Abstract
Introduction
High‐frequency chest wall oscillation (HFCWO) is a commonly prescribed airway clearance technique (ACT) for patients whose ability to expectorate sputum is compromised. This study aimed to assess the effectiveness of a newly developed mobile ACT device (mHFCWO—The Monarch Airway Clearance System) in patients with cystic fibrosis (CF). A standard nonmobile HFCWO device (sHFCWO) was used as a comparator.
Methodology
This was a randomized, open‐label, crossover pilot study. CF patients were treated with each device. Sputum was collected during and after each therapy session, while spirometry tests, Brody score assessment and functional respiratory imaging were performed before and after treatments.
Results
Wet weight of sputum collected during and after treatment was similar for mHFCWO and sHFCWO (6.53 ± 8.55 vs 5.80 ± 5.82; P = .777). Interestingly, the mHFCWO treatment led to a significant decrease in specific airway volume (9.55 ± 9.96 vs 8.74 ± 9.70 mL/L; P < .001), while increasing specific airway resistance (0.10 ± 0.16 vs 0.16 ± 0.23 KPA*S; P < .001). These changes were heterogeneously‐distributed throughout the lung tissue and were greater in the distal areas, suggesting a shift of mucus. Changes were accompanied by an overall improvement in the Brody index (57.71 ± 16.55 vs 55.20 ± 16.98; P = .001).
Conclusion
The newly developed mobile device provides airway clearance for CF patients comparable to a nonmobile sHFCWO device, yielding a change in airway geometry and patency by the shift of mucus from the more peripheral regions to the central airways.
Keywords: cystic fibrosis (CF), functional respiratory imaging (FRI), high‐frequency chest wall oscillation (HFCWO), sputum production
1. INTRODUCTION
The mucociliary escalator and the cough reflex are cornerstone mechanisms in the management of a healthy and functional respiratory system: by removing secretions that could otherwise accumulate in the airways, these processes prevent obstruction and ensure adequate airflow through the lungs. 1 , 2 However, a number of factors can affect these mechanisms, ultimately affecting lung health and function. Indeed, whereas the normal cough reflex can be inhibited by progressive neurodegenerative conditions, the mucus production and composition is often impaired in patients with pulmonary disorders. 2 Cystic fibrosis (CF) is an example of the latter: this autosomal recessive genetic disorder is characterized by abnormally thick bronchial secretions that lead to a defective mucociliary clearance. 3 , 4 The obstruction of the small airways results in chronic infections and exaggerated inflammatory responses, which further increase mucus viscosity as the accumulated neutrophils release their contents, thus creating a vicious circle of obstruction‐infection‐inflammation. 2
Airway clearance techniques (ACTs) are recommended for individuals whose ability to expectorate airway secretions is compromised. These techniques consist of the application of mechanical and/or physical forces that manipulate the airflow, mobilizing the retained secretions and facilitating their evacuation. Several different types of ACTs have been developed and are currently prescribed, such as conventional therapy (which consists of postural drainage, percussion, and vibration), breathing exercises (such as autogenic drainage or active cycles of breathing technique), hand‐held mechanical devices (such as positive expiratory pressure), and wearable mechanical devices. 1 , 2 , 5
A recently developed ACT device (The Monarch Airway Clearance System) is a wearable vest that combines mobility with high‐frequency chest wall oscillations (HFCWO) through the oscillation of eight pulmonary discs over the upper and lower lobes of the lungs. This mobile HFCWO, hereafter referred to as mHFCWO device, has a rechargeable battery and therefore does not need to be plugged into AC power during therapy. The device is user‐friendly, ensures consistent therapy, allows traveling and the performance of multiple activities while on therapy with the expectance to increase patient adherence to ACT. It is as yet unclear what the therapeutic effect of this newly developed mHFCWO is in comparison with standard HFCWO.
Standard HFCWO is a commonly prescribed ACT and consists of an inflatable vest that applies small volume expiratory pulses to the external chest wall, generating high‐velocity expiratory airflow that is thought to mobilize secretions by the sheer force created. This sheer force changes rheology and moves mucus in a cephalad direction during the oscillation. 6 , 7 , 8 , 9 Because of its ease of use and because it can be self‐administered, HFCWO is an attractive alternative to the labor‐intensive and time‐consuming conventional chest physiotherapy. Moreover, a number of studies have shown that HFCWO is at least as effective as conventional methods in the clearance of bronchial secretions, particularly in the case of CF patients. 5 , 10 , 11 , 12 Despite the accepted physiological rationale where expiratory airflow mobilizes mucus secretions, it remains hard to prove this mechanism of action. 13 However, with the emergence of novel imaging techniques such as high‐resolution computed tomography (HRCT) or MRI, it is possible to gain more insight into these changes by ACT's on regional structure such as airway geometry, lobe volumes, and ventilation. 14 , 15 , 16
The aim of this study was first to validate the therapeutic effect of mHFCWO on mucus clearance by comparing the therapy with a standard HFCWO system (The Vest Airway Clearance System, hereafter referred to as sHFCWO). Second, we sought to gain more insight into the mechanism of actions of mHFWCO by using novel outcome parameters through imaging.
2. METHODS
2.1. Study design and population
This was a randomized, open‐label, crossover study to assess the effectiveness of mHFCWO, based on the weight of sputum expectorated (primary outcome). sHFCWO was used as an active comparator. Patients ≥15 years old, with a documented diagnosis of CF (by sweat test and/or genetics) who had daily sputum expectoration (as determined by a treating physician) and required regular home airway therapy, were invited to participate in the study. To be eligible, subjects were also required to be on a stable regimen of CF medication for at least the previous 4 weeks. Patients were excluded if they had a forced expiratory volume in 1 second (FEV1) lower than 30% predicted or higher than 90% predicted an anticipated requirement for hospitalization within the 3 weeks following study start or a history of pneumothorax or haemoptysis requiring embolization within the 6 and 12 months (respectively) before the first study visit. Further exclusion criteria included the inability to perform mHFCWO or sHFCWO therapy as directed, the inability or unwillingness to complete study visits and to provide follow‐up data, the utilization of intravenous antibiotics within the 4 weeks before the first study visit, or an ongoing exacerbation of CF or allergic bronchopulmonary aspergillosis. Pregnant and lactating patients were also excluded from the study, as were patients with a pacemaker or an implantable cardioverter‐defibrillator. Patient eligibility to participate in the study was evaluated at the screening visit, which included the collection of demographic and clinical data (pulmonary‐related medical history and concomitant medication), a physical examination (assessment of vital signals, pregnancy test), standard spirometry tests, and training sessions with both devices.
At the first study visit, patients were randomly allocated to be treated with mHFCWO or sHFCWO, using a computer random number generator. After a washout period of two to 7 days, patients received the alternate treatment. All subjects enrolled in the study performed one‐morning treatment per session, four of them also performed an afternoon treatment. Treatments lasted 30 minutes and were done using intensity settings of 6 to 10, following a regimen described by Kempainen et al 9 In some cases, intensity was adjusted depending on patients’ individual needs. These settings deliver peak pressures of ≈29 cmH2O at 10 Hz for setting 6 up to a maximal peak pressure of ≈45 cmH2O at 15 Hz for setting 10 when the garment perfectly fits on the thorax of the patient. A detailed description of the settings for the sHFCWO is provided by Kempainen et al 9 Before starting the treatment session, all patients inhaled hypertonic saline and that prescribed salbutamol as part of his/her standard of care regime received two puffs of the latter before the administration of hypertonic saline. Patients were supervised by study personnel at the hospital during all treatment sessions to avoid any compliance bias on the outcome parameters.
2.2. Primary outcome
The primary outcome of this study was sputum production consisting of the wet weight of sputum and sputum volume, expectorated during and after therapy. Study participants were instructed to expectorate (and avoid swallowing) all sputum during the 30‐minute therapy and in the 60 minutes that followed.
2.3. Secondary outcomes
2.3.1. Spirometry measures
Spirometry measures included FEV1, forced vital capacity (FVC) and FEV1/FVC (Tiffeneau‐Pinelli index). These parameters were assessed before and at least 150 minutes after each therapy session.
2.3.2. Brody high‐resolution computed tomography scan scores
Low‐dose HRCT scans were taken before and after each session during spirometry‐controlled breath holding at two distinct respiratory phases (functional residual capacity [FRC] and total lung capacity [TLC]). These scans were evaluated by a radiologist (blinded to the date and time of the study), who scored them according to the Brody high‐resolution computed tomography scan scores. 17
2.3.3. Functional respiratory imaging
Functional respiratory imaging (FRI) is a clinically‐validated computational work‐flow in which functional data is added to respiratory anatomical images. Starting from the low‐dose HRCT scans taken at FRC and TLC, geometric changes in airways and lung lobes during a breathing cycle are assessed. Such data is then used in combination with computational fluid dynamics simulations, allowing the analysis of functional information (such as airflow behavior). A detailed description of the FRI methodology is provided by De Backer et al 18 The following FRI parameters were evaluated in this study: airway volume (iVaw) and specific airway volume (siVaw), airway resistance (iRaw) and specific airway resistance (siRaw), lobe volumes, air trapping, and internal airflow distribution. The siVaw and siRaw are normalized measures, calculated by dividing or multiplying (respectively) the correspondent nonspecific parameters by the total lung volume. HRCT scans on which FRI was based were completed before and at least 150 minutes after each therapy session.
2.4. Statistical analysis
Descriptive statistics included N, mean, median, standard deviation, minimum and maximum for continuous variables, and number and percent of patients for categorical variables. A linear mixed‐effects model was used to analyze all data utilizing repeated measures. Data from airway measures (iVaw, siVaw, iRaw, and siRaw) were logarithmically transformed before analysis. A multilevel model, including fixed effects for visit and lobe, was used to incorporate the repeated measurements from the lobes for each subject. Lobe was also included as a random effect within each subject. Heterogeneity across lobes (within‐subject) was modeled using an unstructured variance‐covariance matrix, assuming independence between subjects. The degrees of freedom were computed using the Satterthwaite approximation. All statistical analyses were conducted using R version 3.2.5 or higher (The R Foundation for Statistical Computing, Vienna, Austria).
2.5. Ethical considerations
All patients enrolled in the study signed informed consent. This study was conducted in compliance with the approved protocol, good clinical practice (GCP), Declaration of Helsinki and all applicable regulatory requirements, and was registered at ClinicalTrials.gov with the reference NCT03091062. The study was approved by the local Ethics Committee of the Antwerp University Hospital.
3. RESULTS
Nine patients (six males and three females), with a mean (±standard deviation) age of 25.5 (±5.6) years, were initially enrolled in this study; of these, one (male, 24 years old) dropped out due to inability to comply with the study visit requirements. Since four of the subjects performed an afternoon treatment session based on their individual treatment care regime, only the results of the morning session for all subjects are used for statistical analysis.
The results for sputum production were comparable between the mHFCWO device and sHFCWO. The comparison showed that the two study devices had a similar impact both on sputum wet weight (6.53 ± 8.55 vs 5.80 ± 5.82 g; P = .777), and on its volume (7.27 ± 8.89 vs 6.07 ± 6.15 mL; P = .664). The total amount (wet weight and volume) of sputum expectorated during and after ACT sessions using the mHFCWO and the sHFCWO devices is shown in Figure 1 and Table S1.
Figure 1.
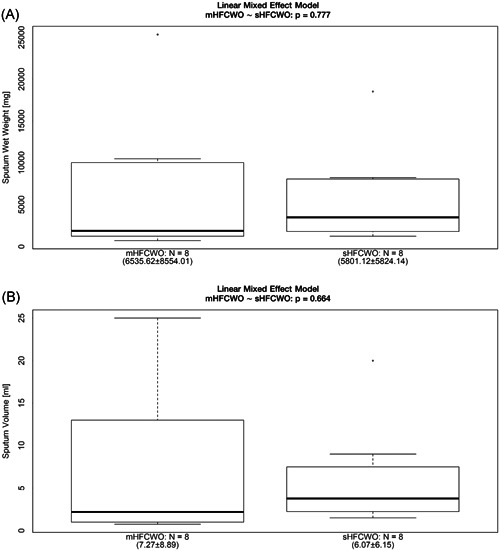
Sputum wet weight (A) and volume (B) expectorated during and after the patients were treated with mHFCWO or sHFCWO therapy. HFCWO, high‐frequency chest wall oscillation
Table 1 depicts the spirometry parameters measured before and after mHFCWO and sHFCWO therapies. Pretherapy and posttherapy values were similar in all cases. Moreover, the change (%) in spirometry values following treatment was similar for the mHFCWO and the sHFCWO systems for FEV1 (1.18 ± 6.60 vs 0.32 ± 7.28; P = .806), FVC (2.45 ± 6.54 vs 1.72 ± 5.13; P = .776), and FEV1/FVC (−1.22 ± 2.92 vs −1.37 ± 5.22; P = .927).
Table 1.
Spirometry parameters (average ± SD) for patients before and after using the mHFCWO and the sHFCWO systems (n = 8 in all cases)
| mHFCWO | sHFCWO | |||||
|---|---|---|---|---|---|---|
| Pretherapy | Posttherapy | P | Pretherapy | Posttherapy | P | |
| FEV1 | ||||||
| L | 2.38 ± 0.75 | 2.40 ± 0.74 | .729 | 2.43 ± 0.81 | 2.42 ± 0.77 | .964 |
| % predicted | 61.07 ± 13.01 | 61.50 ± 12.38 | .750 | 62.22 ± 13.97 | 62.36 ± 13.84 | .925 |
| FVC (L) | 3.72 ± 0.92 | 3.81 ± 0.96 | .283 | 3.81 ± 0.98 | 3.85 ± 0.92 | .408 |
| FEV1/FVC (%) | 64.20 ± 11.51 | 63.56 ± 12.17 | .270 | 63.94 ± 11.35 | 63.33 ± 12.93 | .566 |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HFCWO, high‐frequency chest wall oscillation; SD, standard deviation.
The FRI analyses of mHFCWO sessions showed a significant decrease in the siVaw (9.55 ± 9.96 vs 8.74 ± 9.70 mL/L; P < .001) and a significant increase in the siRaw (0.10 ± 0.16 vs 0.16 ± 0.23 KPA*S; P < .001) after therapy (Figure 2). These changes were also perceptible in terms of nonspecific parameters—iVaw and iRaw—both considering the total lung measures (P = .026 for iVaw and 0.008 for iRaw) and the distal region (P = .028 for iVaw and .010 for iRaw)—Table 2. However, changes in the central region lacked statistical significance, suggesting that the changes occurring in the distal area were greater than those that took place in the central area. Finally, consistent with the changes described, the measure of total lobe volume showed a significant decrease posttherapy (1.21 ± 0.66 vs 1.17 ± 0.65; P = .009)—Table 2.
Figure 2.
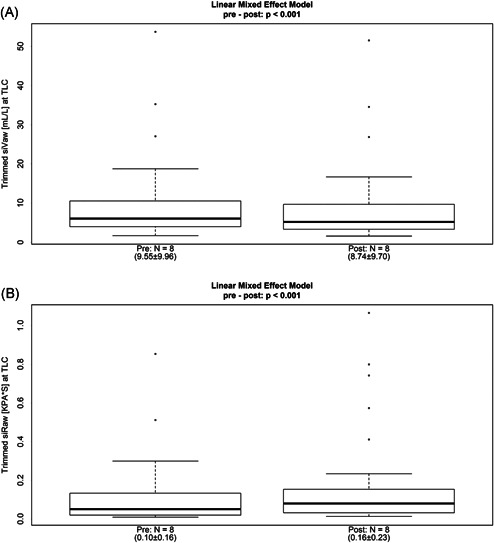
Specific airway volume (A) and specific airway resistance (B) before and after the patients received the mHFCWO therapy. HFCWO, high‐frequency chest wall oscillation
Table 2.
FRI* parameters (average ± SD) before and after using the mHFCWO system (n = 8 in all cases)
| Pretherapy | Posttherapy | P | |
|---|---|---|---|
| siVaw, mL/L | 9.55 ± 9.96 | 8.74 ± 9.70 | <.001 |
| iVaw, mL | |||
| Central | 29.76 ± 8.39 | 28.63 ± 8.06 | .066 |
| Distal | 49.44 ± 50.12 | 44.52 ± 49.64 | .028 |
| Total | 79.20 ± 55.05 | 73.15 ± 53.69 | .026 |
| siRaw (KPA*S) | 0.10 ± 0.16 | 0.16 ± 0.23 | <.001 |
| iRaw (KPA*S/L) | |||
| Central | 0.01 ± 0.01 | 0.01 ± 0.01 | .637 |
| Distal | 0.01 ± 0.01 | 0.02 ± 0.01 | .010 |
| Total | 0.02 ± 0.02 | 0.03 ± 0.02 | .008 |
| iVlobe, L | 1.21 ± 0.66 | 1.17 ± 0.65 | .009 |
Abbreviations: FRI, functional respiratory imaging; HFCWO, high‐frequency chest wall oscillation; SD, standard deviation; TLC, total lung capacity.
Assessed at TLC; iVaw, airway volume; iRaw, airway resistance; iVlobe, lobe volume.
The FRI results described above for the mHFCWO sessions were consistent with those observed when all treatments (mHFCWO and sHFCWO) were pooled into one single group. Pooling of results was done to provide greater insight into the mechanisms of action of HFCWO (Table S2). Indeed, one could detect a significant decrease in siVaw, iVaw and total lobe volume, accompanied by a significant increase in siRaw and iRaw. Moreover, similar to the results for mHFCWO alone, these changes appeared to be greater in the distal areas when compared to the central regions. It should also be highlighted that the analysis of individual responses (Tables S3‐S6) show a spectrum of values changing from only slight alterations (as, for instance, in patient number 005) to more pronounced ones (as, for instance, in patient number 009). Figure 3 illustrates the airway volume change (A) and the mucus shift in central (B) and distal (C) lung regions in the patient who had the greatest change in the airway volume following mHFCWO therapy.
Figure 3.
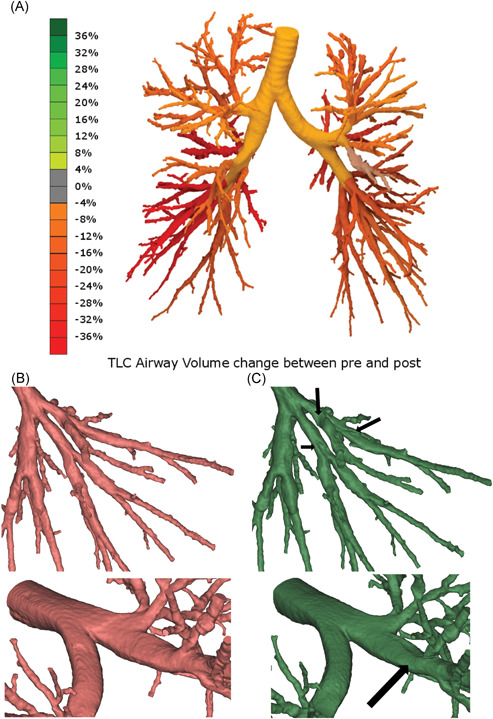
A, Volume change in patient 009 airways: green color indicates dilation whereas red color indicates narrowing (due to mucus transportation). B, Detail of patient 009 distal (lower left lobe) airways before (red) and after (green) mHFCWO treatment; the arrows indicate mucus shift. C, Detail of patient 009 central airways before (red) and after (green) mHFCWO treatment; the arrow indicates mucus shift. HFCWO, high‐frequency chest wall oscillation [Color figure can be viewed at wileyonlinelibrary.com]
Finally, the comparison of the Brody index before and after mHFCWO therapy showed a significant improvement in patient lung status (57.71 ± 16.55 vs 55.20 ± 16.98; P = .001—Figure 4). These results were consistent with those obtained after pooling all treatment sessions (mHFCWO and sHFCWO): 61.34 ± 16.54 vs 58.07 ± 16.09; P = .036.
Figure 4.
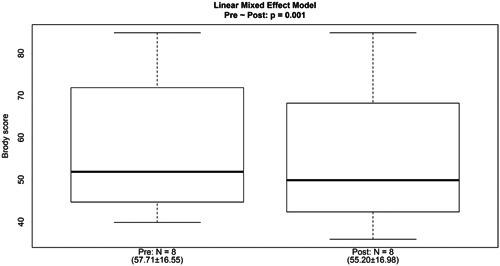
Brody score before and after the patients received the mHFCWO therapy. HFCWO, high‐frequency chest wall oscillation;
4. DISCUSSION
The aim of this randomized, open‐label, crossover pilot study was to validate the therapeutic effect of a new mobile HFCWO on mucus clearance by comparing with a standard HFCWO system (The Vest Airway Clearance System in CF patients who have daily sputum production. All treated patients were analyzed to evaluate weight and volume of expectorated sputum during and after treatment. Standard spirometry assessments, and functional/airways geometry‐related parameters by HRCT were also collected to determine the impact of mHFCWO.
Though airway clearance therapy is generally accepted as an important component of the care regimen for CF patients, and in spite of the physiological rationale supporting the utilization of ACTs, their clinical efficacy is not easily demonstrated. 1 , 13 The primary endpoint in our study, sputum weight, has been used in numerous other studies to evaluate the effectiveness of therapy. 5 No significant differences in wet weight or volume of sputum were found when comparing the two devices. In other words, the sputum production of the mHFCWO is comparable as ACT with the sHFCWO. These results are in line with recent findings of a systematic review evaluating oscillating devices for airway clearance in people with CF. There there was no evidence that one oscillation device is superior to another. 5 However, while a logical outcome for ACT, sputum weight or volume alone does not provide adequate insight into the clearance of the lung posttherapy. 13
In our comparison of spirometry results, comparison of before and after therapy measures showed no significant differences. There were also no differences observed between mHFCWO and sHFCWO. These results are not unique. While spirometry measures are frequently used as endpoints for clinical trials of respiratory patients, these measures have often shown to be insensitive to change resulting from ACT's and therefore are not a good indicator of airway clearance effectiveness. 16 , 19 , 20 , 21 , 22 Other studies suggest that this might also be related with the timing of the measures. Hortal and Hjelte have shown that the optimal time point to accurately assess spirometry parameters after conventional physiotherapy in CF patients varies between individuals. 23 Moreover, these authors have shown that, among adult patients, variation in FEV1 was significant when this parameter was assessed between 30 minutes and 2 hours after therapy but ceased to be significant 3 hours after therapy. 23 As spirometry tests in this study were made at least 2.5 hours after therapy, the assessment timing cannot be excluded as a factor in the lack of significant changes. Therefore, since ACTs are likely to have an effect on airway geometry and patency, our secondary aim of this study was to gain more insight in the mechanism of action of HFCWO through the use of novel imaging techniques as FRI.
Indeed, using FRI to compare airway volume and resistance before and after therapy revealed significant differences. The volume decreased following treatment, whereas the resistance increased. This can be explained by a mucus shift from the periphery (ie, in the region beyond the 10th airway generation, that cannot be imaged because of too low scan resolution) towards the distal and central lung region. The increased amount of mucus in the FRI analyzed area decreases the space available for the air passage and increases its resistance. These differences were accompanied by a consequent decrease in the total lobe volume, therefore promoting a decrease in hyperinflation. Furthermore, it is worth noting that the geometric changes observed in the central and distal parts of the airways were greater for the latter. This suggests that the accumulated mucus in the central airways is cleared as much as possible by coughing during and after the therapy sessions before taking the posttreatment HRCT scan. Interestingly, similar results, showing improvement in FRI parameters, were observed in a sample of patients with acute exacerbation of chronic obstructive pulmonary disease after a single intrapulmonary percussive ventilation session. 16 While the small sample size of this study prevented the observation of significant treatment effects, the authors could nevertheless detect changes in airways calibre and a related reduction in lobar hyperinflation. 16 Airway volume, airway resistance and lobe volume seems to be a reliable indicator of mucus shifting and clearance efficacy in CF and more sensitive to change than FEV1 as reported in previous studies. 14
Last, these changes in FRI parameters were accompanied by a significant improvement in the Brody index, suggesting that changes in airway patency and geometry reflect a positive development in patients’ lung condition.
The present study has some limitations. First, although we observe positive results for mHFCWO regarding mucus clearance, it is a small sample size and larger studies are needed to confirm our results. Second, the intensity of both HFCWO devices required some adjustment, depending on the patient's individual needs. Individual adjustments are standard practice with HFCWO therapy and individualization of ACTs in CF is been seen as GCP. 5 However, we acknowledge that variation in settings could have some effect on results in a small study.
Overall, our results show that the sputum production of the mHFCWO (using The Monarch Airway Clearance System) is comparable to that obtained with a standard HFCWO system that has been in use for more than twenty years. The mechanism of action of the mHFCWO improves mucus transport in patients with CF according to a visual assessment of HRCT images (Brody score) and FRI parameters such as airway volumes and resistance. These latter outcome parameters indicate that the mucus shift from peripheral to more central regions. In conclusion, the new mobile HFCWO device may be an effective option for regular therapy in patients with CF where the mobility offered might help patients to be more adherent to their required therapy regimen.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge Catarina L Santos (W4Research) for medical writing assistance and Prof Wilfried De Backer for the critical discussion of the results and revision of the final manuscript. This work was supported by Hill‐Rom. The sponsor had no role in the collection of data or analysis of study results. Both sponsor and PI have reviewed results. An unbiased analysis and reporting of the results were made.
Leemans G, Belmans D, Van Holsbeke C, et al. The effectiveness of a mobile high‐frequency chest wall oscillation (HFCWO) device for airway clearance. Pediatric Pulmonology. 2020;55:1984–1992. 10.1002/ppul.24784
Meetings at which this work has been presented:
Part of this work was presented as a poster/oral communication at the 32nd North American Cystic Fibrosis Conference, USA, October 2018. Leemans G, De Hondt A, Ides K, et al Evaluation of a mobile HFCWO device in patients with cystic fibrosis. Pediatric Pulmonology. September 2018;53(S2):S1‐S481.
REFERENCES
- 1. Osadnik CR, McDonald CF, Holland AE. Airway clearance techniques for chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2013;7(6):673‐685. 10.1002/14651858.CD008328.pub2 [DOI] [PubMed] [Google Scholar]
- 2. Volsko Ta. Airway clearance therapy: finding the evidence. Respir Care. 2013;58(10):1669‐1678. 10.4187/respcare.02590. http://rc.rcjournal.com/cgi/doi/10.4187/respcare.02590 [DOI] [PubMed] [Google Scholar]
- 3. Flume PA, Robinson KA, O'Sullivan BP, et al. Clinical Practice Guidelines for Pulmonary Therapies Committee . Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54(4):522‐537. 10.1183/09031936.00159111 [DOI] [PubMed] [Google Scholar]
- 4. Elborn JS. Cystic fibrosis. Lancet (London, England). 2016;388(10059):2519‐2531. 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- 5. Wilson A. Oscillating devices for airway clearance in people with cystic fibrosis: a cochrane review summary. Int J Nurs Stud. 2018;88(5):165‐166. 10.1016/j.ijnurstu.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 6. King M, Phillips DM, Gross D, Vartian V, Chang HK, Zidulka A. Enhanced tracheal mucus clearance with high frequency chest wall compression. Am Rev Respir Dis. 1983;128(3):511‐515. 10.1164/arrd.1983.128.3.511 [DOI] [PubMed] [Google Scholar]
- 7. Chang HK, Weber ME, King M. Mucus transport by high‐frequency nonsymmetrical oscillatory airflow. J Appl Physiol. 1988;65(3):1203‐1209. 10.1152/jappl.1988.65.3.1203 [DOI] [PubMed] [Google Scholar]
- 8. Tomkiewicz RP, Biviji A, King M. Effects of oscillating air flow on the rheological properties and clearability of mucous gel simulants. Biorheology. 1994;31(5):511‐520. 10.3233/BIR-1994-31501 [DOI] [PubMed] [Google Scholar]
- 9. Kempainen RR, Milla C, Dunitz J, et al. Comparison of settings used for high‐frequency chest‐wall compression in cystic fibrosis. Respir Care. 2010;55(6):695‐701. [PubMed] [Google Scholar]
- 10. Arens R, Gozal D, Omlin KJ, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med. 1994;150(4):1154‐1157. 10.1164/ajrccm.150.4.7921452 [DOI] [PubMed] [Google Scholar]
- 11. Kluft J, Beker L, Castagnino M, Gaiser J, Chaney H, Fink RJ. A comparison of bronchial drainage treatments in cystic fibrosis. Pediatr Pulmonol. 1996;22(4):271‐274. [DOI] [PubMed] [Google Scholar]
- 12. Hansen L, Warwick W. High‐frequency chest compression system to aid in clearance of mucus from the lung. Biomed Instrum Technol. 1990;24(4):289‐294. [PubMed] [Google Scholar]
- 13. Osadnik CR, McDonald CF, Holland AE. Advances in airway clearance technologies for chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine. 2013;7(6):673‐685. 10.1586/17476348.2013.847368. http://www.ncbi.nlm.nih.gov/pubmed/24224510 [DOI] [PubMed] [Google Scholar]
- 14. Hajian B, De Backer J, Vos W, Van Holsbeke C, Clukers J, De Backer W. Functional respiratory imaging (FRI) for optimizing therapy development and patient care. Expert Review of Respiratory Medicine. 2016;10(2):193‐206. 10.1586/17476348.2016.1136216. http://www.tandfonline.com/doi/full/10.1586/17476348.2016.1136216 [DOI] [PubMed] [Google Scholar]
- 15. Kaireit TF, Sorrentino SA, Renne J, et al. Functional lung MRI for regional monitoring of patients with cystic fibrosis. PLoS One. 2017;12(12):1‐17. 10.1371/journal.pone.0187483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ides K, Vos W, De Backer L, et al. Acute effects of intrapulmonary percussive ventilation in COPD patients assessed by using conventional outcome parameters and a novel computational fluid dynamics technique. Int J Chronic Obstruct Pulm Dis. 2012;7:667‐671. 10.2147/COPD.S29847. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3459658&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21(1):14‐21. 10.1097/01.rti.0000203937.82276.ce [DOI] [PubMed] [Google Scholar]
- 18. De Backer JW, Vos WG, Vinchurkar SC, et al. Validation of computational fluid dynamics in CT‐based airway models with SPECT/CT. Radiology. 2010;257(3):854‐862. 10.1148/radiol.10100322. accessed 2012 May 24. http://www.ncbi.nlm.nih.gov/pubmed/21084417 [DOI] [PubMed] [Google Scholar]
- 19. Osman LP, Roughton M, Hodson ME, Pryor JA. Short‐term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax. 2010;65(3):196‐200. 10.1136/thx.2008.111492. accessed 2012 Sep 13. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2922723&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bingham PM, Lahiri T, Ashikaga T. Pilot trial of spirometer games for airway clearance practice in cystic fibrosis. Respir Care. 2012;57(8):1278‐1284. 10.4187/respcare.01263 [DOI] [PubMed] [Google Scholar]
- 21. Giles DR, Wagener JS, Accurso FJ, Butler‐Simon N. Short‐term effects of postural drainage with clapping vs autogenic drainage on oxygen saturation and sputum recovery in patients with cystic fibrosis. Chest. 1995;108(4):952‐954. 10.1378/chest.108.4.952 [DOI] [PubMed] [Google Scholar]
- 22. Morgan K, Osterling K, Gilbert R, Dechman G. Effects of autogenic drainage on sputum recovery and pulmonary function in people with cystic fibrosis: a systematic review. physiotherapy Canada. Physiotherapie Canada. 2015;67(4):319‐326. 10.3138/ptc.2014-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez Hortal MC, Hjelte L. Time point to perform lung function tests evaluating the effects of an airway clearance therapy session in cystic fibrosis. Respir Care. 2014;59(10):1537‐1541. 10.4187/respcare.02823. http://www.ncbi.nlm.nih.gov/pubmed/24847094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


