Abstract
Huntington's disease (HD) is a devastating, autosomal‐dominant neurodegenerative disease, for which there are currently no disease‐modifying therapies. Clinical trials to replace the damaged striatal medium spiny neurons (MSNs) have been attempted in the past two decades but have met with only limited success. In this study, we investigated whether a clonal, conditionally immortalized neural stem cell line (CTX0E03), which has already shown safety and signals of efficacy in chronic ischemic stroke patients, could rescue deficits seen in an animal model of HD. After CTX0E03 transplantation into the quinolinic acid‐lesioned rat model of HD, behavioral changes were measured using the rotarod, stepping, and staircase tests. In vivo differentiation and neuronal connections of the transplanted CTX0E03 cells were evaluated with immunohistochemical staining and retrograde tracing with Fluoro‐Gold. We found that transplantation of CTX0E03 gave rise to a significant behavioral improvement compared with the sham‐ or fibroblast‐transplanted group. Transplanted CTX0E03 formed MSNs (DARPP‐32) and GABAergic neurons (GABA, GAD65/67) with BDNF expression in the striatum, while cortically transplanted cells formed Tbr1‐positive neurons. Using a retrograde label, we also found stable engraftment and connection of the transplanted cells with host brain tissues. CTX0E03 transplantation also reduced glial scar formation and inflammation, as well as increasing endogenous neurogenesis and angiogenesis. Overall, our results demonstrate that CTX0E03, a clinical‐grade neural stem cell line, is effective for preclinical test in HD, and, therefore, will be useful for clinical development in the treatment of HD patients.
Keywords: CTX0E03, functional recovery, Good Manufacturing Practices (GMP), human neural stem cells, Huntington's disease, intracerebral transplantation
Implantation of the clinical‐grade human neural stem cell line, CTX0E03, leads to behavioral improvement, as well as neuronal differentiation and tissue repair in the quinolinic acid‐lesioned rodent model of Huntington's disease over the period of 12 weeks following implantation.
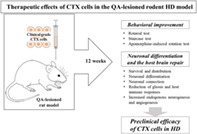
Significance statement.
This study investigated the effects of clinical‐grade neural stem cells manufactured from a conditionally immortalized cell line, CTX0E03, which is currently in a phase IIb clinical trial in the United States in patients with moderate to severe disability after ischemic stroke, on an animal model of Huntington's disease (HD). The results demonstrated that intracerebral transplantation of CTX0E03 cells can lead to significant improvements in the behavioral and pathological deficits of a rodent model of HD by both cell replacement and host cell regeneration mechanisms. Therefore, this study provides a strong basis for clinical development of these cells to treat HD patients in the future.
1. INTRODUCTION
Huntington's disease (HD) is a devastating genetic disease, affecting approximately 5 out of every 100 000 people in the United States, Europe, and Australia. 1 In HD, one of the earliest and major pathologies is the degeneration of the medium spiny neurons (MSNs) in the striatum due to the cytotoxic effects of mutant huntingtin protein. Coupled with pathology at other sites, especially the cortex, MSN degeneration leads to the classical clinical trial of abnormal movements, psychiatric problems, and cognitive deficits. Currently, there is no cure or disease‐modifying therapy available for HD patients. Therefore, stem cells have long been considered a promising therapeutic resource for HD to replace the lost striatal MSNs as well as to modulate several pathogenic pathways through paracrine release of a range of neuroprotective and immune modulatory factors. 2 A significant number of stem/progenitor cells have been studied that include embryonic stem cells; multipotent progenitor cells from the embryo or fetus, which are already partially committed to a neural lineage; cells from the umbilical blood; autologous or allogenic adult stem cells from various tissues; and finally induced pluripotent stem cells. 3 , 4 Among them, neural stem cells (NSCs) have been regarded as a promising option to treat HD as they can survive and differentiate into proper neuronal cell types in vivo. Numerous studies have indicated that NSC can have the potential to replace the damaged MSNs and make functionally active connections to the host neuronal network. Both systemic injection of human NSC in the quinolinic acid (QA) rat model 5 and intrastriatal injection of human NSC in the 3‐NP rat model 6 demonstrated reduced striatal damage and improved locomotor activity. Transplanted mouse NSC from neurospheres could survive in R6/2 transgenic HD model. 7 More recently, intrastriatal transplantation of mouse induced pluripotent stem cell‐derived NSCs (iPSC‐NSC) in YAC128 mice also ameliorated locomotor deficits and differentiated into MSNs. 8 Although various preclinical studies using different types of NSC in different rodent HD models have indicated its potential therapeutic benefits, no one has tested the efficacy of clinical‐grade human NSC, such as CTX cells so far. Clinical trials of intracerebral transplantation using human fetal striatal tissues have demonstrated limited benefits and survival. 2
A sustainable source of ethically approved, safe, and potent stem cells is one of the most important requirements for the development of an effective cell therapy, and this holds for HD as well. CTX0E03 (CTX) is a GMP‐manufactured conditionally immortalized human NSC line 9 , 10 under current clinical trial (PISCES III, NCT03629275) and may serve as a reliable source of NCS for providing beneficial effects in preclinical HD studies. Direct intracerebral implantation of CTX cells in a rodent middle cerebral artery occlusion (MCAO) model of stroke demonstrated reproducible recovery of lesion‐associated behavioral deficits by stimulating reparative mechanisms, such as angiogenesis and neurogenesis in the lesion site. 9 , 11 When CTX cells were placed into the ischemic brain, engulfment of apoptotic cells by macrophages promoted M2 polarization, resulting in tissue repair. 12 Based on this mechanistic understanding, it has been hypothesized that these cells may have benefits in other diseases of the brain, such as HD. To test this hypothesis, we investigated the effect of CTX in the QA‐lesioned rat model of HD. In this model, excitotoxic lesion leads to degeneration of the DARPP‐32 positive cells and an inflammatory response, which correlates with the typical loss of MSNs and neuroinflammation seen in HD patients at postmortem. 13
2. MATERIALS AND METHODS
2.1. Cell preparation
2.1.1. Control fibroblast cell line
Embryonic fibroblasts were obtained from aborted human embryos following approval by the Institutional Review Board of CHA Gangnam Medical Center (Seoul, Korea). Fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) high glucose (Welgene, Korea), supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Sigma‐Aldrich) grown on 0.2% gelatin‐coated culture dish. For the transplantation of fibroblasts, they were dissociated using 0.25% trypsin‐EDTA and resuspended at 100 000 cells/μL in DMEM high glucose with 30 μM Y‐27632 (Tocris) following centrifugation at 1200 rpm for 3 minutes.
2.1.2. CTX (CTX0E03) drug product
CTX is of human origin and was established as a clonal cell line by conditional immortalization with c‐mycERTAM. 14 CTX0E03/06T passage 23 was revived from cryopreservation with a viability of 96%. CTX cells were cultured in a serum‐free medium (RMM) supplemented with epidermal growth factor (EGF, 20 ng/mL, Peprotech), basic fibroblast growth factor (bFGF, 10 ng/mL, Peprotech), and 4‐hydroxytamoxifen (4‐OHT, 10 mM, Sigma) on laminin (20 μg/mL, or 2.28 μg/cm2 AMS Biotech) coated vessels in an incubator at 37°C in a humidified atmosphere containing 5% CO2. The cells were fed every 2 to 3 days and passaged once the cells were 70% to 80% confluent. CTX drug product (DP) cells was prepared from two T‐500 flasks, seeded and grown to 85% confluency for harvest at passage 32. For cell harvest, the medium was aspirated and the adherent cells washed with HBSS (–Ca2+/Mg2+). Cells were then dissociated with TrypZean/EDTA (BE02‐034E Lonza) for 5 minutes before the addition of the DTI/Benzonase inhibitor (Invitrogen R007100; Merck 1.01654.0001). Cells were collected from flasks with 20 mL of RMM+GF+4‐OHT and centrifuged at 500g (eg, 1500 rpm) for 5 minutes at ambient temperature. The cell pellet was resuspended in RMM+GF for trypan blue viability and cell counting, then centrifuged and resuspended in 57.7% HTS (HypoThermosal㉿)‐FRS/DMEM: F12 at a density of 50 000 cells/μL in HTS‐FRS (BioLife Solutions, 101102). Finally, CTX (CTX0E03) DP (CTX0E03/07 passage 32) was prepared at ReNeuron (Guildford, UK) and shipped frozen under controlled conditions to the CHA Stem Cell Institute (Republic of Korea). For use, a vial of CTX0E03 cells was removed from <−135°C storage and thawed immediately in a water bath at 37 (±1)°C for 1 minute before placing on ice for a maximum of 4 hours. Before each use, cells were gently triturated using a pipette. As for the medium control, HTS‐FRS was used.
2.2. QA model of HD and CTX cell transplantation
Animal experiments were performed in accordance with the CHA University Institutional Animal Care and Use Committee (IACUC140013). Male Sprague Dawley rats (Orient Bio Ltd, Seongnam, Korea) were group housed (12:12, light:dark cycle) and fed ad libitum. At 8 weeks of age, 50 rats received a single, unilateral injection of 2 μL volume containing 120 nmol QA (2,3‐pyridinedicarboxylic acid, Sigma) into the right striatum‐stereotaxic coordinates AP = +1.0, ML = +2.5 mm, DV = −5.0 mm. One week after QA lesioning, animals that developed behavioral deficits in the rotarod (the time spent on the rotarod <60 seconds), stepping (the left step numbers <1), and staircase (the number of pellets taken <4) tests were selected for cell transplantation. A total of 32 QA‐lesioned rats underwent stereotaxic injection into the striatum and the cortex with each site receiving 1 μL of the “transplant material” which contained either Media (N = 11, Sham group), 1 × 105/μL Human Fibroblasts (N = 9), or 1 × 105/μL CTX cells (N = 12). This cells were delivered at the coordinates, AP = +1.5, ML = +2.5 mm, DV = −5.0 mm for the striatum and AP = −0.5, ML = +1.5 mm, DV = −2.0 mm for the cortex. All animals received immunosuppressant, Cyclosporine A, by the intraperitoneal route starting at a dose of 10 mg/kg, 2 days prior to the transplantation surgery and then 5 mg/kg daily after surgery until sacrifice (Supporting Information Figure S1).
2.3. Retrograde labeling with Fluoro‐Gold
At 12 weeks post‐transplantation, two rats from the CTX‐transplanted group were injected with 0.5 μL volume of 4% Fluoro‐Gold (FG) into the globus pallidus using the stereotaxic coordinates AP = −1.0, ML = +2.5 mm, DV = −6.0 mm. 15 Animals were sacrificed 1 week after FG injection and processed for immunohistochemistry.
2.4. BrdU administration
At 12 weeks post‐transplantation, three rats from each of the Sham (Media), Fibroblast, and CTX‐transplanted groups were injected with BrdU (Cat no B5002, Sigma‐Aldrich) at a dose of 50 mg/kg twice daily, for 3 days by the intraperitoneal route. 16 After 3 days of BrdU administration, animals were sacrificed and processed for immunohistochemistry.
2.5. Behavioral testing
2.5.1. Rotarod test
The rotarod test was performed at 0 (baseline), 2, 4, 6, 8, 10, and 12 weeks post‐transplantation to assess motor coordination. Baseline was set before cell transplantation, which was performed 7 days after QA lesioning. Rats were placed on the rotating rod with an accelerating speed of 4 to 40 rpm over a period of 3 minutes with a 15 minutes rest period between trials. 17 The time taken for each rat to fall from the rod was recorded over three separate trials and the mean latencies were used for analysis. Rats underwent rotarod test training comprising of three trials per day over three consecutive days prior to QA lesioning. 18 Rats spent on the rotating rod for less than 60 seconds were selected for cell transplantation.
2.5.2. Stepping test
The stepping test was performed at 0 (baseline), 2, 4, 6, 8, 10, and 12 weeks post‐transplantation to assess akinesia. Rats were held on a tabletop in a forelimb stance, with their body at nearly 90° on the table. Once the rats appear relaxed, they were pushed forward to move along the surface of the tabletop. We counted the number of right and left steps while moving across the surface. This test was always performed by the same operator and rats were familiarized with the experimenter's grip prior to testing. During the test, the number of forepaw placements was counted as the rat was moved slowly in both forehand and backhand directions along the edge of a table over a distance of 90 cm in a 5‐second period. 19 Rats used the left step more than one time were not selected for cell transplantation.
2.5.3. Staircase test
The staircase test was performed once a day for three consecutive days at time 0 (baseline), 2, 4, 6, 8, 10, and 12 weeks post‐transplantation to provide a quantifiable measure of fine motor reaching skill in the left affected limb only. The staircase consists of a two‐compartment box with a raised platform and two stairs in the narrow compartment. The left steps on the stairs in the narrow compartment can be reached only with the left paw and the right steps only with the right paw. The eight wells of each step are filled with five 45 mg food pellets. Food was removed from holding cages 24 hours prior to testing and replaced thereafter. Rats underwent a 10‐minutes test period, during which time the number of food pellets retrieved with the affected limb was measured. Rats underwent a training period for the staircase test prior to QA lesioning. Rats that took less than four food pellets were selected for cell transplantation.
2.5.4. Statistical analysis for behavioral test measurements
Analysis of the behavioral data from the rotarod, stepping, and staircase tests was performed using Statistical Analysis System Program (Enterprise 4.1; SAS Korea). The performance measured from the three behavioral tests was analyzed according to the PROC MIXED procedure. For post hoc analysis, Fisher's least significant difference (LSD) was used.
2.6. Immunohistochemistry
2.6.1. Tissue preparation
At 13 weeks post‐transplantation, rats were transcardially perfused with heparinized (5 U/mL) saline (NaCl 0.9%) followed by 4% buffered paraformaldehyde. Brains were removed from their skulls and fixed overnight in 4% buffered paraformaldehyde at 4°C to 8°C, then, transferred to 30% sucrose phosphate buffered saline (PBS) solution for 3 days until they sank and they were then frozen in OCT compound. Forty‐micrometer thick coronal cryosections prepared using a cryotome (CM3050, Leica, Germany) were stored in cryoprotective solution (40% glycerol, 40% ethylene glycol, 0.2 M PBS) at −20°C until use.
2.6.2. CTX cell survival
Cell survival was evaluated with seven free‐floating sections from each of the 12 CTX injected brains. A human‐specific nuclei (hNu) antibody (1:200, MAB1281, Chemicon) was used with conventional single label DAB (3,3′‐diaminobenzidine) immunohistochemistry to detect CTX cells.
2.6.3. CTX cell differentiation
CTX differentiation was analyzed from the transplanted rat brains. Dual label fluorescence immunohistochemistry was performed using antibodies to the human‐specific nuclei 20 (1:200, MAB1281, Chemicon) or human‐specific mitochondria (hMito; 1:200, AB3598, Chemicon) to detect CTX cells, combined with differentiation markers of DARPP‐32 (1:100, #2306, Cell Signalling) for MSNs, GABA (1:1000, A2052, Sigma) and GAD 65/67 (1:200, AB1511, Chemicon) for GABAergic neurons, Tbr1 (1:200, ab31940, Abcam) and BDNF (1:200, Ab1534, Chemicon) for cortical neurons. Goat antimouse IgG‐conjugated Alexa 555 (1:200, Molecular Probes) and goat antirabbit IgG‐conjugated Alexa 488 (1:200, Molecular Probes) were used as secondary antibodies for detection and visualization, and a DAPI counterstain was applied. Images were captured using a confocal laser‐scanning microscope imaging system (Leica TCS SP8, Germany).
2.6.4. Host brain responses
Host brain responses were investigated on the transplanted rat brains. Based on the DAB immunohistochemistry, CTX‐transplanted animals showing a good graft survival with average behavioral scores were chosen (N = 3). As for the other experimental groups, three independent animals with average scores were chosen from the Sham (media, N = 3) and Fibroblast (N = 3) groups. Three independent sections from each animal (ie, a total of nine sections from three brains of each experimental group) were used for immunohistochemical (IHC) analysis per each marker. Fluorescence immunohistochemistry was performed on free‐floating sections using primary antibodies to investigate the glial scar with GFAP IHC (1:200, 556327, BD Bioscience), the microglial response with Iba‐1(1:100, 019‐19741, Wako), the macrophage response with ED1 (1:100, MCA1957GA, Serotec), the inflammatory M1 macrophage phenotype with iNOS (1:100, sc‐650, Santacruz), the anti‐inflammatory M2 phenotype with CD206 (1:100, sc‐34 577, Santacruz), neurogenesis with DCX (1:200, #4604, Cell Signalling) and BrdU (1:100, 555 627,BD Pharmigen), and angiogenesis with Reca‐1 (1:200, ab9774, Abcam). Goat antimouse IgG‐conjugated Alexa 555 (1:200, Molecular Probes) and goat antirabbit IgG‐conjugated Alexa 488 (1:200, Molecular Probes) were used as secondary antibodies, and a DAPI counterstain was applied. Images were captured using a confocal laser‐scanning microscope imaging system (Leica TCS SP8). For BrdU immunohistochemistry, tissue sections underwent pretreatment in 0.1 M sodium citrate buffer for 30 minutes at 100°C before incubation with the antibody. Host responses were quantified using the ImageJ software (NIH). Manual counts were used for collection (ED1, iNOS, CD206 positive cells for macrophage responses; DCX, BrdU‐positive cells forneurogenesis, and Reca‐1 positive blood vessels for angiogenesis) or measurement of the area of staining (GFAP for glial scar and Reca‐1 for angiogenesis) within three separate 100 μm2 regions of interest. For the glial scar, the region of interests (ROIs) of macrophage response and angiogenesis were sampled within the striatum and for neurogenesis, and the same ROIs were applied to each brain within the subventricular zone (SVZ). Data (counts or area) were statistically analyzed using the one‐way analysis of variance (SigmaPlot).
3. RESULTS
3.1. Behavioral improvement following transplantation of CTX cells
To evaluate the therapeutic effect of CTX cells on animal model, we transplanted them into QA‐lesioned rodent model of HD (N = 12). We used media (N = 11, Sham group) and human fibroblasts (N = 9, Fibroblast group) as control groups. On the rotarod, stepping and staircase tests, the CTX treated group exhibited significantly improved performance compared with Sham and Fibroblast only grafted groups, between 8‐ and 12‐weeks post‐transplantation (Figure 1).
FIGURE 1.
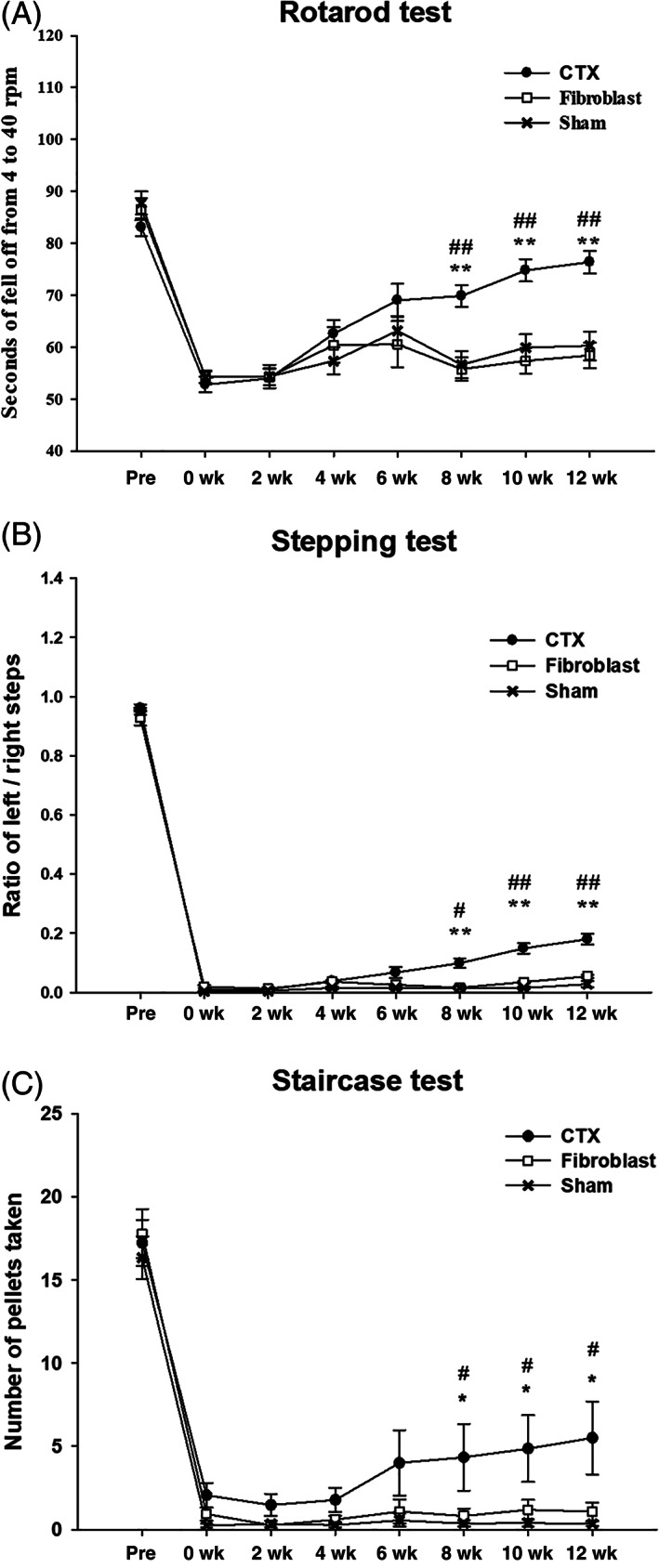
CTX cells significantly improve the behavioral deficits induced by quinolinic acid (QA) lesioning of the striatum. Marked improvement of locomotor activity was observed in the QA‐lesioned rats transplanted with CTX between 8 and 12 weeks post‐transplantation (**P < .001 Sham vs CTX; ##P < .001 Fibroblast vs CTX) on rotarod (A) and stepping (B) tests. In the staircase test (C), significant behavioral improvement was observed in the QA‐lesioned rats with CTX between 8 and 12 weeks after transplantation (*P < .05 Sham vs CTX; #P < .05 Fibroblast vs CTX). Prescore represents the animals before receiving QA and the baseline score represents 7 days after QA lesioning. Subsequent scores represent animals after CTX transplantation
3.2. Survival, distribution, and differentiation of CTX cells in QA‐lesioned brain
To prevent or reduce immune rejection of human‐derived CTX cells when transplanted into a rat HD model, immunosuppressant, Cyclosporine A, was administered intraperitoneally, starting at a dose of 10 mg/kg, 2 days prior to the transplantation surgery and then 5 mg/kg daily after surgery until sacrifice. To determine whether CTX cells were integrated into the QA‐lesioned host brain following transplantation, we performed histological analysis. Serial sections collected from the 12 CTX‐implanted brains, in the range of AP: +1.6 mm and −1.2 mm, were subject to brightfield immunohistochemistry using a human‐specific nuclei antibody (hNu) to detect surviving CTX cells (Figure 2). We detected CTX cells in the transplanted brains at 13 weeks postimplantation that were mostly located close to the site of transplantation, in the range of AP: +0.8 mm and −0.8 mm, in the lesioned striatum (Figure 2) as well as in the overlying cortex. Quantification of CTX survival in the striatum showed that over 10% of the original number of transplanted cells were present at 13 weeks post‐transplantation. Sections from CTX‐transplanted brains were also subject to double‐label fluorescence immunohistochemistry to determine the level of neural differentiation within the graft. To identify the CTX‐derived cells in the transplanted animals, we further performed immunohistochemistry with a series of antibodies against DARPP‐32 (MSN), GABA and GAD 65/67 (GABAergic), BDNF and Tbr1 (glutamatergic). Microscopic analysis revealed that some surviving CTX cells in the lesioned striatum expressed markers specific for striatal neuronal subtypes, such as DARPP‐32, GABA, and GAD 65/67 (Figure 3). In addition, some CTX cells in the striatum expressed the neurotrophic factor, BDNF (Figure 2). Only CTX cells transplanted into the cortex expressed Tbr1, a marker specific for cortical glutamatergic neurons (Figure 4).
FIGURE 2.
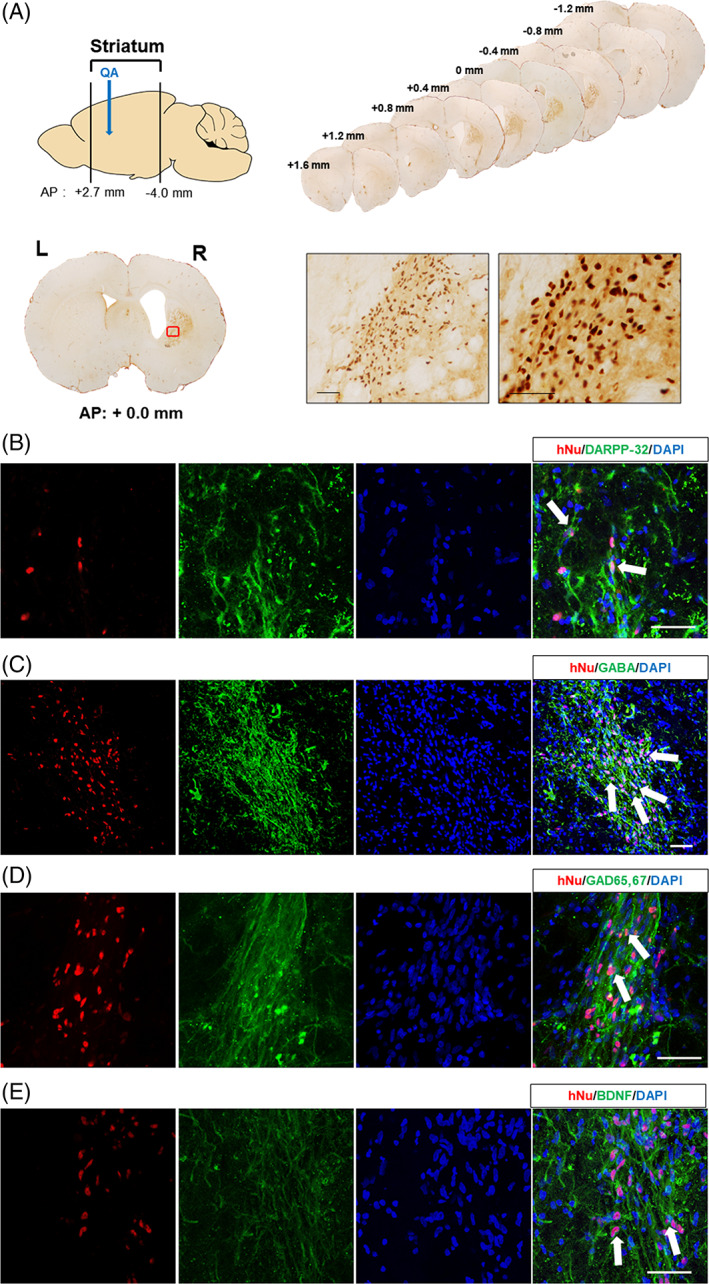
CTX cells can survive and differentiate into striatal neurons in the striatum of quinolinic acid (QA)‐lesioned brain 13 weeks after transplantation. A, Widespread distribution of survived CTX cells were detected using a human nuclei antibody (hNu) and visualized with DAB (3,3′‐diaminobenzidine) in the QA‐lesioned striatum. Scale bar = 50 μm. B‐E, Neuronal differentiation of CTX cells transplanted into the striatum (arrows) was identified by hNu colocalized with neuronal markers, including DARPP‐32 (B), GABA (C), GAD 65/67 (D), and BDNF (E). Scale bar = 50 μm
FIGURE 3.
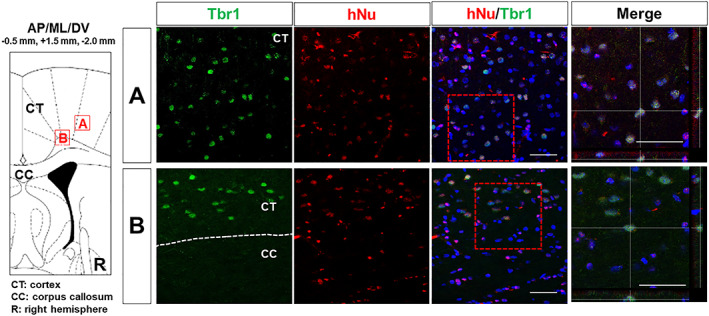
CTX cells implanted in the cortex can differentiate into cortical neurons. Colocalization (yellow) of hNu and Trb1 demonstrates neuronal differentiation of CTX cells in the cortex 13 weeks after transplantation (arrows). Scale bar = 50 μm
FIGURE 4.
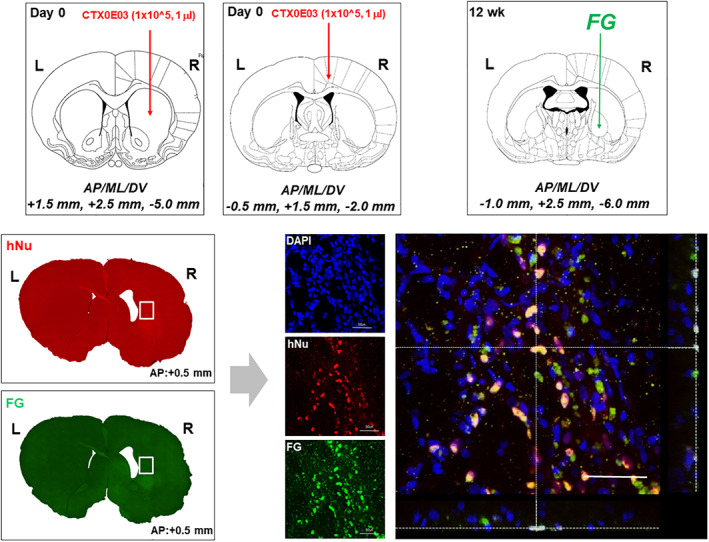
CTX cells can establish striato‐pallidal connection in the quinolinic acid (QA)‐lesioned striatum. Colocalization (yellow) of Fluoro‐Gold (FG) and human nuclei in CTX cells (arrows) within the stratum suggests some restoration of local circuitry. Scale bar = 50 μm
3.3. Neuronal connection between transplanted CTX cells and FG + host brain cells
Microscopic analysis revealed that CTX cells in the striatum showed uptake of FG, which had been injected into the globus pallidus (Figure 4), indicating that CTX cells have established striatal‐pallidal connections.
3.4. Reduction of gliosis and host immune responses following transplantation of CTX cells
First, we investigated glial scar formation. Brain sections of three representative animals from each experimental group were subject to GFAP immunohistochemistry to demonstrate glial scar formation. GFAP staining, quantified by image analysis in the lesioned striatum, was shown to be significantly reduced in CTX‐implanted brains compared with Sham (Media) and Fibroblast treatment (Figure 5A), although we observed small portions of grafted CTX cells turned into GFAP‐positive cells (Supporting Information Figure S2). Second, we examined the microglial response. Brain sections from all three treatment groups were subject to IBA‐1 immunohistochemistry and it was significantly reduced in the CTX‐implanted brains compared with Sham (Media) and Fibroblast treatment (Supporting Information Figure S3). Finally, we quantified the macrophage response to the lesion and graft. Brain sections from all three treatment groups were subject to double‐label immunohistochemistry using a cocktail of anti‐ED1 and anti‐iNOS or anti‐ED1 and anti‐CD206 antibodies to detect pro‐ and anti‐inflammatory macrophage phenotypes, respectively. Quantification of these cell types in the lesioned striatum showed a significant reduction and increase in iNOS/ED1 (M1 proinflammatory) and CD206/ED1 (M2 anti‐inflammatory) cells, respectively, compared with the Sham (media) and Fibroblasts groups (Figure 5B,C).
FIGURE 5.
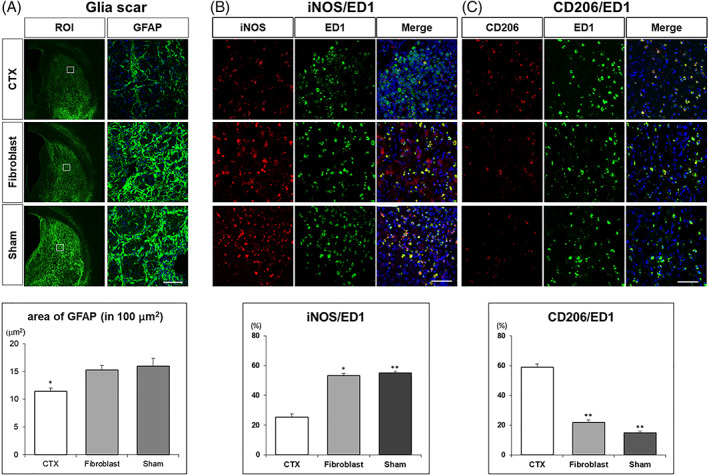
CTX cells decrease gliosis and proinflammatory responses in the quinolinic acid (QA)‐lesioned striatum. A, GFAP staining was significantly reduced in CTX‐implanted brains compared with Sham (Media) and Fibroblast (FB) injected brains (CTX vs Sham P = .004; CTX vs FB P = .014). Scale bar = 50 μm. B, CTX cells reduce a M1 proinflammatory phenotype of macrophage in the QA‐lesioned striatum. The percentage of iNOS/ED1 was reduced in CTX treated brains compared with Sham (Media) and Fibroblast grafted brains (CTX vs Sham P < .001, CTX vs FB P = .017). C, CTX cells increase an M2 anti‐inflammatory phenotype in the QA‐lesioned striatum. CD206/ED1 increased in CTX treated brains compared with Sham (Media) and Fibroblast injected brains (CTX vs Sham P < .001, CTX vs FB P < .001). Scale bar = 50 μm
3.5. Increased endogenous neurogenesis following transplantation of CTX cells
Brain sections collected from animals (ie, all three treatment groups) previously administered with BrdU were subject to double‐label immunohistochemistry with anti‐BrdU and anti‐DCX antibodies. Quantification of BrdU+, DCX+, and DCX+/BrdU+ cells showed an increase in all these phenotypes in the SVZ of CTX‐implanted brains compared with those of the Sham (media) and Fibroblast groups (Figure 6).
FIGURE 6.
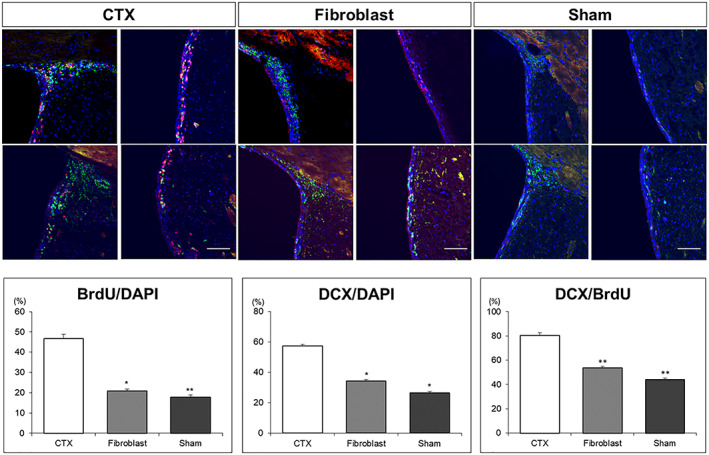
CTX cells promote neurogenesis in the quinolinic acid (QA)‐lesioned brain. Compared with Sham (Media) and Fibroblast injected brains, the proportion of both BrdU and DCX stained cells was increased markedly in the SVZ of CTX treated brains (BrdU/DAPI CTX vs Sham P < .001, CTX vs FB P < .001; DCX/DAPI CTX vs Sham P < .001, CTX vs FB P = .002). The percentage of DCX expressing cells in BrdU‐positive cells in the SVZ was also significantly increased in CTX treated brains (DCX/BrdU CTX vs Sham P = .025, CTX vs FB P = .029). Scale bar = 100 μm
3.6. Increased endogenous angiogenesis following transplantation of CTX cells
Brain sections from all treatment groups were subject to RECA‐1 immunohistochemistry to examine vascularization. RECA‐1 staining, quantified by image analysis, and counts of branch point and microvessels in the lesioned striatum were shown to be significantly increased in the CTX‐implanted brains compared with the Sham (Media) and Fibroblast treated brains (Figure 7).
FIGURE 7.
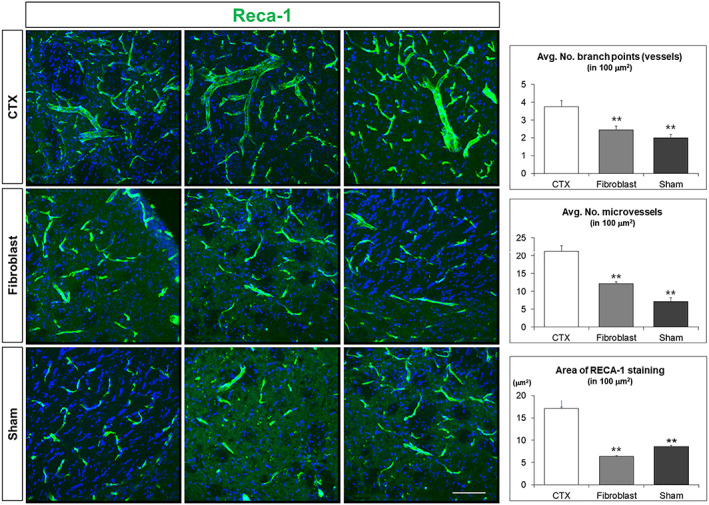
CTX cells promote angiogenesis in the quinolinic acid (QA)‐lesioned brain. RECA‐1 staining demonstrates that the number of branch points and microvessels was all significantly increased in CTX‐implanted brains compared with Sham (Media) and Fibroblast injected brains (P < .001 CTX vs Sham; P < .001 CTX vs FB; branch points P = .019 CTX vs Sham; P < .001 CTX vs FB; microvessels P < .001 CTX vs Sham; P < .001 CTX vs FB). Scale bar = 50 μm
4. DISCUSSION
In this study, we explored the therapeutic effect of CTX, a conditionally immortalized GMP‐manufactured human NSC, in the QA model of HD using various behavioral and IHC analyses. Injection of QA, an intrinsic neuroactive metabolite of the kynurenine pathway, can induce degeneration of striatal GABAergic projection neurons while preserving striatal afferents, which resembles the neuropathologic condition in HD patients. 21 , 22 QA‐lesioned rats is one of the most well‐characterized HD models for motor and cognitive symptoms, including decreased spontaneous locomotion, profound impairment in paw reaching test and place‐learning deficit in the Morris water maze, 23 , 24 , 25 and have been used as a useful model to evaluate the efficacy of stem cell transplantation in terms of behavioral and pathological changes. 26
Rotarod, stepping, and staircase tests were employed to assess motor coordination, akinesia, and fine motor reaching skills, respectively, and the pre‐ and post‐lesioning behavioral deficits following CTX cell transplantation were analyzed compared to those following sham and fibroblast transplantation. The effect of CTX transplantation was also evaluated in terms of cell survival, differentiation, and connectivity as well as their effects on innate regenerative processes such as angiogenesis and neurogenesis and the inflammatory response. As for the target sites for CTX transplantation, the cerebral cortex in addition to the striatum was selected because cortical cell loss can also contribute to motor symptoms, which has been ascribed primarily to the striatum in HD patients. 27 , 28 At the same time, the in vivo potential of CTX cells to differentiate into the region‐specific neurons can be evaluated through this approach.
Behaviorally, we found that the QA‐lesioning–induced behavioral deficits were partially reversed between 8 and 12 weeks after CTX implantation (Figure 1). Although non‐QA‐lesioned animals would be useful as an intertrial test to demonstrate the behavior of “normal” rats, CTX‐implanted rats performed significantly better on all three different behavioral tests employed at these time points, when compared with sham (media) and fibroblast transplants. This delay in benefits is in line with what has been reported previously with CTX transplantation in chronic stroke model. 29 This suggests that the capability of CTX may be attributed to progressive replacement of injured neurons and/or modulation of the host environment to promote regenerative mechanisms rather than through some direct lesion modifying effect.
As shown from IHC analyses, CTX cells can survive, express BDNF, and differentiate into the region‐specific neurons in the striatum (DARPP‐32, GAD65/67) (Figure 2) and in the cortex (Tbr1, Figure 3) in vivo at 13 weeks after transplantation. The presence of FG within the surviving CTX also suggests that these cells can form local circuits (Figure 4). It will be very useful to perform electrophysiology in the future, to confirm the neuronal integration and action potentials of the transplanted cells. All these results suggest that CTX cells have a significant potential as a cell replacement therapy for HD.
Previous reports demonstrated that BDNF‐overexpressing NSCs are neuroprotective in the MCAO model of stroke. 19 Likewise, the current CTX‐transplanted HD model also expressed BDNF, suggesting that production of BDNF from CTX provided neuroprotective effects in HD. This may also explain some of the other changes we observed in the host brain following transplantation.
In neurodegenerative diseases, including HD, the astrocytic and microglial responses are thought to have a role in driving the disease process. 30 , 31 In the present study, we found that CTX cells can modulate a broad spectrum of host cellular responses in a positive way to promote regeneration and repair. In particular, both astrocyte and microglia responses were significantly reduced in brains implanted with CTX. Astrocytes are known to form a glial scar to isolate injured tissue for protecting neurons from contact‐induced apoptosis, and microglia clean up the site of injury by phagocytosis of cellular debris and dead neurons. 32 , 33 We found that CTX transplantation can lead to a significant decrease of glial scar formation (Figure 5A). Interestingly, we also observed small portions of grafted CTX cells turned into GFAP‐positive astroglia cells (Supporting Information Figure S2), suggesting a contribution of graft‐derived glial cells to the rescue of HD phenotypes. 34 CTX transplantation was also shown to modulate macrophages in the lesioned striatum. Macrophages can change their characteristics in response to environmental changes, which result in a dual role in the response to injury. 35 The phagocytic M1 phenotype functions like microglia to remove the post‐injury cellular debris, while the M2 type anti‐inflammatory phenotype secretes a cocktail of anti‐inflammatory mediators that promote wound repair. In the chronic inflammatory environment, the persistent M1 phenotype leads to increased secretion of proinflammatory mediators and enzymes that cause further tissue destruction and injury. Importantly, polarization of M1 to an M2 phenotype is a key event for the resolution of inflammation and the initiation of reparative mechanisms. 36 We found that CTX led to an M1 to M2 polarization as demonstrated by a predominance of a CD206 M2 phenotype and a reduction in the inflammatory iNOS M1 phenotype in the lesioned striatum implanted with CTX (Figure 5B,C). Taken together, it is evident that CTX implantation can not only replace lost cells and make connection but also has the ability to reduce the responses of several cell types involved in neuroinflammation.
Finally, we were able to show that CTX implantation promotes other reparative mechanisms in the brains of HD model. IHC staining using BrdU combined with DCX (a marker of migrating neuroblasts) showed a significant increase in the subventricular zone (SVZ) of CTX‐transplanted brains (Figure 6), which is in line with a previous report showing that there is a significant increase in DCX/Ki67 neuroblasts in CTX‐implanted MCAO brains. 37 In addition, we found that blood vessel formation is significantly increased, as seen with Reca1 staining, in CTX‐transplanted brains (Figure 7), again in line with what is seen with these cells in stroke models. 11
5. CONCLUSION
We have shown that CTX implantation is an effective treatment in the QA model of HD and works through multiple mechanisms, including cell replacement. Transplantation of CTX cells improved lesion‐associated behavioral deficits on the rotarod, stepping, and staircase tests. Characterization of transplanted CTX demonstrated that they could undergo region‐specific differentiation in the striatum and the cortex and establishment of striato‐pallidal connections with the host tissue. Moreover, CTX transplantation reduced the local host inflammatory responses and promoted regenerative mechanisms such as angiogenesis and neurogenesis. Although additional analysis using transgenic HD mouse models will be useful, 38 given all these current results and the fact that the cell has already been subject of clinical trials in stroke, CTX offers great potential as treatment for patients with HD.
6. CONFLICT OF INTEREST
L.S., C.H., R.C., and J.D.S. are employees of ReNeuron. J.S. is the founder and CEO of iPS Bio, Inc. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.S.: conceived the study, designed the experiments, provided funding for the work, wrote the manuscript, supervised the entire work, and approved the final submission of manuscript; J.D.S.: conceived the study, designed the experiments, provided funding for the work, and wrote the manuscript; R.C.: conceived the study, designed the experiments, analyzed the results, and contributed to writing of the manuscript; Y.Y., I.J., H.S.K., S.J., J.E.N., H.J.P.: performed the experiment; L.S.: manufactured and provided CTX0E03 cells for transplantation experiment; C.H.: analyzed the histological data; I.H.P., R.A.B.: analyzed the results and contributed to writing of the manuscript.
Supporting information
Figure S1 Summary of in vivo experimental scheme
Figure S2 Grafted CTX cells form GFAP‐positive astroglia cells. Colocalization of human‐specific mitochondria (hMito) with GFAP indicates the formation of astroglia cells 13 weeks after transplantation. Scale bar 50 μm.
Figure S3 Grafted CTX cells decrease microglial response in the QA‐lesioned striatum. Iba1 staining was significantly reduced in CTX‐implanted brains compared with Sham (Media) and Fibroblast injected brains 13 weeks after transplantation (P = .042 CTX vs Sham; P = .001 CTX vs FB). Scale bar 50 μm.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF‐2017M3A9B4061407), Republic of Korea, and by the internal funding of ReNeuron, United Kingdom, and iPS Bio, Inc, Republic of Korea.
Yoon Y, Kim HS, Jeon I, et al. Implantation of the clinical‐grade human neural stem cell line, CTX0E03, rescues the behavioral and pathological deficits in the quinolinic acid‐lesioned rodent model of Huntington's disease. Stem Cells. 2020;38:936–947. 10.1002/stem.3191
Yongwoo Yoon and Hyun Sook Kim contributed equally to this study.
Funding information National Research Foundation of Korea, Grant/Award Number: NRF‐2017M3A9B4061407; iPS Bio, Inc; ReNeuron, United Kingdom
1. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta‐analysis. Mov Disord. 2012;27:1083‐1091. [DOI] [PubMed] [Google Scholar]
- 2. Connor B. Concise review: the use of stem cells for understanding and treating Huntington's disease. Stem Cells. 2018;36:146‐160. [DOI] [PubMed] [Google Scholar]
- 3. Dunnett SB, Rosser AE. Cell therapy in Huntington's disease. NeuroRx. 2004;1:394‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim HS, Song J. Cell therapy in Huntington's disease In: Demirer T, ed. Progress in Stem Cell Transplantation; 2015:77‐109. https://www.intechopen.com/books/progress‐in‐stem‐cell‐transplantation/cell‐therapy‐in‐huntington‐s‐disease. [Google Scholar]
- 5. Lee ST, Chu K, Park JE, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington's disease rat model. Neurosci Res. 2005;52:243‐249. [DOI] [PubMed] [Google Scholar]
- 6. Ryu JK, Kim J, Cho SJ, et al. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol Dis. 2004;16:68‐77. [DOI] [PubMed] [Google Scholar]
- 7. Johann V, Schiefer J, Sass C, et al. Time of transplantation and cell preparation determine neural stem cell survival in a mouse model of Huntington's disease. Exp Brain Res. 2007;177:458‐470. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Gharaibeh A, Culver R, Stewart AN, et al. Induced pluripotent stem cell‐derived neural stem cell transplantations reduced behavioral deficits and ameliorated neuropathological changes in YAC128 mouse model of Huntington's disease. Front Neurosci. 2017;11:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose‐dependent fashion. Neurorehabil Neural Repair. 2009;23:895‐909. [DOI] [PubMed] [Google Scholar]
- 10. Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first‐in‐man study. Lancet. 2016;388:787‐796. [DOI] [PubMed] [Google Scholar]
- 11. Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013;22:1541‐1552. [DOI] [PubMed] [Google Scholar]
- 12. Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human neural stem cell therapy for chronic ischemic stroke: charting Progress from laboratory to patients. Stem Cells Dev. 2017;26:933‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lelos MJ, Dunnett SB. Generating Excitotoxic lesion models of Huntington's disease. Methods Mol Biol. 1780;2018:209‐220. [DOI] [PubMed] [Google Scholar]
- 14. Pollock K, Stroemer P, Patel S, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143‐155. [DOI] [PubMed] [Google Scholar]
- 15. Conte‐Perales L, Barroso‐Chinea P, Rico AJ, et al. Neuroanatomical tracing combined with in situ hybridization: analysis of gene expression patterns within brain circuits of interest. J Neurosci Methods. 2010;194:28‐33. [DOI] [PubMed] [Google Scholar]
- 16. Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399‐1405. [DOI] [PubMed] [Google Scholar]
- 17. Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920‐1923. [DOI] [PubMed] [Google Scholar]
- 18. Jeon I, Lee N, Li JY, et al. Neuronal properties, in vivo effects, and pathology of a Huntington's disease patient‐derived induced pluripotent stem cells. Stem Cells. 2012;30:2054‐2062. [DOI] [PubMed] [Google Scholar]
- 19. Chang D‐J, Lee N, Choi C, et al. Therapeutic effect of BDNF‐overexpressing human neural stem cells (HB1. F3. BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transplant. 2013;22:1441‐1452. [DOI] [PubMed] [Google Scholar]
- 20. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861‐872. [DOI] [PubMed] [Google Scholar]
- 21. Schwarcz R, Whetsell WO Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon‐sparing lesions in rat brain. Science. 1983;219:316‐318. [DOI] [PubMed] [Google Scholar]
- 22. Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J Neurosci. 1991;11:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Popoli P, Pezzola A, Domenici MR, et al. Behavioral and electrophysiological correlates of the quinolinic acid rat model of Huntington's disease in rats. Brain Res Bull. 1994;35:329‐335. [DOI] [PubMed] [Google Scholar]
- 24. Francis L, Cruz R, Antunez I, et al. Behavior characterization of a model of Huntington's disease in rats, induced by quinolinic acid. Rev Neurol. 2000;30:1016‐1021. [PubMed] [Google Scholar]
- 25. Sanberg PR, Calderon SF, Giordano M, Tew JM, Norman AB. The quinolinic acid model of Huntington's disease: locomotor abnormalities. Exp Neurol. 1989;105:45‐53. [DOI] [PubMed] [Google Scholar]
- 26. McBride JL, Behrstock SP, Chen EY, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J Comp Neurol. 2004;475:211‐219. [DOI] [PubMed] [Google Scholar]
- 27. Rosas HD, Salat DH, Lee SY, et al. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008;131:1057‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thu DC, Oorschot DE, Tippett LJ, et al. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 2010;133:1094‐1110. [DOI] [PubMed] [Google Scholar]
- 29. Smith EJ, Stroemer RP, Gorenkova N, et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30:785‐796. [DOI] [PubMed] [Google Scholar]
- 30. Crotti A, Glass CK. The choreography of neuroinflammation in Huntington's disease. Trends Immunol. 2015;36:364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amor S, Peferoen LA, Vogel DY, et al. Inflammation in neurodegenerative diseases—an update. Immunology. 2014;142:151‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loov C, Hillered L, Ebendal T, et al. Engulfing astrocytes protect neurons from contact‐induced apoptosis following injury. PLoS One. 2012;7:e33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benraiss A, Wang S, Herrlinger S, et al. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat Commun. 2016;7:11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463‐488. [DOI] [PubMed] [Google Scholar]
- 37. Hassani Z, O'Reilly J, Pearse Y, et al. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PLoS One. 2012;7:e50444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reidling JC, Relano‐Gines A, Holley SM, et al. Human neural stem cell transplantation rescues functional deficits in R6/2 and Q140 Huntington's disease mice. Stem Cell Rep. 2018;10:58‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Summary of in vivo experimental scheme
Figure S2 Grafted CTX cells form GFAP‐positive astroglia cells. Colocalization of human‐specific mitochondria (hMito) with GFAP indicates the formation of astroglia cells 13 weeks after transplantation. Scale bar 50 μm.
Figure S3 Grafted CTX cells decrease microglial response in the QA‐lesioned striatum. Iba1 staining was significantly reduced in CTX‐implanted brains compared with Sham (Media) and Fibroblast injected brains 13 weeks after transplantation (P = .042 CTX vs Sham; P = .001 CTX vs FB). Scale bar 50 μm.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


