Abstract
Background
Integrin β3 is involved in tumor and endothelial cell biology as well as in platelet aggregation. Herein, we evaluated the predictive potential of three germline single nucleotide polymorphisms (SNPs) in the integrin β3 gene (rs3809865, rs5918 and rs4642) to predict the risk of venous thromboembolism (VTE) in colorectal cancer (CRC) patients, which is one of the leading causes of death among cancer patients.
Methods
112 patients diagnosed with CRC enrolled in the prospective Vienna Cancer and Thrombosis Study (CATS) were assessed with a median follow-up of 46 months. DNA was isolated from venous blood samples and SNPs were analyzed by the PCR-RFLP method.
Results
VTE occurred in 12% (n = 13) of all patients. The SNPs rs5918 and rs4642 were not associated with VTE risk. For rs3809565, 23% (n = 11) of patients had the A/A genotype, 4% (n = 2) had the A/T genotype, but none (0%) had the T/T genotype. In the univariate analysis, patients with the A/A genotype had a significantly higher risk to develop VTE compared to the other polymorphisms (P = 0.0005 after Fine and Gray). In the multivariable analysis, the predictive value remained significant.
Conclusions
This study identified the rs3809865 A/A genotype as an independent risk factor for VTE in CRC patients. Our findings would help identify high risk patients and would be essential for tailored anticoagulant prophylaxis.
Keywords: Single nucleotide polymorphism, Integrin β3, Venous thromboembolism risk, Colorectal cancer, rs3809565, Anticoagulant prophylaxis
1. Introduction
Venous thromboembolism (VTE) is one of the leading causes of death in cancer patients causing additional life-threatening complications and significantly higher health care costs [1–3]. Specific cancer related patient characteristics such as tumor site, stage at diagnosis, histologic subtype, tumor grade or anticancer treatment with fluoropyrimidines ± bevacizumab or oxaliplatin can promote the development of VTE [4–8]. In colorectal cancer (CRC) patients the estimated risk of developing VTE within 2 years was reported to be 8.2% [9]. Patients with metastatic disease have a 5- to 13-fold higher risk of developing VTE compared to those with localized disease [10,11]. The pathogenesis that leads to a hypercoagulable state in cancer patients is complex and is mediated by various interactions of tumor cells with platelets and endothelial cells, affecting the clotting system [12,13].
Integrin receptors are heterodimeric cell adhesion proteins which consists of an α and β subunit [14]. Integrin β3 is essentially expressed on endothelial cells, platelets, osteoclasts and hematopoietic cells and corresponds to the group of integrins that binds to proteins containing the arginine-glycine-aspartic acid (RGD)-motif [15]. Integrin β3 can form heterodimers with the subunits αV and αIIb. Integrin αVβ3 is expressed by activated endothelial cells and tumor cells [16]. It promotes proangiogenic endothelial cell behavior, such as cell migration, invasion and survival, and is a major mediator of tumor angiogenesis, tumor growth and platelet aggregation [16–20]. Integrin αIIbβ3 is exclusively expressed on platelets and mediates platelet aggregation to promote thrombus formation [20,21].
The paramount role of integrin β3 in thrombus formation prompted us to investigate if there was a correlation between VTE in patients with CRC and the β3 integrin SNPs rs3809865, rs5918 and rs4642. The selection of these SNPs is based on a previous work [22], in which it was investigated if a comprehensive panel of germline SNPs of integrin genes could predict stage-specific time to tumor recurrence in colon cancer. In this post-hoc analysis, we retrospectively analyzed 112 patients recruited in the framework of the prospective Vienna Cancer and Thrombosis Study (CATS) for association of genetic variants in the integrin β3 gene with risk of VTE. 43% (n = 48) of all the patients had a rs3809865 A/A genotype, 45% (n = 50) a A/T genotype and 12% (n = 14) a T/T genotype. VTE occurred in 12% (n = 13) of all patients, 23% (n = 11) of A/A rs3809865 patients, in 4% (n = 2) of A/T patients, but none (0%) patients had the T/T rs3809865 genotype. rs5918 and rs4642 SNPs were not associated with risk of VTE in this population. This study identified the germline polymorphism A/A of rs3809865 as an independent risk factor for VTE in CRC patients. A single laboratory test stratifying high and low risk groups for developing VTE, could lead to prevention strategies, thus, improving quality of life, safe costs and decreasing mortality rates in these patients.
2. Methods
2.1. Patients and study design
112 CRC patients who entered the prospective CATS between February 2004 and June 2011 were analyzed. The study was performed in accordance with the Declaration of Helsinki and was approved by the ethical committee of the institution. Detailed information about the CATS study has been reported previously [23,24]. In brief, patients diagnosed with CRC gave informed consent before venous blood samples were drawn. The overall aim of CATS was defined to identify parameters and biomarkers to predict occurrence of VTE in cancer patients. While the CATS maximum observation period for VTE events is 2 years, the occurrence of objectively confirmed VTE in this analysis was retrospectively extended until August 2013. In CATS, study patients were not routinely screened for VTE, but symptomatic or fatal VTE were classified as events. Diagnosis of VTE was in all cases confirmed by objective medical imaging methods, such as duplex sonography or computer tomographic scans. Each event was discussed and evaluated by an independent adjudication committee. Non symptomatic events, such as accidentally detected VTE in a restaging examination, were considered as an event when the adjudication committee considered them to be of clinical significance. Patients with continuous oral anticoagulation were excluded and no routine thromboprophylaxis for VTE was performed in our study. However, thromboprophylaxis with low-molecular-weight-heparin (LMWH) was allowed in hospitalized patients or after surgery according to clinical practice guidelines. In addition to CATS, this analysis considered additional patient data such as anti-VEGF treatment.
2.2. Blood sampling and SNP genotyping
Venous blood samples were drawn into plasma vacuum tubes (Vacuette; Greiner Bio One, Austria) containing one-tenth volume sodium citrate stock solution at 0.129 mM. To obtain platelet-poor plasma, the citrated blood was centrifuged (ROTANTA/TRC; Hettich, Germany) at 1500 g for 15 minutes, and to obtain platelet free plasma a second centrifugation step (Eppendorf) at 13 400 g for 2 minutes was performed. Plasma aliquots were stored at −80 °C until they were assayed for PCR testing of three SNPs in integrin β3 gene. Samples were coded before laboratory analysis. During analysis researchers and technicians were unaware of the patients’ characteristics at all times. Genotyping was performed applying a combined PCR-restriction fragment length polymorphism approach (PCR-RFLP). In brief, a short sequence including the site in question was amplified using primers binding 66–99 bps upstream and downstream resulting in PCR products 149–158 bps in size. Subsequently, the amplification product was digested using restriction enzymes, which were specifically chosen to cut or not at the site of the polymorphism in question according to genotype. After digestion, the resulting DNA-fragments were separated on an agarose gel. Consequently, lanes with bands in all three size ranges were considered heterozygote samples and lanes with only one band in the 150 bp region or two bands in the 60–100 bp region were considered to be homozygote in respect to the SNP in question. (Primers and enzymes are listed in supplemental Table S1). PCR assays were performed with 1.25 U DreamTaq™, 8 mM (total) dNTPs, 1x DreamTaq™ Green Buffer (Fermentas), 0.5 μM fw-primer and 0.5 μM rv-primer. Annealing temperatures were optimized in advance. Restriction digestions with SNP-specific restriction enzymes were performed according to the recommendations of the manufacturer. DNA-fragments were separated on a pre-stained (GelRED™, GenON) 4% agarose gel at 120 V for 30 min. and visualized on a BioRADChemiDOC XRS system.
2.3. Statistical analysis
Continuous variables were described with median and interquartile range (IQR); nominal variables were described by absolute numbers and percentage. SP-Selectin was compared between genotypes using t-tests on the logarithmised variable. The median follow-up time was estimated using the reverse Kaplan-Meier method [25]. The effects of sex, age, stage, metastatic site, tumor location, BMI and of the polymorphisms rs3809865, rs5918 and rs4642 on the occurrence of VTE was investigated in univariate, bias corrected Fine-Gray models [26] where death was considered as competing event. Due to the small number of events, we only considered multivariable Fine-Gray models including rs389086 and either sex, age, stage, metastatic site, tumor location and BMI. Since Fine-Gray models with death as competing risk do not handle time-dependent variables adequately [27], the effect of the time-dependent variable treatment with bevacizumab and of rs3809865 adjusted for treatment with bevacizumab was estimated in a bias corrected Cox-model where death was considered as censoring event. Here, we assumed that the effect of bevacizumab persisted 4 weeks after the end of therapy. We plotted crude cumulative incidence curves for each level of the polymorphism rs3809865, where death was considered as competing event. Chi-square goodness-of-fit tests were used to test the polymorphisms for Hardy-Weinberg Equilibrium and to compare for each polymorphism the minor allele frequency of our study population with the Global MAF based on 1000 Genomes. All analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study population
Baseline demographic and clinical characteristics of the study population are displayed in Table 1. One-hundred-twelve patients after diagnosis with CRC were prospectively observed. Median age at time of enrolment into CATS was 64 years (IQR, 57–71 years). Median observation time was 1399 days (IQR, 704–2197 days). 42 female patients (37%) and 70 male patients (63%) had been enrolled. Left sided primary tumors, including those originating from the splenic flexure, sigmoid, rectosigmoid junction, or rectum were recorded in 81 cases (72%), while right sided colon cancer was listed in 23 cases (21%). 3 patients (2%) were enrolled with two colorectal primary tumors and 5 (4%) patients were considered as CRC with non-recorded primary origin. Most patients (79%) had locally advanced (stage III) or metastatic disease (stage IV). During the observation period, 49 patients (44%) received the anti-VEGF antibody bevacizumab. Two patients were enrolled into blinded randomized controlled studies considering bevacizumab as the experimental treatment arm. Whether the two patients received bevacizumab is unknown; none of these patients, however, were diagnosed with VTE.
Table 1.
Baseline characteristics of the study population (n = 112).
| Characteristic | Value | Percentage |
|---|---|---|
| Median age at study entry (years) | 64 | |
| IQR | 57–71 | |
| Sex | ||
| Female | 42 | 37% |
| Male | 70 | 63% |
| Site of the primary tumor | ||
| Rectum | 50 | 45% |
| Rectosigmoid junction | 2 | 1.8% |
| Sigmoid colon | 25 | 22% |
| Splenic flexure | 4 | 3.6% |
| Transverse colon | 6 | 5.4% |
| Hepatic flexure | 1 | 0.9% |
| Ascending colon | 7 | 6.3% |
| Cecum | 8 | 7.1% |
| Appendix | 1 | 0.9% |
| Two sites | 3 | 2.7% |
| Unknown | 5 | 4.5% |
| Tumor stage at study entry* | ||
| Stage 0 | 3 | 2.7% |
| Stage I | 9 | 8% |
| Stage II | 11 | 9.8% |
| Stage III | 17 | 15% |
| Stage IV | 72 | 64% |
| Progression of tumor at study entry | ||
| Localized | 40 | 36% |
| Distant metastasis | 72 | 64% |
| Anti-VEGF-A treatment from study entry | ||
| Bevacizumab treatment | 49 | 44% |
| Study patient optional bevacizumab treatment | 2 | 1.8% |
Categorical variables are described with absolute numbers and percentages. Age is described as median with IQR.
For staging UICC staging was used (stage Ia and Ib are summarized as stage I; stage 0 is defined as ypT0pN0M0, R0 - these are patients with rectum carcinoma, who received presurgical radiotherapy).
3.2. Thromboembolic events
VTE occurred in 12% (n = 13) of patients, 38% (n = 5) were diagnosed with stage III and 62% (n = 8) with distant metastatic disease (stage IV). Detailed information on patients with VTE is given in Table 2. 11 patients were male (85%). 9/13 (69%) patients had left sided CRC, while 4/13 (31%) had right sided colon cancer. Of the 49 patients who received a bevacizumab-containing therapy, four developed VTE (8%). At the time of VTE event, all patients had either locally advanced (2/13) or metastatic CRC (11/13). One VTE was associated with a Port-a-Cath (PAC) implant. By August 2013, 62% (n = 69) of patients died and 38% (n = 43) were alive.
Table 2.
Baseline characteristics of VTE patients (N = 13; 11.6%).
| Characteristic | Value | Percentage |
|---|---|---|
| Median age at study entry (years) | 67 | |
| IQR | 62–71 | |
| Sex | ||
| Female | 2 | 15% |
| Male | 11 | 85% |
| Site of cancer | ||
| Rectum | 6 | 46% |
| Rectosigmoid junction | 0 | 0 |
| Sigmoid colon | 2 | 15% |
| Splenic flexure | 1 | 7.7% |
| Transverse colon | 1 | 7.7% |
| Hepatic flexure | 0 | 0 |
| Ascending colon | 1 | 7.7% |
| Cecum | 2 | 0,15 |
| Appendix | 0 | 0 |
| Two sites | 0 | 0 |
| Unknown | 0 | 0 |
| Tumor stage at study entry | ||
| Stage 0 | 0 | 0 |
| Stage I | 1 | 7.7% |
| Stage II | 1 | 7.7% |
| Stage III | 3 | 23% |
| Stage IV | 8 | 62% |
| Progression of tumor at study entry* | ||
| Localized | 5 | 38% |
| Distant metastasis | 8 | 62% |
| Tumor stage when VTE occurred | ||
| Stage 0 | 0 | 0% |
| Stage I | 0 | 0% |
| Stage II | 0 | 0% |
| Stage III | 2 | 15% |
| Stage IV | 11 | 85% |
| Anti-VEGF-A treatment and VTE | ||
| Bevacizumab at time of VTE (+4 weeks) | 4 | 31% |
| In relation to total population with bevacizumab | 4 | 8.2% |
| Site of thrombotic event counted | ||
| Isolated pulmonary vein thrombosis (PE) | 6 | 46% |
| Isolated deep vein thrombosis (DVT) | 6 | 46% |
| Subclavian vein thrombosis (PAC-implant) | 1 | 7.7% |
Categorical variables are described with absolute numbers and percentages. Age is described as median with IQR.
For staging UICC staging was used (stage Ia and Ib are summarized as stage I; stage 0 is defined as ypT0pN0M0, R0 - these are patients with rectum carcinoma, who received presurgical radiotherapy).
3.3. Thromboembolic events and rs3809865 distribution
48 (43%) patients presented the homozygous mutant variant of integrin β3 rs3809865 A/A, 50 patients (45%) were harboring the A/T and 14 (12%) the T/T genotype. This genotype distribution obeyed the genotype count reported in the 1000 Genomes panel (52,91% for the A/A variant, 38,86% for the heterozygote genotype and 8,22% for the T/T variant) [28]. From the 13/112 patients (12%), who developed an objectively confirmed VTE, 11 patients (85%) had an rs3809865 A/A genotype, while 2 (15%) had an A/T genotype. No VTE patient had a homozygous mutant variant rs3809865 T/T.
3.4. Rs3809865 and the risk of VTE
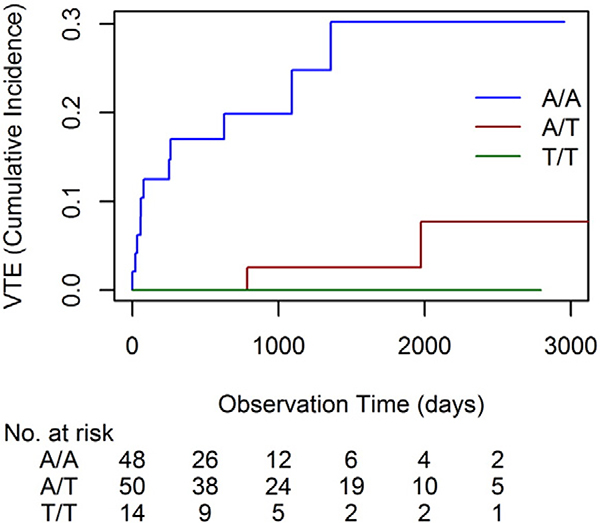
Investigating the effect of rs3809865 polymorphism on the risk of VTE in a univariate Fine and Gray analysis with death as competing event, we found that patients with a rs3809865 A/A genotype had a significantly higher risk to develop VTE compared to those with the polymorphism A/T or T/T (HR = 7.87; 95% CI: 2.31 to 40.00, p = 0.0005). This effect remained significant in the multivariable analysis considering in addition either sex, age, stage, tumor location (left vs. right), metastatic sites, BMI or bevacizumab-containing treatment (Table 3). Cumulative incidence of developing VTE within 2500 days was significantly higher for the homozygous mutant variant rs3809865 A/A with 30% compared to 8% for polymorphism A/T or 0% for polymorphism T/T (Fig. 1). Additional analysis of sP-Selectin and rs3809865 genotype A/A did not show significantly increased sP-Selectin levels in this subgroup of patients (p = 0.6063). Neither sex, age, stage, tumor location, metastatic sites, BMI nor bevacizumab-containing treatment showed a significant association with VTE occurrence in univariate analysis.
Table 3.
Subdistribution hazard ratio for A/A compared to A/T and T/T at rs3809865 (adjusted for either sex, age, stage, metastatic sites, tumor location, BMI or bevacizumab containing treatment).
| Factors | HR | 95% CI | P |
|---|---|---|---|
| Parameters analyzed | |||
| Unadjusted | 7.87 | 2.31–40.00 | 0.0005 |
| Adj. for sex | 7.52 | 2.20–38.46 | 0.0008 |
| Adj. for age | 7.63 | 2.23–40.00 | 0.0007 |
| Adj. for stage *1 | 13.89 | 3.56–76.92 | <.0001 |
| Adj. for metastatic sites | 12.08 | 3.42–66.67 | <.0001 |
| Adj. for tumor location*2 | 7.7 | 2.26–40 | 0.0006 |
| Adj. for BMI | 8.62 | 2.52–45.45 | 0.0003 |
| Adj. for bevacizumab*3 | 8.20 | 2.31–41.67 | 0.0006 |
Stage was determined at CATS admission date. Stage was categorized in stage 1–3 and 4, 3 patients (stage 0) were excluded from analysis.
Three patients with two-sided tumor location were excluded from analysis.
Study patients with unsure treatment of bevacizumab were excluded from the analysis.
Fig. 1. Competing Risk Model:
Cumulative incidence of VTE stratified by rs3809865 polymorphisms (n = 13). Patients with rs3809865 polymorphism A/A (blue) are on a higher risk of developing VTE compared to those with polymorphism A/T (red) or T/T (green).
3.5. Integrin β3 rs5918 or rs4642 and VTE in CRC
DNA of 96 CRC patients, among them DNA from all 13 patients with VTE, was assessable for further SNP germline polymorphism analysis. Integrin β3 rs5918 C > T had no predictive value for VTE (p = 0.2466) (Table 4). Integrin β3 rs4642 polymorphism, which could be analyzed in 88 patients, also did not reveal a significant association with the risk of developing VTE (A/A compared to A/G and G/G, p = 0.9579) (Table 5).
Table 4.
rs5918 polymorphism distribution, p = 0.2466 for polymorphism T/T compared to C/T and C/C.
| rs5918 | Number of Patients (%) | Number of patients with VTE events |
|---|---|---|
| T/T | 75 (78.13%) | 12 (92.31%) |
| C/T | 18 (l8.75%) | 1 (7.69%) |
| C/C | 3 (3.13%) | 0 (0%) |
Table 5.
rs4642 polymorphism distribution, p = 0.9579 for polymorphism A/A compared to A/G and G/G.
| rs4642 | Number of Patients (%) | Number of patients with VTE events |
|---|---|---|
| A/A | 39 (44.32%) | 5 (45.45%) |
| A/G | 33 (37.50%) | 5 (45.45%) |
| G/G | 16 (18.18%) | 1 (9.09%) |
4. Discussion
VTE contributes to elevated morbidity and mortality in cancer patients and so far, several risk factors for VTE were identified in different clinical studies. It was already shown that the risk of VTE in the cohort of patients presented herein (CATS) correlates with tumor stage, metastatic sites, tumor location and chemotherapy (reviewed in [29]). There was no association found between risk of VTE and age, gender or BMI, which is in line with other clinical studies [30,31]. In the present study we observed an almost 8-fold increased risk of VTE in CRC patients carrying the A/A genotype of integrin β3 rs3809865. Of the 112 patients eligible for SNP analysis and VTE risk assessment, almost one quarter of the 48 patients with rs3809865 polymorphism A/A, only 4% of the 50 patients with polymorphism A/T and none of the 14 patients with polymorphism T/T developed VTE during the observation period. In multivariable analysis adjusting for either sex, age, stage tumor location (left vs. right), metastatic sites, BMI or treatment with bevacizumab the predictive value remained significant. The allele frequencies of rs3809865 in our study population did not show a significant deviation from the Hardy-Weinberg Equilibrium (supplemental Table S2).
These results indicate that the hitherto unknown integrin β3 single nucleotide polymorphism rs3809865 can predict the risk of VTE in CRC patients. rs3809865 is located at the 3′UTR region of the integrin β3 gene. The 3′UTR region regulates β3 integrin gene expression and regulation at the mRNA level and interacts with miRNAs [32]. Thus, although not shown here, it is tempting to speculate that rs3809865 might functionally affect miRNA binding and consequently, the protein expression level of the integrin β3 subunit [33–35]. In this context, it was shown before that the miRNAs hsa-mir-506 and hsa-mir-124 bind more stably to the T allele when compared to the A allele of rs3809865 [35], which might lead to an increased expression of integrin β3 in patients harboring the A/A genotype when compared to those with an A/T or T/T rs3809865 genotype [35]. Whether rs3809865 mutant variants, which have been described before to affect miRNA interaction and integrin β3 expression, have a functional impact on platelet or endothelial cell activation is so far unknown and is currently investigated in a subsequent study.
Ligand binding to integrin αIIbβ3 mediates stable platelet adhesion, platelet aggregation, and thrombus formation via binding its ligands fibrinogen, von Willebrand factor, and other RGD-sequence containing matrix proteins. Furthermore, ligand binding triggers an “outside-in” signaling, resulting in platelet spreading, additional granule secretion, stabilization of platelet adhesion, platelet aggregation and clot retraction.
Further on, integrin activation is tightly regulated via intracellular signaling events (inside-out). Talin binds to the cytoplasmic tail of the beta integrin subunit [36], which leads to a conformational change of integrins from an inactive bend formation to an open straighten conformation, which unencrypt the ligand binding site [37–40]. The latter event is a major regulator for platelet activation and aggregation [20]. A decreased expression of integrin αIIbβ3 is associated with an increased bleeding risk and is a characteristic of Glanzmann′s thrombasthenia [41]. Targeting integrin αIIbβ3 affects platelet aggregation [42] and the monoclonal antibody abciximab (a IIb receptor antagonist) is in clinical use during percutaneous coronary intervention for the prevention of cardiac ischemic complications [43]. Although not shown here, this large body of evidence suggests that a miRNA binding site genetic polymorphism might affect β3 integrin expression, interfering with platelet activation.
Patients with advanced disease are on higher risk to develop VTE [10,11]. At study entry more than 80% of CRC patients had advanced disease stages (either stage III or IV). In the multivariable analysis, however, tumor stage did not affect the predictive value of rs3809865 mutant variants for risk of VTE.
The detection of new predictive biomarkers for VTE risk assessment could be an important step forward the improvement in quality of life and overall survival in cancer patients [1–3,44]. Current risk assessment models are based on the testing of multiple blood parameters and clinical risk factors [45–47]. The hypercoagulable state in cancer patients has a complex pathogenesis. A single laboratory parameter with a high predictive value could improve current risk assessment models. Our data provide first evidence that germline variant integrin β3 rs3809865 might predict the risk of VTE in CRC patients. This first observation is currently being validated in an independent prospective translational study PASSION-ATE (NCT02119026), which analyzes biomarkers in metastasized CRC patients during first and second line treatment with bevacizumab and capecitabine plus oxaliplatin or irinotecan.
In summary, we have shown that a single germline variant of a central contributor molecule in tumor-, endothelial cell and platelet biology might play an important role predicting individual risk of VTE in CRC patients. Because prevention of thromboembolism in cancer patients, especially when treated in an out-patient setting, is not generally recommended, identification of high risk patients via a reliable parameter would be essential for tailored anticoagulant prophylaxis. This could reduce VTE rates in high risk patients and avoid unnecessary side-effects and health care costs of anti-coagulant agents in those patients who are at low-risk. Furthermore, the association between integrin subunit genetic variants and risk of VTE supports the functional role of integrins in the pathogenesis of cancer-associated VTE.
Supplementary Material
Acknowledgements
This work has been supported by the Austrian Science Fund (FWF) (Project No. P23199FW).
The study is dedicated to all patients and their family members who participated in CATS. The authors would like to thank Dr. Matthias Unseld for his contribution in preparing the manuscript.
Abbreviations
- SNP
single nucleotide polymorphism
- VTE
venous thromboembolism
- CRC
colorectal cancer
- RFLP
restriction fragment length polymorphism
Footnotes
Disclosure of conflicts of interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.thromres.2015.08.010.
References
- [1].Lyman GH, et al. , American Society of Clinical Oncology Guideline: Recommendations for Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer, J. Clin. Oncol 25 (34) (2007) 5490–5505. [DOI] [PubMed] [Google Scholar]
- [2].A Khorana A, et al. , Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy, J. Thromb. Haemost 5 (3) (2007) 632–634. [DOI] [PubMed] [Google Scholar]
- [3].Lyman GH, et al. , Venous Thromboembolism Risk in Patients With Cancer Receiving Chemotherapy: A Real-World Analysis, Oncologist 18 (12) (2013) 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khorana AA, Connolly GC, Assessing Risk of Venous Thromboembolism in the Patient With Cancer, J. Clin. Oncol 27 (29) (2009) 4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Horsted F, West J, Grainge M, Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis, PLoS Med 9 (7) (2012) e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ahlbrecht J, et al. , Tumor Grade Is Associated With Venous Thromboembolism in Patients With Cancer: Results From the Vienna Cancer and Thrombosis Study, J. Clin. Oncol 30 (31) (2012) 3870–3875. [DOI] [PubMed] [Google Scholar]
- [7].Nalluri S, et al. , Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis, JAMA 300 (19) (2008) 2277–2285. [DOI] [PubMed] [Google Scholar]
- [8].Seng S, et al. , Risk of Venous Thromboembolism in Patients With Cancer Treated With Cisplatin: A Systematic Review and Meta-Analysis, J. Clin. Oncol 30 (35) (2012) 4416–4426. [DOI] [PubMed] [Google Scholar]
- [9].Riedl J, et al. , Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS), Thromb. Haemost. 111 (4) (2014). [DOI] [PubMed] [Google Scholar]
- [10].Chew HK, et al. , Incidence of Venous Thromboembolism and Its Effect on Survival Among Patients With Common Cancers, Arch. Intern. Med 166 (4) (2006) 458. [DOI] [PubMed] [Google Scholar]
- [11].Mandala M, et al. , Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines, Ann. Oncol 22 (Suppl. 6) (2011) vi85–vi92. [DOI] [PubMed] [Google Scholar]
- [12].Falanga A, Marchetti M, Vignoli A, Coagulation and cancer: biological and clinical aspects, J. Thromb. Haemost 11 (2) (2013) 223–233. [DOI] [PubMed] [Google Scholar]
- [13].Lyman GH, A Khorana A, Cancer, clots and consensus: new understanding of an old problem, J. Clin. Oncol 27 (29) (2009) 4821–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van der Flier A, Sonnenberg A, Function and interactions of integrins, Cell Tissue Res 305 (3) (2001) 285–298. [DOI] [PubMed] [Google Scholar]
- [15].Caiado F, Dias S, Endothelial progenitor cells and integrins: adhesive needs, Fibrogenesis Tissue Repair 5 (2012) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Danhier F, Le Breton A, Preat V, RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis, Mol. Pharm 9 (11) (2012. ) 2961–2973. [DOI] [PubMed] [Google Scholar]
- [17].Garmy-Susini B, Varner JA, Roles of integrins in tumor angiogenesis and lymphangiogenesis, Lymphat. Res. Biol 6 (3–4) (2008) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prager GW, Poettler M, Angiogenesis in cancer. Basic mechanisms and therapeutic advances, Hamostaseologie 32 (2) (2012) 105–114. [DOI] [PubMed] [Google Scholar]
- [19].Robinson SD, Hodivala-Dilke KM, The role of β3-integrins in tumor angiogenesis: context is everything, Curr. Opin. Cell Biol 23 (5) (2011) 630–637. [DOI] [PubMed] [Google Scholar]
- [20].Millard M, Odde S, Neamati N, Integrin targeted therapeutics, Theranostics 1 (2011) 154–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].ITGB3 integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) [ Homo sapiens (human) ] Available from: http://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=Graphics&list_uids=36902013 (Dec. 17 (cited 2013 Dec. 22)).
- [22].Bohanes P, et al. , Integrin genetic variants and stage-specific tumor recurrence in patients with stage II and III colon cancer, Pharmacogenomics J 15 (3) (2015) 226–234. [DOI] [PubMed] [Google Scholar]
- [23].Ay C, et al. , High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS), Blood 112 (7) (2008) 2703–2708. [DOI] [PubMed] [Google Scholar]
- [24].Kanz R, et al. , Thrombosis risk and survival in cancer patients with elevated C-reactive protein, J. Thromb. Haemost 9 (1) (2011) 57–63. [DOI] [PubMed] [Google Scholar]
- [25].Schemper M, Smith TL, A note on quantifying follow-up in studies of failure time, Control. Clin. Trials 17 (4) (1996) 343–346. [DOI] [PubMed] [Google Scholar]
- [26].Heinze G, Schemper M, A solution to the problem of monotone likelihood in Cox regression, Biometrics 57 (1) (2001) 114–119. [DOI] [PubMed] [Google Scholar]
- [27].Latouche A, Porcher R, Chevret S, A note on including time-dependent covariate in regression model for competing risks data, Biom. J 47 (6) (2005) 807–814. [DOI] [PubMed] [Google Scholar]
- [28].Mc. Vean, et al. , An integrated map of genetic variation from 1,092 human genomes, Nature 491 (7422) (2012) 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Konigsbrugge O, Pabinger I, Ay C, Risk factors for venous thromboembolism in cancer: novel findings from the Vienna Cancer and Thrombosis Study (CATS), Thromb. Res 133 (Suppl. 2) (2014) S39–S43. [DOI] [PubMed] [Google Scholar]
- [30].Sallah S, Wan JY, Nguyen NP, Venous thrombosis in patients with solid tumors: determination of frequency and characteristics, Thromb. Haemost 87 (4) (2002) 575–579. [PubMed] [Google Scholar]
- [31].Posch F, Thaler J, Zlabinger GJ, Konigsbrugge O, Koder S, Zielinski CC, et al. , Soluble Vascular Endothelial Growth Factor and the Risk of Venous Thromboembolism in Patients with Cancer - Results from the Vienna Cancer and Thrombosis Study, Clin. Cancer Res, 2015. [DOI] [PubMed] [Google Scholar]
- [32].Muller DW, Bosserhoff AK, Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma, Oncogene 27 (52) (2008) 6698–6706. [DOI] [PubMed] [Google Scholar]
- [33].Pillai RS, MicroRNA function: multiple mechanisms for a tiny RNA? RNA 11 (12) (2005) 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gargalionis AN, Basdra EK, Insights in microRNAs biology, Curr. Top. Med. Chem 13 (13) (2013) 1493–1502. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Y, et al. , Genetic variation of ITGB3 is associated with asthma in Chinese Han children, PLoS One 8 (2) (2013) e56914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Prager GW, et al. , CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling, J. Biol. Chem 282 (33) (2007) 24477–24484. [DOI] [PubMed] [Google Scholar]
- [37].Shattil SJ, Kim C, Ginsberg MH, The final steps of integrin activation: the end game, Nat. Rev. Mol. Cell Biol 11 (4) (2010) 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Harburger DS, Bouaouina M, A Calderwood D, Kindlin-1 and −2 Directly Bind the C-terminal Region of β Integrin Cytoplasmic Tails and Exert Integrin-specific Activation Effects, J. Biol. Chem 284 (17) (2009) 11485–11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Montanez E, et al. , Kindlin-2 controls bidirectional signaling of integrins, Genes Dev 22 (10) (2008) 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li Z, et al. , Signaling during platelet adhesion and activation, Arterioscler. Thromb. Vasc. Biol 30 (12) (2010) 2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Phillips DR, Agin PP, Platelet Membrane Defects in Glanzmann’s Thrombasthenia: EVIDENCE FOR DECREASED AMOUNTS OF TWO MAJOR GLYCOPROTEINS, J. Clin. Invest 60 (3) (1977) 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ablooglu AJ, et al. , Antithrombotic effects of targeting alphaIIbbeta3 signaling in platelets, Blood 113 (15) (2009) 3585–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Simoons ML, Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial, Lancet 357 (9272) (2001) 1915–1924. [DOI] [PubMed] [Google Scholar]
- [44].Alcalay A, et al. , Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival,J. Clin. Oncol 24 (7) (2006) 1112–1118. [DOI] [PubMed] [Google Scholar]
- [45].Pabinger I, Thaler J, Ay C, Biomarkers for prediction of venous thromboembolism in cancer, Blood 122 (12) (2013) 2011–2018. [DOI] [PubMed] [Google Scholar]
- [46].Khorana AA, Cancer-associated thrombosis: updates and controversies, ASH Educ. Program Book 2012 (1) (2012) 626–630. [DOI] [PubMed] [Google Scholar]
- [47].Ay C, et al. , Prediction of venous thromboembolism in cancer patients, Blood 116 (24) (2010)5377–5382; NCBI, Database of Short Genetic Variation (dbSNP), http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=4642 (Accessed October 20,2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.