Abstract
Remimazolam (RMZ) is a new and ultra‐fast‐acting, short‐duration intravenous benzodiazepine, a drug class associated with abuse potential. This trial was designed to compare the abuse potential of remimazolam with placebo and midazolam (MDZ), a well‐characterized member of the same pharmacological class in healthy, recreational drug users 18‐55 years‐of‐age, who demonstrated good drug tolerance and were able to discriminate between midazolam and placebo. At equipotent intravenous doses selected to produce effects ranging from mild/moderate to relatively strong sedation without loss of consciousness (RMZ: 5, 10 mg versus MDZ: 2.5, 5 mg), peak scores (Emax or Emin, respectively) for drug liking, good/bad/any effects, and sedation (drowsiness and relaxation) were significantly greater than placebo for both active drugs and were broadly comparable between RMZ and MDZ. In contrast, areas under the effect‐time curves (TA_AUE) were notably lower for RMZ versus MDZ, particularly for measures of good and any effects, reflecting the shorter duration of action and consistent with the more rapid observed plasma clearance for RMZ versus MDZ and the lack of an active RMZ metabolite. Scores for willingness to take drug again were also lower for RMZ versus MDZ, but not significantly so. We concluded that the abuse potential of RMZ is comparable to or lower than that of MDZ, a drug known to have a low potential for intravenous abuse.
Keywords: abuse potential, benzodiazepine, midazolam, randomized clinical trial, remimazolam
Remimazolam is a novel ultra‐short‐acting intravenous benzodiazepine being developed for patients in need of sedation during short medical procedures (procedural sedation), general anesthesia, or intensive care. It is designed to offer a significantly faster and more predictable recovery than currently available drugs in the same class, for example, midazolam, 1 , 2 while maintaining an advantageous safety profile versus propofol. 3 As a methyl ester, remimazolam is rapidly and predictably metabolized by carboxylesterase‐1A to the pharmacologically inactive metabolite (CNS7054), independently of individual variations in the patient's cytochrome oxidase enzyme profile.
Drug abuse is of increasing concern, and benzodiazepines represent a broadly abused class, 4 often in combination with other drugs and often associated with opioid‐related deaths. 5 Current guidelines require that abuse potential be assessed in comparison with an existing drug of the same class. 6 , 7 However, within this class, there is considerable variation in the reported level of abuse, such that drugs with more restricted indications and availability (eg, midazolam) are considerably less frequently abused than more readily available drugs used for chronic treatment of anxiety disorders, for example, diazepam or alprazolam. 8 In addition, the low oral bioavailability of remimazolam necessitated an intravenous comparator. Hence, midazolam was chosen as the most suitable comparator, being a benzodiazepine with similar intended availability (clinic/hospital administration only), having an intravenous formulation available in the United States, and being widely accepted as a standard treatment in the shared indication of procedural sedation. Therefore, this trial was designed to evaluate the effects of remimazolam on abuse‐relevant subjective parameters such as drug liking.
Methods
The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation E6 Guidelines on Good Clinical Practice at a single site in Utah and has been registered under ClinicalTrials.gov with the identifier NCT04110535. The trial protocol was approved by the New England Institutional Review Board, and no major modifications were made thereafter. All subjects gave their informed consent in writing before any trial procedures were performed.
Trial Design
The trial was a phase 1 single‐dose, randomized, double‐blind, placebo‐ and active‐controlled crossover study with a single inpatient treatment visit designed to (1) evaluate absolute abuse potential of remimazolam (RMZ) by comparing the abuse potential of single intravenous doses of RMZ 5 and 10 mg with that of placebo (PBO), (2) evaluate relative abuse potential of RMZ by comparing the abuse potential of single intravenous doses of RMZ 5 and 10 mg with that of equipotent doses of intravenous midazolam (MDZ, active control) 2.5 and 5 mg, (3) assess the safety and tolerability of remimazolam in healthy recreational central nervous system (CNS) depressant users. Doses were selected on the basis of earlier clinical comparisons of sedation. 9
Within 28 days of an outpatient screening (visit 1), subjects were admitted for a 15‐day inpatient treatment visit (visit 2). Admission on day ‐1 was followed by a 3‐day qualification phase from day 1 to day 3 to ensure that subjects were able to (1) discriminate between midazolam and placebo, and (2) tolerate the sedative effects of MDZ (ie, remain conscious). After a rest day (day 4), subjects who passed the qualification phase continued into a 10‐day, 5‐period treatment phase (days 5 to 14). Each period of the treatment phase consisted of a single intravenous dose of trial medication (MDZ, RMZ, or placebo) followed by a 48‐hour washout period. A final outpatient follow‐up visit was performed on days 15 to 17. The maximum duration of participation for any subject was therefore approximately 7 weeks.
Eligible subjects were healthy recreational CNS depressant users between 18 and 55 years old with a body mass index (BMI) of ≥19.0 to ≤33.0 kg/m2. CNS depressant use had to include at least 10 lifetime nontherapeutic experiences, of which ≥1 experience had to be within 8 weeks and ≥1 experience using benzodiazepines within 1 year of screening. Female subjects of childbearing potential were required to be using an adequate method of birth control. Principle exclusion criteria included a history of drug (except nicotine) or alcohol dependence per Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision 10 within 12 months of screening and any self‐reported dependence (except nicotine or caffeine) or a history of treatment for substance use disorders (except nicotine) at any time.
Pharmacodynamic Assessments
Subjective pharmacodynamic (PD) measures were recorded using a visual analog scale (VAS) from 0 to 100 on a computerized interface; subjects were appropriately trained beforehand. As described in Table 1, parameters included bipolar measures of drug liking (both at this moment and overall), and sedation (alertness/drowsiness and agitation/relaxation) as well as unipolar measures of good effects, bad effects, any effects, and desire to take the drug again. Memory and amnestic effects were assessed based on paired associates learning (PAL) to measure visual memory and new learning as described elsewhere. 11 , 12
Table 1.
Description of PD Parameters
| Parameter | Outcome Measures | Scale | Question | Minimum | Neutral | Maximum |
|---|---|---|---|---|---|---|
| Drug liking (at this moment) | Emin, Emax, TA‐AUE, Emax/min | VAS, bipolar | At this moment, my liking for this drug is: | 0 (strong dislike) | 50 | 100 (strong like) |
| Drug liking (overall) | Emax/Emin | VAS, bipolar | Overall, my liking for this drug is: | 0 (strong dislike) | 50 | 100 (strong like) |
| Take drug again | Emax | VAS, unipolar | I would take this drug again: | 0 (definitely not) | — | 100 (definitely so) |
| Good effects | Emax, TA‐AUE | VAS, unipolar | At this moment, I feel good drug effects: | 0 (not at all) | — | 100 (extremely) |
| Bad effects | Emax, TA‐AUE | VAS, unipolar | At this moment, I feel bad drug effects: | 0 (not at all) | — | 100 (extremely) |
| Any effects | Emax, TA‐AUE | VAS, unipolar | At this moment, I feel any drug effects: | 0 (not at all) | — | 100 (extremely) |
| Alertness/drowsiness | Emin, TA‐AUE | VAS, bipolar | At this moment, my mental state is: | 0 (very drowsy) | 50 | 100 (very alert) |
| Agitation/relaxation | Emin, TA‐AUE | VAS, bipolar | At this moment, my mood is: | 0 (very relaxed) | 50 | 100 (very agitated) |
| PAL | Emax, TA‐AUE | Total error score | NA | 0 | — | — |
Emax, maximum observed effect; Emin, minimum observed effect; NA, not applicable; PAL, paired associates learning; TA‐AUE, area under the effect time curve; VAS, visual analog scale.
PD end points included (1) maximum recorded effect score (Emax) for all measures except sedation (ie, drug liking, overall drug liking, desire to take drug again, good effects, bad effects, any effects, and PAL), (2) minimum recorded effect score (Emin) for sedation (alertness/drowsiness and agitation/relaxation) and drug liking, and (3) time‐averaged score calculated as the area under the effect time curve (TA‐AUE) to 8 hours posttreatment for all measures except overall drug liking, which was assessed based on Emax/Emin and desire to take the drug again. Although all end points were considered in the assessment, drug liking Emax was considered the primary end point for the calculation of power and for the assessment of eligibility because it is a broadly validated and sensitive measure of abuse potential. 13 , 14
Safety Measures
Standard safety assessments were performed including adverse event questioning (incidence, frequency, severity) and vital signs (pulse, blood pressure, respiratory rate, O2 saturation) at all visits and from predose to 8 hours postdose and at follow‐up/end of trial. Laboratory parameters (hematology, clinical laboratory, urinalysis), physical examinations, and 12‐lead electrocardiograms (ECGs) were taken at screening/admission and follow‐up/end of trial, as was suicidal behavior (assessed using the Columbia‐Suicide Severity Rating Scale). Oral temperature was recorded at screening, predose, and at end of trial. The need for airway interventions was assessed based on supplemental oxygen use (predose to 120 minutes postdose) and continuous pulse oximetry/telemetry (predose to 240 minutes postdose). Concomitant medication use and spontaneous AE reporting were recorded from admission to the end of trial.
Qualification and Treatment Allocation
Human abuse potential trials typically include both therapeutic and supratherapeutic doses. 6 However, because higher doses of RMZ result in loss of consciousness, supratherapeutic doses would prevent completion of the PD assessments. Therefore, treatment doses of 5 and 10 mg RMZ (and the equivalent 2.5 and 5 mg MDZ, respectively) were chosen, as these were expected to induce mild to relatively strong sedation without loss of consciousness. Those subjects who were more sensitive to the sedative effects (ie, lost consciousness) were excluded de facto via the qualification phase requirement to complete PD assessments; in fact, this only applied to 2 subjects. Patients in the qualification phase were assigned a qualification randomization number, generated by the unblinded site statistician, corresponding to either 2.5 mg MDZ on day 1 and PBO on day 2 or the other way around. Eligible subjects received 5 mg MDZ on day 3, followed by a 48‐hour washout period prior to the treatment phase.
An individual subject's qualification for the treatment phase was based on a combination of the bipolar VAS of Emax drug liking (range, 0 to 100; neutral value, 50) and on the subject's midazolam tolerance (ability to remain conscious). A subject's Emax drug Liking for the 2.5‐mg MDZ dose had to be ≥65 points in first 60 minutes and at least ≥15 points greater than the placebo score; the absolute placebo response had to be ≥40 to ≤60 points. For the tolerability assessment of 5 mg MDZ, the subjects had to be able to complete a PD assessment within 60 minutes postdose (ie, was conscious or could be roused), and the oxygen saturation was not permitted to fall below 90% for more than 60 seconds at a time.
Qualified subjects were then assigned a treatment phase randomization number, generated by the unblinded site statistician, and were allocated to 1 of 10 treatment sequences in two 5 × 5 Williams squares including 5 and 10 mg RMZ, 2.5 and 5 mg MDZ, and PBO.
Statistics
The very low oral bioavailability of RMZ required the use of an intravenous comparator. Although appropriate drug‐liking data are available for the commonly used short‐acting benzodiazepine alprazolam, no intravenous formulation was available in the United States. Alternatively, MDZ is a similar short‐acting benzodiazepine with a suitable intravenous formulation. Its restricted intended availability (clinics and hospitals only) also makes MDZ an appropriate comparator. However, there is a lack of drug‐liking VAS data for MDZ, and so the sample size calculation was based on data for alprazolam. A total of 30 subjects was sufficient for 80% power to detect a clinically significant 15‐point difference 15 between treatments in drug‐liking VAS Emax (assuming an intersubject SD of 14.5). To ensure study validity (MDZ versus PBO), using a 0.05 2‐sided significance, a total of 30 completed subjects would allow 90% power of detecting a significant difference in drug‐liking VAS, assuming an effect, that is, (μ1 ‐ μ2)/s, of at least 1.2, that is, a 20% difference.
A mixed‐effects model for a crossover study was used for PD treatment comparisons. The model included treatment, period, sequence, and first‐order carryover effect as fixed effects and subject nested within treatment sequence as a random effect. Baseline (predose) measurement was included as a covariate, where applicable. The carryover effect was found to be nonsignificant at the 25% level; therefore, the term was dropped from the model. Data were assessed for normality and analyzed nonparametrically, if appropriate. Assessment of the trial's validity was based on each dose of intravenous MDZ versus PBO. The study was to be considered valid if either dose of MDZ was statistically different from PBO on the primary end point (drug‐liking VAS Emax). The assessment of RMZ's abuse potential was based on each dose of RMZ versus PBO (absolute abuse potential) as well as RMZ 5 mg versus MDZ 2.5 mg and RMZ 10 mg versus MDZ 5 mg (relative abuse potential).
The statistical analysis and reporting were done using SAS for Windows version 9.4 or higher (SAS Institute, Inc., Cary, North Carolina). PK parameter calculations were done using Phoenix WinNonlin version 6.3 or higher (Pharsight, Inc., Mountain View, California).
Results
A summary of subject participation is given in the Consort Diagram (Supplemental Figure 1). In short, between June 23 and October 6, 83 eligible subjects were enrolled in the qualification phase. Of these, 40 subjects qualified (ie, were able to distinguish between placebo and MDZ and had good drug tolerability), were randomized into the ITT population, and received trial medication in the treatment phase.
In the ITT, 30 (75.0%) were male, 38 (95.0%) were white, 2 (5.0%) were black, mean ± SD age was 27.1 ± 5.14 years, and BMI was 24.40 kg/m2 (19.9 to 32.7 kg/m2). As per protocol, all subjects were recreational drug users with a history of benzodiazepine abuse. In the 60 days prior to screening, 36 subjects (90%) used marijuana, 30 (75%) used alprazolam (benzodiazepine), 17 (42.5%) used acetaminophen/hydrocodone (opioid), 16 (40%) used acetaminophen/oxycodone (opioid), and 15 (37.5%) used diazepam (benzodiazepine). In all, 39 (97.5%) reported drinking alcohol, and 33 (82.5%) were smokers. As per Food and Drug Administration guidance, subjects were selected because they generally preferred benzos for recreational use, but most recreational drug users are polysubstance users so it is normal that many would also abuse other substances.
One subject in the ITT had to be withdrawn by the Investigator (because of confrontational behavior and failing to follow the study restrictions) after completing only the first treatment period (RMZ 5 mg) and was therefore excluded from the PD analyses (completer set).
Pharmacodynamics
Trial Validity
For drug liking, comparison of least‐squares (LS) mean Emax (primary end point) scores, the 95%CIs of the difference between MDZ versus PBO did not include zero (see Table 2, difference in LS mean), that is, both doses of MDZ scored significantly higher than PBO. The study was therefore considered valid. Scores for overall drug liking (Emax and Emin), desire to take drug again (Emax), good/bad/any effects (Emax and TA_UAE), and PAL (Emax) were also significantly higher for MDZ than PBO. Consistent with this, Emin scores for alertness/drowsiness and agitation/relaxation were significantly lower for MDZ versus PBO.
Table 2.
Comparison of Key Abuse Liability Parameters—VAS Measures (Completer Set, n = 39)
| Comparison | Drug Liking (at This Moment) | Drug Liking Overall | Take Drug Again | Effects — Good | Effects — Bad | Effects — Any | Agitation/Relaxation | Alertness/Drowsiness | PAL Total Errors | |
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute values, mean (SD) | ||||||||||
| Emax | ||||||||||
| PBO | 53.1 (8.1) | 52.8 (14.1) | 17.1 (28.2) | 6.9 (19.0) | 0.2 (0.8) | 6.6 (18.0) | — | — | 16.7 (17.5) | |
| RMZ 5 | 77.7 (14.1) | 61.8 (17.2) | 36.9 (35.5) | 64.5 (24.1) | 15.0 (20.7) | 67.0 (22.3) | — | — | 27.1 (21.0) | |
| RMZ 10 | 79.8 (15.1) | 67.3 (18.7) | 49.2 (34.0) | 70.9 (22.8) | 30.7 (34.4) | 73.1 (18.8) | — | — | 36.2 (21.5) | |
| MDZ 2.5 | 78.6 (14.0) | 67.3 (17.2) | 56.4 (33.2) | 65.6 (24.7) | 12.9 (21.9) | 67.2 (22.9) | — | — | 36.7 (20.7) | |
| MDZ 5 | 81.5 (11.7) | 69.3 (16.2) | 58.5 (32.4) | 72.9 (20.6) | 27.9 (33.4) | 76.1 (18.8) | — | — | 55.4 (14.5) | |
| Emin | ||||||||||
| PBO | 45.9 (13.4) | 51.8 (13.1) | — | — | — | — | 41.1 (14.0) | 49.1 (16.3) | — | |
| RMZ 5 | 43.8 (15.2) | 57.2 (16.8) | — | — | — | — | 18.7 (12.4) | 24.2 (15.8) | — | |
| RMZ 10 | 42.4 (15.3) | 58.5 (20.6) | — | — | — | — | 15.1 (11.8) | 16.4 (13.4) | — | |
| MDZ 2.5 | 46.2 (11.3) | 63.9 (18.5) | — | — | — | — | 16.7 (11.5) | 18.1 (13.9) | — | |
| MDZ 5 | 44.7 (13.8) | 62.3 (15.5) | — | — | — | — | 12.6 (9.9) | 15.4 (12.4) | — | |
| TA_AUE | ||||||||||
| PBO | −6.9 (60.4) | — | — | 6.3 (21.5) | 0.1 (0.3) | 5.7 (19.8) | 6.3 (77.6) | 19.4 (124.5) | 12.1 (95.0) | |
| RMZ 5 | 22.2 (66.4) | — | — | 55.0 (87.2) | 6.1 (15.3) | 50.3 (67.3) | −23.1 (61.0) | −13.4 (119.7) | −6.6 (76.8) | |
| RMZ 10 | 28.3 (57.7) | — | — | 71.0 (86.2) | 13.1 (36.9) | 72.4 (84.7) | −22.9 (37.1) | −29.9 (67.7) | 21.1 (82.6) | |
| MDZ 2.5 | 41.5 (64.0) | — | — | 100.0 (110.3) | 11.1 (28.7) | 95.5 (90.5) | −34.5 (63.3) | −62.7 (86.3) | 48.6 (106.2) | |
| MDZ 5 | 48.4 (52.3) | — | — | 112.9 (82.3) | 20.5 (33.8) | 119.9 (89.6) | −65.2 (58.2) | −66.7 (103.9) | 51.1 (55.8) | |
| Difference in LS mean | ||||||||||
| Emax | — | |||||||||
| MDZ 2.5 | vs PBO | 25.53 a | 14.45 a | 39.17 a | 58.61 a | 12.66 a | 60.54 a | — | — | 19.9 a |
| MDZ 5 | vs PBO | 28.32 a | 16.35 a | 41.09 a | 65.71 a | 27.75 a | 69.24 a | — | — | 38.6 a |
| RMZ 5 | vs PBO | 24.57 a | 8.97 a | 19.81 a | 57.39 a | 14.57 a | 60.32 a | — | — | 10.2 a |
| RMZ 10 | vs PBO | 26.69 a | 14.43 a | 32.09 a | 63.83 a | 30.35 a | 66.44 a | — | — | 19.3 a |
| RMZ 5 | vs MDZ 2.5 | −0.95 | −5.48 | −19.35 a | −1.23 | 1.91 | −0.22 | — | — | −9.6 a |
| RMZ 10 | vs MDZ 5 | −1.63 | −1.92 | −9.01 | −1.88 | 2.60 | −2.80 | — | — | −19.4 a |
| Emin | ||||||||||
| MDZ 2.5 | vs PBO | 0.22 | 11.99 a | — | — | — | — | −24.31 a | −30.87 a | — |
| MDZ 5 | vs PBO | −1.33 | 10.34 a | — | — | — | — | −28.46 a | −33.55 a | — |
| RMZ 5 | vs PBO | −2.13 | 5.36 | — | — | — | — | −22.40 a | −24.63 a | — |
| RMZ 10 | vs PBO | −3.65 | 6.62 a | — | — | — | — | −26.02 a | −32.56 a | — |
| RMZ 5 | vs MDZ 2.5 | −2.35 | −6.63 a | − | — | — | — | 1.91 | 6.23 a | — |
| RMZ 10 | vs MDZ 5 | −2.32 | −3.72 | — | — | — | — | 2.45 | 0.99 | — |
| TA_AUE | ||||||||||
| MDZ 2.5 | vs PBO | 48.47 a | — | — | 93.83 a | 10.95 a | 89.95 a | −41.21 a | −81.53 a | 36.1 |
| MDZ 5 | vs PBO | 55.20 a | — | — | 106.30 a | 20.48 a | 114.01 a | −71.51 a | −86.16 a | 38.6 a |
| RMZ 5 | vs PBO | 29.08 a | — | — | 48.59 a | 5.96 | 44.55 a | −29.51 a | −32.36 | −19.9 |
| RMZ 10 | vs PBO | 35.20 a | — | — | 64.89 a | 12.97 a | 66.96 a | −29.12 a | −48.66 a | 7.9 |
| RMZ 5 | vs MDZ 2.5 | −19.39 | — | — | −45.24 a | −4.99 | −45.50 a | 11.70 | 49.17 a | −55.9 a |
| RMZ 10 | vs MDZ 5 | −20.00 | — | — | −41.41 a | −7.51 | −47.05 a | 42.39 a | 37.50 | −30.7 |
Emax, maximum observed effect; Emin, minimum observed effect; MDZ, midazolam; PAL, paired associates learning; PBO, placebo; RMZ, remimazolam; TA, time‐averaged area under the effect curve; VAS, visual analog scale.
P < .05.
Drug Liking and Desire to Take Drug Again
Peak (Emax) drug‐liking scores (at that moment) were significantly greater for both doses of RMZ than PBO and were comparable to equipotent doses of MDZ (see Table 2), comparison of LS means. However, the drug‐liking effect was more short‐lived for RMZ (see Figure 2) than for equivalent MDZ doses, resulting in lower areas under the time‐effect curve (TA‐AUC); see Table 2. Drug‐liking scores (overall) did not appear dose dependent for RMZ or MDZ and were numerically lower for RMZ than for the corresponding MDZ doses, though not significantly.
Figure 2.
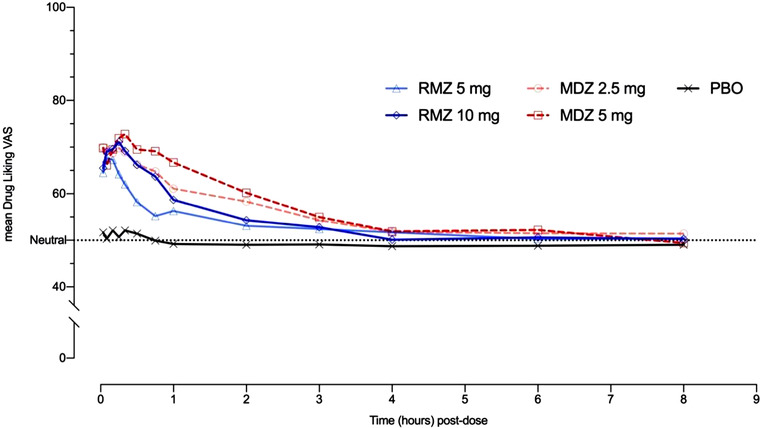
Drug‐liking scores over time by treatment. MDZ, midazolam; PBO, placebo; RMZ, remimazolam; VAS, visual analog scale. Dotted line indicates score = 50, that is, the neutral value for bipolar measures.
Peak scores for desire to take drug again were significantly higher for all doses of active drugs versus PBO and appeared dose dependent for RMZ. Desire to take drug again was both statistically and clinically significantly lower for RMZ 5 mg than the equivalent MDZ 2.5 mg dose; this difference was less pronounced and not significant at the higher doses.
Perceived Drug Effects
Peak (Emax) scores for perceived drug effects (“good,” “bad,” “any”) were significantly higher for active treatments than PBO and were comparable between RMZ and MDZ. “Good” and “any” effects were weak in the PBO dose period and were significantly stronger and only marginally influenced by dose for both RMZ and MDZ (Table 2, Figure 1). Bad effects were weak, and dose dependent for both RMZ and MDZ and appeared comparable between paired doses. Time‐averaged scores for RMZ and MDZ were also higher than PBO and appeared dose dependent. Perceived “good” or “any” effects were lower for RMZ over time (approximately 40% to 50%, although not statistically significant) compared with the equivalent MDZ doses; this difference was less pronounced for “bad” effects (Table 2).
Figure 1.
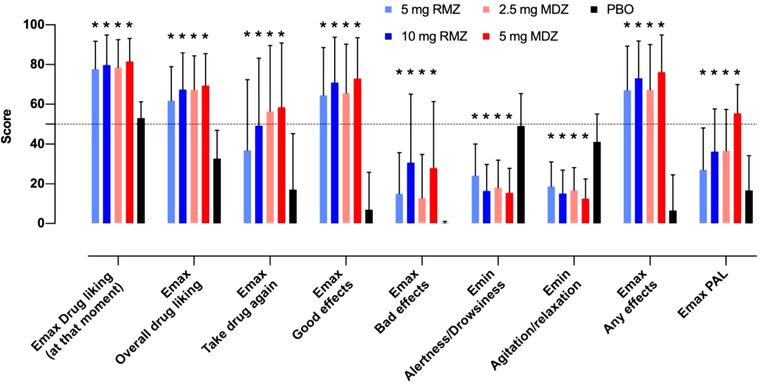
Overview of peak PD responses to RMZ versus MDZ and placebo. MDZ, midazolam; PBO, placebo; PD, pharmacodynamics; RMZ, remimazolam. Error bars indicate standard deviation of the mean. Dotted line indicates score = 50, that is, the neutral value for bipolar measures *Significant difference (P < .05) versus placebo.
Sedative Effects
Peak perceived sedation, as assessed by Emin agitation/relaxation and alertness/drowsiness scores, was generally comparable between MDZ and RMZ (Table 2, Figure 1), although subjects reported slightly (but significantly) lower scores (greater sedation) in the low‐dose MDZ versus low‐dose RMZ. In contrast to peak sedation scores, however, time‐averaged scores for agitation/relaxation and alertness/drowsiness were notably higher (ie, less relaxed and less drowsy) for RMZ versus corresponding MDZ doses as expected because of the fast clearance, resulting in rapid resolution of RMZ activity. Interestingly, although peak relaxation and drowsiness was greater in MDZ versus RMZ, and depressed level of consciousness and somnolence (adverse events) were both more common in the RMZ groups versus MDZ (see Supplemental Table 1).
Memory/Amnestic Effects
The Emax total error scores in the PAL test were significantly higher than placebo for both active drugs, indicating significant amnestic effects of both RMZ and MDZ; however, the Emax scores after RMZ were significantly lower than after MDZ (Table 2), showing these amnestic effects of RMZ to be less than those of MDZ. Comparison of time‐average scores revealed a broadly similar profile as for Emax, although LS mean scores were even lower for RMZ 5 mg than for placebo and statistical significance was restricted to the comparisons of MDZ 5 mg versus PBO, and RMZ 5 mg versus MDZ 2.5 mg. Interestingly, it was noted that amnesia was only reported as an adverse event in the RMZ treatment groups (5 mg: 1/40, 2.5%; 10 mg: 3/40, 7.7%).
Safety
Remimazolam was well tolerated, both at the 5‐mg and 10‐mg doses. Almost all subjects (39/40, 98%) experienced at least 1 treatment‐emergent adverse event (TEAE), although these were mostly mild (Supplemental Table 1). No fatal, serious, or severe adverse events were reported during the treatment phase. In general, the AE profiles were comparable between RMZ and MDZ, despite minor differences in somnolence, depressed level of consciousness, and amnesia as described above.
Beyond these differences, TEAEs in the MedDRA SMQ “Drug abuse, dependence and withdrawal [broad],” were slightly more common after MDZ than RMZ, particularly “feeling abnormal,” “feeling drunk,” “feeling of relaxation,” and “euphoric mood.”
There were no clinically significant changes in laboratory or ECG parameters during the treatment phase, and no subjects required airway interventions. Both RMZ and MDZ lead to transitory increases in heart rate (RMZ 5 and 10 mg: 17 and 20 bpm versus MDZ 2.5 and 5 mg: 13 and 15 bpm) starting at about 2 minutes postdose with a duration of about 15 minutes; this is a known effect of midazolam 16 and therefore expected with remimazolam.
Discussion
In accordance with the draft guidelines at the time, this study of the human abuse potential of single doses of intravenous remimazolam was performed in recreational CNS depressant users, the most likely abuse population. 6 Opioid users were not excluded because 30% or more were also benzodiazepine users 17 and the combined use posed an additional risk. Statistically significant differences in drug liking in subjects known to be able to discriminate between PBO and MDZ demonstrated the validity of the study design. Furthermore, although a direct comparison is problematic, the magnitude of peak subjective effects for MDZ also appears broadly consistent with those of other benzodiazepines, for example, alprazolam. 18 Equipotency of the paired active sedative doses in this trial (RMZ 5 mg versus MDZ 2.5 mg and RMZ 10 mg versus MDZ 5 mg) was predicted by earlier results. 9 Although drug effects on drowsiness and relaxation were slightly greater for MDZ versus RMZ, relevant adverse events were slightly more common in RMZ versus MDZ; on balance, therefore, equipotency was considered confirmed.
In this study, assessments of positive abuse‐relevant parameters were analyzed for peak (Emax) and time‐averaged (TA_AUE) subjective effects. Both RMZ and MDZ showed clear and broadly comparable increases in peak drug liking and “good effects” when compared with placebo. Likewise, amnestic effects were also increased versus placebo. Interestingly, although PAL total error scores were significantly lower for RMZ versus MDZ, the adverse event of amnesia was only reported for RMZ. PAL assesses learning of new information, whereas amnesia reported as an AE likely refers to anterograde amnesia, that is, the former refers to the actual test (PAL), whereas the latter focuses on the subjective experience; however, both are indicative of the same phenomenon. Overall, abuse‐relevant positive effects did not appear to be dose dependent, indicating that the effect ceiling had already been reached for abuse‐related effects, and supporting the suitability of the selected doses for assessment of abuse potential. Peak “bad effects” were weak but greater than placebo for both active treatments, at least at the higher dose, and they appeared to be dose dependent at the tested doses, indicating that maximum negative effects may not have been reached. Higher doses were not feasible in this case, however, because they would likely lead to loss of consciousness.
In contrast to peak effects, time‐averaged “good” effects were significantly weaker for RMZ than MDZ. The difference in time‐averaged subjective effects is consistent with the known differences in pharmacokinetics between RMZ and MDZ, that is, the considerably shorter t1/2 of 0.6 to 0.8 hours for RMZ versus 1.5 to 2.5 hours for MDZ, 9 , 19 resulting in shorter durations for RMZ drug effects, the putative cause of the lower overall drug‐liking scores and the lower willingness to take drug again (both were significant at the lower dose).
Griffiths and Wolf describe a drug's abuse liability as being the product of (1) its reinforcing effects and (2) its positive subjective effects. 20 Self‐administration was not included in the study design, and as such these results do not permit direct assessment of reinforcement. However, our results showed lower willingness to take RMZ again versus MDZ despite broadly comparable peak effects, therefore suggesting that the shorter duration of RMZ's effects resulted in weaker reinforcement for RMZ, at least versus intravenous MDZ. This is consistent with the analysis of potentially abuse‐related adverse events reported by recreational drug users in this trial, which showed a marginally greater incidence of potentially abuse‐related adverse events following MDZ administration versus RMZ.
Data from the Drug Abuse Warning Network, 8 showing that the vast majority of benzodiazepine abuse is associated with medium duration, orally bioavailable rather than intravenous drugs. The number of emergency department visits linked with injectable benzodiazepines such as midazolam was exceptionally small, and because remimazolam has extremely poor oral bioavailability (publication in preparation), this effectively limits its abuse via the oral route.
Because of significantly greater drug liking, “good effects,” and a positive willingness to take drug again compared with PBO, it may be concluded that RMZ does have abuse potential via injection. However, considering the significantly lower time‐averaged positive effects and the relatively low willingness to take drug again compared with MDZ, we conclude that the abuse potential for RMZ is comparable to or lower than that of MDZ, a drug known to have a low potential for intravenous abuse.
Conflicts of Interest
Frank Schippers, MD, Marija Pesic, PhD, and Thomas Stoehr, PhD, are employees of Paion AG and own shares/stock options. Robert Saunders, PhD, is a consultant medical writer under contract with Paion AG with no conflicting sources of income. Keith Borkett was an employee of Paion AG during the conduct of the study and is currently self‐employed with no conflicting sources of income. Lynn Webster and Shawn Searle were employees of PRA, the contract research organization that conducted the clinical trial.
Funding
The clinical trial CNS7056‐014 was sponsored by PAION AG, the developer of remimazolam.
Author Contributions
F.S., M.P., K.B., S.S., L.W., and T.S. contributed to the conception and design of the work; the acquisition, analysis, or interpretation of data for the work. R.S., F.S., M.P., K.B., S.S., L.W., and T.S. contributed to drafting the work and revising it critically for important intellectual content and finally approved the version to be published. All authors agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Sharing
The data are not available in a repository, but requests can be directed to t.stoehr@paion.com.
Supporting information
Supporting Information
Supporting Information
References
- 1. Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137‐146. [DOI] [PubMed] [Google Scholar]
- 2. Borkett KM, Riff DS, Schwartz HI, et al. A Phase IIa, randomized, double‐blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771‐780. [DOI] [PubMed] [Google Scholar]
- 3. Bansal S, Singhal SJJoC, Research D. Remimazolam (CNS 7056): an emerging sedative and general anaesthetic. J Clin Diagn Res. 2018;12(3):UE01‐UE03. [Google Scholar]
- 4. Hughes A, Williams M, Lipari R, Bose J, Copello E, Kroutil LJNDR. Prescription drug use and misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016:A1‐A24.
- 5. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US FDA . Assessment of abuse potential of drugs—draft guidance In: U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research. Rockville, MD: U.S. Department of Health and Human Services; Food and Drug Administration Center for Drug Evaluation and Research; https://www.safetypharmacology.org/FDA_DraftGuide_DrugAbuse.pdf. Published 2010. Accessed May 24, 2020. [Google Scholar]
- 7. US FDA . Assessment of abuse potential of drugs—guidance for industry In: U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; https://www.fda.gov/media/116739/download. Published 2017. Accessed May 24, 2020. [Google Scholar]
- 8. Drug Abuse Warning Network, 2011 : National Estimates of Drug‐Related Emergency Department Visits: Center for Behavioral Health Statistics and Quality (CBHSQ) Substance Abuse and Mental Health Services Administration (SAMHSA). Rockville, MD: Center for Behavioral Health; Statistics and Quality Substance Abuse and Mental Health Services Administration; https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. Published 2013. Accessed May 24, 2020. [Google Scholar]
- 9. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo‐ and midazolam‐controlled phase I single ascending‐dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274‐283. [DOI] [PubMed] [Google Scholar]
- 10. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 11. Rusted JM, Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology (Berl). 1988;96(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 12. Sahakian BJ, Morris RG, Evenden JL, et al. A comparative study of visuospatial memory and learning in Alzheimer‐type dementia and Parkinson's disease. Brain. 1988;111(Pt 3):695‐718. [DOI] [PubMed] [Google Scholar]
- 13. Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70(3 suppl):S13‐S40. [DOI] [PubMed] [Google Scholar]
- 14. Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 suppl):S41‐S54. [DOI] [PubMed] [Google Scholar]
- 15. Schoedel K, Shram M, Levy‐Cooperman N, et al. Defining clinically important differences in subjective abuse potential measures. Poster presented at 74th Annual Meeting of the College on Problems of Drug Dependence, 2012.
- 16. Win NN, Fukayama H, Kohase H, Umino M. The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth Analg. 2005;101(1):97‐102. [DOI] [PubMed] [Google Scholar]
- 17. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine‐type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy‐Cooperman N, Schoedel KA, Chakraborty B, Blum D, Cheng H. Abuse liability assessment of eslicarbazepine acetate in healthy male and female recreational sedative users: a phase I randomized controlled trial. Epilepsy Behav. 2016;61:63‐71. [DOI] [PubMed] [Google Scholar]
- 19. Wockhardt UK Ltd . Summary of Product Characteristics—midazolam 5mg/ml solution for injection or infusion. http://www.mhra.gov.uk/spc-pil/index.htm?prodName=MIDAZOLAM%205MG/ML%20SOLUTION%20FOR%20INJECTION%20OR%20INFUSION&subsName=MIDAZOLAM&pageID=SecondLevel. Accessed October 14, 2019. [Google Scholar]
- 20. Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol. 1990;10(4):237‐243. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


