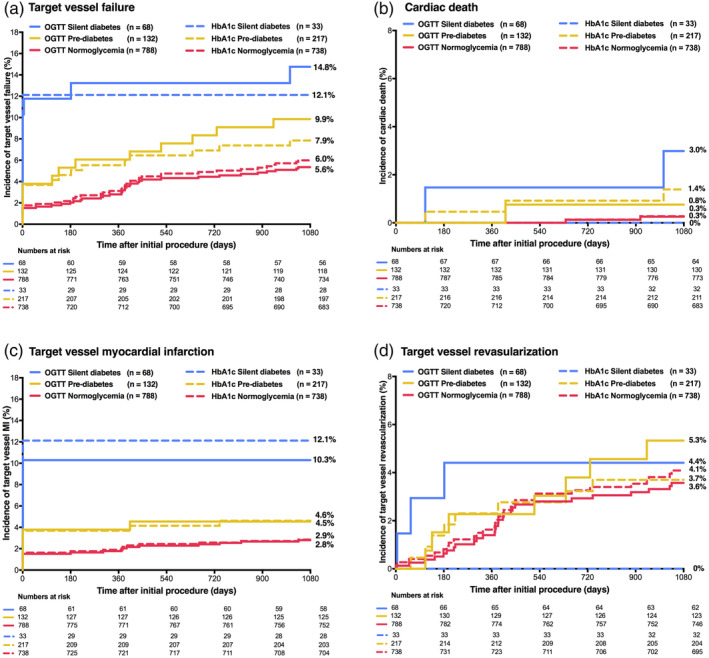
Main clinical endpoint target vessel failure and components at 3‐year follow‐up. HbA1c, glycemic state based on glycated hemoglobin and fasting plasma glucose levels; OGTT, glycemic state based on oral glucose tolerance testing
Multivariable analysis showed after adjusting for the confounders history of previous MI and total stent length that abnormal glucose metabolism detected by OGTT was independently associated with the main endpoint TVF (adjusted HR:2.20, 95%‐CI:1.32–3.65). In addition, silent diabetes and prediabetes (separately assessed) were also found to be independently associated with TVF (adjusted HR:2.52, 95%‐CI:1.26–5.03, and adjusted HR:2.00, 95%‐CI:1.07–3.74).
3.3. Abnormal glucose metabolism, based on HbA1c and FPG
When using the alternative diagnostic approach based on HbA1c and FPG, the rates of TVF in patients with silent diabetes, prediabetes, and normoglycemia were 12.1, 7.9, and 6.0%, respectively (plog‐rank = 0.23). There was a significantly higher rate of target vessel MI in patients with silent diabetes as compared to patients with normoglycemia (12.1 vs. 2.9%; HR:4.44, 95%‐CI:1.53–12.95, p = .006). This difference was driven by a higher rate of periprocedural MI, related to the index PCI procedure (12.1 vs. 1.6%; HR:7.56, 95%‐CI:2.44–23.44, p < .001; Table 2). Figure 1 displays the time‐to‐event curves of TVF and its components, and Table 2 reports data of a landmark analysis from 48 hr to 3‐year follow‐up.
3.4. Abnormal glucose metabolism, based on OGTT or HbA1c and FPG
When using either approach to diagnose abnormal glucose metabolism, TVF occurred in 8.8% of patients with abnormal glucose metabolism versus 5.5% of patients with normal glucose metabolism (unadjusted HR:1.64 95%‐CI:1.01–2.68); this difference in TVF was mainly driven by a significant difference in (periprocedural) MI that occurred during the first 48 hr after the index PCI. After adjusting for confounders, multivariable analysis showed no significant difference in TVF between both metabolic groups (adjusted HR:1.54 95%‐CI:0.94–2.52). Table 3 shows further clinical outcomes, which were similar between groups.
Table 3.
Clinical event rates at 3‐year follow‐up categorized by abnormal glucose metabolism diagnosed by either diagnostic approach
| Based on OGTT or HbA1c and FPG | P log‐rank | HR (95%CI) | ||
|---|---|---|---|---|
| Abnormal glucose metabolism | Normal glucose metabolism | |||
| Total n = 988 | n = 330 | n = 658 | ||
| Target vessel failurea | 29 (8.8) | 36 (5.5) | 0.044 | 1.64 (1.01–2.68) |
| Death | 6 (1.8) | 13 (2.0) | 0.87 | 0.92 (0.35–2.42) |
| Cardiac death | 3 (0.9) | 2 (0.3) | 0.21 | 2.99 (0.50–17.88) |
| Any MI | 18 (5.5) | 24 (3.7) | 0.18 | 1.51 (0.82–2.79) |
| Target vessel MI | 17 (5.2) | 18 (2.7) | 0.051 | 1.90 (0.98–3.70) |
| Periprocedural MI | 15 (4.5) | 9 (1.4) | 0.002 | 3.34 (1.46–7.63) |
| Revascularization, any | 26 (7.9) | 50 (7.6) | 0.88 | 1.04 (0.65–1.66) |
| Target vessel revascularization | 13 (4.0) | 25 (3.8) | 0.92 | 1.04 (0.53–2.03) |
| MACE | 29 (8.8) | 47 (7.2) | 0.33 | 1.26 (0.79–2.00) |
Note: Data are n(%).
Abbreviations: CI, confidence interval; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; OGTT, oral glucose tolerance testing.
Target vessel failure was the main study endpoint.
4. DISCUSSION
4.1. Main findings
Three years after PCI with contemporary DES, patients with previously unknown silent diabetes and prediabetes—as detected by OGTT—showed higher rates of adverse clinical events than patients with normoglycemia. In patients with silent diabetes, the higher incidence of the main composite endpoint TVF was driven by periprocedural MI and cardiac death. In patients with prediabetes, the difference was driven by a higher rate of periprocedural MI. Multivariable analyses showed that both, silent diabetes and pre‐diabetes, were independently associated with TVF. During the second and third year of follow‐up, adverse event rates were quite low. A landmark analysis showed that there were no significant between‐group differences in TVF and target vessel MI from 48 hr to 3 years. This reveals that the aforementioned differences in clinical endpoints at 3‐year follow‐up were mainly based on events that had occurred during the first 48 hours. Although from 48 hours to 3‐year follow‐up the incidence of cardiac death was higher in patients with silent diabetes, there was no significant difference in efficacy endpoint target vessel revascularization between the three patient groups. The low revascularization rates support the efficacy of PCI with new‐generation DES in patients with (previously unknown) abnormal glucose metabolism. While the 3‐year repeat revascularization rates were low in patients with an abnormal glucose metabolism, further follow‐up will be of interest to assess whether these patients may have an increased risk of neoatherosclerosis formation, and very late lesion recurrence and repeat target lesion revascularization inside the previously implanted stents.
When using the alternative diagnostic approach based on HbA1c and FPG to classify the glycemic state of patients without known diabetes, we found no difference in TVF between the three metabolic groups, but periprocedural MI occurred significantly more often in patients with silent diabetes than in patients with normoglycemia. Our findings suggest that the use of HbA1c and FPG to classify metabolic states might be less suitable than OGTT to identify patients with an abnormal glucose metabolism and an increased event risk following PCI.
4.2. Previous studies
Three‐year outcome data of patients with previously unknown abnormal glucose metabolism, who underwent PCI with new‐generation DES, is scarce. In patients undergoing coronary angiography—not necessarily having obstructive coronary disease—Schnell et al. found a higher 3‐year mortality in the presence of an impaired glycemic control (determined by OGTT) versus normoglycemia (8.3 vs. 5.5%).15 Furthermore, they noted a relationship between the extent of coronary disease and the mean difference between the FPG and the 2‐hour post‐load plasma glucose level. A prognostic role of this parameter was suggested, as “non‐survivors” had higher baseline values than “survivors” at 3‐year follow‐up. In contrast, patients with different extent of coronary disease showed similar HbA1c levels.15
Aggarwal et al. assessed 1,686 patients with STEMI and found a 3‐year mortality of 27.3% in patients with known diabetes and 24.0% in patients with silent diabetes (detected by HbA1c or FPG), which both were significantly higher than the mortality in patients with normoglycemia (11.1%). In patients with prediabetes (12.1%), the corresponding mortality rate was similar to the rate in normoglycemic patients.16 Mortality rates in that study were high, as compared to the present study, which may be due to differences in patient populations and stents used. As that study assessed patients treated from 2005 to 2012 in a tertiary center for PCI, parts of the study population must have been treated with devices other than new‐generation DES (stent types were not specified).16 In our study, we observed a somewhat higher mortality in patients with silent diabetes, diagnosed by OGTT, versus normoglycemia.
In patients with a history of diabetes, PCI with newer generation DES have more favorable outcomes than PCI with bare metal stents,17 but the event rates are still higher than in nondiabetic patients. An example may be the BIOSCIENCE randomized trial, which investigated the safety and efficacy of a biodegradable polymer‐coated sirolimus‐eluting stent (Orsiro) and a durable polymer‐coated everolimus‐eluting stent (Xience Prime/Xpedition, Abbott Vascular, Santa Clara, CA) and found that the 1‐year rate of the primary clinical endpoint (target lesion failure: cardiac death, target vessel MI, or clinically indicated target lesion revascularization) was significantly higher in patients with known diabetes than in non‐diabetic patients (10.1 vs 5.7%).6
In an analysis of the randomized TWENTE trial, Tandjung et al. investigated 626 patients treated with second‐generation DES in whom HbA1c data were available. The rate of periprocedural MI was found to be highest in patients with silent diabetes (13.6%), as compared to patients with known diabetes (6.1%), and normoglycemia (3.7%). It was suggested that the higher incidence of periprocedural MI may reflect microvascular dysfunction or obstruction in patients with silent diabetes, potentially caused by periprocedural microembolization of atherothrombotic debris.8
Previous research suggested several pathophysiological mechanisms to explain the increased adverse event risk in patients with an impaired glucose tolerance. Platelet hyper‐activity, endothelial dysfunction, increased monocyte activation (resulting in more inflammation), and an increased thrombogenicity are assumed to be relevant. These factors may promote a hypercoagulable state and the formation of complex atherosclerotic lesions, predisposing patients to the occurrence of adverse cardiovascular events, such as periprocedural myocardial infarction or target lesion recurrence.4, 18
Identifying patients with an impaired glucose tolerance is a first step that may ultimately lead to tailored treatment in order to lower the event risk. Different diagnostic approaches are available, such as OGTT, FPG, and HbA1c, which are generally accepted as being equally appropriate for diagnosing diabetes.5 While HbA1c is more convenient and less susceptible to day‐to‐day fluctuations during stress and illness, it may be affected by ethnicity, pregnancy status, anemia, and hemoglobinopathies. In addition, data from the National Health and Nutrition Examination Survey of the United States showed that sole use of HbA1c identifies undiagnosed diabetes only in one‐third of the patients, who are classified as diabetic based on OGTT, while the combined use of FPG and HbA1c identified one out of two patients.19 Data from the present analysis corroborate these findings, as at baseline we found HbA1c combined with FPG identified only half as many subjects with silent diabetes than OGTT. Furthermore, when classifying patients using OGTT, the patients with silent diabetes had a higher 3‐year TVF risk than normoglycemic patients, while this was not seen when using HbA1c with FPG to classify them. This suggests that patients with an abnormal glucose metabolism and an increased event risk might be missed when using HbA1c with FPG as the diagnostic tool. Therefore, despite being less convenient, OGTT may be considered as the optimal diagnostic technique to detect abnormal glucose metabolism. Nevertheless, if OGTT cannot be done, the assessment of HbA1c with FPG may be a valuable alternative.
4.3. Limitations
This is a prespecified secondary analysis of data from a prospective randomized clinical trial. Therefore, all findings should be considered hypothesis generating. The number of adverse events was rather low. Nevertheless, 3‐year follow‐up was almost complete (99.8% of all 988 participants), the study was monitored, and the adverse events were assessed by an independent external clinical event committee. As OGTT was performed 4–6 weeks after the index procedure, it was inevitable that patients who died during that period could not be included in the BIO‐RESORT Silent Diabetes study. But this applies only to very few patients, as in BIO‐RESORT the all‐cause mortality was low during this short period.9 The timing of OGTT was chosen, to avoid any disturbances caused by procedure‐ and disease‐related stress or repair processes after an MI, and for logistic reasons. The presence of cardiogenic shock at admission was not recorded in our database. We included patients treated at a single center, but this high‐volume tertiary center for cardiac interventions was the highest enrolling site of the BIO‐RESORT randomized trial, and it exclusively serves a large region in the east of the Netherlands with a rather unselected patient referral. It would have been interesting to compare participants in the study with non‐participants, but OGTT was only performed in study participants and the availability of HbA1c and FPG differed between populations.
5. CONCLUSIONS
In patients without known diabetes, abnormal glucose metabolism by OGTT was independently associated with higher 3‐year TVF rates after PCI with contemporary DES. This difference was driven by periprocedural MI and cardiac death. After the first 48 hr, the rates of TVF, target vessel MI, and target vessel revascularization were low and did not differ significantly between metabolic groups.
CONFLICT OF INTEREST
CvB reports that the research department of Thoraxcentrum Twente has received research grants provided by Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. All other authors declared that they have no conflict of interest. The BIO‐RESORT study was equally funded by Biotronik, Boston Scientific, and Medtronic.
Supporting information
Appendix S1: Supporting information
Ploumen EH, Buiten RA, Kok MM, et al. Three‐year clinical outcome in all‐comers with “silent” diabetes, prediabetes, or normoglycemia, treated with contemporary coronary drug‐eluting stents: From the BIO‐RESORT Silent Diabetes study. Catheter Cardiovasc Interv. 2020;96:E110–E118. 10.1002/ccd.28536
Funding information Biotronik; Boston Scientific Corporation; Medtronic
REFERENCES
- 1. Doerr R, Hoffmann U, Otter W, et al. Oral glucose tolerance test and HbA₁c for diagnosis of diabetes in patients undergoing coronary angiography: the silent diabetes study. Diabetologia. 2011;54:2923‐2930. [DOI] [PubMed] [Google Scholar]
- 2. Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140‐2144. [DOI] [PubMed] [Google Scholar]
- 3. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527‐1532. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Classification and diagnosis of diabetes: standard of medical care in diabetes‐2019. Diabetes Care. 2019;42:S13‐S27. [DOI] [PubMed] [Google Scholar]
- 6. Franzone A, Pilgrim T, Heg D, et al. Clinical outcomes according to diabetic status in patients treated with biodegradable polymer sirolimus‐eluting stents: prespecified subgroup analysis of the BIOSCIENCE trial. Circ Cardiovasc Interv. 2015;8:e002319. [DOI] [PubMed] [Google Scholar]
- 7. Kok MM, von Birgelen C, Sattar N, et al. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention. 2018;14:e1049‐e1056. [DOI] [PubMed] [Google Scholar]
- 8. Tandjung K, van Houwelingen KG, Jansen H, et al. Comparison of frequency of periprocedural myocardial infarction in patients with and without diabetes mellitus to those with previously unknown but elevated glycated hemoglobin levels (from the TWENTE trial). Am J Cardiol. 2012;110:1561‐1567. [DOI] [PubMed] [Google Scholar]
- 9. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegredable polymer everolimus‐eluting and sirolimus‐eluting stents versus durable polymer zotarolimus‐eluting stents in allcomers with coronary artery disease (BIO‐RESORT): a three‐arm, randomised, non‐inferiority trial. Lancet. 2016;388:2607‐2617. [DOI] [PubMed] [Google Scholar]
- 10. von Birgelen C, Kok MM, Sattar N, et al. “Silent” diabetes and clinical outcome after treatment with contemporary drug‐eluting stents. The BIO‐RESORT silent diabetes study. J Am Coll Cardiol Cardiovasc Intv. 2018;11:448‐459. [DOI] [PubMed] [Google Scholar]
- 11. Buiten RA, Ploumen EH, Zocca P, et al. Thin, very thin, or ultrathin strut biodegradable‐ or durable‐polymer‐coated drug‐eluting stents, in all comers. J Am Coll Cardiol Cardiovasc Intv. 2019;12(17):1650‐1660. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 13. Cutlip DE, Windecker S, Mehran R, et al. Academic research consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344‐2351. [DOI] [PubMed] [Google Scholar]
- 14. Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity: addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871‐874. [DOI] [PubMed] [Google Scholar]
- 15. Schnell O, Doerr R, Lodwig V, Weissmann J, Lohmann T. A 3‐year follow‐up of the silent diabetes study. Diabetologia. 2014;57:2596‐2598. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assesment of glucose metabolism in patients with ST‐segment elevation myocardial infarction. Am J Cardiol. 2016;117:749‐753. [DOI] [PubMed] [Google Scholar]
- 17. Bangalore S, Toklu B, Feit F. Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus: can newer generation drug‐eluting stents bridge the gap? Circ Cardiovasc Interv. 2014;7:518‐525. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca(2+) homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2001;280:H1480‐H1489. [DOI] [PubMed] [Google Scholar]
- 19. Cowie CC, Rust KF, Byrd‐Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988‐2006. Diabetes Care. 2010;33:562‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


