Abstract
Aim
To investigate the long‐term efficacy and safety of dapagliflozin as an adjunct to adjustable insulin in adults with type 1 diabetes (T1D) and inadequate glycaemic control.
Materials and Methods
Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes (DEPICT‐2) was a placebo‐controlled, double‐blind, multicentre, phase III study of adults with T1D (HbA1c 7.5%‐10.5%) randomized (1:1:1) to receive dapagliflozin 5, 10 mg, or placebo. The efficacy and safety of dapagliflozin over 52 weeks were exploratory endpoints in this extension to DEPICT‐2.
Results
Of 813 participants randomized, 88.2% completed the study. From baseline to 52 weeks, dapagliflozin 5 and 10 mg were associated with reduction in HbA1c (difference [95% CI] vs. placebo: −0.20% [−0.34, −0.06] and −0.25% [−0.38, −0.11], respectively) and adjusted mean percentage change in body weight (difference [95% CI] vs. placebo: −4.42% [−5.19, −3.64] and −4.86% [−5.63, −4.08], respectively). Serious adverse events were reported in the dapagliflozin 5, 10 mg, and placebo groups (32 [11.8%], 19 [7.0%] and 16 [5.9%], respectively). The proportion of hypoglycaemic events was similar across groups; severe hypoglycaemia was uncommon. More participants with events adjudicated as definite diabetic ketoacidosis (DKA) were in the dapagliflozin 5 and 10 mg groups versus placebo (11 [4.1%], 10 [3.7%] and 1 [0.4%], respectively); the majority of events were mild or moderate in severity and all were resolved with treatment.
Conclusions
Dapagliflozin led to long‐term reductions in HbA1c and body weight in adults with T1D, but increased DKA risk compared with placebo.
Keywords: dapagliflozin, DEPICT‐2, SGLT2 inhibitor, type 1 diabetes
1. INTRODUCTION
In type 1 diabetes (T1D), poor glycaemic control is associated with an increased risk of microvascular and macrovascular complications. 1 Insulin‐independent adjunct therapies could help in achieving glycaemic control in people with T1D. Dapagliflozin, a sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor, was recently approved in Europe at a dose of 5 mg for use as an oral adjunct to adjustable insulin in adults with T1D and a body mass index (BMI) of ≥27 kg/m2, and in Japan (5 mg or uptitrated to 10 mg if needed) regardless of BMI, when insulin alone does not provide adequate glycaemic control. 2 , 3 , 4 Sotagliflozin, a combined SGLT1/2 inhibitor, also received approval in Europe for those with T1D and a BMI of ≥27 kg/m2. 5
The European and Japanese approvals of dapagliflozin in T1D were based on results obtained in the Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes (DEPICT‐1 and ‐2) phase III programme; Food and Drug Administration approval was not granted based on these same data. The DEPICT‐1 and ‐2 trials had similar designs, but participants were from different geographical regions. In DEPICT‐1, participants were randomized at 138 participating sites across 17 countries. 6 The majority of people in DEPICT‐1 were of European descent, whereas in DEPICT‐2 ~ 20% were from the Asia‐Pacific region (predominantly Japan). 6 , 7 , 8 Daily dapagliflozin 5 and 10 mg administration was associated with reductions in HbA1c from baseline to 24 and 52 weeks compared with placebo in DEPICT‐1, and from baseline to 24 weeks in DEPICT‐2. 8 Secondary efficacy outcomes including body weight reduction were also improved in the DEPICT studies. Dapagliflozin was well tolerated, with no increase in the risk of hypoglycaemia compared with placebo, but with a higher incidence of events adjudicated as definite diabetic ketoacidosis (DKA). 6 , 7 , 8 All DKA events were resolved with treatment. 8
Here, we present results of the 52‐week DEPICT‐2 study, consisting of the 24‐week short‐term period plus a 28‐week extension, which aimed to assess the long‐term efficacy and safety of dapagliflozin in people with T1D. Safety data are aggregated for the 56‐week study period, including the 4‐week follow‐up period.
2. MATERIALS AND METHODS
2.1. Study design and procedures
DEPICT‐2 (NCT02460978) was a phase III, randomized, double‐blind, three‐arm, parallel‐group, placebo‐controlled trial conducted at 148 participating sites across 13 countries, namely, Argentina, Belgium, Canada, Chile, Germany, Japan, the Netherlands, Poland, the Russian Federation, Sweden, Switzerland, the UK and the United States. 8
This study was performed in accordance with the Declaration of Helsinki and is consistent with Good Clinical Practice guidelines as defined by the International Council on Harmonisation and applicable regulatory requirements. The study was approved by the institutional review boards and independent ethics committees for all participating centres. Written informed consent was obtained for all participants.
In brief, this study comprised an 8‐week lead‐in period to optimize diabetes management based on individual needs and treatment tolerability, followed by a 24‐week, double‐blind treatment period; a 28‐week, participant‐ and site‐blinded extension phase, during which participants continued to receive their randomized treatment; and a 4‐week follow‐up period.
During the 8‐week lead‐in period, the variability in blood glucose profiles was assessed and the frequency of hypoglycaemic episodes monitored. On completing the lead‐in period, participants entered the 24‐week, double‐blind treatment period and were randomly assigned (1:1:1) to dapagliflozin 5 mg, dapagliflozin 10 mg, or placebo using an interactive voice response system. Randomization was stratified by current use of continuous glucose monitoring (CGM), method of insulin administration and baseline HbA1c.
Participants completing the 24‐week double‐blind period continued their assigned medication to week 52. Throughout the study, insulin dose could be adjusted according to self‐monitored blood glucose readings, local guidance and individual circumstances. To minimize the risk of DKA, reductions in insulin doses of >20% were not recommended.
2.2. Study participants
Adults (aged 18‐75 years) were eligible for inclusion in DEPICT‐2 if they had a diagnosis of T1D with HbA1c 7.7%‐11.0% at screening/enrolment and 7.5%‐10.5% at randomization; had used insulin for ≥12 months prior to screening; had been receiving at least three insulin injections per day if following a multiple daily injections regimen or were on an insulin pump (continuous subcutaneous insulin infusion) at a dose of ≥0.3 U/kg/day; their method of insulin administration had not changed for ≥3 months; and had a BMI of ≥18.5 kg/m2. People with a history of type 2 diabetes (T2D) or previous use of any SGLT2 inhibitor were excluded. Detailed inclusion and exclusion criteria were described previously. 8
2.3. Outcome measures
2.3.1. Efficacy
The long‐term efficacy of dapagliflozin was exploratory in the 52‐week DEPICT‐2 study. Efficacy endpoints at 52 weeks included adjusted mean change from baseline in HbA1c, adjusted mean percentage change from baseline in the total daily insulin dose (TDD), adjusted mean percentage change from baseline in body weight, proportion of participants achieving an HbA1c reduction ≥0.5% without a severe hypoglycaemic event, and adjusted mean change from baseline in fasting plasma glucose (FPG). Adjusted mean change in seated systolic blood pressure (SBP) was assessed only among participants with hypertension at baseline (defined as SBP ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg at baseline). Investigators and patients were not masked to adjusted mean change from baseline in HbA1c during the 28‐week extension.
2.3.2. Safety
Safety assessments included adverse events (AEs), serious AEs (SAEs), physical examination findings, vital signs, laboratory tests and electrocardiograms. AEs of special interest (AESIs) included hypoglycaemia, events adjudicated as definite DKA, genital infections, urinary tract infections, volume depletion, fractures, worsening renal function, hypersensitivity and cardiovascular AEs.
Hypoglycaemia was assessed as the proportion of participants with an event and the frequency and severity of the events. Hypoglycaemic events were defined according to the American Diabetes Association (ADA) classification criteria. 9 Severe hypoglycaemia was defined as an event that resulted in unconsciousness because of hypoglycaemia or requiring the assistance of another person to actively administer carbohydrate, glucagon, or to take other corrective actions to promote neurological recovery. 8
Participants were advised how to identify potential signs and symptoms of DKA and were provided with blood ketone monitors and instructions for use. Participants recorded home ketone values (β‐hydroxybutyrate) and relevant risk factors in a diary, and were advised to contact the study site if their self‐measured blood ketone reading was ≥0.6 mmol/L. Potential events of DKA were identified by the investigator based on symptoms, diagnoses or home ketone values, as described previously. 8 Queries were also raised by the sponsor based on predefined Medical Dictionary for Regulatory Activities preferred terms for AEs potentially indicating DKA. An independent, blinded, DKA adjudication committee classified all potential events of DKA as definite, possible or unlikely DKA. Definite DKA was defined using the ADA consensus criteria and included laboratory‐confirmed acidosis (pH < 7.3 and/or bicarbonate <18 mEq/L); signs and symptoms of DKA were part of a well‐documented diagnosis but were not explicitly required. 8 , 10 , 11 To avoid missing any potential euglycaemic DKA events, glucose was not part of the diagnostic criteria. There were no adjudication criteria for possible and unlikely DKA events as these were not explicitly defined.
2.4. Statistical analysis
Determination of sample size, randomization schedule and stratification factors have been described previously. 8 Efficacy analyses were performed on all participants in the full analysis set (randomized patients who received ≥1 dose of study medication) who had baseline and any postbaseline assessment. Analyses on the full analysis set were based on the randomized treatment. Analytical methods for efficacy outcomes in the 24‐week treatment period have been reported previously. 8 For all efficacy outcomes, adjusted mean (standard error [SE]) change or adjusted mean percentage change (SE) from baseline were assessed for all postbaseline visits, except the post‐treatment follow‐up visit at week 56 wherein only mean change from baseline was calculated. TDD was determined using the midpoint of the highest and lowest patient‐recorded insulin dose ranges for each week except for weeks 2‐10, 12‐22 and 24‐56, during which every insulin dose was recorded. If both basal and bolus daily dose data were available, these were converted into weekly ranges and then combined for analysis. For weeks 1‐2, 10‐12 and 22‐24, the average insulin dose was determined.
Logistic regression models were used where the endpoint was a proportion of those achieving a specified target at week 52, with adjustment for randomization stratification factors and baseline values. A longitudinal repeated‐measures approach using direct likelihood was performed for analyses of variables in terms of change or percent change (using log transformation for the endpoint) from baseline for the dapagliflozin treatment groups versus placebo, as described previously. 6 The mixed models included terms for the baseline value, treatment, study week, randomization stratification factor, interaction between study week and treatment, and interaction between study week and baseline value.
For missing data at week 52, the last observation carried forward method was used. Odds ratios (ORs) based on logistic regression for each endpoint and the 95% confidence intervals (CIs) were determined for dapagliflozin versus placebo. The 52‐week efficacy analyses were considered exploratory as multiplicity adjustments were not performed. Therefore, P‐values were not calculated for treatment group comparisons.
Safety outcomes were assessed using the safety analysis set, which included all participants who had received ≥1 dose of study medication. Analyses on the safety analysis set are based on actual treatment received. Safety was assessed from treatment initiation to the end of the 28‐week long‐term extension plus the 30‐day post‐treatment follow‐up period. Data were summarized descriptively and no statistical tests were performed to compare rates between treatment groups.
3. RESULTS
3.1. Participants
Of the 813 participants randomized in the study, 717 (88.2%) completed the 24‐week study and entered the 28‐week extension (Figure S1). In the full analysis set, 231 (85.2%), 226 (83.7%) and 216 (79.4%) participants receiving dapagliflozin 5, 10 mg, and placebo completed the 52‐week treatment period, respectively. Baseline characteristics and demographics for all randomized participants have been reported previously (Table S1). 8
3.2. Efficacy outcomes
Treatment with dapagliflozin 5 or 10 mg was associated with a reduction in HbA1c over 52 weeks, with a similar decrease observed in both groups (Figure 1A). Adjusted mean change in HbA1c (SE) from baseline to week 52 was −0.11% (0.05) for dapagliflozin 5 mg, −0.16% (0.05) for dapagliflozin 10 mg, and 0.09% (0.05) for placebo. The difference (95% CI) versus placebo was −0.20% (−0.34, −0.06) and − 0.25% (−0.38, −0.11) for dapagliflozin 5 and 10 mg, respectively.
FIGURE 1.
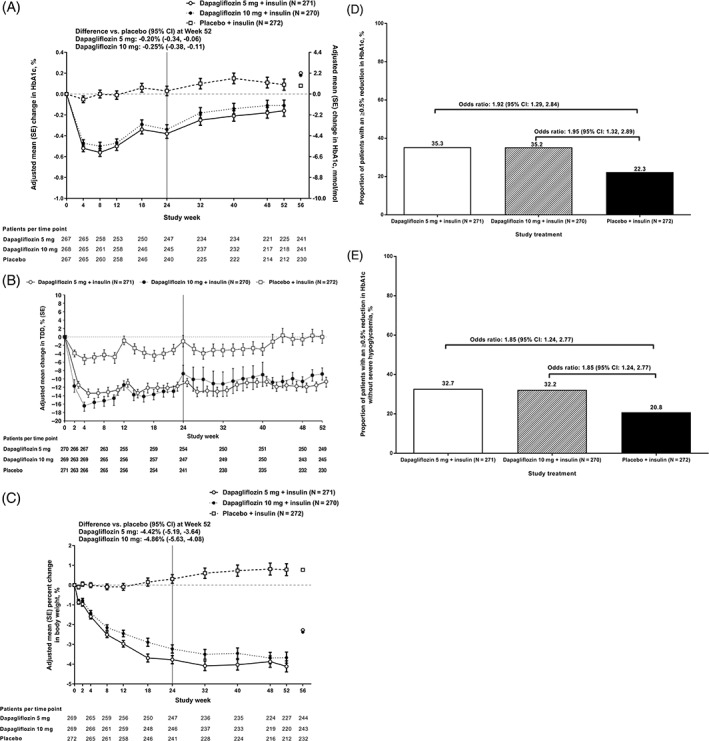
(A) Change in HbA1c (%) over 52 weeks; (B) change in total daily insulin dose (TDD) (%) over 52 weeks; (C) change in total body weight (%) over 52 weeks; (D) proportion of participants achieving an HbA1c reduction of ≥0.5%; (E) proportion of participants achieving an HbA1c reduction of ≥0.5% without severe hypoglycaemia (%) over 52 weeks. Week 0‐52 data show adjusted mean change from baseline (standard error); week 56 data are only mean change from baseline. Data are for all participants in the full analysis set. The study's primary and secondary endpoints were assessed in the 24‐week treatment period (indicated by a black line at week 24), and exploratory endpoints were assessed at week 52 in the long‐term 28‐week extension. HbA1c (%): mean (standard deviation [SD]) at baseline was 8.44 (0.69), 8.42 (0.69) and 8.41 (0.64) for dapagliflozin 5, 10 mg and placebo, respectively. Body weight (%): mean (SD) at baseline was 78.7 (17.4), 80.1 (18.3) and 78.9 (18.9) kg for dapagliflozin 5, 10 mg and placebo, respectively. TDD, U (SD) at baseline was 58.19 (27.93), 58.68 (28.26) and 56.57 (25.23) for dapagliflozin 5, 10 mg, and placebo, respectively
In patients treated with dapagliflozin 5 and 10 mg, an initial decrease in TDD was observed during the 24‐week treatment period, with values remaining lower compared with placebo over the 28‐week extension (Figure 1B).
Reductions in body weight observed in both dapagliflozin treatment groups at 24 weeks were sustained over the 52‐week treatment period (Figure 1C). At week 52, mean (SD) body weight was 76.0 (16.5), 76.6 (18.5) and 78.6 (18.1) kg in the dapagliflozin 5, 10 mg, and placebo groups, respectively. Adjusted mean percent change in body weight (SE) from baseline to week 52 was −3.67% (0.28), −4.11% (0.28) and 0.78% (0.30) for dapagliflozin 5, 10 mg, and placebo, respectively (difference [95% CI] vs. placebo, dapagliflozin 5 mg: −4.42% [−5.19, −3.64]; and dapagliflozin 10 mg: −4.86% [−5.63, −4.08]; Figure 1C).
The proportion of participants achieving an HbA1c reduction of ≥0.5% after 52 weeks was higher in participants receiving dapagliflozin 5 or 10 mg compared with those receiving placebo (35.3%, 35.2% and 22.3%, respectively; Figure 1D). The OR (95% CI) versus placebo for achieving an HbA1c reduction of ≥0.5% was 1.92 (1.29, 2.84) and 1.95 (1.32, 2.89) for dapagliflozin 5 and 10 mg, respectively. The proportion of participants achieving an HbA1c reduction of ≥0.5% without a severe hypoglycaemia event after 52 weeks was higher in the dapagliflozin 5 and 10 mg groups compared with placebo (32.7%, 32.2% and 20.8%, respectively; Figure 1E). The OR (95% CI) versus placebo for achieving an HbA1c reduction of ≥0.5% without experiencing severe hypoglycaemia was the same for both dapagliflozin 5 and 10 mg doses (1.85 [1.24, 2.77]).
At week 52, reductions in FPG were observed in the dapagliflozin 5 and 10 mg groups compared with placebo, with a difference versus placebo (95% CI) of −1.14 mmol/L (−1.85, −0.43) and − 1.04 mmol/L (−1.76, −0.32) for dapagliflozin 5 and 10 mg, respectively (Table S2).
Among participants having hypertension at baseline, there was a numerical reduction in SBP in the dapagliflozin treatment groups compared with the placebo group at week 52, although the 95% CIs included zero for the higher dose of dapagliflozin with a difference versus placebo (95% CI) of −6.76 mmHg (−12.93, −0.58) and − 3.34 mmHg (−10.06, 3.38) for dapagliflozin 5 and 10 mg, respectively (Table S3).
3.3. Safety outcomes
The proportion of participants with at least one AE over the 52‐week treatment period plus 30‐day follow‐up was similar in the dapagliflozin 5, 10 mg, and placebo groups (82.3%, 75.9% and 75.4%, respectively; Table 1). AEs leading to study discontinuation occurred in 8.9%, 6.3% and 6.6% of participants in the dapagliflozin 5, 10 mg, and placebo groups, respectively (Table 1). The most frequently reported AEs (≥5% in any treatment group) by preferred term are shown in Table S4, and included nasopharyngitis (21.0%, 23.3% and 25.4%), upper respiratory tract infection (9.6%, 6.3% and 5.5%), pollakisuria (8.1%, 5.2% and 2.6%) and headache (6.3%, 6.7% and 4.8%) in the dapagliflozin 5, 10 mg, and placebo groups, respectively.
TABLE 1.
Safety summary
| Dapagliflozin 5 mg + insulin (N = 271) | Dapagliflozin 10 mg + insulin (N = 270) | Placebo + insulin (N = 272) | |
|---|---|---|---|
| AEs | |||
| ≥1 AE | 223 (82.3) | 205 (75.9) | 205 (75.4) |
| ≥1 AE related to the study drug | 88 (32.5) | 83 (30.7) | 49 (18.0) |
| AE leading to discontinuation of study drug | 24 (8.9) | 17 (6.3) | 18 (6.6) |
| AESI a | |||
| Genital infection | 30 (11.1) | 28 (10.4) | 10 (3.7) |
| Hypersensitivity | 27 (10.0) | 18 (6.7) | 21 (7.7) |
| Urinary tract infection | 25 (9.2) | 14 (5.2) | 18 (6.6) |
| Hypotension/dehydration/hypovolemia | 10 (3.7) | 3 (1.1) | 5 (1.8) |
| Fractures | 8 (3.0) | 5 (1.9) | 4 (1.5) |
| Renal impairment/failure | 3 (1.1) | 1 (0.4) | 1 (0.4) |
| Cardiovascular events | 1 (0.4) | 3 (1.1) | 3 (1.1) |
| SAEs | |||
| ≥1 SAEs | 32 (11.8) | 19 (7.0) | 16 (5.9) |
| ≥1 SAEs related to the study drug | 15 (5.5) | 7 (2.6) | 4 (1.5) |
| SAEs leading to discontinuation of study drug | 17 (6.3) | 5 (1.9) | 6 (2.2) |
| Death | 1 (0.4) b | 0 | 0 |
| Hypoglycaemia | |||
| ≥1 SAE of hypoglycaemia | 5 (1.8) | 1 (0.4) | 2 (0.7) |
| SAE of hypoglycaemia leading to discontinuation of study drug | 2 (0.7) | 1 (0.4) | 1 (0.4) |
| Ketone‐related events | |||
| ≥1 ketone‐related SAEs | 15 (5.5) | 7 (2.6) | 1 (0.4) |
| Ketone‐related SAE leading to discontinuation of study drug | 12 (4.4) | 4 (1.5) | 0 |
All data are n (%). Safety was assessed over 52 weeks of treatment plus 30 days post‐treatment.
Abbreviations: AE, adverse event; AESI, adverse event of special interest; SAE, serious adverse event.
The total number of participants with an event, organized by system organ class.
One death in the dapagliflozin 5 mg group was because of an SAE that was not related to the study treatment.
The proportion of participants with at least one SAE was higher in the dapagliflozin treatment groups versus placebo (Table 1). The summary of observed SAEs is presented in Table S5. AESIs are shown in Table 1. Genital infections were the most common AESI, with vulvovaginal mycotic infections observed in 10 (3.7%), 12 (4.4%) and five (1.8%), and fungal genital infections in six (2.2%), seven (2.6%) and zero participants in the dapagliflozin 5, 10 mg, and placebo groups, respectively. The second most common AESI was hypersensitivity, which was observed in 10.0%, 6.7% and 7.7% of participants in the dapagliflozin 5, 10 mg, and placebo groups, respectively, most of which were skin‐related AEs such as rash and dermatitis.
A summary of hypoglycaemic events observed in the dapagliflozin and placebo groups is provided in Table 2. Severe hypoglycaemia was uncommon, with a similar number of participants in the dapagliflozin 5, 10 mg, and placebo groups experiencing an event (Table 2); SAEs of hypoglycaemia were rare in all treatment groups (Table 1). Very few participants in any treatment group experienced SAEs of hypoglycaemia leading to study discontinuation (Table 1).
TABLE 2.
Summary of hypoglycaemic events
| Dapagliflozin 5 mg + insulin (N = 271) | Dapagliflozin 10 mg + insulin (N = 270) | Placebo + insulin (N = 272) | |
|---|---|---|---|
| [246.0 patient‐years] | [246.3 patient‐years] | [239.6 patient‐years] | |
| Total number of hypoglycaemic events, n | 7998 | 8321 | 8054 |
| Participants with ≥1 events, n (%) | 231 (85.2) | 234 (86.7) | 237 (87.1) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 3251.27 | 3378.74 | 3361.82 |
| Severe hypoglycaemia | |||
| Number of events, n | 97 | 84 | 65 |
| Number of participants with ≥1 events, n (%) | 24 (8.9) | 26 (9.6) | 23 (8.5) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 39.43 | 34.11 | 27.13 |
| Documented symptomatic hypoglycaemia | |||
| Number of events, n | 6121 | 6494 | 6228 |
| Number of participants with ≥1 events, n (%) | 225 (83.0) | 221 (81.9) | 229 (84.2) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 2488.25 | 2636.89 | 2599.63 |
| Asymptomatic hypoglycaemia | |||
| Number of events, n | 1487 | 1564 | 1548 |
| Number of participants with ≥1 events, n (%) | 108 (39.9) | 138 (51.1) | 114 (41.9) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 604.48 | 635.06 | 646.15 |
| Probable symptomatic hypoglycaemia | |||
| Number of events, n | 119 | 105 | 125 |
| Number of participants with ≥1 events, n (%) | 23 (8.5) | 37 (13.7) | 25 (9.2) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 48.37 | 42.64 | 52.18 |
| Relative hypoglycaemia | |||
| Number of events, n | 119 | 64 | 82 |
| Number of participants with ≥1 events, n (%) | 24 (8.9) | 21 (7.8) | 32 (11.8) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 48.37 | 25.99 | 34.23 |
| Other hypoglycaemia | |||
| Number of events, n | 55 | 10 | 6 |
| Number of participants with ≥1 events, n (%) | 8 (3.0) | 7 (2.6) | 5 (1.8) |
| Exposure‐adjusted incidence rate, per 100 patient‐years including recurrences | 22.36 | 4.06 | 2.50 |
Safety was assessed over 52 weeks of treatment plus 30 days post‐treatment. All reported hypoglycaemic events with onset within 4 days of the last day of treatment are included. Hypoglycaemia categorization is based on the ADA classifications.
The proportion of participants with an event adjudicated as definite DKA was higher in both the dapagliflozin 5 and 10 mg groups than in the placebo group (4.1%, 3.7% and 0.4%, respectively; Table 3). There was a higher incidence of events adjudicated as definite DKA in continuous subcutaneous insulin infusion versus multiple daily injection users in any treatment group (16 [5.8%] vs. six [1.1%], respectively). There was no difference in the proportion of participants with an event adjudicated as definite DKA in any treatment group when stratified by baseline HbA1c <9.0% and ≥9.0% (17 [2.7%] and five [2.8%], respectively). The majority of events adjudicated as definite DKA were either mild or moderate in severity. The most common identified primary causes of events adjudicated as definite DKA in the dapagliflozin treatment groups were insulin pump failure or missed insulin dose. All DKA events resolved with treatment.
TABLE 3.
Summary of events adjudicated as diabetic ketoacidosis (DKA)
| Dapagliflozin 5 mg + insulin (N = 271) | Dapagliflozin 10 mg + insulin (N = 270) | Placebo + insulin (N = 272) | |
|---|---|---|---|
| Participants with events sent for adjudication, n (%) | 27 (10.0) | 23 (8.5) | 11 (4.0) |
| Participants with definite DKA, n (%) | 11 (4.1) | 10 (3.7) | 1 (0.4) |
| Number of events of definite DKA, n | 11 | 10 | 1 |
| Incidence rate, per 100 patient‐years | 4.47 | 4.06 | 0.42 |
| Severity of event as adjudicated, n (%) a | |||
| Mild | 4 (36.4) | 4 (40.0) | 0 |
| Moderate | 5 (45.5) | 4 (40.0) | 1 (100.0) |
| Severe | 2 (18.2) | 2 (20.0) | 0 |
| Number of events of euglycaemic DKA, n b | 3 | 2 | 0 |
| Primary cause of definite DKA events, n (%) a | |||
| Insulin pump failure | 1 (9.1) | 3 (30.0) | 0 |
| Missed insulin dose | 4 (36.4) | 1 (10.0) | 0 |
| Severe illness | 1 (9.1) | 1 (10.0) | 0 |
| Not identified | 4 (36.4) | 1 (10.0) | 1 (100.0) |
| Other | 1 (9.1) | 4 (40.0) | 0 |
| Mean percent TDD reduction compared with baseline for week before definite DKA events, % | −17.35 | −19.49 | −4.19 |
| Mean percent TDD reduction compared with baseline at end of treatment period in participants with definite DKA events, % | −12.26 | −23.00 | 5.13 |
| Events adjudicated as not DKA | |||
| Participants with possible DKA, n (%) | 8 (3.0) | 4 (1.5) | 2 (0.7) |
| Number of events of possible DKA, n | 9 | 4 | 2 |
| Participants with unlikely DKA, n (%) | 10 (3.7) | 10 (3.7) | 9 (3.3) |
| Number of events of unlikely DKA, n | 20 | 27 | 21 |
Safety was assessed over 52 weeks of treatment plus 30 days post‐treatment.
Abbreviation: TDD, total daily insulin dose.
Percentages are based on the total number of events of definite DKA in each treatment group.
Based on patients’ own meter reading.
There were no notable differences in laboratory variables from baseline to week 52 among the three treatment groups, with the exception of an expected increase in urinary glucose in the dapagliflozin treatment groups (data not shown). There were no notable differences in vital signs and electrocardiogram data among the three treatment groups (data not shown).
4. DISCUSSION
In this long‐term extension of the DEPICT‐2 study, dapagliflozin as an adjunct to insulin was associated with a reduction in HbA1c over 52 weeks of randomized treatment. The overall efficacy and safety profile of dapagliflozin observed in this study were similar to that seen in the DEPICT‐1 52‐week study. 6 As the study population in the present study included a high number of Japanese people in addition to those of European descent, these results support the long‐term benefit of dapagliflozin in people with T1D of different ethnicities and countries of origin.
Reduction in HbA1c was less pronounced at week 52 compared with week 24. 8 The sustainability of the glycaemic response of SGLT2 inhibitors over time needs further exploration in real‐world studies, but the unmasking of the change from baseline in HbA1c after the first 24 weeks in this study may have impacted the results observed at week 52.
Weight management in people with T1D is important as intensive insulin therapy commonly results in weight gain. 12 SGLT2 inhibitors are often associated with weight loss in people with T1D because of a reduction in fat mass related to caloric loss from increased urinary glucose excretion (UGE). 13 , 14 Consistent with these findings, and in addition to the benefit of HbA1c reduction, treatment with dapagliflozin resulted in weight loss of ~4% after 24 weeks that was maintained for up to 52 weeks.
Fear of hypoglycaemia can be a barrier to treatment intensification in T1D. 15 , 16 Intensification of insulin therapy and introduction of adjunct therapies are often associated with an increased risk of hypoglycaemia. 17 , 18 In the present study, consistent with findings in the DEPICT‐1 52‐week study, 6 the proportion of participants experiencing severe hypoglycaemia was low in all treatment groups and did not increase in those receiving dapagliflozin versus placebo, despite the improvement in HbA1c seen with dapagliflozin treatment. This may be partly because of the reduction in TDD afforded by the insulin‐independent mode of action of dapagliflozin, where the increase in UGE is dependent on blood glucose concentration. 19 Dapagliflozin can reduce the magnitude of plasma glucose fluctuations, with a low intrinsic potential for hypoglycaemia. This was supported by CGM data obtained in the DEPICT trials, where no notable differences were observed at week 24 with regards to the percentage of glucose values within the ranges indicating hypoglycaemia (≤3.9 mmol/L [≤70 mg/dL] or ≤3.0 mmol/L [≤54 mg/dL]) over 24 hours in people who received dapagliflozin compared with placebo. 20 The consensus recommendation from the Advanced Technologies & Treatments for Diabetes expert panel states that CGM data should be considered in conjunction with HbA1c for the assessment of glycaemic status and therapy adjustment for people with T1D. 21 , 22
The risk of DKA has been shown to increase with the use of SGLT1/2 and SGLT2 inhibitors in people with T1D. 23 , 24 As a key clinical strategy, healthcare providers and people with T1D should be educated regarding the symptoms and risks, and provided with appropriate support before the initiation of SGLT2 inhibitors to help recognize and manage DKA. 13 , 22 The importance of ketone monitoring and appropriate reductions in insulin dose need further attention in current diabetes practice to mitigate DKA risk. 25 Interventions such as the STOP DKA protocol could be useful to prevent DKA if ketone levels rise. 26 In the present study, there was an increase in events adjudicated as definite DKA in those receiving dapagliflozin at 52 weeks (4.1%, 3.7% and 0.4% for dapagliflozin 5, 10 mg, and placebo, respectively) compared with definite DKA events at 24 weeks (2.6% and 2.2% for dapagliflozin 5 and 10 mg vs. 0% for placebo, respectively). 6 The risk of DKA appeared to be constant between the first 24 weeks and the 28‐week extension of DEPICT‐2, so the risk did not diminish over time. Consistent with the DEPICT‐1 study, all DKA events resolved with treatment. 6 , 7
The DEPICT‐1 and ‐2 studies featured education on how to recognize symptoms of DKA, and guidance for patients to contact the investigators if ketones were even mildly elevated applied across both clinical teams and study participants. Despite these initiatives, the risk of DKA remained higher in those receiving dapagliflozin versus placebo. As outlined in the European Summary of Product Characteristics for dapagliflozin, patients with T1D should be evaluated for their DKA risk and educated on how to recognize signs and symptoms before initiating dapagliflozin; in cases where DKA is suspected or diagnosed, dapagliflozin treatment should be stopped immediately. 2
The use of SGLT1/2 and SGLT2 inhibitors in clinical practice must be evaluated against the background of increased DKA risk, emphasizing the need for careful patient selection and education by the prescribing physician to optimize treatment benefits and minimize the risk of DKA. Patients most probable to display a positive benefit/risk profile with dapagliflozin treatment include those with a BMI ≥27 kg/m2, those with low DKA risk profiles who utilize relevant education relating to DKA risk mitigation strategies, those who are receiving established, stable and optimized insulin therapy, with insulin doses of >0.5 units/kg body weight/day, and those who do not use insulin pumps. 25 , 27
Our results also add to data already reported for other SGLT1/2 and SGLT2 inhibitors in T1D therapy. Changes in HbA1c, TDD and body weight observed in the present study were consistent with those reported for the two higher doses of empagliflozin (10 and 25mg) in the EASE Phase III programme 28 and for sotagliflozin in the inTandem1 and 2 studies. 29 , 30 Although improvements in glycaemic control and weight loss without an increase in the risk of hypoglycaemia have been associated with dapagliflozin, 24 an increased risk of DKA that does not appear to diminish over time has also been observed. 31 The benefit/risk profile of dapagliflozin in the treatment of T1D should be evaluated further in real‐world settings to determine whether the numerical attenuation seen in HbA1c over time is present outside the clinical trial setting and to improve understanding of how to mitigate the risk of DKA in clinical practice.
The present study has some limitations. First, insulin was not titrated using a protocol‐mandated algorithm, which may have masked the full glycaemic potential of dapagliflozin. Second, the way in which insulin dose was captured and analysed differed in the short‐term and long‐term periods, making comparisons between treatment groups throughout the 52 weeks difficult. Finally, people with certain co‐morbidities such as unstable or rapidly progressing renal disease were excluded, which may affect applicability to some populations.
In conclusion, dapagliflozin as an add‐on to adjustable insulin in people with T1D showed long‐term reductions in HbA1c and sustained weight loss. Dapagliflozin was well tolerated in this geographically diverse population, with no increased risk of hypoglycaemia. These findings are consistent with those from previous long‐term studies of SGLT2 inhibitors in people with T1D and in those with T2D. Although dapagliflozin was associated with increased DKA, this risk was manageable and all events were resolved with treatment. It is important that the risk of DKA is considered when treating adults with T1D with dapagliflozin, and that risk mitigation strategies are implemented, including active patient selection and education.
CONFLICT OF INTEREST
C.M. has served as a consultant or advisory board member for ActoBio Therapeutics, AstraZeneca, Boehringher Ingelheim, Eli Lily and Company, Merck Sharp and Dohme Ltd., Novo Nordisk, Roche and Sanofi, and has also received honorarium from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Novo Nordisk and Sanofi. G.R. received honorarium for conducting this clinical trial, as well as from AstraZeneca, Eli Lilly, Novo Nordisk, and Merck Sharp and Dohme, and participated in advisory boards for Merck Sharp and Dohme, AstraZeneca, Eli Lilly and Novo Nordisk. E.A. has participated on advisory panels for Alcon, Astellas Pharma, AstraZeneca, Eli Lilly, Kowa Pharmaceutical, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanofi and Terumo Corporation, has received honoraria for lectures from Astellas Pharma, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, and Sanofi, and scholarship grants from Astellas Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Shionogi, Sumitomo Dainippon Pharma and Takeda Pharmaceutical. M.L. has received research grants from AstraZeneca, DexCom, Novo Nordisk and Pfizer, and has been a consultant for or received honoraria from AstraZeneca, DexCom, Eli Lilly, Medtronic, MSD, Novo Nordisk and Rubin Medical. P.D. serves on the advisory boards of AstraZeneca, Novo Nordisk, Sanofi, Boehringer Ingelheim, Merck, Intarcia and AbbVie, and has received research grants from all of these companies, apart from Intarcia. M.P. serves on the advisory boards of Sanofi, Medtronic, Novo Nordisk, Eli Lilly and Pfizer, has received research grants from Medtronic, Novo Nordisk, Roche, Eli Lilly, Merck, Sanofi, Pfizer, Bristol‐Myers Squibb, OPKO, Dexcom, Insulet and Lexicon, and honorarium, consultation and speaker bureau from Sanofi, Medtronic, Novo Nordisk, Eli Lilly, Pfizer, RSP Systems, Qulab Medical, AstraZeneca, Lilly and Insulet, and, in addition, is a stockholder for DreaMed Diabetes, NG Solutions and NutriTeen Professionals. N.A., N.I. and F.T. are employees of AstraZeneca. M.F.S. is an employee of Bayer. At the time of the study, M.F.S. was an employee of AstraZeneca. No other conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
C.M., P.D., E.A., M.P., N.A., F.T. and M.L. contributed to the study concept and design, analysis and interpretation of the study data. G.R., C.M., E.A., M.L., P.D., N.A., N.I., M.F.S., M.P. and F.T. contributed to the drafting of the manuscript and critical revision for intellectual content. C.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
DATA AVAILABILITY
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The authors thank the participating adults, their families and all investigators involved in this study. Medical writing support was provided by Jonathon Ackroyd, inScience Communications, Springer Healthcare, and was funded by AstraZeneca. This study was presented at the 18th International Congress of Endocrinology (ICE) Congress, 1‐4 December 2018, Cape Town, South Africa, and the European Association for the Study of Diabetes, 55th Annual Meeting, 16‐20 September 2019, Barcelona, Spain. Post hoc analyses of DEPICT‐2 have been presented as abstracts at American Diabetes Association (ADA) 79th Scientific Sessions, 7‐11 June 2019, San Francisco, California. This study was funded by AstraZeneca and Bristol‐Myers Squibb. A complete list of the DEPICT‐2 Investigators can be found in the supporting information.
Mathieu C, Rudofsky G, Phillip M, et al. Long‐term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 52‐week results from a randomized controlled trial. Diabetes Obes Metab. 2020;22:1516–1526. 10.1111/dom.14060
Funding information AstraZeneca; Bristol‐Myers Squibb
REFERENCES
- 1. Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EMC. Forxiga 5 mg film‐coated tablets: European Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/2865/smpc. Accessed August 28, 2019.
- 3.Japanese Ministry of Health Labour and Welfare. Forxiga 5 and 10 mg product label. 2019; https://www.info.pmda.go.jp/go/pack/3969019F1027_2_10/?view=frame&style=XML&lang=ja. Accessed December 20, 2019.
- 4. Araki E, Watada H, Uchigata Y, et al. Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT‐5): 52‐week results from a randomized, open‐label, phase III clinical trial. Diabetes Obes Metabol. 201922(4):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency. New add‐on treatment to insulin for treatment of certain patients with type 1 diabetes. 2019. https://www.ema.europa.eu/en/news/new-add-treatment-insulin-treatment-certain-patients-type-1-diabetes. Accessed April 30, 2020.
- 6. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of Dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care. 2018;41(12):2552‐2559. [DOI] [PubMed] [Google Scholar]
- 7. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24 week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):864‐876. [DOI] [PubMed] [Google Scholar]
- 8. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of Dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care. 2018;41(9):1938‐1946. [DOI] [PubMed] [Google Scholar]
- 9. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 201336(5):1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61‐e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799‐812. [DOI] [PubMed] [Google Scholar]
- 13. Riddle MC, Cefalu WT. SGLT inhibitors for type 1 diabetes: an obvious choice or too good to be true? Diabetes Care. 2018;41(12):2444‐2447. [DOI] [PubMed] [Google Scholar]
- 14. Li K, Xu G. Safety and efficacy of sodium glucose co‐transporter 2 inhibitors combined with insulin in adults with type 1 diabetes: a meta‐analysis of randomized controlled trials. J Diabetes. 2019;11(8):645‐655. [DOI] [PubMed] [Google Scholar]
- 15. Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902‐1912. [DOI] [PubMed] [Google Scholar]
- 17. Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and safety of Liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat‐to‐target randomized trial. Diabetes Care. 2016;39(10):1702‐1710. [DOI] [PubMed] [Google Scholar]
- 18. Ahren B, Hirsch IB, Pieber TR, et al. Efficacy and safety of Liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693‐1701. [DOI] [PubMed] [Google Scholar]
- 19. Henry RR, Strange P, Zhou R, et al. Effects of Dapagliflozin on 24‐hour glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2018;20(11):715‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mathieu C, Dandona P, Phillip M, et al. Glucose variables in type 1 diabetes studies with Dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT‐1 and ‐2. Diabetes Care. 2019;42(6):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 21. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta‐analysis of randomised controlled trials. BMJ. 2019;365:l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCrimmon RJ, Henry RR. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia. 2018;61(10):2126‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans M, Hicks D, Patel D, Patel V, McEwan P, Dashora U. Optimising the benefits of SGLT2 inhibitors for type 1 diabetes. Diabetes Ther. 2020;11(1):37‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium‐glucose co‐transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab. 2019;21(10):2192‐2202. [DOI] [PubMed] [Google Scholar]
- 27. Yanai H, Adachi H, Hakoshima M, Katsuyama H. An effective insulin therapy in combination with sodium‐glucose cotransporter 2 inhibitors. J Clin Med Res. 2019;11(1):76‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560‐2569. [DOI] [PubMed] [Google Scholar]
- 29. Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with Sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care. 2018;41(9):1981‐1990. [DOI] [PubMed] [Google Scholar]
- 30. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the north American in Tandem1 study. Diabetes Care. 2018;41(9):1970‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20(3):620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


