Abstract
It is unknown if the cardioprotective and renal effects of glucagon‐like peptide‐1 receptor agonists are consistent across blood pressure (BP) categories in patients with type 2 diabetes and at high risk of cardiovascular events. Using data from the LEADER (9340 patients) and SUSTAIN 6 (3297 patients) trials, we evaluated post hoc the cardiorenal effect of liraglutide and semaglutide on major adverse cardiovascular events (MACE) and nephropathy by baseline BP categories using a Cox proportional hazards model (treatment and subgroup as factors; adjusted for cardiorenal risk factors). Data from the two trials were analysed separately. In the LEADER and SUSTAIN 6 trials, the prevalence of stage 1 hypertension was 30% and 31%, respectively, and of stage 2 hypertension 41% and 43%, respectively. There was no statistical heterogeneity across the BP categories for the effects of liraglutide (P = .06 for MACE; P = .14 for nephropathy) or semaglutide (P = .40 for MACE; P = .27 for nephropathy) versus placebo. This implies that liraglutide and semaglutide may be beneficial for patients with type 2 diabetes, irrespective of their baseline BP.
Keywords: blood pressure, cardiovascular, liraglutide, MACE, semaglutide
1. INTRODUCTION
Elevated blood pressure (BP) is very common in people with type 2 diabetes (T2D), and increases the risk of cardiovascular (CV) and renal events in this population. 1 , 2 Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) have been shown to reduce the incidence of CV and renal events in people with T2D or at risk of CV disease. 3 , 4 These agents have also been shown to reduce BP versus placebo, insulin and sulphonylureas, 5 with greater reductions observed in those with higher baseline BP (liraglutide vs. placebo). 6 Whether GLP‐1 RAs exert CV and renal event reduction in a consistent fashion across the spectrum of baseline BP remains unknown. Therefore, we studied this question in the LEADER 7 and SUSTAIN 68 trials, through separate post hoc analyses.
2. METHODS
The design, baseline patient characteristics and primary results of the LEADER 7 and SUSTAIN 6 8 trials have been reported previously. Briefly, they were global, double‐blind, placebo‐controlled, randomized CV outcomes trials of subcutaneously injected liraglutide (LEADER) and semaglutide (SUSTAIN 6), in patients with T2D (HbA1c ≥ 7.0%) and high CV risk. Institutional review boards or ethics committees for each centre approved the trial protocols and all patients provided informed consent. 7 , 8 The key inclusion criteria were age ≥ 50 years with ≥1 co‐existing CV condition (coronary heart disease, cerebrovascular disease, chronic kidney disease stage ≥3, or chronic New York Heart Association class II or III heart failure) or age ≥ 60 years with ≥1 CV risk factor. In both trials, CV risk factors included microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle‐brachial index of <0.9. 7 , 8
Patients were followed for up to 5 years in the LEADER trial (N = 9340; median time 3.8 years), and in the SUSTAIN 6 trial all patients were followed for ~ 2 years (N = 3297; median time 2.1 years). 7 , 8 The primary composite outcome in both trials was the first occurrence of major adverse cardiovascular events (MACE: CV death, non‐fatal myocardial infarction [MI] or non‐fatal stroke). 7 , 8 Secondary outcomes included a composite renal outcome of new‐onset persistent macroalbuminuria, persistent doubling of serum creatinine level, the need for continuous renal replacement therapy or death from renal disease. Expanded MACE included the events within primary MACE in addition to revascularization and hospitalization for heart failure or unstable angina. CV and renal events were adjudicated by an external, blinded, independent, expert committee. BP was measured at baseline and designated clinic visits (at least annually) according to the usual practice at the investigator's site 7 , 8 (further details are available in the Supplementary Appendix, see the supporting information).
2.1. Statistics
In this post hoc analysis, the effects of liraglutide and semaglutide on the primary CV and secondary new or worsening nephropathy outcomes were evaluated by baseline American College of Cardiology/American Heart Association‐defined BP categories 9 : normal (<120/80 mmHg), elevated (systolic 120‐129 mmHg and diastolic <80 mmHg), stage 1 hypertension (systolic 130‐139 mmHg or diastolic 80‐89 mmHg) and stage 2 hypertension (systolic ≥140 mmHg or diastolic ≥90 mmHg). The mean of two BP measurements taken at the randomization visit (baseline) was used to assign the BP category. A Cox proportional hazards model, with treatment and BP category as factors and the interaction between both, was used to calculate the hazard ratio (HR) and 95% confidence interval (CI). The model was adjusted for baseline characteristics related to cardiorenal risk (age, antihyperglycaemic medications, diabetes duration, geographic region, history of MI or stroke, renal function as measured by estimated glomerular filtration rate, sex, and smoking status [smoking status was omitted for endpoints with low frequency in SUSTAIN 6]). An interaction P‐value of <.05 was considered significant. Analysis of expanded MACE, hospitalization for heart failure, CV death and all‐cause mortality was also performed using the Cox proportional hazards model as described. The analysis of MACE and nephropathy was repeated with patients further categorized by angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) use at baseline. For all tests, P‐values were not adjusted for multiple comparisons. Quadratic spline regression applied using a Cox proportional hazard model was used to analyse the treatment differences in time to first MACE by systolic and diastolic BP on a continuous scale.
To further test potential interactions between BP categories and treatment, the Gail‐Simon test (for qualitative interactions) was applied in the Cox proportional hazard model, where a P‐value of <.05 would indicate that the direction of treatment effect differed in one or more subgroups versus the remaining subgroups, e.g. that a treatment increased the risk in one subgroup, while simultaneously decreasing risk in another subgroup for an endpoint. The Gail‐Simon test differed from the interaction test (which measured quantitative interactions), wherein small P‐values would indicate that the magnitude of the treatment effects differed between subgroups. All analyses were performed using the statistical software package SAS version 9.4.
3. RESULTS
All patients randomized in the LEADER (n = 9340) and SUSTAIN 6 (n = 3297) trials were included in these analyses. The baseline characteristics of patients in each BP category in LEADER and SUSTAIN 6 are shown in Tables S1 and S2, respectively. In the LEADER and SUSTAIN 6 trials, the prevalence of stage 1 hypertension was 30% and 31%, respectively, and the prevalence of stage 2 hypertension was 41% and 43%, respectively.
3.1. Cardiorenal efficacy across BP categories in LEADER and SUSTAIN 6
Figure 1 depicts the HRs for the primary MACE and renal outcomes in LEADER (Figure 1A) and SUSTAIN 6 (Figure 1B) trials across the BP categories studied. In both instances, treatment with liraglutide or semaglutide versus placebo was associated with a consistent reduction in cardiorenal outcomes with no evidence of statistical heterogeneity. Although there appeared to be a trend with liraglutide versus placebo towards a lower relative risk reduction in those with normal BP (compared with those with stage 1 or 2 hypertension), this was not statistically significant (P‐interaction = .06). The Gail‐Simon test for qualitative interaction revealed no significant interaction for the primary MACE or nephropathy outcomes in LEADER or SUSTAIN 6, respectively (Figure 1A,B).
FIGURE 1.
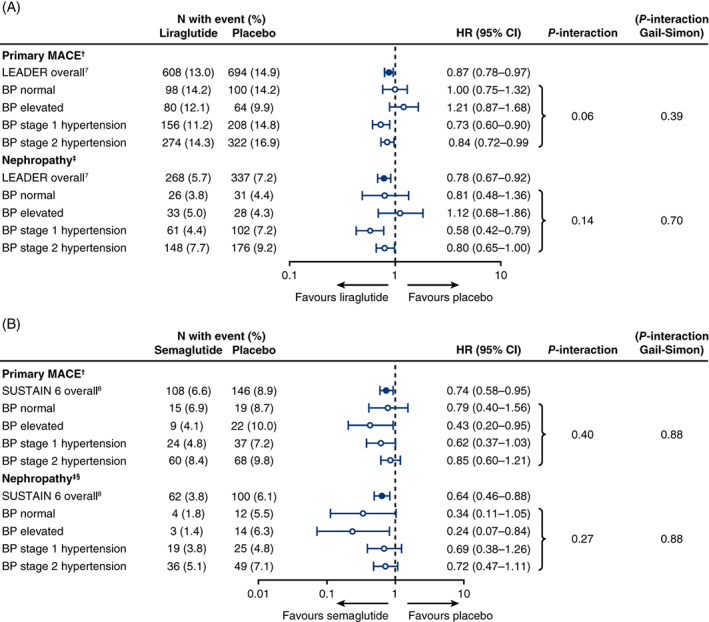
Cardiorenal outcomes by baseline blood pressure (BP) category, adjusted for baseline variables related to cardiorenal risk, in the LEADER (A) and SUSTAIN 6 trials (B). †Primary major adverse cardiovascular events (MACE): composite of cardiovascular death, non‐fatal myocardial infarction (MI) and non‐fatal stroke. Analysis adjusted for baseline characteristics related to cardiorenal risk (age, antihyperglycaemic medications, diabetes duration, geographic region, history of MI or stroke, renal function as measured by estimated glomerular filtration rate, sex, and smoking status). ‡Nephropathy (new or worsening): new or persistent macroalbuminuria, doubling of serum creatinine, end‐stage kidney disease or death from kidney disease. §Analysis adjusted as for MACE, with the omission of smoking status because of a low number of events. BP categories were defined as follows: normal = systolic blood pressure (SBP) < 120 mmHg, diastolic blood pressure (DBP) 80 mmHg; elevated = SBP 120‐129 mmHg and DBP < 80 mmHg; stage 1 hypertension = SBP 130‐139 mmHg or DBP 80‐89 mmHg; stage 2 hypertension = SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. CI, confidence interval; HR, hazard ratio
Additional outcomes, including expanded MACE, hospitalization for heart failure and all‐cause mortality, are depicted in Tables S3 and S4, and generally supported a conclusion of consistent benefit for liraglutide and semaglutide across the BP categories. For the expanded MACE outcome (which included CV death, non‐fatal MI, non‐fatal stroke, coronary revascularization, hospitalization for unstable angina or heart failure), the P‐value for interaction was .048 in LEADER, and was non‐significant in SUSTAIN 6. Analysis by ACEi/ARB use at baseline in both trials also revealed a consistent benefit across all categories (Figures S1 and S2).
Analysis of systolic and diastolic BP at baseline as continuous variables confirmed a consistent benefit of liraglutide or semaglutide within the quartile boundaries (i.e. end of Q1 to beginning of Q3), in which 50% of the events occurred (Figure 2).
FIGURE 2.
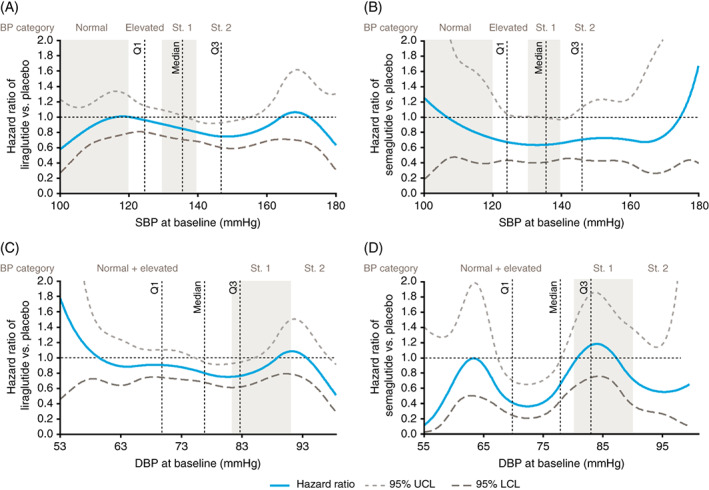
Quadratic spline regression treatment differences in time to first major adverse cardiovascular events (MACE), according to baseline systolic blood pressure (SBP) (A and B) and diastolic blood pressure (DBP) (C and D), in the LEADER (A and C) and SUSTAIN 6 (B and D) trials. Primary MACE: composite of cardiovascular death, non‐fatal myocardial infarction (MI) and non‐fatal stroke. Q1, one quarter of patients had a lower blood pressure (BP) value than this. Median, half of patients had a lower BP value than this. Q3, three‐quarters of patients had a lower BP value than this. BP categories were defined as follows: normal = SBP < 120 mmHg, DBP 80 mmHg; elevated = SBP 120‐129 mmHg and DBP < 80 mmHg; stage 1 hypertension = SBP 130‐139 mmHg or DBP 80‐89 mmHg; stage 2 hypertension = SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. LCL, lower confidence limit; Q, quartile; St., stage; UCL, upper confidence limit
4. DISCUSSION
GLP‐1 RAs are known to have multiple favourable cardiometabolic effects on glycaemia, weight and blood pressure. 3 , 7 , 8 While the CV benefits of these therapies are unlikely to be solely dependent on these effects 10 , 11 and are also suggested to be modulated by direct vasculoprotective/antiatherosclerotic pathways, 12 , 13 questions have been raised about whether these therapies exert cardiorenal benefits in the setting of adequate risk factor control. The present analyses, from two large and contemporary randomized controlled trials, suggest that liraglutide and semaglutide provide similar benefits, both quantitatively and qualitatively, on major cardiorenal outcomes in people with T2D across the spectrum of baseline BP values. Even in patients with normal BP on entering both of these trials, these benefits were seen for both GLP‐1 RAs. These data, taken together with prior analyses from LEADER and SUSTAIN 6 showing efficacy across the spectrum of lipid levels 11 , 13 and body mass index, 14 suggest that these therapies should be considered as complementary to traditional risk factor modification for risk reduction in people with T2D.
Indeed, when examining data related to BP, it can be important to consider traditional risk factor modifications in terms of medications frequently prescribed for this purpose, such as ACEis and ARBs. We noted that the benefits with GLP‐1 RAs were consistently observed in users and non‐users of ACEis/ARBs, suggesting that the cardiorenal benefits of liraglutide and semaglutide are probably additive to that of renin‐angiotensin system blockade. Future studies could further explore this to determine exactly how GLP‐1 RAs confer such cardiorenal benefits, independently of the renin‐angiotensin system.
Limitations of this post hoc analysis include that it was a retrospective analysis of data from two different trials, not powered to look at endpoints in these subgroups, and only baseline and not in‐trial BP categories were considered. Furthermore, BP was recorded using different techniques, as per the usual practice at each site, which may have had an impact on the measurements. In addition, the use of antihypertensive medication during the trial was at the discretion of the investigator and was not examined as a time‐varying covariate. Competing risk factors (e.g. non‐CV death) may have also impacted the results, and there was no adjustment for biomarkers in this analysis, as has been performed in other similar analyses. 15
In conclusion, in both LEADER and SUSTAIN 6, liraglutide and semaglutide showed no heterogeneity of efficacy in CV and renal outcomes, irrespective of baseline BP categories and of ACEi/ARB use.
CONFLICT OF INTEREST
LAL reports consultant and speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier; and research grant or support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk and Sanofi. SCB reports personal fees and other from Abbott, AstraZeneca, Boehringer Ingelheim, BMS, Cellnovo, Diartis, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi‐aventis, Schering‐Plough, Servier and Takeda; and other from Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia‐Med, Medscape, All‐Wales Medicines Strategy Group, National Institute for Health and Care Excellence (NICE) UK and Glycosmedia. DLB discloses the following relationships: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care and TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor‐in‐Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Level Ex, MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; Trustee: American College of Cardiology; and unfunded research: FlowCo, Merck, Novo Nordisk, and Takeda. JBB's contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen; he reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; he is a consultant to Cirius Therapeutics Inc, CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health; he holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health; and he is supported by grants from the National Institutes of Health (UL1TR002489, U01DK098246, UC4DK108612 and U54DK118612), PCORI and ADA. CDM reports research grants to institution and/or consulting honoraria from Amgen, Boehringer Ingelheim, CSL Behring, OctaPharma and Quark Pharmaceuticals. REP reports research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Sanofi‐Aventis US LLC and Takeda; is a speaker for AstraZeneca, Novo Nordisk and Takeda; and a consultant for AstraZeneca, Boehringer Ingelheim, Eisai, Inc., GlaxoSmithKline, Janssen Scientific Affairs LLC, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Pfizer and Takeda; all payments are made directly to his employer (Florida Hospital/AdventHealth). SR is an employee of and shareholder in Novo Nordisk. MSR is an employee of Novo Nordisk. HV was an employee of Novo Nordisk at the time of manuscript development. SV reports consultant and speaker fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Valeant, Amgen, HLS Therapeutics and Sun Pharma; and research support from Boehringer Ingelheim, Eli Lilly and Amgen.
AUTHOR CONTRIBUTIONS
Design (of the post hoc analysis): SV, LAL, DLB, CDM, SR, MSR, HV, SCB, JBB and REP. Conduct/data collection: LAL, SCB, JBB and REP. Analysis: SR. Manuscript writing: LAL and SV, who contributed equally to writing the first full draft of this paper.
Supporting information
Table S1 Baseline characteristics according to baseline blood pressure: the LEADER trial
Table S2. Baseline characteristics according to baseline blood pressure: the SUSTAIN 6 trial
Table S3. Additional CV outcomes and all‐cause death by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the LEADER trial
Table S4. Additional CV outcomes and all‐cause death by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the SUSTAIN 6 trial
Figure S1. Cardiorenal outcomes by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the LEADER trial by ACEi/ARB use
Figure S2. Cardiorenal outcomes by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the SUSTAIN 6 trial by ACEi/ARB use
ACKNOWLEDGMENTS
The authors thank Emre Yildirim (Novo Nordisk) for reviewing this manuscript. Editorial support (limited to formatting, collation and incorporation of co‐author comments) was provided by Gillian Groeger, PhD, and Izabel James, MBBS, both from Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. Part of the data described in the article were previously presented at the American Heart Association Congress (11 November 2018, Chicago, IL, USA).
Leiter LA, Bain SC, Bhatt DL, et al. The effect of glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: Analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:1690–1695. 10.1111/dom.14079
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14079.
Funding information LEADER and SUSTAIN 6 were sponsored by Novo Nordisk and are registered with ClinicalTrials.gov (NCT01179048 and NCT01720446, respectively).
DATA ACCESSIBILITY
The data used for this manuscript are available on reasonable request from the corresponding author.
REFERENCES
- 1. Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601‐610. [DOI] [PubMed] [Google Scholar]
- 2. Sun D, Zhou T, Heianza Y, et al. Type 2 diabetes and hypertension. Circ Res. 2019;124:930‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776‐785. [DOI] [PubMed] [Google Scholar]
- 4. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 5. Sun F, Wu S, Guo S, et al. Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2015;110:26‐37. [DOI] [PubMed] [Google Scholar]
- 6. Wijkman MO, Dena M, Dahlqvist S, et al. Predictors and correlates of systolic blood pressure reduction with liraglutide treatment in patients with type 2 diabetes. J Clin Hypertens. 2019;21:105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 9. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 10. Sharma A, Verma S. Mechanisms by which glucagon‐like‐peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44:93–102. [DOI] [PubMed] [Google Scholar]
- 11. Verma S, Leiter LA, Mazer CD, et al. Liraglutide reduces cardiovascular events and mortality in type 2 diabetes mellitus independently of baseline low‐density lipoprotein cholesterol levels and statin use. Circulation. 2018;138:1605‐1607. [DOI] [PubMed] [Google Scholar]
- 12. Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15‐30. [DOI] [PubMed] [Google Scholar]
- 13. Rakipovski G, Rolin B, Nohr J, et al. The GLP‐1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma S, Bain SC, Bhatt DL, et al. The glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide improve cardiovascular and renal outcomes across most body mass index categories in type 2 diabetes: results of the LEADER and SUSTAIN 6 trials. Circulation. 2018;138(Supp 1):14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergmark BA, Scirica BM, Steg PG, et al. Blood pressure and cardiovascular outcomes in patients with diabetes and high cardiovascular risk. Eur Heart J. 2018;39:2255‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics according to baseline blood pressure: the LEADER trial
Table S2. Baseline characteristics according to baseline blood pressure: the SUSTAIN 6 trial
Table S3. Additional CV outcomes and all‐cause death by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the LEADER trial
Table S4. Additional CV outcomes and all‐cause death by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the SUSTAIN 6 trial
Figure S1. Cardiorenal outcomes by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the LEADER trial by ACEi/ARB use
Figure S2. Cardiorenal outcomes by baseline BP category, adjusted for baseline variables related to cardiorenal risk, in the SUSTAIN 6 trial by ACEi/ARB use
Data Availability Statement
The data used for this manuscript are available on reasonable request from the corresponding author.


