Abstract
Epacadostat is a potent and highly selective inhibitor of indoleamine 2,3‐dioxygenase 1 (IDO1). Here we report results from the open‐label, dose‐escalation, Phase 1b ECHO‐110 study evaluating epacadostat plus atezolizumab in patients with previously treated Stage IIIB/IV nonsmall cell lung cancer (NSCLC). Eligible patients had received ≥1 prior line of platinum‐based chemotherapy (≥2 cycles) and no prior checkpoint/IDO inhibitors treatment. Oral epacadostat (25, 50, 75, 100, 200 or 300 mg) was administered twice daily (BID) with intravenous atezolizumab 1,200 mg every 3 weeks (Q3W). Primary endpoints were safety, tolerability and dose‐limiting toxicities (DLTs). Twenty‐nine patients received ≥1 dose of treatment. The maximum tolerated dose of epacadostat was not reached. Two patients had DLTs: one patient with Grade 3 dehydration and hypotension (epacadostat 200 mg BID); one patient with Grade 3 hyponatremia and Grade 4 autoimmune encephalitis (epacadostat 300 mg BID). Twenty‐three patients (79%) had treatment‐related adverse events (AEs); seven patients (24%) experienced Grade 3/4 events; five patients (17%) discontinued treatment due to treatment‐related AEs. No fatal treatment‐related AEs occurred. One patient achieved a partial response (objective response rate, 3%), which was maintained for 8.3 months; eight patients had stable disease. Baseline tumoral programmed cell death ligand 1 (PD‐L1) and IDO expression were low among patients with evaluable samples (1 of 23 expressed PD‐L1; 5 of 17 expressed IDO). Epacadostat pharmacokinetics was comparable to historical controls. Epacadostat, at doses up to 300 mg BID, combined with atezolizumab 1,200 mg Q3W was well tolerated in patients with previously treated NSCLC, although clinical activity was limited.
Keywords: epacadostat, atezolizumab, nonsmall cell lung cancer, combination
Short abstract
What's new?
There has been considerable interest in investigating combination treatment strategies that target separate but complementary immune‐evasion pathways, in patients with advanced non‐small‐cell lung cancer (NSCLC). The goal is to enhance the efficacy of immune checkpoint inhibitors. In this study, the authors found that combining the IDO1 enzyme inhibitor epacadostat with the PD‐L1 checkpoint inhibitor atezolizumab was generally well tolerated. However, clinical activity was limited. These results provide important insights into the challenges associated with developing combination immunotherapies in NSCLC.
Abbreviations
- AE
adverse event
- ALK
anaplastic lymphoma kinase
- BID
twice daily
- CI
confidence interval
- DLT
dose‐limiting toxicity
- EGFR
epidermal growth factor receptor
- IC
immune cell
- IC50
half‐maximal inhibitory concentration
- IDO1
indoleamine 2,3‐dioxygenase
- KRAS
KRAS proto‐oncogene, GTPase
- MTD
maximum tolerated dose
- NSCLC
nonsmall cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed cell death 1 protein
- PD‐L1
programmed cell death ligand 1
- PFS
progression‐free survival
- PK
pharmacokinetic
- Q3W
every 3 weeks
- RECIST
Response Evaluation Criteria in Solid Tumors
- TC
tumor cell
- TKI
tyrosine kinase inhibitor
- TME
tumor microenvironment
Introduction
In recent years, anti‐programmed cell death 1 protein (PD‐1)/programmed cell death ligand 1 (PD‐L1) monoclonal antibodies have provided breakthrough treatment options for patients with advanced or metastatic nonsmall cell lung cancer (NSCLC).1, 2, 3 Atezolizumab is a PD‐L1 inhibitor that has been approved as monotherapy for the treatment of patients with previously treated metastatic NSCLC or in combination with chemotherapy in patients with newly diagnosed metastatic nonsquamous NSCLC.3 However, many patients do not respond to PD‐L1 monotherapy, or develop disease progression after an initial response, possibly due to other immune evasion mechanisms besides checkpoint pathways.4, 5, 6 Therefore, there has been considerable interest in investigating combination treatment strategies targeting complementary and distinct immune evasion pathways to enhance response.
Indoleamine 2,3‐dioxygenase 1 (IDO1) is an intracellular enzyme primarily expressed in tumor cells, endothelial cells, dendritic cells and macrophages within the tumor microenvironment (TME).7, 8 IDO1 catalyzes the first and rate‐limiting step in the tryptophan–kynurenine catabolism pathway,9 inducing suppression of effector T cells and activation of immunosuppressive cells (regulatory T cells),10 myeloid‐derived suppressor cells,11 and tumor‐associated macrophages.12 These changes contribute to immunosuppression within the TME, and, as a result, coexpression of IDO1 and PD‐L1 has been associated with poor prognosis in patients with advanced NSCLC.13 Taken together, these findings underpin the rationale for investigating IDO1 as a potential therapeutic target in NSCLC.
Epacadostat is a potent and highly selective inhibitor of the IDO1 enzyme.14 In preclinical studies, epacadostat was shown to decrease tryptophan metabolism and have immunostimulatory effects on the TME, including enhanced T‐cell and natural killer cell proliferation, decreased dendritic cell apoptosis and reduced regulatory T‐cell expansion.15, 16, 17 Furthermore, encouraging tumor suppression results were observed with an IDO1 inhibitor plus an anti‐PD‐L1 antibody in a murine melanoma model.16 Optimal IDO1 inhibition with epacadostat was reported at doses that achieved steady‐state predose exposures that exceeded the half‐maximal inhibitory concentration (IC50).17 In the first‐in‐human Phase 1 study, epacadostat monotherapy was well tolerated at doses up to 700 mg twice daily (BID) in patients with advanced solid malignancies; doses ≥100 mg BID were shown to achieve predose exposure above the IC50 at steady state.18 Collectively, these findings supported the investigation of epacadostat with PD‐1/PD‐L1 blockers, such as atezolizumab.
Here we report the results of ECHO‐110 an open‐label Phase 1b study that evaluated epacadostat plus atezolizumab in patients with previously treated advanced or metastatic NSCLC.
Materials and Methods
Patients
Eligible patients included adults with histologically or cytologically confirmed Stage IIIB/IV NSCLC who had measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1, a life expectancy of at least 12 weeks, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and an archival tumor specimen or willingness to undergo a pretreatment tumor biopsy. Patients must have received ≥1 prior line of standard platinum‐based chemotherapy for ≥2 cycles (inclusive of treatment in adjuvant setting). Patients must not have received prior checkpoint inhibitor therapy or IDO inhibitors. Patients with tumors bearing driver mutations, including anaplastic lymphoma kinase (ALK) rearrangement or epidermal growth factor receptor (EGFR) mutation, should have had disease progression on two targeted tyrosine kinase inhibitors (TKIs). Patients with symptomatic central nervous system metastases were excluded.
Study design and treatment
This study was planned to include a 3 + 3 epacadostat dose escalation in combination with atezolizumab, followed by three dose‐expansion cohorts. During dose escalation, patients received epacadostat 25, 50, 75, 100, 200 or 300 mg administered orally BID in combination with atezolizumab 1,200 mg intravenously every 3 weeks (Q3W). Patients could continue receiving the combination treatment in continuous 21‐day cycles as long as they benefited from treatment per the investigator's medical judgment and did not meet study withdrawal/discontinuation criteria, such as confirmed radiographic disease progression per modified RECIST 1.1 or unacceptable toxicity that did not resolve in 4 weeks. Exceptions for patients to continue treatment were possible at the discretion of the investigator upon consultation with the sponsor.
On June 12, 2017, approximately 2.5 years after initiating the study, the sponsor (Incyte Corporation, Wilmington, DE), collaboration partner (Genentech, Inc., South San Francisco, CA) and study investigators mutually decided to terminate patient enrollment based on slow recruitment and diverging development strategies for epacadostat and atezolizumab. Therefore, the planned dose‐expansion portion of the study was not conducted.
The study protocol and amendments were approved by independent ethics committees or institutional review boards at each study site. Study conduct conformed to Good Clinical Practice guidelines and ethical requirements outlined in the Declaration of Helsinki. All patients provided written informed consent prior to any study procedures.
Objective and assessments
The primary objective of this study was to assess the safety, tolerability and dose‐limiting toxicities (DLTs) of a pharmacologically active dose of epacadostat administered in combination with atezolizumab in patients with previously treated Stage IIIB/IV NSCLC. Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events Version 4.0. Immune‐mediated AEs were defined as any AEs of potential immunologic etiology requiring treatment with systemic corticosteroids. DLTs included any protocol‐defined AEs occurring up to and including study Day 21, regardless of attribution to study treatment. Such AEs could include, for example, Grade 4 thrombocytopenia or neutropenia lasting >7 days; febrile neutropenia; most Grade 3/4 nonhematologic toxicities or Grade ≥2 episcleritis, uveitis or iritis. Additionally, a DLT could be any toxicity that led to patients being unable to receive 75% of epacadostat or 1 dose of atezolizumab during the DLT observation period or any treatment‐related toxicity that resulted in a delay of >3 weeks in starting Cycle 2.
Dose escalation was permitted if no more than 0 or 1 of the first 3 or 6 evaluable patients, respectively, experienced a DLT. If >1 or ≥2 of the first 3 or 6 evaluable patients in a dose cohort, respectively, had a DLT, the next lower dose of epacadostat was deemed the maximum tolerated dose (MTD).
Secondary endpoints included objective response rate (ORR), duration of response, progression‐free survival (PFS) and duration of disease control. Tumor response was assessed every 6 weeks by the investigator per RECIST 1.1. Modified RECIST was used to guide treatment if imaging showed progressive disease (PD); clinically stable patients could continue study treatment at the investigator's discretion until confirmatory tumor assessment ≥4 weeks later.
Exploratory endpoints included overall survival (OS), pharmacokinetics (PK) of epacadostat and atezolizumab and baseline PD‐L1 and IDO1 expression. Predose and postdose blood samples were collected at protocol‐defined time points for PK assessments. PD‐L1 expression on immune and tumor cells was evaluated using the SP142 immunohistochemistry assay and scored as described previously.19, 20 IDO1 expression in tumor cells was evaluated using the SP260 antibody clone (Indivumed GmbH, Hamburg, Germany); a composite H score ≥1 was used as an arbitrary cutoff for IDO1 positivity.
Statistical analysis
Planned enrollment in the dose‐escalation phase was up to 48 patients to determine the MTD of epacadostat when administered in combination with atezolizumab. All enrolled patients who received ≥1 dose of the study treatment were included in the safety and efficacy analyses. Those who provided ≥1 postdose blood sample were evaluable for PK assessments. Descriptive statistics were used to summarize findings where appropriate.
PK data were analyzed using a model‐independent approach (i.e., noncompartmental analysis) with commercial software (Phoenix WinNonlin v7, Certara USA, Princeton, NJ). Predose (trough) samples were analyzed with an assigned time point of 0. Nominal times after dosing for postdose samples were used for PK analysis when available.
Trial registration and availability of data and material
This trial is registered in the National Institutes of Health clinical trials database (NCT02298153). The data sets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Results
Patients
Between February 25, 2015, and June 16, 2017, 29 patients were enrolled. Most patients received platinum‐based chemotherapy in conjunction with a folic acid analogue as prior treatment. All patients were PD‐1/PD‐L1 blockade and IDO blockade naïve. Median age was 63 years (range, 45–78). The majority of patients were male (66%) and white (83%) and had ECOG PS of 1 (72%) (Table 1). Adenocarcinoma was the most common histology (69%). All patients had known EGFR and ALK status (3% and 7%, respectively, were mutated), and 21 had known KRAS proto‐oncogene, GTPase (KRAS) status at baseline (28% were mutated). Of 23 patients who were evaluable for PD‐L1 expression, 1 had positive PD‐L1 expression on tumor cells (TC2) and 5 others had positive expression on immune cells (all IC1). Seventeen patients had IDO1 expression results; results were positive for 5 patients and negative for 12 patients. Three patients had both positive PD‐L1 expression (on either tumor or immune cells) and positive IDO1 expression. The median number of prior therapies for advanced disease was 1 (range, 0–6).
Table 1.
Baseline demographics and disease characteristics
| Variable | Total (N = 29) |
|---|---|
| Age, median (range), years | 63 (45–78) |
| Male, n (%) | 19 (66) |
| Race (%) | |
| White | 24 (83) |
| Black/African American | 3 (10) |
| Other | 2 (7) |
| ECOG PS, n (%) | |
| 0 | 8 (28) |
| 1 | 21 (72) |
| Histopathology, n (%) | |
| Adenocarcinoma | 20 (69) |
| Squamous | 2 (7) |
| Adenosquamous (mixed) | 1 (3) |
| Bronchoalveolar | 1 (3) |
| Other | 5 (17) |
| PD‐L1 status, n (%)1 | |
| TC3 or IC3 | 0 |
| TC2/3 or IC2/3 | 1 (3) |
| TC1/2/3 or IC1/2/3 | 6 (21) |
| TC0 and IC0 | 17 (59) |
| Unknown | 6 (21) |
| IDO1 status, n (%)2 | |
| Positive (H score ≥ 1) | 5 (17) |
| Negative (H score < 1) | 12 (41) |
| Unknown | 12 (41) |
| EGFR mutated, n (%) | 1 (3) |
| KRAS mutated, n (%) | 8 (28) |
| ALK rearrangement, n (%) | 2 (7) |
| PD‐L1 positive and IDO1 positive, n (%) | 3 (10) |
| No. of prior therapies for advanced/metastatic disease, n (%) | |
| 0 | 1 (3)3 |
| 1 | 17 (59) |
| 2 | 5 (17) |
| ≥3 | 6 (21) |
| Prior treatment with TKI, n (%) | 6 (21) |
| Prior surgery, n (%) | 14 (48) |
| Prior radiation, n (%) | 13 (45) |
| History of smoking, n (%) | 22 (76) |
Abbreviations: ALK, anaplastic lymphoma kinase; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IC, immune cell; IDO1, indoleamine 2,3‐dioxygenase 1; KRAS, KRAS proto‐oncogene, GTPase; PD‐L1, programmed cell death 1 ligand 1; TC, tumor cell; TKI, tyrosine kinase inhibitor.
Twenty‐three patients were available and evaluable for PD‐L1 expression. Tumor cells expressing PD‐L1 were scored as a percentage of total tumor cells: TC3 ≥50%, TC2 ≥5% and <50%, TC1 ≥1% and <5% and TC0 <1%. Tumor‐infiltrating immune cells expressing PD‐L1 were scored as a percentage of tumor area: IC3 ≥10%, IC2 ≥5% and <10%, IC1 ≥1% and <5% and IC0 <1%.19, 20
IDO1 expression was evaluated in tumor cells; a composite H score ≥1 was used as an arbitrary cutoff for IDO1 positivity.
This patient received platinum in the adjuvant setting.
Three patients were treated with epacadostat 25 mg BID, four with 50 mg BID, four with 75 mg BID, five with 100 mg BID, seven with 200 mg BID and six with 300 mg BID. As of the November 8, 2017, data cutoff, all patients had discontinued the combination treatment due to PD (n = 24), AEs (n = 2), physician decision (n = 2) or patient decision (n = 1). The median duration of epacadostat treatment was 43 days (range, 8–362 days). Most patients received ≤4 doses of atezolizumab. The median follow‐up was 27 weeks (range, 7–93 weeks).
Safety
Two patients had DLTs: one patient receiving epacadostat 200 mg BID plus atezolizumab 1,200 mg Q3W experienced Grade 3 dehydration and Grade 3 hypotension; one patient receiving epacadostat 300 mg BID plus atezolizumab 1,200 mg Q3W experienced Grade 3 hyponatremia and Grade 4 autoimmune encephalitis. All DLTs resolved except for the Grade 4 encephalitis, which decreased to Grade 3 after treatment with antibiotics plus methylprednisolone for 8 days followed by an oral prednisone taper that was ongoing at the time of the patient's death due to disease progression 6 weeks later. An MTD was not determined.
Treatment‐related AEs occurred in 23 patients (79%); the most common AEs (reported in ≥15% of patients) were fatigue (38%), decreased appetite (17%), nausea (17%) and rash (17%) (Table 2). Grade ≥3 treatment‐related AEs were observed in seven patients (24%); events occurring in >1 patient were hypotension, lipase increased and rash (n = 2 each). Treatment‐related AEs led to dose interruption in five patients (17%) and dose reduction in one patient (3%). Five patients (17%) discontinued treatment because of treatment‐related AEs, including autoimmune encephalitis, hyponatremia and hypotension in one patient; infusion‐related reaction and throat tightness in one patient; and increased lipase, pneumonitis and maculopapular rash in one patient each. No treatment‐related AEs led to death.
Table 2.
Most common treatment‐related AEs occurring in ≥10% of all patients
| AE, n (%) | Total (N = 29) | Epacadostat dose (+atezolizumab 1,200 mg Q3W) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg BID (n = 3) | 50 mg BID (n = 4) | 75 mg BID (n = 4) | 100 mg BID (n = 5) | 200 mg BID (n = 7) | 300 mg BID (n = 6) | |||||||||
| All grade | Grade ≥31 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | All grade | Grade ≥3 | |
| Total | 23 (79) | 7 (24) | 1 (33) | 0 | 2 (50) | 1 (25) | 4 (100) | 1 (25) | 4 (80) | 1 (20) | 6 (86) | 2 (29) | 6 (100) | 2 (33) |
| Fatigue | 11 (38) | 0 | 1 (33) | 0 | 0 | 0 | 2 (50) | 0 | 2 (40) | 0 | 4 (57) | 0 | 2 (33) | 0 |
| Rash2 | 5 (17) | 2 (7) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 1 (20) | 2 (29) | 1 (14) | 2 (33) | 0 |
| Nausea | 5 (17) | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 2 (40) | 0 | 1 (14) | 0 | 1 (17) | 0 |
| Decreased appetite | 5 (17) | 0 | 1 (33) | 0 | 1 (25) | 0 | 2 (50) | 0 | 0 | 0 | 1 (14) | 0 | 0 | 0 |
| Vomiting | 3 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 0 | 1 (14) | 0 | 1 (17) | 0 |
| Chills | 3 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (43) | 0 | 0 | 0 |
| Pyrexia | 3 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (29) | 0 | 1 (17) | 0 |
| Weight decreased | 3 (10) | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (29) | 0 | 0 | 0 |
| Dysgeusia | 3 (10) | 0 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (29) | 0 | 0 | 0 |
| Dyspnea | 3 (10) | 0 | 0 | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 | 2 (29) | 0 | 0 | 0 |
Abbreviations: AE, adverse event; BID, twice daily; MedDRA, Medical Dictionary for Regulatory Activities.
Other Grade ≥3 treatment‐related AEs not listed include hypotension and lipase increased (n = 2 each) and autoimmune encephalitis, dehydration, hyponatremia, hypophosphatemia, infusion‐related reaction and pneumonitis (n = 1 each).
Rash includes the following MedDRA preferred terms: rash, rash maculopapular and rash pruritic.
Three patients (10%) experienced immune‐mediated AEs, including one patient with Grade 3 maculopapular rash (epacadostat 100 mg BID), one patient with Grade 3 infusion‐related reaction and Grade 2 throat tightness (epacadostat 200 mg BID) and one patient with Grade 4 autoimmune encephalitis (epacadostat 300 mg BID).
Antitumor activity
Objective response was observed in one patient (ORR, 3%) who was treated with epacadostat 100 mg BID plus atezolizumab 1,200 mg Q3W (Fig. 1 ); the response was partial per RECIST 1.1 and maintained for 8.3 months. This patient presented with Stage IV disease, large cell neuroendocrine histology, had no prior treatment with TKI, had wild‐type EGFR, no ALK rearrangement and unknown KRAS mutation status. Additionally, this patient had prior radiation and surgery and tested negative for both PD‐L1 and IDO1 expression. Eight patients had stable disease as their best response. The median duration of disease control was 4.1 months (95% confidence interval [CI], 2.3–11.2 months). Overall, the median PFS was 1.4 months (95% CI, 1.3–1.4 months) and the median OS was 12.7 months (95% CI, 3.4–17.2 months).
Figure 1.
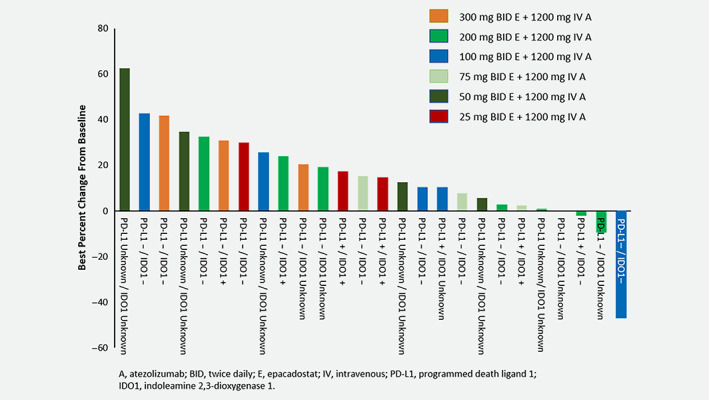
Best percentage change from baseline in target lesions for patients with postbaseline assessments. Patients are listed in order of best percent change from baseline per RECIST 1.1. Epacadostat dose, PD‐L1 status and IDO1 status are listed for each patient.
Pharmacokinetics and immunogenicity
Epacadostat PK values at steady state (Supporting Information Table S1 ) demonstrated a dose‐dependent plasma exposure (area under the concentration vs. time curve and maximum observed plasma concentration [C max]), with time of observed C max at 2 h. For patients treated with epacadostat 25, 50, 75, 100 and 200 mg BID, 0/3, 3/4, 0/4, 3/5 and 6/7, respectively, achieved Cycle 1 Day 8 (steady‐state) predose exposure above the IC50 determined in the nonclinical model.17 Since no patients treated with epacadostat 300 mg BID had Cycle 1 Day 8 samples, Cycle 2 Day 1 was evaluated and 2/2 patients achieved this level of exposure.
The PK measurements and rates of antidrug antibody associated with atezolizumab in this study were comparable to those previously observed with monotherapy (Incyte, data on file).
Discussion
Epacadostat at doses up to 300 mg BID in combination with atezolizumab 1,200 mg Q3W was generally well tolerated in patients with previously treated Stage IIIB/IV NSCLC. Among the 29 patients enrolled in this study, 2 had DLTs and no MTD was determined. No new safety signals were detected for either epacadostat or atezolizumab, as reported AEs in this study were similar compared to AEs reported for either epacadostat or atezolizumab as monotherapy.18, 20 For example, fatigue, nausea and decreased appetite were the most frequently reported AEs in patients enrolled in this study and in patients receiving epacadostat monotherapy.18 A similar AE profile was also experienced by patients receiving atezolizumab monotherapy, as decreased appetite, dyspnea and nausea were the most frequently reported AEs in the POPLAR study.20
Antitumor response was observed in only one patient (partial response). This could be, in part, due to the majority of enrolled patients having known negative baseline PD‐L1 expression (PD‐L1 expression on <1% of tumor and immune cells). It is possible that this is related to emerging data for enriched response to PD‐1 monotherapy in patients with PD‐L1 expressing NSCLC, such that patients with negative PD‐L1 expression were preferentially considered for investigational combination treatments such as in this study. Because of the small study sample size and high proportion of patients having unknown IDO1 expression, the significance of such a biomarker in patients with NSCLC remains to be elucidated. Identification of IDO1 expression in later studies of epacadostat was performed using an RNAscope assay, which provided greater sensitivity of detection and a greater dynamic range of IDO1 expression compared to the immunohistochemistry approach utilized in this study.21, 22
The PK characteristics of epacadostat were generally consistent with those of epacadostat monotherapy and epacadostat plus a PD‐1 inhibitor reported in previous studies.18, 23 Atezolizumab PK measurements in this study were also similar to those previously reported for the monotherapy. Together, these findings suggest that adding atezolizumab to epacadostat had no effect on the PK of either drug. Additionally, most patients treated with epacadostat 50, 100, 200 and 300 mg BID achieved Cycle 1 Day 8 (steady‐state) or Cycle 2 Day 1 pre‐dose exposure above the IC50 determined in the nonclinical model.17
As previously mentioned, the current study (ECHO‐110) was terminated early due to slow recruitment and diverging development strategies for epacadostat and atezolizumab. The combination was not further evaluated in this study or elsewhere in the epacadostat development program.
At the time of this publication, the pivotal Phase 3 ECHO‐301/KEYNOTE‐252 study evaluating epacadostat 100 mg BID plus pembrolizumab 200 mg Q3W in patients with unresectable or metastatic melanoma did not meet the primary endpoint of improving PFS in the overall population compared to pembrolizumab monotherapy.23 Given these results, the utility of combining epacadostat (at doses that provide steady state trough concentrations exceeding IC50) and inhibitors of the PD‐1/PD‐L1 pathway remains unclear. Of note, the dose of epacadostat (100 mg BID) in this melanoma study differed from the doses described in this report (50, 100, 200 and 300 mg BID), as independent studies were used establish recommended dose for further development in combination with pembrolizumab versus atezolizumab.
As nearly all patients in this study were PD‐L1 negative and efficacy was limited in this population, we conclude that epacadostat plus PD‐1/PD‐L1 blockade is not effective to be pursued in PD‐L1 negative patients. Whether this treatment strategy in PD‐L1 expressing NSCLC, or potentially in combination with platinum‐based chemotherapy, has an application in NSCLC depends on future results from the ongoing, randomized, Phase 2 studies ECHO‐305 (NCT03322540) and ECHO‐306 (NCT03322566).
Conflict of interests
M.D.H. reports grants, nonfinancial support and personal fees from Bristol‐Myers Squibb; consultancy fees from Merck, Genentech/Roche, Nektar, Syndax, Mirati, Immunai and Shattuck Labs; travel support/honoraria from A7, Eli Lilly, Merck and Bristol‐Myers Squibb; consultancy fees and nonfinancial support from AstraZeneca outside the submitted work. M.D.H. also has a patent PCT/US2015/062208 licensed to PGDx. S.G. reports research funding to his institution from Bristol‐Myers Squibb, Iovance, Genentech/Roche and Takeda/Ariad; and consultancy fees from NextCure, Nektar and Bristol‐Myers Squibb. L.Q.M.C. reports minor personal consulting advisory board participation and fees from Genentech, AstraZenenca, Merck, Bristol‐Myers Squibb, Pfizer and Novartis outside the submitted work; research grant funding to her institution from Incyte and Genentech for the conduct of this study; research grant funding to her institution from Bristol‐Myers Squibb, AstraZeneca, Pfizer and Merck outside the submitted work. M.G. reports research support to his institution from Genentech. M.M.A. reports grants and personal fees from Genentech, Bristol‐Myers Squibb and AstraZeneca; personal fees from Merck, Maverick, Blueprint Medicines, Syndax, Ariad, Nektar and Gritstone and grants from Lilly outside the submitted work. E.C. reports being an employee of Genentech and owns stock in Genentech/Roche. X.G. is an employee of Incyte and owns stock in Incyte. G.Z. is an employee of Incyte and owns stock in Incyte. C.W. is an employee of Incyte and owns stock in Incyte. L.L. is an employee of Incyte and owns stock in Incyte. R.S.H. reports research support for clinical trials to her institution from Genentech/Roche, Novartis, Agios, Daichii Sankyo, Corvus, Mirati, Abbvie, Eli Lilly, Exelixis and Turning Point Therapeutics; consulting honoraria from Novartis, Boehringer Ingelheim, Tarveda, Apollomics and Chugai outside the submitted work.
Supporting information
Table S1 Epacadostat pharmacokinetics at steady state
Acknowledgements
Editorial assistance was provided by Ann T. Yeung, PhD, CMPP and Michael R. Convente, PhD, of Scientific Pathways, Inc (Warren, NJ) and funded by Incyte Corporation.
Laura Q. M. Chow's current address is: University of Washington, Seattle, Washington
References
- 1. Opdivo (nivolumab) [prescribing information]. Princeton, NJ: Bristol‐Myers Squibb, 2020. [Google Scholar]
- 2. Keytruda (pembrolizumab) [prescribing information]. Whitehouse Station, NJ: Merck Sharp & Dohme Corporation, 2019. [Google Scholar]
- 3. Tecentriq (atezolizumab) [prescribing information]. South San Francisco, CA: Genentech, Inc., 2019. [Google Scholar]
- 4. Drake CG. Combination immunotherapy approaches. Ann Oncol 2012;23(suppl 8):viii41‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khalil DN, Smith EL, Brentjens RG, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13:273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen DC, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 7. Théate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2,3 dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res 2015;3:161–72. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Q, Kuang DM, Wu Y, et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor‐associated macrophages. J Immunol 2012;188:1117–24. [DOI] [PubMed] [Google Scholar]
- 9. Prendergast GC, Malachowski WJ, Mondal A, et al. Indoleamine 2,3‐dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol 2018;336:175–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munn DH, Mellor AL. Indoleamine 2,3‐dioxygenase and tumor‐induced tolerance. J Clin Invest 2007;117:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmgaard RB, Zamarin D, Li Y, et al. Tumor‐expressed IDO recruits and activates MDSCs in a Treg‐dependent manner. Cell Rep 2015;13:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang XF, Wang HS, Wang H, et al. The role of indoleamine 2,3‐dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP‐1 cells. Cell Immunol 2014;289:42–8. [DOI] [PubMed] [Google Scholar]
- 13. Kozuma Y, Takada K, Toyokawa G, et al. Indoleamine 2.3‐dioxygenase 1 and programmed cell death‐ligand 1 co‐expression correlates with aggressive features in lung adenocarcinoma. Eur J Cancer 2018;101:20–9. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010;115:3520–30. [DOI] [PubMed] [Google Scholar]
- 15. Moon YW, Hajiar J, Hwu P, et al. Targeting the indoleamine 2,3‐dioxygenase pathway in cancer. J Immunother Cancer 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spranger S, Koblish HK, Horton B, et al. Mechanism of tumor rejection with doublets of CTLA‐4, PD‐1/PD‐L1, or IDO blockade involves restored IL‐2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2014;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine‐2,3‐dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO‐expressing tumors. Mol Cancer Ther 2010;9:489–98. [DOI] [PubMed] [Google Scholar]
- 18. Beatty GL, O'Dwyer PJ, Clark J, et al. First‐in‐human phase 1 study of the oral inhibitor of indoleamine 2,3‐dioxygenase‐1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 2017;23:3269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ventana PD‐L1 (SP142) Assay. Tucson, AZ: Ventana Medical Systems, 2018. https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp142-assay2.html. [Google Scholar]
- 20. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated on‐small cell lung cancer (POPLAR): a multicenter, open‐label, phase 2 randomized controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- 21. Mitchell TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open‐label phase I/II trial (ECHO‐202/KEYNOTE‐037). J Clin Oncol 2018;36:3223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pratta M. RNAscope as a platform to detect IDO1 expression in tumor tissue sections. J Clin Oncol 2018;36(5 Suppl):116. [Google Scholar]
- 23. Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus pembrolizumab alone in patients with unresectable or metastatic melanoma: results of the phase 3 ECHO‐301/KEYNOTE‐252 study. J Clin Oncol 2018;36(15 Suppl):108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Epacadostat pharmacokinetics at steady state


