Abstract
We describe a procedure to quantify emissions of chemicals for environmental protection, assessment, and management purposes. The procedure uses production and use volumes from registration dossiers and combines these with Specific Environmental Release Category data. The procedure was applied in a case study. Emission estimations were made for chemicals registered under the European Union chemicals regulations for industrial chemicals (Registration, Evaluation, Authorisation and Restriction of Chemicals [REACH]) and for the active ingredients of medicines and crop protection products. Emissions themselves cannot be validated. Instead, emission estimates were followed by multimedia fate modeling and mixture toxic pressure modeling to arrive at predicted environmental concentrations (PECs) and toxic pressures for a typical European water body at steady state, which were compared with other such data. The results show that screening‐level assessments could be performed, and yielded estimates of emissions, PECs, and mixture toxic pressures of chemicals used in Europe. Steady‐state PECs agreed fairly well with measured concentrations. The mixture toxic pressure at steady state suggests the presence of effects in aquatic species assemblages, whereby few compounds dominate the predicted impact. The study shows that our screening‐level emission estimation procedure is sufficiently accurate and precise to serve as a basis for assessment of chemical pollution in aquatic ecosystems at the scale of river catchments. Given a recognized societal need to develop methods for realistic, cumulative exposures, the emission assessment procedure can assist in the prioritization of chemicals in safety policies (such as the European Union REACH regulation), where “possibility to be used safely” needs to be demonstrated, and environmental quality policies (such as the European Union Water Framework Directive), where “good environmental quality” needs to be reached. Environ Toxicol Chem 2020;39:1839–1851. © 2020 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Emission, Environmental release category, Exposure, Mixture toxic pressure, Chemical safety, Environmental quality
Environmental decision support requires attention for more than 350 000 chemicals and their mixtures. Any assessment would ask for assessment of Drivers and Pressures (emissions) and then Status and Impacts, to steer the societal Responses (according to the Drivers, Pressures, State, Impact, and Response [DPSIR] model, causal analysis).
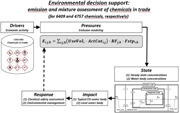
INTRODUCTION
Human activity inevitably results in possibly non‐negligible emissions of thousands of chemical substances. A recent inventory showed that more than 350 000 chemicals are currently registered globally (Wang et al. 2020), and numbers and amounts of chemicals in use are expected to increase (United Nations Environment Programme 2013; European Chemicals Agency 2016b; Bernhardt et al. 2017). This resulted in a call for comprehensive risk assessments, which would require quantification of emissions, exposures, and risks for large numbers of chemicals and their mixtures. Freshwater ecosystems are among the most human‐impacted habitats, and chemical pollution is one of the main drivers of deterioration of freshwater biodiversity (Vörösmarty et al. 2010). This has triggered the development of preventive and curative regulatory measures. In terms of prevention, chemical substances can be registered and marketed only after an ex ante chemical safety assessment has demonstrated the possibility that (the registered tonnage of) the chemical can be used safely. Under the European Union Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation (European Commission 2006), for example, exposure concentrations (predicted environmental concentration [PEC]) are compared with critical effect concentrations (predicted no‐effect concentration [PNEC]); the quotients (PEC/PNEC) are to remain below 1 for all possible uses of chemicals, commonly assessed individually.
In the present study, we expand on the ex ante assessment of net chemical pollution by considering the derivation of PECs for a large number of compounds from 1) amounts of chemicals used, and 2) fractions thereof released into the environment. This enables estimation of PECs by means of multimedia fate modeling and, subsequently, by a prospective assessment of the risk of net chemical pollution. The latter results are useful to evaluate whether the chemical safety policies are sufficient, or whether the environmental quality is affected by mixtures. For the aquatic compartment, the European Union Water Framework Directive is the regulatory framework that has been implemented to protect or restore water quality (European Commission 2000). We used the Water Framework Directive definition of good water quality status as benchmark to evaluate the PECs and the cumulation of risks of chemical pollution. Calls to account for mixtures for all policy frameworks have been voiced by scholars and regulatory bodies alike (European Commission 2017; Kortenkamp and Faust 2018; Drakvik et al. 2020).
The assessment of emissions is currently the least developed step in the comprehensive assessment of chemical pollution risks. The main driver for the research of the present study was to develop a practice‐oriented procedure by which the environmental release rates of large numbers of chemicals can be estimated, such that they can be used in chemical safety assessments and environmental quality assessments, for prioritization of risk‐preventing and/or curative management efforts. Prospective modeling of ecological impacts of chemical substances starts with estimating the amounts of chemicals that are released to the environment as a consequence of production and downstream use in the production–use–waste chain. Emitted masses, combined with characteristics of the compounds and the environment (e.g., the breakdown rates and the volume of environmental compartments, respectively), result in exposure concentrations, either in steady state (derived from multimedia fate modeling; Mackay 1991) or for local water bodies (derived from a dedicated model; Kapo et al. 2016). Combining these data with knowledge of the toxicities of individual chemicals subsequently allows us to quantify the impacts of chemicals, alone or in mixtures. The information gained helps us to prioritize water bodies regarding the expected pollution pressures as well as those chemicals contributing most to the pollution, and to focus environmental protection and management (Tsakiris 2015; Boelee et al. 2019).
We developed a new emission estimation procedure by which release rates (into air, water, and soil) of chemicals are estimated from registered use volumes, using additional information submitted by registrants in registration dossiers (i.e., information about the intended uses and about the physical and chemical properties of the chemical). Commonly, available data only provide gross insights in potentially emitted fractions of chemicals. In a case study, we succeeded in using those data for 6409 chemicals and we applied further exposure and impact assessment modeling for the 4757 chemicals with sufficient data for all analysis steps. We developed an estimation procedure capable of delivering the release rates necessary for comprehensive (and practicable) impact assessments of chemicals produced and used in Europe. Because predicted emissions themselves cannot be validated at the European scale, we tested the resulting release rates for plausibility and functionality by comparing predicted and observed concentrations and toxic pressure data obtained from other studies.
The principal research question addressed in the present study was whether emission rates of chemicals into the environment can be estimated with sufficient accuracy and precision for environmental risk assessments of chemicals and their mixtures and for prioritization of chemicals, as a basis for regulation and management. Our aims were to: 1) describe a generic emission estimation approach that was developed to estimate emission rates of chemicals; 2) apply and test this emission estimation approach in a case study that involves a large fraction of the chemicals used in the European Union (REACH chemicals, pesticides, and pharmaceuticals), by comparing the outcomes of the emission model with data from other studies (involving both modeled and monitored data); 3) analyze the outcomes of the case study regarding the issue of predicted impacts of chemical pollution (as total mixture); and 4) evaluate the results regarding potential utility for the prioritization of chemicals for management (chemical and environmental quality policies).
The results of the use of the outcomes of the present study for water body–specific chemical pollution assessments and the implications for water quality protection and management have been published elsewhere (Posthuma et al. 2019b; Van Gils et al. 2020).
MATERIALS AND METHODS
The analyses consisted of 3 main steps: 1) emission modeling, starting from data on masses of chemicals in trade in Europe; 2) exposure modeling, resulting in predicted mixture exposure concentrations of studied chemicals in a “typical European Union water body”; and 3) the assessment of the (mixture) toxic pressure of the predicted exposure concentrations.
Emission modeling
We developed a new substance‐flow estimation procedure (emission model) that enables accounting for the differences in release rates patterns covering all possible uses in the entire life cycle of chemicals. Release rates differ across compounds. That is, crop protection products used in open field applications are released almost entirely to crops and soils, “down‐the‐drain” household product chemicals find their ways to the environment via sewage collection and treatment systems, and other chemicals are made for use as parts of durable products, from which very variable amounts are released into the environment.
The new emission model is based on the generic emission estimation method developed in the 1980s by Van der Poel and Ros (1995). This method was used in the first European Union System for the Evaluation of Substances (EUSES) model for regulatory chemical substances evaluation (Vermeire et al. 1997). It was later reworked into the format of Specific Environmental Release Category (spERC) tables (European Chemical Industry Council 2012), which are currently used in chemical safety assessments under REACH.
The new emission model considers all life stages of a chemical and the generic characteristics of the stages, coded and defined as follows.
Life cycle stages I ‐ 1 and I ‐ 2 (production and transport)
Small, but significant amounts of chemical may be released to air, (waste)water, or soil directly from the first life cycle stage, during manufacturing or transport, although exceptions with specific point‐source releases may occur (e.g., Lindim et al. 2015, 2016a). Manufacturing may or may not take place in Europe. Fractions of the overall tonnage, manufactured in Europe, were obtained from various dossiers and are accounted for in the emission estimation.
Life cycle stage I ‐ 3 (processing)
Chemicals are rarely used without further processing. Generally, chemicals are formulated into products and distributed to other places in or outside Europe.
Life cycle stage II (use)
During service life, chemicals are used in different ways and released to different degrees. For most chemicals, the largest releases take place during service life.
Life cycle stage III (recycling)
Small but increasing fractions of chemicals produced and used are recycled; these data were combined with modeling chemical release from sewage treatment plants for chemicals passing through the plants, as appropriate. Total emission into the environment was thus modeled as the product of use volume, release fraction, and fraction not retained in the process of waste treatment, using literature‐based estimated emission fractions (Table 1):
| (1) |
where E i,j,k denotes the European Union‐wide emission rate [M/T] of substance i from use j into environmental medium k, represents the volume of substance i, used in activity category j [M], RF j,k is the fraction [−] of this use that is released into medium k, and Fstp i,k [T−1] denotes the fraction of substance i released to medium k after sewage treatment, as predicted by the sewage treatment plant model SimpleTreat, recommended in the REACH Guidance (European Chemicals Agency 2016a). Equation 1 was initially applied to 14 000+ chemical substances known to be currently used in Europe (REACH chemicals, pesticides, and pharmaceuticals), but due to missing data needed to derive PECs, the environmental–chemical analyses proceeded with 6409 compounds. Even for these relatively data‐rich compounds, neither total amounts used, nor fractions applied in specific uses are available to allow precise emission estimation according to Equation 1. Knowledge of amounts produced and used is generally confidential and unavailable for research. Fractions applied in specific uses are usually unknown, even to those who file registration dossiers. Therefore, the full emission model of Equation 1 could mainly be applied to chemical substances of which 1) the total amounts produced and used, and 2) the fractions of this use, applied in specific uses, are known or assumed, that is, for pharmaceuticals and pesticides. For the REACH substances it was necessary to categorize the 169 spERCs (Supplemental Data, Table SI‐1), claimed to be “best possible estimates” (European Chemical Industry Council 2012), into the 12 composite main uses listed in Table 1, and to assign release‐category averages to each chemical that, according to our expert judgement, belonged predominantly to this release category.
Table 1.
Estimated emission fractions (%) in the various uses and life cycle stages of chemicals, based on published and unpublished reports
| Activity category | Release % | To air % | To water % | To soil % | |
|---|---|---|---|---|---|
| Stage I | Use 1: Manufacturinga | 0.4 | 0.2 | 95 | 5 |
| Stage I | Use 2: Distribution/formulationa | 0.5 | 60 | 39 | 1 |
| Stage I | Use 3: Industrial processinga | 0.5 | 59 | 35 | 6 |
| Stage II | Use 4: Use in agricultureb | 100 | 15 | 1 | 84 |
| Stage II | Use 5: Use in medicinec | 12 | 0.0 | 100 | 0 |
| Stage II | Use 6: Wide‐dispersive use “down‐the‐drain”a | 100 | 0.0 | 100 | 0 |
| Stage II | Use 7: Other wide‐dispersive usesa | 100 | 73 | 11 | 16 |
| Stage II | Use 8: Wide‐dispersive “low‐release” usesa | 5 | 73 | 11 | 16 |
| Stage II | Use 9: Use as fueld | 0.04 | 96 | 4 | 0 |
| Stage II | Use 10: Other stage II usesa | 0.5 | 45 | 39 | 15 |
| Stage III | Use 11: Treatment/recyclinga | 5 | 33 | 33 | 33 |
| Stage III | Use 12: Solid waste disposala | 10 | 0.0 | 0.0 | 100 |
| Use‐weighted averages, based on selected SpERCs | 25 | 63 | 31 | 6 | |
European Chemical Industry Council 2012.
Fent et al. 2006; Van der Aa et al. 2008; International Commission for the Protection of the Rhine against Pollution 2009.
B. Dmytraz, Conservation of Clean Air and Water in Europe, Brussels, Belgium; confidential information from Chemical Safety Reports submitted to European Chemical Agency for the purpose of registrating petroleum products under the REACH regulation, personal communication to D. van de Meent 2008.
SpERC = Specific Environmental Release Category. Releases may not sum to 100% due to rounding error.
Exposure modeling
European Union risk assessment under REACH prescribes that registrants of new chemicals provide an explicit estimation of what environmental exposure concentrations are to be expected after the chemical is brought onto the European Union market. We have adopted this line of reasoning, including the use of terminology that comes with it, particularly of the use of the word “expected.” We use this word in the present study in its statistical meaning: the (statistical) expectation of the true, but unknown value that a random variable may take in a specific situation. In using the word “expected,” we further follow Aldenberg et al. (2002) who named the outcome of the Van Straalen–Aldenberg convolution integral of Equation 2 as “expected risk.” In European Union risk assessment, such “predictions” or “expectations” relate to standardized emission/exposure/effect circumstances (termed in the present study “typical European Union water body”), which is based on the ‘Unit World’ concept that was introduced in the late 1970s by Baughman and Lassiter (Mackay and Paterson 1984). Expected exposure concentrations (PECs) of chemicals in a typical European Union water body' (sensu REACH) were calculated from estimated emission rates using the multimedia mass balance model SimpleBoxTreat. Specifically, SimpleBoxTreat4Solutions was constructed as a simplified (spatially and temporally invariable) version of the spatially explicit model for European water bodies as created for the European Union project SOLUTIONS (Brack et al. 2015; Van Gils et al. 2019, 2020). It is a combination of the emission estimation model just described with the environmental fate simulation models SimpleBox Ver 4 (Hollander et al. 2016) and SimpleTreat Ver 4 (Struijs et al. 2016; Lautz et al. 2017), and a further simplification to a 3‐compartment (air/water/soil) version as applied in the KnowSEC decision support system developed at the German Federal Environment Agency (UBA; Striffler and Wassermann 2015). We used integrated emission + exposure modeling to test the usefulness and plausibility of the outcomes of the emission model.
Using emission estimates (Equation 1), we used SimpleBoxTreat4Solutions to simulate emissions of chemicals at so‐called local and regional spatial scales, and calculated expected steady‐state concentrations of chemicals in a “generic receiving environment.” This offered the opportunity to validate the emission + exposure model results against concentrations reported in other studies, because emission estimates themselves cannot be validated directly. Subsequently, by combination with expected critical effect concentrations, expected toxic pressures (defined in the following section, Toxic pressure modeling) were derived, which offered us the opportunity to validate predicted (mixture) toxic pressures against such data from other studies.
Toxic pressure modeling
We adopted the method of toxic pressure calculation as described by Van Straalen (2002) and Aldenberg et al. (2002), who quantified “toxic pressure” as the probability that ambient exposure concentrations would exceed concentration levels that are considered to be “riskful” (Solomon and Takacs 2002). In the present study, we derived toxic pressure as the probability that expected concentrations of one or more chemicals in typical European Union water would be greater than a selected critical effect concentration. We chose to use the hazardous concentration for 50% of the tested species (HC50), based on median effect concentration (EC50) data for tested subsets of species (i.e., HC50‐EC50). This toxic pressure parameter empirically relates to impacts on ecological status in aquatic ecosystems (Posthuma et al. 2019a).
Following Van Straalen (2002) and Aldenberg et al. (2002), we derived toxic pressure from the convolution integral of 2 distributions, that is, the probability density function of exposure concentrations (from the exposure modeling) and the cumulative probability function of critical effect concentrations (from Posthuma et al. 2019b), known as the Van Straalen–Aldenberg convolution integral:
| (2) |
with:
| (3) |
and
| (4) |
where and represent the probability density function of toxicologically standardized exposure concentrations (in water) and the cumulative distribution function of toxicologically standardized acute EC50 values, respectively (Figure 1).
Figure 1.
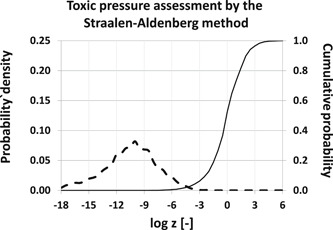
Graphical illustration of the Van Straalen–Aldenberg convolution integral (Equation 2). Probability of exceedance of critical effect concentrations in water is obtained by evaluating the product of the probability density function of exposure concentrations (dotted line) and the cumulative distribution function of critical effect concentrations (black line) at all possible values of the standardized concentration z.
Values of sd(logEC50), needed in Equation 4, are often not known with great precision for many chemicals, because of insufficient numbers of experimental toxicity data (Posthuma et al. 2019b). Following the practice of Posthuma et al. (2019b), we assigned the value 0.7 to across‐species standard deviations of all individual chemicals, for the purpose of toxic pressure calculation.
Case study
The complete model (covering emissions, fate, and mixture impacts) could be completed for 4757 chemical substances, representative of the substances currently used in the European Union as described in Van Gils et al. (2019).
Data on the amounts of substances used were collected from various sources, as follows.
REACH substances
Amounts produced and marketed to regulatory authorities are part of the obligatory submission of registration information. The information is confidential, to be used only for registration purposes (e.g., European Chemicals Agency 2019). We obtained so‐called European Union tonnages (defined as ) from registration dossier data for use as research data from the registration dossiers (submitted until April 2015).
Active ingredients of medicines
Amounts of more than 1000 individual pharmaceuticals sold in Sweden and the United Kingdom were obtained from public sources (combined data from Boxall et al. 2012 and M. Rahmberg, IVL, Stockholm, Sweden, personal communication to D. van de Meent 2015).
Additional sales data for a lesser numbers of pharmaceuticals, sold in Austria, Switzerland, Germany, France, and the Netherlands, were obtained from various other public reports (Fent et al. 2006; Van der Aa et al. 2008; International Commission for the Protection of the Rhine against Pollution 2009). No more sufficiently detailed or more recent use data were publicly available for our study.
Active ingredients of crop protection products
Amounts of active ingredients of crop protection products are monitored by individual European Union member states and are reported to the European Statistics Agency EUROSTAT. This agency combines the confidential original data into main categories of active ingredients. Original sales data of pesticides could not be made available for the present study. Instead, estimations made by the Joint Research Center of the European Commission by means of the so‐called harvested area approach (Sala et al. 2014, 2015) were used. Use volumes of over 400 active ingredients of crop protection products, used in different European Union countries, were thus obtained.
Releases to air, water, and soil were estimated according to the procedure described previously (in the Toxic pressure modeling section, Equation 1). Expected exposure concentrations in European Union air, water, and soil were modeled with the exposure model mentioned previously in the Toxic pressure modeling section. Expected toxic pressures, for each of the substances and of all substances together, were calculated with the Van Straalen and Aldenberg's method, as described previously (in the Toxic pressure modeling section, Equations (2), (4)). Specific steps for the 3 compound groups were needed, as follows:
REACH substances
Fractions released to air, water, and soil in the different life cycle stages, aggregated across the 12 composite main uses of Table 1, were calculated by means of Equation 1, starting with the 12 861 compounds registered until April 2015. Unfortunately, fractions of chemicals used in the 169 spERC uses are not listed in REACH registration dossiers, and cannot be used to estimate emission rates with Equation 1. For the release assessment, use‐volume weighted average release fractions were therefore assumed for all REACH substances in all activity categories.
Pharmaceuticals
Pharmaceuticals are assumed to be used entirely according to stage II, use 5 (use in medicine). Based on a publication by Lindim et al. (2016b), who studied releases of 56 pharmaceuticals in Sweden, we have assigned a release fraction of 12% of the human consumption rate, which is assumed to be released entirely to sewage treatment plants.
Crop protection products
Pesticides are assumed to be used entirely according to stage II, use 4 (use in agriculture). Based on the studies of Sala et al. (2014, 2015), who applied the so‐called harvested area approach to estimate releases to air, water, plants, and soil, we assigned the emission fractions listed in Table 2: 100% release to the environment (of which 15% to air, 1% to water, and 84% to soil), without wastewater treatment.
Table 2.
Numbers and volumes of chemical substances used in Europe, and their expected concentrations and per‐chemical and mixture toxic pressures in a “typical European Union water body” at steady state
| Unit | REACH | Pharma | Pesticide | All | |
|---|---|---|---|---|---|
| No. of substances | — | 3940 | 397 | 420 | 4757 |
| European Union‐wide use volume | Ton yr−1 | 2.7E+08 | 2.5E+04 | 1.8E+05 | 2.7E+08 |
| Emission to environment | Ton yr−1 | 5.4E+07 | 3.6E+03 | 1.8E+05 | 5.4E+07 |
| Median concentration in European Union water | g/L−1 | 1.0E‐09 | 5.0E‐12 | 4.0E‐09 | 1.0E‐9 |
| Average acute EC50 | g/L−1 | 1.6E‐02 | 7.3E‐03 | 1.7E‐03 | 1.5E‐02 |
| Median toxic pressure (=Pr{Cw > EC50}) | — | 7E‐25 | 2E‐38 | 9E‐16 | 7E‐25 |
| Mixture pressure (=Pr{Cw > EC50}) | — | 3.6% | 0.0% | 0.1% | 3.7% |
| Fraction TP(EC50) < 1.0E‐6 | — | 99.2% | 100.0% | 96.7% | 99.1% |
| Pareto ratio | — | 99.6%/0.4% | 99.8%/0.2% | 98%/2% | 99%/1% |
Cw = predicted exposure concentration in water at steady state; EC = effect concentration (here median of the EC50s of the tested species); TP = toxic pressure (multisubstance potentially affected fraction [msPAF]‐EC50); REACH = Registration, Evaluation, Authorisation and Restriction of Chemicals.
In the present case study, (expected) steady‐state concentrations were modeled for all chemicals (REACH substances, pharmaceuticals, and pesticides). In the real‐world situation, steady states are not expected for all chemicals (i.e., particularly not for agriculturally used pesticides). We interpret steady‐state solutions of the multimedia model as predictors of the usually uncertain (or unknown, even) space‐ and time‐average value of all possible concentrations in the hypothetical European Union water body. Finally, the case study results were compared with data sets for aquatic exposure concentrations and for toxic pressure, to evaluate whether the emission model (which itself can hardly be validated) could provide useful assessment information.
RESULTS
Overview
The numbers of chemicals in various subgroups, the amounts of chemicals used and their estimated emission rates, the expected exposure concentrations, and the expected toxic pressures are summarized in Table 2 (for further details, see Supplemental Data, Table SI‐2).
European Union‐wide emission rates to (continental) air, water, and soil were estimated for 4757 compounds with complete data (3940 REACH chemicals, 397 pharmaceuticals, and 420 pesticides with data for emission, concentration, and impact modeling). European Union‐wide use volumes and expected emission masses, exposure concentrations in water, and expected per‐chemical toxic pressures on aquatic life in the typical European Union water body all varied by several orders of magnitude. The predicted mixture toxic pressure, expressed as the multisubstance potentially affected fraction (msPAF)‐EC50 was estimated as 3.7% in the typical European Union water body, with a dominant contribution of the REACH chemicals (which also represented by far the largest relative mass). The relative contributions of the different chemicals to the mixture toxic pressure at the EC50 level were calculated, and expressed as Pareto ratios for the total mixture and for the mixtures for each studied compound group. Clearly, for each group of chemicals (REACH substances, pharmaceuticals, and pesticides), a relatively small fraction of the chemicals was responsible for a large fraction of the toxic pressure. Similar observations have been made in other fields of science (Muller 2016) and have been termed the 80–20 rule, or (more correctly), the Pareto principle (Wikipedia 2020).
Amounts used and rates of emission
For reasons of confidentiality, European Union tonnages of individual chemical substances cannot be shared for REACH chemicals. Information on amounts of chemicals in trade is shown in Figure 2A as a histogram. Emission rates were estimated for all chemical substances represented in Figure 2B and are listed in Supplemental Data, Table SI‐2.
Figure 2.
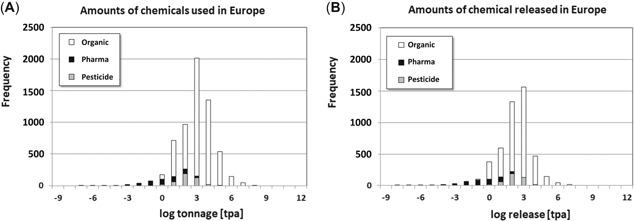
Registered amounts of chemical substances used in Europe (A) and estimated amounts of chemicals emitted to the environment (B). Black = pharmaceuticals; gray = pesticides; white = monoconstituent organics. Use volumes and releases range over 12 orders of magnitude: from as little as kg/yr (pharmaceuticals) to over a million tons/yr (monoconstituent organics).
REACH substances
According to the REACH registrations per April 2015, a total mass of 3.14 × 109 tons of chemical substances is marketed annually in the European Union. European Union tonnages were obtained for 5592 different chemical substances (representing 2.2 × 109 tons, or 70% of the European Union total mass). Of these, 3940 substances (2.7 × 108 tons or 8.5% of European Union total mass) were relatively simple monoconstituent organic substances (in REACH terminology), of which expected emissions and steady‐state concentrations in a typical European Union water body could be predicted by means of the combined emission and exposure model.
Pharmaceuticals
Active ingredients of medicines are produced and used in much lower numbers and masses compared with REACH substances. Use volumes of 397 different pharmaceuticals, representing 2.5 × 105 tons (0.01% of European Union total mass) were available, and used in the combined emission and exposure model.
Pesticides
The same (lower numbers and masses compared with REACH) also holds for active ingredients of crop protection products. Use volumes for 420 chemicals, representing 1.8 × 105 tons (0.006% of European Union total mass) were available, and used in the combined emission and exposure model.
Predicted emissions encompass narrower tonnage ranges than use volumes, as shown by comparing Figure 2A and B. Estimated emission rates span a range of roughly 10 orders of magnitude: from less than 1 kg/yr to over 1 million tons/yr.
Exposure concentrations
Expected steady‐state concentrations for the typical European Union water body are shown in Figure 3A, together with the information on species sensitivities (at the level of acute EC50s) as obtained from Posthuma et al. (2019b) and shown in Figure 3B. Expected steady‐state concentrations in the European Union water for the studied 4757 compounds span roughly the same range as emission masses. Apparently, variance in “fate” (as modeled by multimedia environmental fate modeling) contributes little to variance in exposure, compared with the contributions of variances in tonnages and emissions. Expected exposure concentrations range from femtograms/liter to milligrams/liter (Figure 3A). This is in agreement with earlier observations of Zijp et al. (2014), who predicted similar concentration distributions in river Rhine water, and found these to match available concentration measurements. It is also in agreement with recent findings from the SOLUTIONS project (Van Gils et al. 2019, 2020), which reported similar concentration distributions for currently used organic chemicals in various other river catchments. Note the small overlap between the distribution of exposure concentrations in Figure 3A and the distribution of critical effect concentrations in Figure 3B, which provides a visual indication of the probability of occurrence of harmful concentrations of chemicals.
Figure 3.
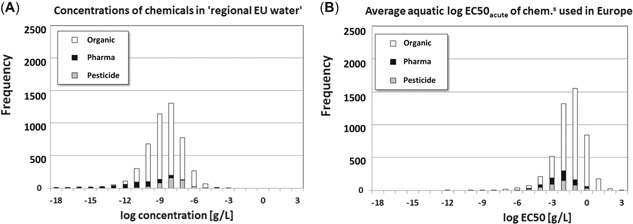
Expected steady‐state concentrations of chemical substances in a “typical European Union water body,” as defined in European Chemicals Agency (2016; A, present study), compared with the average aquatic log median effect concentration (EC50; acute) for the studied chemicals (B, from Posthuma et al. 2019b). Note the small overlap between the 2 types of distributions, as in Figure 1. Black = pharmaceuticals; gray = pesticides; white = monoconstituent organics.
Toxic pressures
Expected probabilities that critical effect concentrations of chemicals would be exceeded by concentrations of chemicals in a typical European Union water body appear to be below 5%, in the present study relating to exceedance of the median EC50 of tested species (Table 2 and Figure 4).
Figure 4.
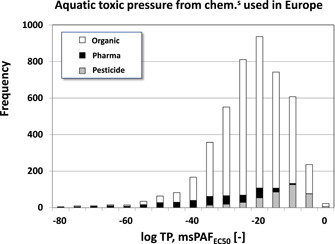
Distribution of expected toxic pressures (TPs) of chemicals used in Europe, calculated from the overlap of the exposure and (median effect concentration [EC50]) effect distributions established for each chemical according to Figure 3. Black = pharmaceuticals; gray = pesticides; white = monoconstituent organics. msPAF = multisubstance potentially affected fraction.
Earlier referred to as “ecological risk” (Van Straalen 2002) or “expected risk” (Aldenberg et al. 2002), we refer to this outcome as “(mixture) toxic pressure,” as suggested by Traas et al. (2002). Toxic pressure is a metric with which compounds or water bodies can be ranked regarding toxicity. The mixture toxic pressure of the typical European water body at steady state in an exposure assessment that considers approximately 8.5% of the mass of chemicals used in Europe was calculated as 3.7%, that is, the probability that steady‐state exposures would exceed the acute EC50 of a median‐sensitive species is 3.7%. Estimated toxic pressures at steady state are, logically, lower for separate chemicals, and higher if all chemicals would have been modeled. Most chemicals have exposure concentrations in water that result in negligible “expected risk” at the EC50 level. Toxic pressures at the EC50 level of over 99% of the chemicals tested were below the value of 10−6 (Table 2): it is less than 0.0001% probable that concentrations greater than the EC50 of a median‐sensitive species will be found, which is the same as saying that it is 0.0001% probable to encounter a species for which the EC50 would be exceeded in the typical European Union water body. The outcome of the calculations is that higher toxic pressures at the EC50 level and steady state are to be expected for less than 1% of the chemicals that are currently used in the European Union.
It is worth noting that, out of the 4757 chemicals considered, only 23 individual chemicals (17 REACH chemicals and 6 pesticides) contributed substantially to the 3.7% EC50‐based mixture toxic pressure found in the present case study. Table 2 lists the associated Pareto ratios found in our study for REACH chemicals (99.6/0.4), pharmaceuticals (99.8/0.2), and pesticides (98/2).
DISCUSSION
Overview
The present study is the first to provide an approach to and an analysis of the emissions of chemical pollutants at the European scale, evaluated from calculated steady‐state concentrations in a typical European water body for chemicals currently in trade. The results are based on a combination of techniques that are all well founded in the scientific literature and used in risk assessment practices (spERC data multimedia, effect, and mixture models). The steps performed resemble those of regulatory prospective chemical safety assessment (here, e.g., REACH), and expand on that by attempting to consider chemical pollution as a whole, as most recently emphasized by Wang et al. (2020). The steps can also be expanded with emission locator mapping and hydrological modeling to predict spatiotemporally explicit compound concentrations and (mixture) toxic pressures, like higher tier assessments reported elsewhere (Posthuma et al. 2019b; Van Gils et al. 2019). Outcomes can help in assessing whether “safe use of chemicals” (as meant by the European Union REACH regulation, for example) can be achieved and whether the environment goal of “good ecological status” (as meant by the European Union Water Framework Directive) can be maintained or reached. We specifically aimed at practical utility for both types of assessments. We recognize that each model step, and all required data, can be refined for any individual chemical and region, as has been done by others for specific compounds or compound groups (Lindim et al. 2016a, 2016b; Oldenkamp et al. 2019). The aim of achieving insights into the chemical pollution problem as a whole called for some simplifying steps, and the use of a relatively simple approach to emission estimation. Nonetheless, the results allow for some key observations and some conclusions on various aspects of our study, as well as some uncertainties that need further improvements.
Comparing outcomes with regulatory goals
Our case study yielded results of the kind needed for comprehensive assessment of chemical pollution for a region, based on a relevant fraction of chemicals used in a region. Such results allow one to assess the mixture toxic pressure in a typical European water body, and to rank the chemicals contributing most to such pressure. The results (Table 2) indicate that the current use of industrial chemicals, plant protection products, biocides, and pharmaceuticals leads to exposure concentrations that could cause significant effects on aquatic ecosystems, because the probability of exceeding the median EC50 of tested species was 3.7% (msPAF‐EC50 = 3.7%, for all studied chemicals). This is far higher than the toxic pressure threshold value utilized in chemical safety assessment/chemical, which is defined as msPAF‐no‐observed‐effect concentration = 5% and is used as a science‐based threshold to define the PNEC. Studies by Malaj et al. (2014) and Posthuma et al. (2019b) confirm that water body‐ or area‐specific exposures of single chemicals and mixtures indeed suggest that a nontoxic environment (European Commission 2014; Munthe et al. 2019) or toxic‐free environment (European Commission 2019) seems far away. Thus, the comprehensive assessment of emissions, fate, and mixture impacts serves to provide the kinds of outputs needed to provide a basis for prospective decision support and prioritization of actions to forward the goal of the zero‐pollution policy ambition. Such an assessment is a potentially valuable addition to current practices, which often neglect mixtures (Geiser 2015) even while mixtures form the common real‐life exposure situation in the field (Brack et al. 2019). With the outcomes in kind being potentially useful, there is still a need to consider issues such as precision and accuracy, dependent on the context of use of the outcomes.
Accuracy, precision, and uncertainty in toxic pressure calculation
Exposure metrics are often used in the practice of chemical safety assessments, in the format of PECs being compared with PNECs, as the PEC/PNEC ratio (European Commission 2006) of individual chemicals. The accuracy of predicted exposures is thus key for decisions to allow chemicals on the market. We acknowledge that emissions cannot be estimated with great certainty, by virtue of basing the modeling on available principles and data, because key information on the uses of chemicals is unavailable especially in REACH registration dossiers. As a result, the precision of the emission estimation is necessarily poor. However, estimated emission rates may be accurate, in the sense that the emission model yields outcomes that are right on average. First, an indirect judgment of emission model accuracy can be made by comparing predicted with measured environmental concentrations. Such an assessment has been made using a much more detailed hydrologic model by Van Gils et al. (2019), who showed that PECs—obtained with integrated emission + exposure modeling—were on average similar to measured environmental concentrations (MECs) for 226 substance/basin combinations for which both PECs and MECs were available, whereas for 65 and 90% of cases, the under‐ or overprediction of PECs for individual substances was within 1 and 2 orders of magnitude, respectively.
It should be noted that the MECs themselves may not reflect true (i.e., accurate) exposure due to temporal variability. That is, the MEC for a chemical in a water body can vary widely when determined before or after pesticide spraying or before or after a rain event causing city runoff. The accuracy of the PEC estimation of the present study is further similar to independently obtained results for pharmaceuticals (Oldenkamp et al. 2018). Earlier studies (Zijp et al. 2014), and preliminary assessments with Monte Carlo simulations (data not shown) suggested that the PECs for individual chemicals may range over nearly 1 order of magnitude as a result of uncertain emission estimations. Given the many orders of magnitude difference in exposures across chemicals (Figure 3), these outcomes show that, although the emission model outcomes are of low precision, they are accurate enough to serve as a starting point for toxic pressure calculation.
Second, it is important to note that uncertain emission estimation does result in the shown uncertainty in exposure assessment, but not in uncertain (mixture) toxic pressures. When calculated from overlap of distributions of exposure concentrations and critical effect concentrations, as in Equation 2, decreased precision (i.e., increased uncertainty) of emission estimation and hence, of expected exposure concentrations, results in increased toxic pressure, rather than in uncertain toxic pressure. This can be seen from Figure 1: greater uncertainty in emissions leads to wider concentration distributions, with enhanced probability of having greater‐than‐average exposure concentrations. Use of this method of calculation (Equation 2) directly translates uncertainty in emissions into enhanced probability of exceeding the EC50, and thus in greater (mixture) toxic pressure. Although neither Van Straalen (2002) nor Aldenberg et al. (2002) have mentioned this factor in their studies, the original procedure of toxic pressure calculation automatically accounts for uncertainty in exposure estimation.
Third, the core aim of environmental protection and management—a nontoxic or toxic‐free environment (European Commission 2014, 2019)—is the absence of or negligible net impacts (in the present study, in mixtures). Comparison of mixture toxic pressures derived from PECs or MECs from other studies provides another way to indirectly judge the emission modeling approach. This should be done by acknowledging the Pareto ratios found in the present study and when based on MECs (e.g., Backhaus and Karlsson 2014; Vallotton and Price 2016). That is, considering greater numbers of chemicals does not always result in finding greater (mixture) toxic pressures. When the Pareto principle is applied, and when the 20% most important chemicals have been looked at already, not much additional toxic pressure knowledge is to be expected from considering the remaining 80%.
Fourth, the outcomes of the present study were compared with other toxic pressure assessments (Table 3; expressed as msPAF‐EC50; as fraction, varying between 0 and 1). The present study yielded a mixture toxic pressure for 4757 compounds of 3.7% for a typical European Union water body (based on msPAF‐EC50). That compares well with the mixture toxic pressures found for Europe and specific European basins, which are oin the same order of magnitude, or lower when considering the 95th percentile estimate of daily predicted values over 1 yr (i.e., mixture toxic pressures in a basin are higher than the 50th percentile of msPAF‐EC50 over sites for 18 of 365 d). Note that accounting for spatiotemporal variation also suggests the presence of lower and higher mixture toxic pressures (Table 3, columns with 5th and 95th percentile of msPAF‐EC50 over sites). The outcomes are also in line with a large data set of measured environmental concentrations for the Netherlands, despite the large variability in number of compounds measured/sample (between 1 and 263). In summary, the comparison indicates that the emission model is accurate in helping to obtain the necessary insights into the expected level of the ambient mixture toxic pressure and into compounds likely contributing the least and the most.
Table 3.
Comparison between mixture toxic pressure for the “typical European Union water body” (present study) and mixture toxic pressures (msPAF‐EC50) reported for large numbers of water bodies in major catchments in Europe (PEC‐based) and the same for a set of monitoring data for Dutch surface waters (MEC‐based)
| Compounds (no.) | Sites (no.) | msPAF‐EC50 (p values over sites) | |||||
|---|---|---|---|---|---|---|---|
| Surface water (system) | Exposure | P5 | P50 | P95 | Reference | ||
| Typical European Union water body | PEC, steady state | 4757 | 1 | 3,7E‐02 | Present study | ||
| Europea | PEC‐local (P95yr) | 1806 | 22 728 | 3,0E‐11 | 2,0E‐03 | 5,3E‐02 | Van Gils et al. 2019; Posthuma et al. 2019b |
| Danubea | PEC‐local (P95yr) | 1806 | 3477 | 1,0E‐07 | 5,0E‐03 | 6,1E‐02 | Van Gils et al. 2019; Posthuma et al. 2019b |
| Rhinea | PEC‐local (P95yr) | 1806 | 813 | 6,0E‐05 | 1,4E‐02 | 1,7E‐01 | Van Gils et al. 2019; Posthuma et al. 2019b |
| Spanish basinsa | PEC‐local (P95yr) | 1806 | 696 | 5,0E‐06 | 2,0E‐03 | 3,2E‐02 | Van Gils et al. 2019; Posthuma et al. 2019b |
| The Netherlands | MEC | 1–263 | 5939 | 1,0E‐03 | 1,3E‐02 | 9,4E‐02 | Posthuma et al. 2016 |
Mixture exposures judged on the basis of the local‐P95 (95th percentile) of PECs for chemicals as predicted for all days of a year for each site (exposure is higher during ~18 d of a year), based on Van Gils et al. 2019.
PEC = predicted environmental concentration; MEC = measured environmental concentration; msPAF = multisubstance potentially affected fraction; P5 = 5th percentile; P50 = 50th percentile; P95 = 95th percentile.
Given the findings summarized in Table 3, it may be concluded that there is indirect evidence that the emission estimation procedure helps to provide insights into the average values of possible real‐life emissions and associated effects. That is, despite large uncertainties in the model and the input data, it appears that the accuracy of the emission prediction can yet be considered sufficient for screening‐level calculation of mixture toxic pressures of ambient mixtures of chemicals.
Prioritization of chemicals for management
The key problems of chemical safety assessment policies have always been 1) how to judge “the universe of chemicals” (Wang et al. 2020), and 2) how to judge sufficiently safe use of all chemicals. The former problem was addressed by introducing prioritization principles, where the potentially worst chemicals are identified, grouped, and tested for hazardous properties first. The present approach addresses both questions, and this resulted in a corroboration of the finding of a suite of other studies in which it was often found that mixture impacts can commonly be attributed to a relatively low number of chemicals (see, e.g., Backhaus and Karlsson 2014 for pharmaceuticals in European coastal waters, Vallotton and Price 2016 for pesticides in the United States, and Posthuma et al. 2016 for a suite for chemicals in Dutch surface waters). Our case study also reconfirms one of the earliest findings of this kind (Harbers et al. 2006), that generally only small fractions of the chemicals produced, used, and emitted are responsible for the larger part of the direct ecological impacts for a particular situation. The results provide both a signal of insufficient protection (see preceding discussion) as well as a very “sharp” prioritization of chemicals regarding potential effects that occur via direct exposure to species assemblages. As discussed also by Posthuma et al. (2019b), the prioritization should as yet not be interpreted as absolute ranking of the 4757 compounds. Instead, as derived from preliminary Monte Carlo simulations (data not shown), the absolute rank order of chemicals may vary in this respect, but chemicals with toxic pressures greater than 10−6 appear to remain above this negligibility threshold in all ranking simulations under uncertainty; chemicals with toxic pressures less than 10−6 appear to be of low priority, regardless of uncertainty in emission estimations.
The outcome of our study clearly illustrates the Pareto principle, namely, that few causes often explain a large part of the result. In toxic pressure calculation, this goes well beyond the classical Pareto 80–20 rule. We observed Pareto ratios (Table 2) that were systematically larger: for pesticides a 98–2 rule was found, whereas for the “ordinary” (monoconstituent) REACH chemicals and the pharmaceuticals, even greater ratios were found. This pattern should be conceptually expected from the probability calculation that underlies the toxic pressure derivation by the Van Straalen–Aldenberg integral (overlaps of tails of distributions). This statistical reason has not been recognized so far. However, many studies report such findings for a wide array of contexts (Newman 2005; Muller 2016; West 2017), and—in hindsight—it would have been surprising to find nonskewed outcomes. The most unlikely outcome would be that mixtures in the field would be equitoxic, that is: composed such that all chemicals contribute equally to the mixture effect everywhere. For the present results and accuracy level, we suggest using the outcomes in practice by considering 3 classes: even despite some uncertainties, the method can help us to identify a class of chemicals unlikely to pose harm in the European Union water body, a class for which this is possible (and depending on circumstances), and a class that likely poses harm. Such assessments have also been made, based on different degrees of model focus (e.g., emissions, hazard) and approaches, and for other endpoints (Ahrens et al. 2017; Oltmanns et al. 2018; Schulze et al. 2018; Posthuma et al. 2019b), given that prioritization is key to managing more than 350 000 chemicals (Wang et al. 2020).
Suitability for regulatory decision‐making
We found that the kinds of outputs of the emission model (after combination with exposure and effect models) are suitable for 2 regulatory contexts, namely, chemical safety assessment and environmental quality assessment and management. We conclude from the present study that the emission estimation procedure is sufficiently accurate and precise for use in screening‐level chemical safety assessments of substances and their mixtures (as required in REACH and Life Cycle Impact Assessment) and for screening‐level assessment of mixture toxic pressures at local spatial scales (as needed for assessment of “good ecological status” under the European Union Water Framework Directive). Refined approaches may be developed for chemical safety assessment of the assemblage of substances produced and used, or they have already been developed for environmental quality assessments (Water Framework Directive; Van Gils et al. 2020), such that more precise information can be generated for situations where the screening‐level assessment suggest non‐negligible exposures, risks, or impacts. The main finding of the present study is that it has demonstrated how uncertain emission estimates for a large number of chemicals can be used to assess and understand expected impacts of expected exposure concentrations of currently used chemicals, with emphasis on the novel insights in the consequences of mixtures. We emphasize that toxic pressure calculation owes this to consequent application of the Van Straalen–Aldenberg convolution integral, and that the final interpretation of our results can be understood from 2 considerations: 1) Valerie Forbes' statement (Forbes 2010) that “although we may be right on average, we may be wrong most of the time”; and 2) George E.P. Box's teaching (Box 1979) that “all models are wrong, but some are useful.”
CONCLUSIONS
We developed a new model to estimate emission rates of—in principle—all chemicals in commerce. We found that application of the model to the chemicals typically used in Europe resulted in sufficient precision and accuracy to derive screening‐level statements on expected impacts of the use of these chemicals on aquatic ecosystems and on ranking the relative contributions of individual chemicals to the net expected impacts. Our study demonstrates how, for the purpose of environmental risk assessment and management of (mixtures of) chemicals, one can afford to be “wrong” (not precise) most of the time, provided that one is “right” (accurate) on average. The outcomes are useful for screening‐level chemical safety assessment (REACH) and water quality assessment (Water Framework Directive) purposes at the European scale. The results provide information to help in prioritizing chemicals for chemical safety policies, and for water bodies and chemicals within water bodies for water quality management.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at https://doi.org/10.1002/etc.4801.
Disclaimer
The present study was performed under the strict confidentiality agreement on production and import/export masses between the Dutch competent authority for the European Union Registration, Evaluation, Authorisation and Restriction of Chemicals regulation and the Dutch National Institute for Public Health and the Environment (RIVM; https://echa.europa.eu/legal-notice). This agreement states, among other factors, that “[. .] the replication, in whole or in substantial part, of the ECHA databases is prohibited, unless ECHA's prior written permission is given. All requests shall be submitted to ECHA through the information request forms on ECHA's contact page.”
Author Contributions Statement
D. van de Meent, D. de Zwart, and L. Posthuma designed the study, D. van de Meent and D. de Zwart ran the data collection and analyses, D. van de Meent wrote the article, D. de Zwart, L. Posthuma checked the article, and all co‐authors finalized the manuscript.
Supporting information
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Acknowledgment
The present study was supported by, and prepared as result of, the SOLUTIONS project (European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement 603437). Contributions of the SOLUTIONS research team, and especially the SOLUTIONS‐modelers team, have been indispensable to reach the present results. We thank K. Kramer for critical reading and useful comments. Special thanks are due to J. van Gils, who has been leading the integrated SOLUTIONS modeling, and who ran the spatiotemporal‐specific model analyses. The data source (Registration, Evaluation, Authorisation and Restriction of Chemicals [REACH]‐chemicals: European Chemicals Agency) can be found at http://echa.europa.eu/. Further data providers are gratefully acknowledged. The comments of 2 SOLUTIONS reviewers on an earlier version of this manuscript (I. Cousins and M. Rahmberg) are gratefully acknowledged.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (Leo.Posthuma@rivm.nl).
REFRENCES
- Ahrens A, Moilanen M, Martin S, Garcia‐John E, Sättler D, Bakker J, Reihlen A, Wind T, Tolls J. 2017. European Union regulators and industry agree on improving specific environmental release categories: Report from the Exchange Network for Exposure Scenarios Specific Environmental Release Category Workshop, May 13, 2016. Integr Environ Assess Manag 13:815–820. [DOI] [PubMed] [Google Scholar]
- Aldenberg T, Jaworska JS, Traas TP. 2002. Normal species sensitivity distributions in probabilistic ecological risk assessment In Posthuma L, Suter GW, Traas TP, eds, Species Sensitivity Distributions in Ecotoxicology. Lewis, Boca Raton, FL, USA, pp 49–102. [Google Scholar]
- Backhaus T, Karlsson M. 2014. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res 49:157–165. [DOI] [PubMed] [Google Scholar]
- Bernhardt ES, Rosi EJ, Gessner MO. 2017. Synthetic chemicals as agents of global change. Front Ecol Environ 15:84–90. [Google Scholar]
- Boelee E, Geerling G, Van der Zaan B, Blauw A, Vethaak AD. 2019. Water and health: From environmental pressures to integrated responses. Acta Trop 193:217–226. [DOI] [PubMed] [Google Scholar]
- Box GEP. 1979. Robustness in the strategy of scientific model building In Launer RL, Wilkinson GN, eds, Robustness in Statistics. Academic, Cambridge, MA, USA, pp 201–236. [Google Scholar]
- Boxall ABA, Rudd MA, Brooks BW. 2012. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ Health Perspect 120:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack W, Altenburger R, Schüürmann G, Krauss M, López Herráez D, van Gils J, Slobodnik J, Munthe J, Gawlik BM, van Wezel A, Schricks M, Hollender J, Tollefsen KE, Mekeyan O, Dimitrov S, Bunke D, Cousins I, Posthuma L, de Aragao Umbezeiro G. 2015. The SOLUTIONS project: Challenges and responses for present and future emerging pollutants in land and water resources management. Sci Total Environ 503–504:22–31. [DOI] [PubMed] [Google Scholar]
- Brack W, Hollender J, de Alda ML, Müller C, Schulze T, Schymanski E, Slobodnik J, Krauss M. 2019. High‐resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environ Sci Eur 31:62. [Google Scholar]
- Drakvik E, Altenburger R, Aoki Y, Backhaus T, Bahadori T, Barouki R, Brack W, Cronin MTD, Demeneix B, Bennekou SH, van Klaveren J, Kneuer C, Kollosa‐Gehring M, Lebret E, Posthuma L, Reiber L, Rider C, Ruegg J, Bergman A. 2020. Statement paper on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ Int 134:105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Chemical Industry Council . 2012. Specific environmental release categories (SpERCs). Chemical safety assessments, supply chain communication and downstream user compliance. CEFIC Internal Document of the SpERC Core Team. Brussels, Belgium.
- European Chemicals Agency . 2016a. Environmental exposure assessment In Guidance on Information Requirements and Chemical Safety Assessment. Ver 3.0. Report ECHA‐16‐G‐03‐EN. Helsinki, Finland. [Google Scholar]
- European Chemicals Agency . 2016b. Information on chemicals. Helsinki, Finland. [cited 2018 March 6]. Available from: http://echa.europa.eu/information-on-chemicals
- European Chemicals Agency . 2019. Legal notice. Helsinki, Finland. Available from: https://echa.europa.eu/legal-notice
- European Commission . 2000. Directive 2000/60 of the European Parliament and of the Council of 23 October 2000, establishing a framework for community action in the field of water policy. Official J Eur Commun 43:L327. [Google Scholar]
- European Commission . 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Brussels, Belgium.
- European Commission . 2014. Living well, within the limits of our planet. General Union Environment Action Programme to 2020. Brussels, Belgium.
- European Commission . 2017. Study for the strategy for a non‐toxic environment of the 7th Environment Action Programme. Final Report. Brussels, Belgium.
- European Commission . 2019. Communication from the Commission to the European Parliament, the European Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. COM (2019) 640 final. Brussels, Belgium.
- Fent K, Weston AA, Caminada D. 2006. Ecotoxicology of human pharmaceuticals—Review. Aquat Toxicol 76:122–159. [DOI] [PubMed] [Google Scholar]
- Forbes VA. 2010. Platform Presentation. SETAC North America, Long Beach, CA, USA. [Google Scholar]
- Geiser K. 2015. Chemicals Without Harm. Policies for a Sustainable World. MIT, Cambridge, MA, USA. [Google Scholar]
- Harbers J, Huijbregts MAJ, Pothuma L, Van de Meent D. 2006. Estimating the impact of high‐production‐volume chemicals on remote ecosystems by toxic pressure calculation. Environ Sci Technol 40:1573–1580. [DOI] [PubMed] [Google Scholar]
- Hollander A, Schoorl M, van de Meent D. 2016. SimpleBox 4.0: Improving the model while keeping it simple. Chemosphere 148:99–107. [DOI] [PubMed] [Google Scholar]
- International Commission for the Protection of the Rhine against Pollution . 2009. Auswertungsbericht Humanarzneimittel. Anlage zum Bericht no. 182. Koblenz, Germany, p 10.
- Kapo KE, Deleo PC, Vamshi R, Holmes CM, Ferrer D, Dyer SD, Wang X, White‐Hull C. 2016. iSTREEM®: An approach for broad‐scale in‐stream exposure assessment of “down‐the‐drain” chemicals. Integr Environ Assess Manag 12:782–792. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M. 2018. Regulate to reduce chemical mixture risk. Science 361:224–226. [DOI] [PubMed] [Google Scholar]
- Lautz LS, Struijs J, Nolte TM, Breure AM, Van der Grinten E, Van de Meent D, Van Zelm R. 2017. Evaluation of SimpleTreat 4.0: Simulations of pharmaceutical removal in wastewater treatment plant facilities. Chemosphere 168:870–876. [DOI] [PubMed] [Google Scholar]
- Lindim C, Cousins IT, vanGils J. 2015. Estimating emissions of PFOS and PFOA to the Danube River catchment and evaluating them using a catchment‐scale chemical transport and fate model. Environ Pollut 207:97–106. [DOI] [PubMed] [Google Scholar]
- Lindim C, van Gils J, Cousins IT. 2016a. Europe‐wide estuarine export and surface water concentrations of PFOS and PFOA. Water Res 103:124–132. [DOI] [PubMed] [Google Scholar]
- Lindim C, van Gils J, Georgieva D, Mekanyan O, Cousins IT. 2016b. Evaluation of human pharmaceutical emissions and concentrations in Swedish river basins. Sci Total Environ 572:2008–2015. [DOI] [PubMed] [Google Scholar]
- Mackay D. 1991. Multimedia Environmental Models, the Fugacity Approach. Lewis, Boca Raton, Fl, USA. [Google Scholar]
- Mackay D, Paterson S. 1984. Spatial concentration distributions. Environ Sci Technol 18:207A–214A. [DOI] [PubMed] [Google Scholar]
- Malaj E, Von der Ohe PC, Grote M, Kühne R, Mondy CP, Usseglio‐Polatera P, Brack W, Schäfer RB. 2014. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci USA 111:9549–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NZ. 2016. Power laws and air pollution. Environ Model Assess 21:31–52. [Google Scholar]
- Munthe J, Lexén J, Skårman T, Posthuma L, Brack W, Altenburger R, Brorstrom‐Lunden E, Bunke D, Faust M, Rahmberg M, Slleuwert F, Slobodnik J, van Gils J, van Wezel A. 2019. Increase coherence, cooperation and cross‐compliance of regulations on chemicals and water quality. Environ Sci Eur 31:64. [Google Scholar]
- Newman MEJ. 2005. Power laws, Pareto distributions and Zipf's law. Contemp Physics 46:323–351. [Google Scholar]
- Oldenkamp R, Beusen AHW, Huijbregts MAJ. 2019. Aquatic risks from human pharmaceuticals—Modelling temporal trends of carbamazepine and ciprofloxacin at the global scale. Environ Res Lett 14:034003. [Google Scholar]
- Oldenkamp R, Hoeks S, Čengić M, Barbarossa V, Burns EE, Boxall ABA, Ragas AMJ. 2018. A high‐resolution spatial model to predict exposure to pharmaceuticals in European surface waters: ePiE. Environ Sci Technol 52:12494–12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmanns J, Licht O, Bitsch A, Bohlen ML, Escher SE, Silano V, MacLeod M, Serafimova R, Kass GEN, Merten C. 2018. Development of a novel scoring system for identifying emerging chemical risks in the food chain. Environ Sci Processes Impacts 20:340–353. [DOI] [PubMed] [Google Scholar]
- Posthuma L, Brack W, van Gils J, Focks A, Müller C, de Zwart D, Birk S. 2019a. Mixtures of chemicals are important drivers of impacts on ecological status in European surface waters. Environ Sci Eur 31:71. [Google Scholar]
- Posthuma L, De Zwart D, Keijzers R, Postma J. 2016. Water systems analysis with the ecological key factor ‘toxicity’. Part 2. Calibration. Toxic pressure and ecological effects on macrofauna in the Netherlands. STOWA, Amersfoort, The Netherlands.
- Posthuma L, van Gils J, Zijp MC, van de Meent D, de Zwart D. 2019b. Species sensitivity distributions for use in environmental protection, assessment, and management of aquatic ecosystems for 12 386 chemicals. Environ Toxicol Chem 38:905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala S, Benini L, Mancini L. 2014. Methodology for building LCA‐compliant national inventories of emissions and resource extraction. JRC Science and Policy Reports JRC92036. Publication Office of the European Union, Luxembourg.
- Sala S, Benini L, Mancini L, Pant R. 2015. Integrated assessment of environmental impact of Europe in 2010: Data sources and extrapolation strategies for calculating normalisation factors. Int J Life Cycle Assess 20:1568–1585. [Google Scholar]
- Schulze S, Sättler D, Neumann M, Arp HPH, Reemtsma T, Berger U. 2018. Using REACH registration data to rank the environmental emission potential of persistent and mobile organic chemicals. Sci Total Environ 625:1122–1128. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Takacs P. 2002. Probabilistic risk assessment using species sensitivity distributions In Posthuma L, Suter GW, II, Traas TP, eds, Species Sensitivity Distributions in Ecotoxicology. Lewis, Boca Raton, FL, USA, pp 285–313. [Google Scholar]
- Striffler A, Wassermann D. 2015. KnowSEC, A web system for managing data on substances. 2015‐03‐02. Denkbares, Wurzberg, Germany. [cited 2018 May 16]. Available from: http://www.bfr.bund.de/cm/349/knowsec-a-web-system-for-managing-data-on-substances.pdf
- Struijs J, Van de Meent D, Schowanek D, Buchholz H, Patoux R, Wolf T, Austin T, Tolls J, van Leeuwen K, Galay‐Burgos M. 2016. Adapting SimpleTreat for simulating behaviour of chemical substances during industrial sewage treatment. Chemosphere 159:619–627. [DOI] [PubMed] [Google Scholar]
- Traas TP, Luttik R, Mensink H. 2002. Mapping risks of heavy metals to birds and mammals using species sensitivity distributions In Posthuma L, Suter GW II, Traas TP, eds, Species Sensitivity Distributions in Ecotoxicology. Lewis Publishers, Boca Raton, FL, USA, pp 403–420. [Google Scholar]
- Tsakiris G. 2015. The status of the European waters in 2015: A review. Environ Proc 2:543–557. [Google Scholar]
- United Nations Environment Programme . 2013. Global chemicals outlook. Towards sound management of chemicals, Geneva, Switzerland. [Google Scholar]
- Vallotton N, Price PS. 2016. Use of the maximum cumulative ratio as an approach for prioritizing aquatic coexposure to plant protection products: A case study of a large surface water monitoring database. Environ Sci Technol 50:5286–5293. [DOI] [PubMed] [Google Scholar]
- Van der Aa NGFM, Kommer GJ, De Groot GM, Versteegh JFM. 2008. Pharmaceuticals in drinking water sources (In Dutch: Geneesmiddelen in bronnen voor drinkwater Monitoring, toekomstig gebruik en beleidsmaatregelen). Report 609715002/2008. RIVM, Bilthoven, The Netherlands.
- Van der Poel P, Ros JPM. 1995. Emissions of chemicals In Van Leeuwen CJ, Hermens JLM, eds, Risk Assessment of Chemicals. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Van Gils J, Posthuma L, Cousins IT, Brack W, Altenburger R, Baveco H, Focks A, Greskowiak J, Kühne R, Kutsarova S, Lindim C, Markus A, Van de Meent D, Munthe J, Schueder R, Schüürmann G, Slobodnik J, De Zwart D, Van Wezel A. 2020. Computational material flow analysis for thousands of chemicals of emerging concern in European waters. J Hazard Mater 397:122655. [DOI] [PubMed] [Google Scholar]
- Van Gils J, Posthuma L, Cousins IT, Lindim C, De Zwart D, Bunke D, Kutsarova S, Muller C, Munthe J, Slobodnik J, Brack W. 2019. The European Collaborative Project SOLUTIONS developed models to provide diagnostic and prognostic capacity and fill data gaps for chemicals of emerging concern. Environ Sci Eur 31:72. [Google Scholar]
- Van Straalen NM. 2002. Theory of ecological risk assessment based on species sensitivity distributions In Posthuma L, Suter GW, II, Traas TP, eds, Species Sensitivity Distributions in Ecotoxicology. Lewis, Boca Raton, FL, USA, pp 37–48. [Google Scholar]
- Vermeire TG, Jager DT, Bussian B, Devillers J, den Haan K, Hansen B, Lundberg I, Neissen H, Robertson S, Tyle H, van der Zandt PT. 1997. European Union system for the evaluation of substances (EUSES). Principles and structure. Chemosphere 34:1823–1836. [DOI] [PubMed] [Google Scholar]
- Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Reidy Leirman C, Davies PM. 2010. Global threats to human water security and river biodiversity. Nature 467:555–561. [DOI] [PubMed] [Google Scholar]
- Wang Z, Walker GW, Muir DCG, Nagatani‐Yoshida K. 2020. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol 54:2575–2584. [DOI] [PubMed] [Google Scholar]
- Wikipedia . 2020. Pareto Principle. [cited 2020 May 15]. Available from: https://en.wikipedia.org/wiki/Pareto_principle
- West G. 2017. Scale: The Universal Laws of Growth, Innovation, Sustainability, and the Pace of Life in Organisms, Cities, Economies, and Companies. Penguin, New York, NY, USA. [Google Scholar]
- Zijp MC, Posthuma L, Van de Meent D. 2014. Definition and applications of a versatile chemical pollution footprint methodology. Environ Sci Technol 48:10588–10597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (Leo.Posthuma@rivm.nl).


