Abstract
Objectives
Temporary, percutaneous peripheral nerve stimulation (PNS) has been shown to provide analgesia for acute postoperative pain, postamputation pain, and low back pain. The implanted device stimulates the neural target for up to 60 days at which point the leads are extracted. Patients have demonstrated prolonged analgesia continuing after extraction of the leads. The purpose of this case series is to demonstrate peripheral neural targets that could feasibly be used to treat various pain syndromes prevalent in the oncologic population.
Materials and Methods
A temporary, percutaneous PNS was implanted under ultrasound guidance in 12 oncologic chronic pain patients seen in an outpatient pain clinic who had failed medical and/or interventional management. The device was implanted for up to 60 days. Clinical progress of pain and functional capacity was monitored through regular clinical visits.
Results
The case series presents seven successful cases of implementation of the PNS to treat oncologic pain. Three of these cases demonstrate targeting of proximal spinal nerves to treat truncal neuropathic pain and lumbar radicular pain. The four remaining cases demonstrate successful targeting of other peripheral nerves and brachial plexus. We also share five failed cases without adequate pain relief with PNS.
Conclusions
PNS has potential uses in the treatment of oncologic pain. Further high‐quality studies should be designed to further elucidate use of the PNS to treat oncologic pain.
Keywords: Oncologic pain, peripheral nerve stimulator, post‐herpetic neuralgia, post‐mastectomy pain, post‐thoracotomy pain
INTRODUCTION
Of the 14 million cancer patients in the United States, up to as many as 40% have pain as a significant symptom 1. Neuropathic pain in oncologic patients is due to both the disease itself and associated treatments. Tumors may invade or compress nerves 2, 3. Surgical resection can result in nerve transection and scar tissue formation. Commonly used chemotherapy agents such as vinca‐alkaloids, taxanes, platinum‐based agents, anti‐microtubule agents, and anti‐angiogenesis agents are known to cause neuropathy in 30–70% of patients, often of dose‐limiting severity 4. Lastly, radiation therapy can cause fibrosis causing tissue and nerve damage 2, 4.
The use of a temporary, percutaneous peripheral nerve stimulator (PNS) (SPRINT® PNS system, SPR Therapeutics, Cleveland, OH, USA) for controlling neuropathic pain has been supported clinically in the literature. The device has received FDA‐approval for implantation for up to 60‐days as treatment for both acute and chronic pain. In addition to providing analgesia during stimulation, a unique feature of the device is that it has shown to continue to provide analgesia after extraction of the PNS leads. Currently, data are limited to one randomized‐control trial in postamputation pain 5, and multiple case series in axial low back pain 6, 7, acute postoperative pain after rotator cuff surgery 8, total knee arthroplasty 9, and foot surgery 10. Analgesic benefits up to 12 months 5 and 4 months 6, 7 after extraction of the PNS were noted in postamputation and low back pain patients, respectively.
Experience for the use of this device is developing. In the oncologic population, minimal information is known regarding concerns or appropriate targets for pain relief. While controlled trials may be difficult to perform in the oncologic population, clinical experience is valuable to improve patient care. We highlight interesting cases and observed limitations of this therapy in this population and propose recommendations for the use of PNS in chronic intractable neuropathic pain related to cancer.
PNS being discussed in this article is a form of peripheral neuromodulation directly stimulating nerves that are the source of the patient's pathology. In these cases, the stimulator will be located proximally along the anatomical pathway of the nerve innervating the region of pain. The stimulation will not necessarily occur at the direct region of pain, although the paresthesia experienced should cover the painful region. Initial devices required surgical implantation with placement of leads in direct contact with peripheral nerves. Newer devices such as the percutaneous PNS demonstrated in this study allow percutaneous implantation under visual guidance under fluoroscopy or ultrasound. This technology needs to be differentiated from another form of peripheral neuromodulation called peripheral nerve field stimulation (PNfS). This procedure involves stimulating the subcutaneous tissue directly in the region of the pain. This stimulation acts on the various afferent peripheral nerves innervating the region and altering their nociceptive pathways 11.
MATERIALS AND METHODS
Patient Selection
This was an institutional review board (IRB) approved single‐center retrospective analysis of patients at Memorial Sloan Kettering Cancer Center (MSKCC), approved via a waiver for informed consent and supported by MSKCC support grant (P30 core grant). Data from all consecutive cases with management of cancer‐related pain using the PNS device at MSKCC outpatient interventional pain clinic from September 2017 through June 2019 were included in the study.
Technique
A portable ultrasound (GE LOGIQ P9™, Chicago, IL, USA) and either linear probe (8–10 Hz) for femoral nerve, suprascapular nerve, and brachial plexus or curvilinear probe (1–5 MHz) for the proximal spinal nerves and sciatic nerve was used to visualize the nerve. Additionally, fluoroscopy was used to confirm appropriate positioning of the leads targeting proximal spinal nerves. First patient was positioned to optimize visualization of the target nerve. Once the neural target was appropriately visualized by ultrasound, an 18‐gauge stimulating needle (SPR Therapeutics) was inserted into the skin and directed towards the target nerve. Electrical stimulation was initiated once the 18‐gauge stimulating needle was within 1–1.5 cm of the nerve. Per manufacturer guidelines (SPR Therapeutics), a pulsed, square waveform was applied at a frequency of 100 Hz using the manufacturer‐provided pulse generator (SPR Therapeutics). The amplitude (range: 0.2–20 mA) and pulse duration (range: 2–100 μs) were titrated until “comfortable sensations” were produced in the distribution of the nerve covering the targeted region of pain. The 18‐gauge stimulating needle was repositioned as necessary to produce the desired “comfortable sensation.”
At this point, the inner cannula of the stimulating needle was removed, with the outer cannula stabilized. Then, the 12.5 cm, 20 G introducer needle was introduced into the cannula using Seldinger technique deploying the stimulating lead. The external portion of the stimulating lead was connected to the pulse generator. The lead and pulse generator were then secured to the skin using sterile occlusive dressing (provided by SPR Therapeutics).
Follow‐Up
After the implantation procedure, patients continued to follow‐up at the outpatient interventional pain clinic as clinically indicated per discretion of the pain provider. Patients were able to adjust the amplitude and pulse duration on their own according to a preprogramed scale. Patients continued to receive continuous, pulsed, square‐waveform stimulation until extraction of the leads. If needed, the amplitude and pulse duration were reprogrammed by the clinician and device representatives to again achieve the desired “comfortable sensation” at clinic visits. While the PNS was implanted and thus receiving stimulation, patients were contacted weekly via telephone or clinic visit to record the efficacy of treatment as measured by improved pain score, decreased use of analgesics, and functional improvement. Successful response to the PNS was defined as a 50% or greater relief of pain based on the analog pain score during both the stimulation phase and continuing after extraction of the leads compared to baseline prior to implantation.
At the end of the stimulation phase, patients had the PNS extracted. This occurred at 60 days as planned for all but one patient, who needed to have the leads removed earlier due to an urgent need for an MRI. The patients were then continued to be followed in the outpatient clinic to monitor for continued response after extraction of the device. The duration of continued analgesia of 50% or greater was also measured as a secondary outcome.
RESULTS
Tables 1 and 2 describe the clinical experience of seven patients with neuropathic pain who received successful benefit from the PNS. Two patients were treated for upper extremity radicular pain, three were treated for lower extremity neuropathic pain, and two were treated for truncal neuropathic pain. As mentioned earlier, the peripheral nerve stimulators were implanted for a duration of 60 days in all cases except one where it had to be extracted early due to urgent need for an MRI. Patient demographics, clinical pathology, and analgesic outcomes are shown in Tables 1 and 2.
Table 1.
Clinical Summary and Management of Successful Cases Prior to PNS.
| Patient number | Neural target | Pain syndrome | Clinical summary | Active treatments | Failed treatments | Diagnostic interventions |
|---|---|---|---|---|---|---|
| 1 | Proximal T2 and T4 spinal nerves | Post‐mastectomy pain syndrome | 46 F with breast cancer s/p mastectomy c/b post‐mastectomy pain with intercostobrachial neuralgia for 8 years | Amitriptyline 75 mg | Medical: Pregabalin, gabapentin, mexiletine, opioids | None |
| 2 | Proximal T7 spinal nerve | Post‐herpetic neuralgia | 54 M with CLL with T7 and T8 post‐herpetic neuralgia presenting with neuralgia and allodynia anterior and posterior chest wall for eight months | None | Medical: Pregabalin, gabapentin, transdermal lidocaine, and opioid | Intercostal nerve block and cryoablation (<1 week of pain relief) |
| 3 | Proximal L2 and L3 Spinal Nerve | L2‐3 radiculopathy | 56 M with psoas muscle sarcoma s/p radiation and resection with anterolateral thigh neuralgia | Pregabalin 50 mg bid, buprenorphine 5 mg | Medical: Gabapentin, duloxetine, mexiletine, opioids | Sympathetic block (relief for several weeks) |
| 4 | Suprascapular nerve | C4‐6 radiculopathy | 54 M with RCC with metastasis causing C4‐6 nerve root compression presenting with shoulder and posterior back pain for two months refractory to surgical decompression and fusion, and radiation | None | Medical: Pregabalin, duloxetine, and opioids; Interventional: Cervical medial branch block C4‐6, epidural steroid injections, glenohumeral joint injection, subacromial bursa injection, surgical decompression and fusion | C5 nerve root block (provided one day of relief) |
| 5 | Brachial plexus via supraclavicular approach | C5‐8 radiculopathy | 71 F with vaginal cancer with metastasis encasing brachial plexus nerve roots s/p radiation presenting with right arm and shoulder pain | Oxycodone 5 mg PRN, fentanyl patch 12.5 mcg/h | Medical: Pregabalin, gabapentin, and duloxetine | Suprascapular nerve block (100% pain relief) |
| 6 | Sciatic nerve | L5‐S1 radiculopathy | 71 M with lung adenocarcinoma with metastasis to L5 vertebral body s/p resection c/b L5‐S1 post‐surgical radiculopathy presenting with calf pain, foot pain, and inability to walk | Oxymorphone PRN | Medical: Pregabalin, gabapentin, and long‐acting opioids; Interventional: SCS trial | None |
| 7 | Femoral nerve | Femoral nerve neuropathy | 25 F with left anterior thigh myxoid liposarcoma s/p resection presenting with chronic pain two years after resection | Tizanidine, ibuprofen, hydrocodone‐paracetamol PRN | Medical: Gabapentin; Interventional: Steroid block of left anterior cutaneous branch of femoral nerve | None |
Table 2.
Analgesic Results and Clinical Outcomes From Successful PNS.
| Patient number | Neural target | Pain score before PNS | Pain score during stimulation | Pain score after extraction | Clinical outcome |
|---|---|---|---|---|---|
| 1 | Proximal T2 and T4 Spinal Nerves | 10 | 1 | 1 | Six weeks of analgesia after extraction followed by return to baseline pain. |
| 2 | Proximal T7 Spinal Nerve | 10 | 1 | 5 | 12 months of analgesia after extraction** |
| 3 | Proximal L2 and L3 Spinal Nerve | 10 | 5 | 5 | Four months of analgesia after extraction. Patient then received SCS |
| 4 | Suprascapular nerve | 8 | 1 | 2 | No recurrence of pain at 18 months after extraction** |
| 5 | Brachial plexus via supraclavicular approach | 8 | 2* | 2 | Pain controlled after extraction. Due to progression of disease patient is not candidate for re‐implantation after MRI. Patient passed away shortly after extraction |
| 6 | Sciatic nerve | 8 | 2 | 4 | Six months of analgesia after extraction and regained ability to walk for 20 minutes. Duration of effects limited by progression of disease requiring transition to hospice care** |
| 7 | Femoral nerve | 9 | 3 | 3 | Eight months of analgesia after extraction. Patient received steroid injection of left anterior cutaneous branch of femoral nerve with 95% pain relief** |
| Average | 9.0 | 2.1 | 3.1 | ||
| Standard deviation | 1.0 | 1.5 | 1.6 |
PNS removed at 45 days due to urgent need for MRI.
Continue to have analgesia at the publication of this article.
Patients 1–3 had specific proximal spinal nerves targeted using the paravertebral and/or neuroforaminal approach. Ultrasound was used to visualize the transverse process and the needle was advanced in a lateral to medial direction to target the specific proximal spinal nerve. The approach for targeting the thoracic proximal spinal nerve in Patient 2 is shown in Fig. 1 and that for targeting the lumbar proximal spinal nerves in Patient 3 is shown in Figs. 2 and 3. In Patient 4, the suprascapular nerve was targeted under the transverse ligament at the suprascapular notch of the scapula using a medial to lateral approach. In Patient 5, the brachial plexus block was performed at the supraclavicular level (Figs. 4 and 5). The sciatic nerve was blocked at the popliteal fossa in Patient 6 and the femoral nerve at the femoral crease in Patient 7. Additionally, we describe five patients in Table 3 that did not have adequate pain relief from the PNS.
Figure 1.
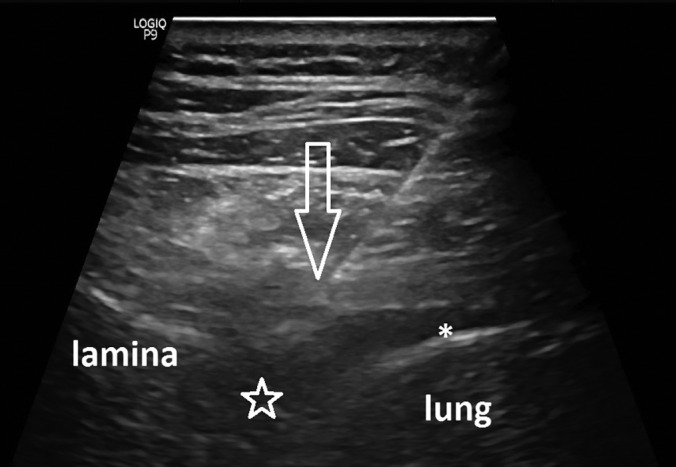
Image of the paravertebral approach to targeting the proximal T7 spinal nerve. Needle has traversed costotransverse ligament and is approaching spinal nerve (not seen). Arrow = needle tip, * = pleural space with small amount of pleural fluid, star = neuroforaminal space of T7.
Figure 2.
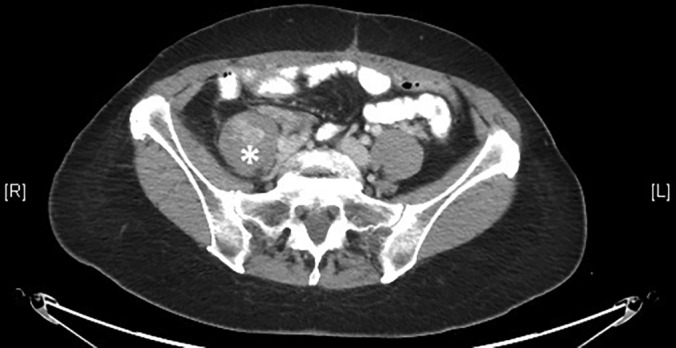
Computed tomographic image of pelvis showing spindle cell sarcoma in right psoas. Mass was excised and radiated prior to placement of PNS. * = sarcoma.
Figure 3.
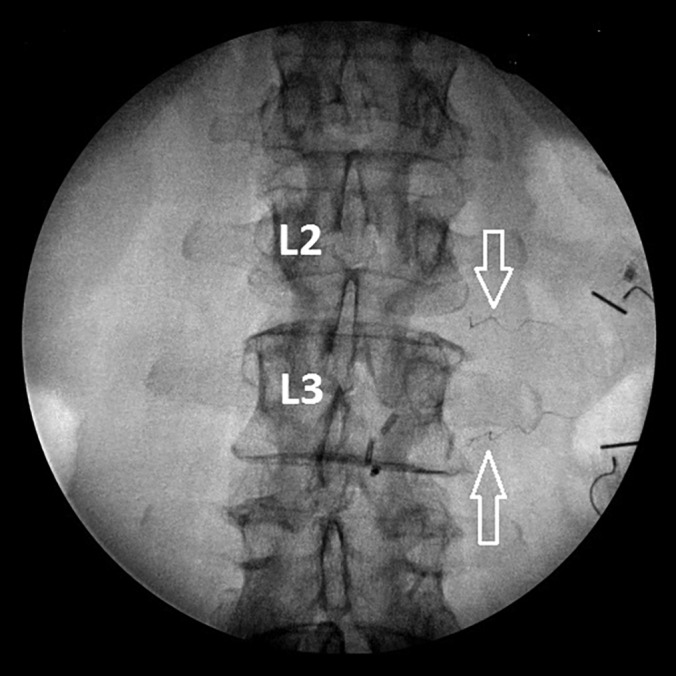
Peripheral nerve stimulator leads shown targeting proximal L2 and L3 spinal nerves. Leads were placed under ultrasound guidance and confirmed under fluoroscopy. Arrows = PNS leads.
Figure 4.
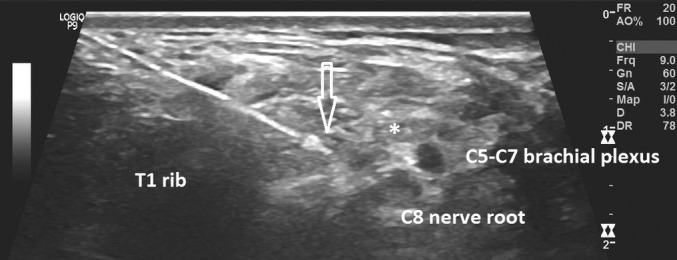
Ultrasound image of introducer demonstrating lead position for supraclavicular brachial plexus stimulation. * = tumor encasing brachial plexus, arrow = needle introducer tip.
Figure 5.
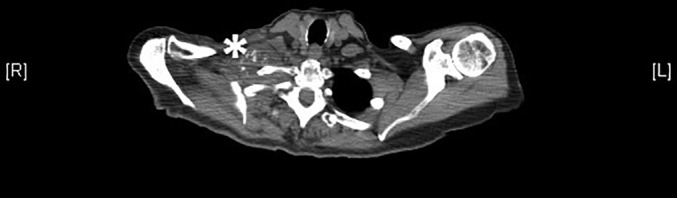
Axial CT image at T1 level showing metastatic mass encasing brachial plexus. * = metastatic mass.
Table 3.
Description of PNS Cases Without Prolonged Analgesia.
| Pathology | Intervention | Reason unsuccessful |
|---|---|---|
| Surgical resection of calf sarcoma | Sciatic nerve in popliteal fossa | 10–15% improvement during stimulation |
| Surgical resection of foot sarcoma | Sciatic nerve in popliteal fossa | 10–15% improvement during stimulation |
| Surgical resection of finger sarcoma | Median nerve at carpal tunnel | Lead removed due to discomfort with stimulation |
| Surgical resection of humeral metastatic disease | Brachial plexus | Lead removed due to aggravation of pain from stimulation |
| Surgical resection of sacral sarcoma and reconstruction | Sciatic nerve at gluteal level* | 20% improvement during stimulation |
Fractured lead at extraction.
DISCUSSION
The mechanism of action of PNS is a topic of ongoing research, with likely both centrally and peripherally mediated effects. One theory suggests that the effect of PNS may be carried out via the gate control theory similar to dorsal column stimulation, which causes stimulation of the A‐beta fibers as they traverse the dorsal columns. Comparatively, PNS leads to activation of A‐beta fibers at the location of the peripheral leads with orthodromic activation of the A‐beta fibers as the travel back to the spinal cord 12, 13. Stimulation of A‐beta neurons leads to excitation of inhibitory dorsal horn interneurons, which in turn inhibit the transmission of nociceptive, small‐diameter A‐delta and C nerve fibers. Some of these effects may be mediated through the effects of neuropeptides such as substance P and calcitonin gene‐related peptides 12.
A second paradigm focuses on the local effects at the site of peripheral stimulation. Chemical mediators, such as neurotransmitters and endorphins, may play a key role in transmission of pain signals by increasing local blood flow 14. Animal models have demonstrated that nerve injury leads to localized inflammatory changes such as edema, ischemia, and increased vascular permeability 14. Studies have suggested that PNS may reduce the levels of these biochemical mediators thus producing their analgesic effect 12, 13. This theory is supported by a study that demonstrated increased latency of afferent signals via A and C nerve fibers when stimulated by electrical stimulation. This effect was most significant on small‐diameter fibers, which primarily carry nociceptive signals 15. Further research in this area should help elucidate the roles of these various theories in PNS.
The first three patients in our case series demonstrate novel targeting of proximal spinal nerves at the thoracic and lumbar levels with the temporary PNS. Prior studies using this device have targeted peripheral nerves and the brachial plexus to treat pain in the extremities 5, 8, 9, 10. Our approach introduces a unique approach for treating truncal pain using this device by allowing selective targeting of dermatomal patterns as we have demonstrated with post‐mastectomy pain syndrome and post‐herpetic neuralgia. Patient 1 with post‐mastectomy pain syndrome had pain return to baseline six weeks after extraction of the PNS. She continues to have her pain medically managed with amitriptyline as she was prior to PNS implantation. Patient 2 with post‐herpetic neuralgia has not had recurrence of pain for 12 months after lead extraction. It is unclear whether the PNS continues to have residual analgesic effect or post‐herpetic neuralgia pain has resolved on its own. Patient 3 with neuralgia after sarcoma resection had four months of relief after lead extraction. The patient subsequently chose to receive a spinal cord stimulator after return of pain, which continues to provide analgesia at the time of this writing.
Patient number 4 demonstrates the first use of PNS leads targeting the suprascapular nerve for analgesia in acute or chronic cancer pain settings. Targeting the suprascapular nerve for a patient with disease near the spinal cord reflects the understanding of referral pain patterns. While there was not a direct lesion on the peripheral nerve, stimulation of the nerve improved pain occurring at the spinal cord level. This idea underscores the use of visceral and somatic referral patterns as potential targets for PNS. This patient continues to have residual analgesia at the time of this writing, totaling 18 months thus far.
Patient number 5 in our case series demonstrates the first time PNS was successfully used to target the brachial plexus in an active cancer pain patient. In a prior study, analgesia for rotator cuff surgery was successfully performed by targeting the C5 proximal spinal nerve, superior trunk, and the middle trunk 8. Unfortunately, Patient 5 had progression of her malignancy leading to urgent need for an MRI requiring extraction of the leads at 45 days, 15 prior to the target of 60 days. The patient then passed away shortly after from progression of her disease with continued analgesia after extraction. This case highlights a potential use of temporary PNS in end‐of‐life scenarios.
Patient 6 demonstrated successful targeting of the sciatic nerve at the popliteal fossa. Prior attempts at doing this approach have led to subjective cramping sensations, lead dislodgement, and spontaneous lead fractures during use, and lead fractures during extraction 10. Our patient did not experience any of these complications. This patient has had six months of residual analgesia after extraction that still persists. Lastly, the femoral nerve was successfully targeted in Patient 7 as has been demonstrated in postamputation pain 5 and for postsurgical pain in total‐knee arthroplasty 9. This patient continues to have analgesia in the distribution of the femoral nerve. The patient continues to have pain in distribution of lateral femoral cutaneous nerve, which has been treated with repeated peripheral nerve steroid injections.
In our experience, there were five PNS cases that did not lead to significant improvement in pain after extraction of leads. Three of these cases failed due to inadequate analgesia during stimulation. Two other cases failed due to discomfort or aggravation from lead stimulation. Discomfort and aggravation of pain are known side effects associated with other neurostimulation techniques such as spinal cord stimulators. These patients were offered other forms of stimulation and intrathecal drug delivery, which neither patient was interested in. Both patients are currently maintained on oral opioids.
Furthermore, in this cohort, we had one lead fracture, which is one of the most common complications with this device 8, 9, 10. Retained fragments only have conditional MRI compatibility, which should be a significant concern in the oncologic population. The patient was informed of the complication and potential risks associated with MRI‐imaging. Thus far, we have not had any complications related to MRI‐imaging. A prior study on fracture rates using the same helical‐coiled leads in other devices showed an 7.5% incidence of lead fracture on extraction 10.
The retained leads are restricted to a static magnetic field of 1.5 T, maximum spatial field gradient of 20 T/m, and maximum whole body averaged specific absorption rate (SAR) of 2 W/kg. Of these three criteria, the greatest limitation is likely to be due to the 1.5 T restriction 16. Over the past decade, 3 T MRI are becoming increasingly common in clinical practice. Market data from 2004 showed that 25% of new MRI machines purchased featured 3 T magnets 17, 18. Although we were unable to find more recent data, that number is likely higher now. The restriction on the maximum spatial gradient and SAR is less likely to place significant restrictions. The maximum spatial gradient determines the translational force applied to the retained lead. The maximum spatial gradient even for typical 3 T MRI will have a maximum spatial gradient of about 10 T/m 16, which is significantly less than the limit. Additionally, gradient experienced by the retained lead could be significantly less if it is not within the core of the magnet itself 16, 17, as would be the case in a patient receiving a brain MRI with a retained lead in the popliteal fossa. The restriction of SAR limits the permissible amount of energy that can be transferred to the body tissue over a designated amount of time preventing excessive heating of the implanted device and body tissue. The SAR can be reduced by extending the duration of the MRI scan allowing extra time for cooling 17.
Potential Future Applications in Oncologic Pain
Persistent Pain Post‐Breast Cancer Treatment
Breast cancer is the most commonly diagnosed cancer among women with five‐year survival greater than 90% 19. The prevalence of persistent pain after breast cancer treatment is 25–60% 20, 21. Three of the common causes of chronic pain in these patients include intercostobrachial neuralgia from transection of the intercostobrachial nerve during surgery, radiation‐induced cutaneous changes, and radiation‐induced inflammation 20. Current literature on treatment modalities is limited. However, some studies suggest that early intervention, including improved perioperative analgesia can reduce incidence. One prospective, randomized, double‐blinded, placebo‐controlled trial showed that one‐time preoperative paravertebral blocks with local anesthetic at levels T1‐5 before breast cancer surgery reduced risk of persistent pain at three and six months postoperative by 32.6% and 40.5%, respectively 21. We feel PNS of the proximal spinal nerves introduces a novel modality to consider in treating this patient population, as we demonstrated with Patient 1 in our case series.
Postherpetic Neuralgia
Similar to patients with post‐mastectomy related pain, patients with post‐herpetic neuralgia (PHN) have potential PNS targets at the proximal spinal nerve level. The annual incidence of herpes zoster is about 3.4 cases per 1000 people 22 and even higher in the cancer population. The incidence in patients with hematologic malignancies is 4.8 times higher and that in patients with solid tumors is 1.9 times higher. The relative risk of developing herpes zoster increases with increasing levels of immunosuppression such as in patients on chemotherapy or corticosteroids 23. Approximately, 20% of all patients with herpes zoster will continue to have pain three months and 15% two years after a herpes zoster episode 22. We feel a temporary PNS system may be an ideal treatment for PHN since a majority of patients are expected to have resolution of their symptoms within three to six months, as was demonstrated in the case of Patient 2.
Postthoracotomy Pain Syndrome
Lung cancer is among the most common cancers in the United States, often treated via surgical resection. Chronic post‐thoracotomy pain syndrome affects 30–60% 24, 25, 26 of patients and is debilitating in about 5% 25. Currently, there is a lack of literature on management strategies for chronic post‐thoracotomy pain. We hypothesize that targeting the thoracic proximal spinal nerves using a paravertebral approach as we did in Patient 1 and 2 in this case series could provide analgesia in this unique patient population as these same neural targets have been shown to be effective in treating acute postoperative pain after a thoracotomy 25.
Surgical Resections of Cancer
Many patients who undergo surgical resection of cancer present to chronic pain specialists for management of chronic postoperative pain 27, 28. The pathophysiology of pain after resection likely has significant overlap with post‐amputation pain, which has been shown to be successfully treated with PNS in a prior randomized, placebo‐controlled, double‐blinded study 5. However, in our case series, only two out of six cases with post‐sarcoma resection pain responded well to the PNS. Three of the failures were due to lack of analgesic response and one due to discomfort with stimulation. Only one of our cases involved a resection of bone (finger amputation). The remaining five cases involved soft‐tissue resection. Furthermore, patients with subacute pain, less than one year since surgery, had an improved outcome relative to patients with longstanding pain symptoms.
Electrical Stimulation and Progression of Cancer
Pain providers should be aware of the theoretical risk of exacerbation of malignancy associated with electrical stimulation. This has mostly been addressed in the context of transcutaneous electrical nerve stimulation (TENS). The rationale behind this concern is that electrical stimulation may stimulate DNA synthesis and cell replication leading to increased tumor growth. However, as of now, there is no literature to substantiate this theory. Pain providers should be aware of this issue to help address patient concerns 29.
Limitations and Future Directions
The results and conclusions that can be drawn from this study are limited by the case series nature of the data. Two prior studies using this PNS have studied acute postoperative pain using randomized, crossover studies 8 10 and one prior study looked at chronic postamputation pain using a randomized, controlled trial 5. Future research should focus on generating additional high‐quality evidence to help identify criteria predictive of successful response to PNS. In the data provided here, five out of 12 patients did not have successful response to PNS, which leaves significant space for improvement. The prior RCT on postamputation pain had a sample size of 25 patients and targeted femoral and sciatic nerves 5. Future studies could similarly focus on specific neural targets and pathology. A larger sample size should be employed to facility subgroup analysis. Thus far, limited research has been done on clinical factors that may influence efficacy of PNS. Two particular factors that might be relevant are patient body mass index and psychiatric co‐morbidities. Extremes of BMI in either direction may make it hard to target the nerve and increase risk of lead migration during the stimulation phase. Psychiatric co‐morbidities are often considered relative contraindications to spinal cord stimulation due to reduced efficacy in this patient population, which may extend to PNS. Additionally, studies with larger sample sizes would be indicated to identify risk of rare complications such as infection or hematoma.
CONCLUSIONS
This case series demonstrates that the temporary, percutaneous PNS has several feasible targets to treat acute and chronic oncologic pain. Our data introduce novel targeting of proximal spinal nerves and corroborates prior data demonstrating targeting of various peripheral nerves. Further randomized, placebo‐controlled, blinded trials will need to be designed to determine PNS efficacy in various oncologic pathologies. Temporary, percutaneous PNS is a promising and evolving field but needs significant research to help develop clinical guidelines before it can become a routine part of the pain providers regular armamentarium.
Authorship Statement
Ojas Mainkar helped write the paper. Che Antonio Sollo helped perform the cases and write the paper. Grant Chen helped perform the cases and edit the paper. Aron Legler helped perform the cases and edit the paper. Amitabh Gulati helped perform the cases and helped write the paper.
COMMENTS
The authors present a small case series using peripheral neurostimulation for cancer‐related pain. This under‐recognized and underserved population will benefit from the expansion of treatment options. This report advances that objective.
William Rosenberg, MD
Kansas City, MO, USA
***
The main conclusion one can draw from this article – the electrical neuromodulation is a valid option for patients with cancer pain. Even though the first patient that underwent spinal cord stimulation in 1967 by Shealy was suffering from cancer pain, the neurostimulation for pain is used today almost exclusively for patients with pain due to non‐malignant causes. Two main reasons for avoiding neurostimulation in patients with cancer pain have been their short life expectancy (that makes conventional neuromodulation non‐cost‐effective) and the need in MRI scanning (for which the previous neuromodulation devices were a contraindication).
However, the technology advances have addressed these concerns, and the devices that the authors used for percutaneous peripheral nerve stimulation in this series of cancer pain patients are different from conventional neuromodulation systems: they are not intended for permanent implantation and are removed after up to 60 days of continuous use.
With neuromodulation devices becoming more affordable and MRI compatible (conditionally approved) their use in cancer pain patients may be explored further, perhaps with associated decrease in opioid consumption and reduction in situations that would necessitate neurodestructive interventions. The experience of the authors should prompt a change in the therapy paradigm for management of cancer pain, introducing neurostimulation of peripheral nerves, dorsal root ganglia, spinal cord and brain as a valid option before, instead of, or in addition to other pain‐relieving approaches.
Konstantin Slavin, MD
Chicago, IL, USA
***
This provides an excellent review of cancer pain and the introduction of various PNS treatment options. This small case series offers real world experience that may spark further interest in the field of cancer pain intervention. Due to the medically challenging nature of the patient population, large scale randomized controlled trials may otherwise not be feasible.
Sean Li, MD
Shrewsbury, NJ, USA
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: The authors have no sources of funding to declare for this manuscript.
Conflict of Interest: Amitabh Gulati is a consultant for Medtronic as well as a scientific advisor for Flowonix, Advanced Infusion Solutions, Enso Relief, and Centrexion. The other authors of the paper have no conflicts of interest to report.
[The copyright line for this article was changed on 18 May 2020 after original online publication.]
REFERENCES
- 1. Paice J, Portenoy R, Lacchetti C et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:3325–3345. 10.1200/jco.2016.68.5206. [DOI] [PubMed] [Google Scholar]
- 2. Portenoy R. Treatment of cancer pain. Lancet 2011;377:2236–2247. 10.1016/s0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 3. Deer T, Mekhail N, Provenzano D et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The neuromodulation appropriateness consensus committee. Neuromodulation 2014;17:515‐550. [DOI] [PubMed] [Google Scholar]
- 4. Clark M. Minimizing risk of cancer therapeutics. Phys Med Rehabil Clin N Am 2018;29:701–719. 10.1016/j.pmr.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 5. Gilmore C, Ilfeld B, Rosenow J et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo‐controlled trial. Region Anesth Pain Med 2019;44:637–645. [DOI] [PubMed] [Google Scholar]
- 6. Gilmore C, Kapural L, McGee M, Boggs J. Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low Back pain provides sustained relief. Neuromodulation 2018;22:615–620. 10.1111/ner.12854. [DOI] [PubMed] [Google Scholar]
- 7. Kapural L, Gilmore C, Chae J et al. Percutaneous peripheral nerve stimulation for the treatment of chronic low Back pain: Two clinical case reports of sustained pain relief. Pain Pract 2017;18:94–103. 10.1111/papr.12571. [DOI] [PubMed] [Google Scholar]
- 8. Ilfeld B, Finneran J, Gabriel R et al. Ultrasound‐guided percutaneous peripheral nerve stimulation: neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof‐of‐concept. Reg Anesth Pain Med 2019. 10.1136/rapm-2018-100121. [DOI] [PubMed] [Google Scholar]
- 9. Ilfeld B, Ball S, Gabriel R et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation 2018;22:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilfeld B, Gabriel R, Said E et al. Ultrasound‐guided percutaneous peripheral nerve stimulation. Reg Anesth Pain Med 2018;43:580–589. 10.1097/aap.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deer T, Levy R, Verrills P, Mackey S, Abejon D. Perspective: Peripheral nerve stimulation and peripheral nerve field stimulation birds of a different feather. Pain Med 2015;16:411–412. 10.1111/pme.12662. [DOI] [PubMed] [Google Scholar]
- 12. Chakravarthy K, Nava A, Christo P, Williams K. Review of recent advances in peripheral nerve stimulation (PNS). Curr Pain Headache Rep. 2016;20:60. 10.1007/s11916-016-0590-8. [DOI] [PubMed] [Google Scholar]
- 13. Deer T, Jain S, Hunter C, Chakravarthy K. Neurostimulation for intractable chronic pain. Brain Sci 2019;9:23 10.3390/brainsci9020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chakravarthy K, Xing F, Bruno K et al. A review of spinal and peripheral neuromodulation and Neuroinflammation: Lessons learned thus far and future prospects of biotype development. Neuromodulation 2018;22:235–224. [DOI] [PubMed] [Google Scholar]
- 15. Torebjork HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatr 1974;37:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panych L, Madore B. The physics of MRI safety. J Magn Reson Imaging 2017;47:28–43. 10.1002/jmri.25761. [DOI] [PubMed] [Google Scholar]
- 17. Jerrolds J, Keene S. MRI safety at 3T versus 1.5T. Internet J World Health Soc Politics 2009;6:1‐8. [Google Scholar]
- 18. Tanenbaum L. 3T MRI in clinical practice. Appl Radiol 2005;34 https://appliedradiology.com/articles/3t-mri-in-clinical-practice. [Google Scholar]
- 19. Tait R, Zoberi K, Ferguson M et al. Persistent post‐mastectomy pain: Risk factors and current approaches to treatment. J Pain 2018;19:1367–1383. 10.1016/j.jpain.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang K, Yee C, Tam S et al. Prevalence of pain in patients with breast cancer post‐treatment: A systematic review. Breast 2018;42:113–127. 10.1016/j.breast.2018.08.105. [DOI] [PubMed] [Google Scholar]
- 21. Qian B, Fu S, Yao Y, Lin D, Huang L. Preoperative ultrasound‐guided multilevel paravertebral blocks reduce the incidence of postmastectomy chronic pain: A double‐blind, placebo‐controlled randomized trial. J Pain Res. 2019;12:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson R, Rice A. Postherpetic neuralgia. N Engl J Med 2014;371:1526–1533. 10.1056/nejmcp1403062. [DOI] [PubMed] [Google Scholar]
- 23. Habel L, Ray G, Silverberg M et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev 2012;22:82–90. 10.1158/1055-9965.epi-12-0815. [DOI] [PubMed] [Google Scholar]
- 24. Hetmann F, Kongsgaard U, Sandvik L, Schou‐Bredal I. Post‐thoracotomy pain syndrome and sensory disturbances following thoracotomy at 6‐ and 12‐month follow‐ups. J Pain Res 2017;10:663–668. 10.2147/jpr.s126639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bendixen M, Jørgensen O, Kronborg C, Andersen C, Licht P. Postoperative pain and quality of life after lobectomy via video‐assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol 2016;17:836‐844. [DOI] [PubMed] [Google Scholar]
- 26. Humble S, Dalton A, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post‐surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain 2014;19:451–465. 10.1002/ejp.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeMoss P, Ramsey L, Karlson C. Phantom limb pain in pediatric oncology. Front Neurol 2018;9:219. 10.3389/fneur.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelan J, Davis L. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol 2018;36:188–193. 10.1200/jco.2017.75.1743. [DOI] [PubMed] [Google Scholar]
- 29. Rennie S. Electrophysical agents ‐ contraindications and precautions: An evidence‐based approach to clinical decision making in physical therapy. Physiother Can. 2010;62:1–80. 10.3138/ptc.62.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


