Abstract
Estrogen has been shown not only to reduce the incidence of colorectal cancer but also gastric cancer (GC). Polymorphisms in estrogen receptor β gene, ESR2, correlate with colorectal cancer survival. To better understand the role of ESR2 in GC, genomic DNA extracted from 169 Japanese patients and 172 patients from Los Angeles County (LAC) was analyzed for association of overall survival (OS) with three ESR2 polymorphisms, which are of biological significance using multivariable Cox proportional hazard regression. ESR2 rs1271572 (C > A) and rs3020443 (T > G) had univariate and multivariable associations with OS in the Japanese cohort, whereas the C allele of ESR2 rs2978381 (T > C) predicted favorable OS in the Japanese cohort but worse OS in the LAC cohort. The interaction term of the ESR2 rs2978381 and cohort group reached statistical significance. Our study provides evidence that genetic variations in ESR2 gene are significantly associated with survival in patients with locally advanced GC.
INTRODUCTION
Estrogen functions are mediated by two subtypes of nuclear receptors, estrogen receptor α and estrogen receptor β (ERβ), which transduce extracellular signals into transcriptional response by binding to estrogen response elements as homodimers or heterodimers either directly in promoter regions or indirectly through alternative DNA elements such as regions of activator protein 1 or specificity protein 1.1–3 The estrogen receptor α and ERβ have different tissue distributions and are sometimes coexpressed within the same tissue and cell type. The ERβ activates transcription of various targets upon binding to 17β-estradiol or related ligands and is also the most abundantly expressed sex-steroid hormone receptor in the gut.
Loss of ERβ expression correlates with higher grades, malignant transformation and advanced Dukes stage of colorectal cancer.4–6 Multiple review articles have indicated that the loss of ERβ expression is a common step in the development of colonic carcinoma,7,8 suggesting that ERβ is responsible for the protective effect of estrogens against colorectal carcinogenesis. An analysis of prospective data from the Women’s Health Initiative have shown that the use of postmenopausal hormone replacement therapy significantly reduces the relative risk of developing colorectal cancer.9 Moreover, several studies support evidence that hormone therapy reduces the incidence and risk of death from colon cancer.10–17 In gastric adenocarcinoma it has been shown that ERβ is expressed more abundantly than estrogen receptor α.18–23 Some studies demonstrated that ERβ overexpression was associated with lower tumor stage, lack of perineural invasion and favorable survival time in gastric cancer (GC), whereas other studies reported the inverse associations.18–20,22–30 Epidemiologic studies have indicated the reduced incidence of GC by the protective effect of estrogen.31,32 However, despite the abundance of literature the association between estrogen and prognosis in GC remains inconclusive.
Several single-nucleotide polymorphisms (SNPs) in the ERβ gene, ESR2, located in a promoter region have been shown to be associated with colorectal cancer survival.33 However, to the best of our knowledge, this is the first study evaluating the associations between the ERβ gene and prognosis in GC. We hypothesized that SNPs in ESR2 might be related with overall survival (OS) in patients with GC. We therefore tested the prognostic value of previously reported SNPs with known biological significance within a promoter region of ESR2, namely rs1271572, rs2978381 and rs3020443, in two independent cohorts of patients with locally advanced GC.
MATERIALS AND METHODS
Eligible patients
This study enrolled two independent cohorts. For the Japanese cohort, a total of 169 patients with histologically confirmed (stage I to stage IV with no distant metastases; AJCC 6th) gastric adenocarcinoma treated with surgery alone or surgery plus fluoropyrimidine-based adjuvant chemotherapy in Fukushima Red Cross Hospital (Fukushima) or Kitasato University East Hospital (Sagamihara) between 1991 and 2011 were included. For the Los Angeles County (LAC) cohort, a total of 172 patients with histologically confirmed (stage I to stage IV with no distant metastases; AJCC 6th) gastric adenocarcinoma treated with surgery alone or surgery plus fluoropyrimidine-based adjuvant (radio)-chemotherapy in multiple centers in LAC between 1992 and 1997 were included. Japanese patients were followed clinically every 3 months for the first 2 years and then every 6 months. Japanese patient data were collected retrospectively through chart review. Patients in LAC were participants in a case–control study.34 Pathologic stage was assigned according to tumor–node–metastasis classification, sixth edition. All tissue analyses in the current study were carried out at the University of Southern California/Norris Comprehensive Cancer Center following approval by the University of Southern California Institutional Review Board of Medical Sciences. All patients signed an informed consent for the analysis of molecular correlates.
DNA extraction and genotyping
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissues of 138 out of 169 Japanese patients and from peripheral whole blood of the other patients, using the QIAmp Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol (www.qiagen.com). PCR-based direct DNA sequence analysis using ABI 3100 A Capillary Genetic Analyzer and Sequencing Scanner v1.0 (Applied Biosystems, Life Technologies, Grand Island, NY, USA) was performed for genotyping the SNPs. Both forward and reverse primers of Table 1 were used for DNA amplification for each polymorphism. A tenth of the samples in each cohort were randomly selected and examined for quality control. The genotyping quality control by direct DNA sequencing provided a genotype concordance of 99% or more. All analyzed SNPs were genotyped with a success rate of > 97%. In case of failure, extracted genomic DNA had limited quantity and/or poor quality. The investigator analyzed the SNPs blindly to the clinical data set. This study was conducted adhering to the REporting recommendations for tumor MARKer prognostic studies (REMARK).
Table 1.
Analyzed polymorphisms in genes encoding ERβ and their functional significance
| rs number | Location of polymorphism | Base exchange | Published data | Forward primer (5′–3′) Reverse primer (5′–3′) |
MAFa |
|---|---|---|---|---|---|
| rs1271572 | Promoter chromosome 14:64295199 | C > A | Associated with breast and prostate cancer risk (J Biomed Sci 2013;20:32; Cancer Res 2000;60:702) T/T genotype inhibits ESR2 expression (J Biomed Sci 2013;20:32) |
GCAGCTGTTGCTGATGAAAA CCCCCTCGTCTTCCTCTATT |
0.46 |
| rs2978381 | Promoter chromosome 14:64299934 | T > C | Associated with colorectal cancer survival (Cancer Res 2013;73:767) | GCCAGGATCTCTGCATTCTC AGGCTGAGGAAGCACTTGAC |
0.50 |
| rs3020443 | Promoter chromosome 14:64325622 | T > G | Associated with colorectal cancer survival (Cancer Res 2013;73:767) | GGAGAAAAAGGTTTAGGATGTGA TTATCAGTGGACCCATGCAA |
0.19 |
Abbreviation: MAF, Minor allele frequency.
Frequency from Ensemble database: http://www.ensembl.org/index.html.
Statistical analysis
The primary endpoint of this study was OS, which was defined as the period from the date of surgery or diagnosis to death in both cohorts. If there was no observed event, the endpoint was censored at the last time of contact or follow-up.
χ2-tests were performed to examine differences in baseline patient characteristics between the two cohorts. Exact test was performed to test allelic distribution of all SNPs in each race/ethnic group for deviation from Hardy–Weinberg equilibrium. Linkage disequilibrium among candidate SNPs for each race/ethnic group was assessed using D′ and r2 values, and the haplotype frequencies were inferred using Haploview version 4.2 (www.broad.mit.edu/mpg/haploview).
Kaplan–Meier curves and log-rank tests were conducted for univariate analysis of the association between the ESR2 SNPs and OS using codominant, dominant or recessive genetic models when appropriate. The baseline demographic and clinical characteristics that remained significantly associated with endpoint in the model selection procedures (P < 0.1) were included in multivariable analysis of the SNPs and clinical outcome. Concordant probability estimates for the Cox proportional hazards model were calculated to evaluate the incremental contribution in discrimination provided by the ESR2 SNPs over the baseline prognostic markers in both cohorts.35 With the sample size of 169 patients in the Japanese cohort and 172 patients in the LAC cohort, we would have 80% power to identify the SNPs with a hazard ratio of 1.95–2.20 and 1.70–1.94, respectively, and minor allele frequency of > 10% using a two-sided log-rank test. To simplify the scenarios of power calculation, we only considered the dominant model of inheritance. All tests were two-sided at a 0.05 significance level and performed by using the SAS statistical package version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The baseline characteristics in both cohorts were summarized in Table 2. The median follow-up periods were 4.0 years in the Japanese cohort and 8.3 years in the LAC cohort. The median OS was 5.7 years for the Japanese cohort compared with 2.6 years for the LAC cohort. The clinicopathologic characteristics and outcome in both cohorts varied considerably. In brief, compared with the Japanese cohort, the LAC cohort comprised younger patients, more advanced T- and N-categories, a higher percentage of proximal-located and poorly differentiated cancer as well as a worse general condition. In the Japanese cohort, age, performance status, stage, T-/N-category and tumor site were significantly associated with OS (Supplementary Table 1). On the other hand, in the LAC cohort, race, stage, T-/N-category and tumor differentiation grade were significantly correlated with OS (Supplementary Table 2).
Table 2.
Baseline clinical characteristics of Japanese and LAC patient cohorts
|
Japanese (N = 169) |
LAC (N = 172) |
P-valuea | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | |||||
| Male | 109 | 64 | 115 | 67 | 0.65 |
| Female | 60 | 36 | 57 | 33 | |
| Age (year) | |||||
| Median (range) | 67 (31–88) | 61 (26–74) | < 0.001 | ||
| < 65 | 65 | 38 | 101 | 59 | < 0.001 |
| ⩾ 65 | 104 | 62 | 71 | 41 | |
| Stage | |||||
| I – II | 81 | 48 | 84 | 49 | 0.34 |
| III–IV | 88 | 52 | 74 | 43 | |
| Unknownb | 14 | 8 | |||
| T-category | |||||
| T1 – T2 | 78 | 46 | 54 | 31 | 0.017 |
| T3 – T4 | 91 | 54 | 108 | 63 | |
| Unknownb | 10 | 6 | |||
| N-category | |||||
| N0 – N1 | 121 | 72 | 82 | 48 | 0.003 |
| N2 – N3 | 48 | 28 | 66 | 38 | |
| Unknownb | 24 | 14 | |||
| Primary tumor site | |||||
| Proximal | 46 | 27 | 77 | 45 | < 0.001 |
| Distal | 123 | 73 | 95 | 55 | |
| Tumor differentiation | |||||
| Differentiated | 68 | 40 | 44 | 26 | 0.018 |
| Undifferentiated | 101 | 60 | 114 | 66 | |
| Unknownb | 14 | 8 | |||
| Ethnicity | |||||
| Asian | 169 | 100 | 30 | 17 | NA |
| Caucasian | 90 | 52 | |||
| Hispanic | 38 | 22 | |||
| African American | 14 | 8 | |||
Based on χ2-test or Wilcoxon test, whichever was appropriate.
Excluded in χ2-test.
The allelic frequencies for all SNPs were within the probability limits of Hardy–Weinberg equilibrium (Exact test, P > 0.05) in each race/ethnic group. No significant linkage disequilibrium was found in the Japanese cohort. In the LAC cohort, linkage disequilibrium was found between ESR2 rs1271572 and ESR2 rs2978381 in Caucasians (D′ = 0.90, r2 = 0.55). Haplotype analysis was constructed for those two polymorphisms; however, it showed no significant results. In addition, significantly different allelic distributions in ESR2 rs2978381 and ESR2 rs3020443 were found between the two cohorts (Supplementary Table 3).
Univariate and multivariable analyses in the Japanese cohort
In the Japanese cohort, all of the three ESR2 SNPs were significantly associated with OS in univariate analysis. ESR2 rs1271572 (C > A) and rs3020443 (T > G) remained statistically significant in multivariable analysis when adjusted by age, performance status, stage and tumor site. The A allele of ESR2 rs1271572 predicted shorter OS, whereas the G allele of ESR2 rs3020443 was significantly associated with longer OS. The C allele of ESR2 rs2978381 (T > C) correlated with significantly favorable OS in univariate analysis but this was not statistically significant in multivariable analysis. Concordant probability estimates was 0.712 (s.e., 0.024) in the multivariable model for OS when only baseline tumor characteristics were included. The Concordant probability estimates increased to 0.736 (SE, 0.022) and 0.726 (SE, 0.023) when ESR2 rs1271572 or ESR2 rs3020443 was added to the model, respectively.
Univariate and multivariable analyses in the LAC cohort
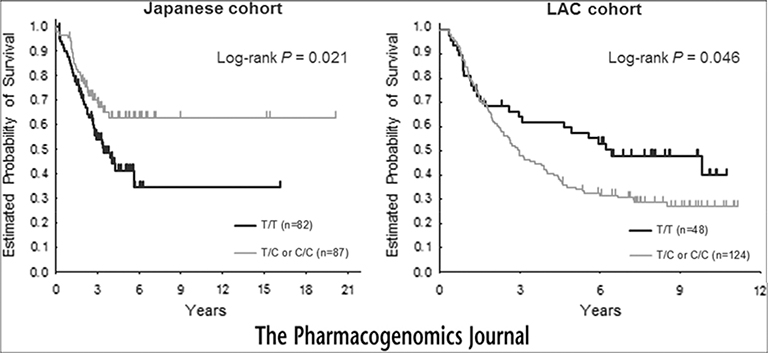
In the LAC cohort, only the ESR2 rs2978381 was significantly associated with OS. Patients with the C allele of ESR2 rs2978381 had significantly worse OS than those with T/T genotype in both univariate and multivariable analyses (Table 3). Concordant probability estimates were 0.683 (SE, 0.022) and 0.705 (SE, 0.023) in the multivariable model for OS, including baseline tumor characteristics only and after adding ESR2 rs2978381 into the model, respectively. Interestingly, the C allele was associated with worse OS in the LAC cohort but with better OS in the Japanese cohort (Figure 1).
Table 3.
Associations between ESR2 SNPs and overall survival in the Japanese and LAC cohorts
| Genotype |
Japanese cohort (N = 169) |
LAC cohort (N = 172) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariate analysis |
Multivariable analysisa |
Univariate analysis |
Multivariable analysisa |
|||||||||
| N | 5-year survival rate ± SE | HR (95% CI) | P-value | HR (95% CI) | P-value | N | 5-year survival rate ± SE | HR (95% CI) | P-value | HR (95%CI) | P-value | |
| ESR2 rs1271572 | 0.0051 | 0.025 | 0.82 | 0.36 | ||||||||
| C/C | 59 | 0.73 ± 0.06 | 1 | 1 | 59 | 0.42 ± 0.06 | 1 | 1 | ||||
| C/A | 73 | 0.44 ± 0.07 | 2.59 (1.40, 4.81) | 2.32 (1.22, 4.40) | 74 | 0.38 ± 0.06 | 0.96 (0.63, 1.45) | 1.03 (0.62, 1.70) | ||||
| A/A | 35 | 0.40 ± 0.10 | 2.44 (1.23, 4.87) | 2.34 (1.14, 4.78) | 34 | 0.51 ± 0.09 | 0.84 (0.50, 1.43) | 0.68 (0.37, 1.27) | ||||
| C/A or A/A* | 108 | 0.41 ± 0.06 | 2.54 (1.41, 4.57) | 0.0012 | 2.33 (1.27, 4.27) | 0.007 | 108 | 0.42 ± 0.05 | 0.92 (0.62, 1.36) | 0.67 | 0.89 (0.56, 1.43) | 0.64 |
| ESR2 rs2978381 | 0.065 | 0.40 | 0.084 | 0.077 | ||||||||
| T/T | 82 | 0.41 ± 0.06 | 1 | 1 | 48 | 0.58 ± 0.07 | 1 | 1 | ||||
| T/C | 69 | 0.64 ± 0.06 | 0.55 (0.33, 0.93) | 0.75 (0.44, 1.27) | 86 | 0.30 ± 0.05 | 1.67 (1.05, 2.65) | 1.80 (1.08, 2.99) | ||||
| C/C | 18 | 0.57 ± 0.13 | 0.66 (0.30, 1.48) | 0.64 (0.28, 1.47) | 38 | 0.46v0.08 | 1.34 (0.76, 2.34) | 1.41 (0.71, 2.81) | ||||
| T/C or C/C* | 87 | 0.63 ± 0.06 | 0.58 (0.36, 0.93) | 0.021 | 0.72 (0.44, 1.18) | 0.19 | 124 | 0.35 ± 0.04 | 1.56 (1.00, 2.44) | 0.046 | 1.71 (1.04, 2.81) | 0.036 |
| ESR2 rs3020443 | 0.0040 | 0.043 | 0.54 | 0.090 | ||||||||
| T/T | 119 | 0.44 ± 0.05 | 1 | 1 | 97 | 0.45 ± 0.05 | 1 | 1 | ||||
| T/G* | 42 | 0.75 ± 0.07 | 0.39 (0.20, 0.76) | 0.50 (0.25, 0.98) | 62 | 0.37 ± 0.06 | 1.12 (0.77, 1.64) | 1.46 (0.94, 2.26) | ||||
| G/G* | 7 | 9 | ||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; LAC, Los Angeles County. Based on the log-rank test in the univariate analysis and Wald test in the multivariable analysis within Cox regression model.
Adjusted for age, performance status, stage and tumor site in the Japanese cohort; adjusted for stage, T-category, N-category, and tumor differentiation and stratified by race in the LAC cohort. Bold characters; significant.
Combined in the analysis in the dominant genetic model.
Figure 1.
Probability of overall survival by ESR2 rs2978381 in the Japanese and LAC cohorts, (left) Japanese cohort, (right) LAC cohort.
Interaction test between ESR2 polymorphism and cohort
We performed an exploratory interaction test between the three ESR2 SNPs and cohort group based on a likelihood ratio test in a multivariable Cox proportional hazards regression model adjusting for T- and N-categories and stratified by race. All three SNPs had a significantly different effect on survival in the Japanese and LAC cohorts. The Interaction term of the ESR2 rs2978381 and cohort group reached statistical significance (adjusted P = 0.021). Moreover, ESR2 rs1271572 and rs3020443 also showed a significant interaction with OS (adjusted P = 0.023 and 0.015, respectively).
DISCUSSION
Our study demonstrates that genetic variations in a promoter region of the ESR2 gene may predict prognosis in patients with locally advanced GC. These results also provide preliminary evidence, suggesting that the prognostic value of ESR2 gene variations may vary in an ethnic-dependent manner.
We found that all analyzed ESR2 SNPs were significantly associated with OS in univariate analysis among Japanese patients with locally advanced GC. Moreover, ESR2 rs1271572 and rs3020443 remained statistically significant in multivariable analysis. Functional variations in the promoter region of the ESR2 gene encoding ERβ have been reported to influence the expression of the gene and function of the protein. The 5′-UTR of the ESR2 gene is plentiful in CpG islands and permits several ERβ transcript variants.36 It has been shown that hypermethylation of the CpG islands near the untranslated exon 0 N of the ESR2 gene is linked to transcriptional inactivation of ERβ in several cancers.1,37,38 Moreover, SNPs in the promoter 0 N region of the ESR2 gene may affect cancer risk.19,39 The functionality of ESR2 rs3020443 has not been well characterized. However, the T/T genotype of ESR2 rs1271572 was reported to inhibit expression of its gene by downregulating transcriptional activity of the promoter 0 N,40 suggesting that the T allele of ESR2 rs1271572 may lead to a decrease in the transcriptional activity of the ERβ gene promoter 0 N. The functional mechanism of ESR2 rs2978381 is still not well understood. However, this SNP is located near ESR2 rs2987983, which has been identified as a putative susceptibility SNP for cancers and is also located among binding sites for a number of transcription factors.41,42 Given the location of the SNP, it is biologically plausible that this variation may have an impact on the gene transcription. Further mechanistic studies confirming the functional role of these polymorphisms are highly warranted.
In this study, the ESR2 rs2978381 predicted OS in both Japanese and US patients with GC. Furthermore, the effect of C allele of the SNP on survival was statistically significant in both cohorts and showed an inverse correlation. This phenomenon may result from the diversity in allele frequency that produces different patterns of survival association of the allele across different ethnic groups.5 In our study, a significant difference in allele frequency of the SNP was observed (Supplementary Table 3). On the other hand, this finding may be attributed to regional differences not only in biology but also in etiology of gastric carcinogenesis underlying the different GC subtypes observed in Japan and the United States. Preliminary interaction tests revealed that all three candidate SNPs showed significant interactions between the cohorts, indicating that ESR2 gene variations may affect prognosis of GC patients in a regional- or ethnic-dependent manner. GC can be classified at least into three principal subtypes based on clinical and epidemiological data in addition to gene expression analysis.43 Distal intestinal GC is strongly associated with chronic inflammation related to Helicobacter pylori infection.7,8 For gastroesophageal junction or proximal GC, inflammation due to chronic gastric acid secretion may be the driving force in carcinogenesis.44,45 In Asians, the intestinal type is more prevalent than the diffuse type,43,45 which is associated with higher rate of ERβ expression.20,22,23 Expression rate of ERβ by immunohistochemistry in GC has been shown to be higher in Asians than in Western populations.20,22,27 In our study, Japanese patients had a significantly higher incidence of more differentiated cancer than US patients, implying that the influence of estrogen may differ according to the cohorts. These GC-specific diversities based on physiologic and clinicopathologic backgrounds among regions may account for the different effect of the ESR2 gene in this study.
Our findings may also have clinical implications for future treatment insofar that the estrogen pathway could serve as a potential target for adjuvant treatment strategies to improve survival in patients with GC. A meta-analysis of epidemiologic studies has shown that postmenopausal hormone replacement therapy reduces the incidence of GC.46 It is likely that the use of estrogen before activation of aberrant pathways will protect against developing GC; however, it is uncertain whether estrogen will be able to restrain tumor progression when precancerous processes are already initiated. In addition, given the opposite effect of ERβ gene variation observed in our study, GC patients may respond differently to estrogen pathway-targeted therapy according to their race. Moreover, genetic variations in ESR2 may also be of predictive significance enabling us to select individuals who achieve maximum benefit from intensive chemotherapies. Further clinical validation studies not only in adjuvant GC but also in metastatic GC are needed to confirm these presumptions.
In an exploratory subgroup analysis, the worse survival associated with the C allele of the ESR2 rs2978381 SNP was also observed among Caucasian patients (N = 90) in the LAC cohort (adjusted hazard ratio 1.51, P = 0.23). However, this association did not differ significantly compared with the association in the Japanese cohort, which is likely due to reduced sample size. Body mass index (BMI) had a positive association with plasma estradiol (E2) levels.3 Moreover, the plasma E2 levels in postmenopausal women can be affected by adiposity, ethnicity and lifestyle factors such as diet and smoking.3,47,48 It is well established that plasma E2 levels are positively associated with BMI in Asian and Caucasian populations. The higher E2 levels in the US population in each gender may be related to the higher BMI compared with those in Japanese.49,50 The difference of E2 levels derived from environmental and non-genetic factors may impact the protective effect of estrogen in GC, probably explaining the findings in our study. In addition, estrogen is considered to have a protective role against development of GC, leading to a globally significant lower incidence and delayed onset in females compared with males.51,52 We therefore performed a sub-analysis by gender to evaluate a gender-specific difference in the prognostic impact of 40 the ERβ gene SNPs. However, no clear gender-related effect of ESR2 variations could be observed in the Japanese and US cohorts (Supplementary Table 4).
This study is hypothesis-generating and has a number of limitations that need to be considered. The number of patients in each cohort is modest for a polymorphism study and may lack the adequate statistical power to investigate the associations with clinical outcome. In addition, selection bias cannot be excluded due to the retrospective study design. One hundred and nine (64%) out of 169 Japanese patients received adjuvant chemotherapy, which was not associated with OS. Information about adjuvant chemotherapy was missing in approximately half of the patients of the US cohort. Nevertheless, our analysis allowed us to assume that ESR2 gene variations may serve as prognostic markers in an ethnic-dependent manner although we cannot exclude that the results were attributable to chance. Owing to small sample size of Asian patients in the LAC cohort, we were not able to validate the associations between LAC Asian patients and Japanese patients (data not shown). Therefore, translational studies including larger patient cohorts with same ethnicity are necessary to validate our findings.
In conclusion, our results provide evidence that genetic variations in a promoter region of the ESR2 gene are significantly associated with survival in patients with locally advanced GC. Our data also suggest that the prognostic value of ESR2 gene variations may vary in an ethnic-dependent manner. Further functional correlative preclinical analyses and external clinical validation studies are needed to confirm our results.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly funded by the NIH grant P30CA14089–27S1 and Yvonne Bogdanovich. It was performed in the Sharon A Carpenter Laboratory at University of Southern California/Norris Comprehensive Cancer Center and in memory of David Donaldson. Martin D Berger received a grant from the Swiss Cancer League (BIL KLS-3334–02-2014). Stefan Stremitzer is a recipient of an Erwin Schrödinger fellowship of the Austrian Science Fund (J3501-B13).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website (http://www.nature.com/tpj)
REFERENCES
- 1.Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC et al. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene 2003; 22: 7600–7606. [DOI] [PubMed] [Google Scholar]
- 2.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 2000; 275: 5379–5387. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 2010; 66: 33–38. [DOI] [PubMed] [Google Scholar]
- 4.Castiglione F, Taddei A, Rossi Degl’Innocenti D, Buccoliero AM, Bechi P, Garbini F et al. Expression of estrogen receptor beta in colon cancer progression. Diagn Mol Pathol 2008; 17: 231–236. [DOI] [PubMed] [Google Scholar]
- 5.Evans DM, Cardon LR. A comparison of linkage disequilibrium patterns and estimated population recombination rates across multiple populations. Am J Hum Genet 2005; 76: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am J Hum Genet 2007; 80: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002; 2: 28–37. [DOI] [PubMed] [Google Scholar]
- 8.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 9.Hartz A, He T, Ross JJ. Risk factors for colon cancer in 150,912 postmenopausal women. Cancer Causes Control 2012; 23: 1599–1605. [DOI] [PubMed] [Google Scholar]
- 10.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand JS, Jacobs EJ, Campbell PT, McCullough ML, Teras LR, Thun MJ et al. Colorectal cancer incidence and postmenopausal hormone use by type, recency, and duration in cancer prevention study II. Cancer Epidemiol Biomarkers Prev 2009; 18: 2835–2841. [DOI] [PubMed] [Google Scholar]
- 12.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000; 404: 398–402. [DOI] [PubMed] [Google Scholar]
- 13.de Bruin WC, Wagenmans MJ, Board PG, Peters WH. Expression of glutathione S-transferase theta class isoenzymes in human colorectal and gastric cancers. Carcinogenesis 1999; 20: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 14.Moon YW, Park S, Sohn JH, Kang DR, Koo JS, Park HS et al. Clinical significance of progesterone receptor and HER2 status in estrogen receptor-positive, operable breast cancer with adjuvant tamoxifen. J Cancer Res Clin Oncol 2011; 137: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 15.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 2005; 11: 4741–4748. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Barnes CJ, Kumar R. Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cells. Clin Cancer Res 2004; 10: 3621–3628. [DOI] [PubMed] [Google Scholar]
- 17.Chan JA, Meyerhardt JA, Chan AT, Giovannucci EL, Colditz GA, Fuchs CS. Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol 2006; 24: 5680–5686. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol 2002; 128: 319–324. [DOI] [PubMed] [Google Scholar]
- 19.Sun YH, Yang B, Wang XH, Xu CL, Gao XF, Gao X et al. Association between single-nucleotide polymorphisms in estrogen receptor beta gene and risk of prostate cancer. Zhonghua wai ke za zhi [Chinese journal of surgery] 2005; 43: 948–951. [PubMed] [Google Scholar]
- 20.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol 2007; 33: 195–201. [DOI] [PubMed] [Google Scholar]
- 21.Guo JL, Xu CY, Jiang ZN, Dong MJ, Xie SD, Shen JG et al. Estrogen receptor beta variants mRNA expressions in gastric cancer tissues and association with clinicopathologic parameters. Hepatogastroenterology 2010; 57: 1584–1588. [PubMed] [Google Scholar]
- 22.Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY et al. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol 2010; 17: 2503–2509. [DOI] [PubMed] [Google Scholar]
- 23.Ryu WS, Kim JH, Jang YJ, Park SS, Um JW, Park SH et al. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol 2012; 106: 456–461. [DOI] [PubMed] [Google Scholar]
- 24.Theodoropoulos GE, Lazaris AC, Panoussopoulos D, Davaris P, Golematis BC. Significance of estrogen receptors and cathepsin D tissue detection in gastric adenocarcinoma. J Surg Oncol 1995; 58: 176–183. [DOI] [PubMed] [Google Scholar]
- 25.Matsui M, Kojima O, Kawakami S, Uehara Y, Takahashi T. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today 1992; 22: 421–425. [DOI] [PubMed] [Google Scholar]
- 26.Woo IS, Park MJ, Choi SW, Kim SJ, Lee MA, Kang JH et al. Loss of estrogen receptor-alpha expression is associated with hypermethylation near its ATG start codon in gastric cancer cell lines. Oncol Rep 2004; 11: 617–622. [PubMed] [Google Scholar]
- 27.Chandanos E, Rubio CA, Lindblad M, Jia C, Tsolakis AV, Warner M et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. Gastric Cancer 2008; 11: 168–174. [DOI] [PubMed] [Google Scholar]
- 28.Karat D, Brotherick I, Shenton BK, Scott D, Raimes SA, Griffin SM. Expression of oestrogen and progesterone receptors in gastric cancer: a flow cytometric study. Br J Cancer 1999; 80: 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Poulsom R, Wright NA, Sheppard MC, Langman MJ. Differential expression of oestrogen receptor and oestrogen inducible genes in gastric mucosa and cancer. Gut 1997; 40: 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan AM, Collins D, Baird AW, Winter DC. Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol 2009; 307: 19–24. [DOI] [PubMed] [Google Scholar]
- 31.Maguire P, Margolin S, Skoglund J, Sun XF, Gustafsson JA, Borresen-Dale AL et al. Estrogen receptor beta (ESR2) polymorphisms in familial and sporadic breast cancer. Breast Cancer Res Treat 2005; 94: 145–152. [DOI] [PubMed] [Google Scholar]
- 32.Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Ann Epidemiol 2006; 16: 908–916. [DOI] [PubMed] [Google Scholar]
- 33.Passarelli MN, Phipps AI, Potter JD, Makar KW, Coghill AE, Wernli KJ et al. Common single-nucleotide polymorphisms in the estrogen receptor beta promoter are associated with colorectal cancer survival in postmenopausal women. Cancer Res 2013; 73: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001; 12: 721–732. [DOI] [PubMed] [Google Scholar]
- 35.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005; 92: 965–970. [Google Scholar]
- 36.Li LC, Yeh CC, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor beta promoter. Biochem Biophys Res Commun 2000; 275: 682–689. [DOI] [PubMed] [Google Scholar]
- 37.Li LC, Chui R, Nakajima K, Oh BR, Au HC, Dahiya R. Frequent methylation of estrogen receptor in prostate cancer: correlation with tumor progression. Cancer Res 2000; 60: 702–706. [PubMed] [Google Scholar]
- 38.Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S et al. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5’-untranslated region in human epithelial ovarian carcinoma. Cancer Sci 2008; 99: 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risk M-GCoGSfMHTRBC. Polymorphisms in genes of the steroid receptor superfamily modify postmenopausal breast cancer risk associated with menopausal hormone therapy. Int J Cancer 2010; 126: 2935–2946. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Liang Y, Qiu J, Zhang L, Chen X, Luo X et al. Significance of rs1271572 in the estrogen receptor beta gene promoter and its correlation with breast cancer in a southwestern Chinese population. J Biomed Sci 2013; 20: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treeck O, Elemenler E, Kriener C, Horn F, Springwald A, Hartmann A et al. Polymorphisms in the promoter region of ESR2 gene and breast cancer susceptibility. J Steroid Biochem Mol Biol 2009; 114: 207–211. [DOI] [PubMed] [Google Scholar]
- 42.Thellenberg-Karlsson C, Lindstrom S, Malmer B, Wiklund F, Augustsson-Balter K, Adami HO et al. Estrogen receptor beta polymorphism is associated with prostate cancer risk. Clin Cancer Res 2006; 12: 1936–1941. [DOI] [PubMed] [Google Scholar]
- 43.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011; 17: 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265: 1287–1289. [PubMed] [Google Scholar]
- 45.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2012; 21: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu AH, Stanczyk FZ, Seow A, Lee HP, Yu MC. Soy intake and other lifestyle determinants of serum estrogen levels among postmenopausal Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev 2002; 11: 844–851. [PubMed] [Google Scholar]
- 48.Kim C, Golden SH, Mather KJ, Laughlin GA, Kong S, Nan B et al. Racial/ethnic differences in sex hormone levels among postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab 2012; 97: 4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R et al. Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clinica chimica acta 2008; 398: 43–47. [DOI] [PubMed] [Google Scholar]
- 50.Vaidya D, Dobs A, Gapstur SM, Golden SH, Cushman M, Liu K et al. Association of baseline sex hormone levels with baseline and longitudinal changes in waist-tohip ratio: Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond) 2012; 36: 1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer 2002; 5: 213–219. [DOI] [PubMed] [Google Scholar]
- 52.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut 2009; 58: 16–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.