Abstract
Dupilumab is a fully human monoclonal antibody directed against the interleukin (IL)‐4 receptor α subunit (IL‐4Rα) of IL‐4 heterodimeric type I and type II receptors that mediate IL‐4/IL‐13 signaling through this pathway. Blockade of these receptors broadly suppresses type 2 inflammation associated with atopic/allergic diseases, including atopic dermatitis and asthma. Six phase 1 studies investigated the pharmacokinetics, pharmacodynamics, safety, and tolerability of dupilumab in healthy subjects. Two randomized, double‐blind, placebo‐controlled, sequential studies assessed safety and tolerability of single escalating dupilumab doses administered intravenously or subcutaneously (one included various racial groups, and one included exclusively Japanese subjects); 3 randomized, parallel‐group, single‐dose studies compared the pharmacokinetic profiles of different dupilumab products and formulations after single subcutaneous doses; and one study assessed dupilumab administered as fast versus slow subcutaneous injections. Dupilumab concentrations in serum were measured in all studies, and total immunoglobulin E (IgE) and thymus‐ and activation‐regulated chemokine (TARC) concentrations were measured in 2 studies as pharmacodynamic markers. Across the phase 1 studies, dupilumab exhibited target‐mediated pharmacokinetics consisting of parallel linear and nonlinear elimination, with the target‐mediated phase highly dominated by nonlinearity at lower drug concentrations. Systemic exposure and tolerability of dupilumab were consistent irrespective of differences in product, formulation, or racial background. Dupilumab reduced circulating concentrations of total IgE and TARC, indicating blockade of IL‐4Rα–mediated signaling. Dupilumab had a favorable safety profile across the wide range of doses administered. Together, these findings support the continued development and use of dupilumab in treatment of type 2 diseases.
Keywords: dupilumab, type 2 immune diseases, healthy subjects, pharmacokinetics, pharmacodynamics
The prevalence of type 2 inflammatory diseases (primarily atopic or allergic diseases such as asthma, atopic dermatitis [AD], and eosinophilic esophagitis) is increasing worldwide. 1 , 2 Asthma affects approximately 300 million people worldwide, 3 and the prevalence of AD has tripled in industrialized countries during the past 3 decades, with up to 30% of children and 10% of adults being affected. 4 Similarly, chronic rhinosinusitis has been reported to affect approximately 15% of the general population, of whom 20% to 40% have concomitant nasal polyposis, 5 and eosinophilic esophagitis has a prevalence of 13‐49 per 100 000 and an incidence of new cases up to 20 per 100 000 per year. 6 In addition to causing significant health problems for sufferers, type 2 diseases are associated with significant social and economic burdens. 3 , 7 , 8 , 9 Furthermore, patients are often affected by more than 1 of these diseases, which exhibit an increased incidence of atopy/allergy and/or eosinophilia, suggesting a shared underlying mechanism. 10 , 11 It is now recognized that interleukin (IL)‐4 and IL‐13 are among the central drivers in the pathophysiology of type 2 inflammatory diseases, including asthma, AD, nasal polyposis, and eosinophilic esophagitis. 1 , 11 This has focused attention on the use of inhibitors of these pathways in the management of these diseases.
Dupilumab is a fully human VelocImmune‐derived monoclonal antibody obtained from mice in which precise large‐scale in situ replacements of the immunoglobulin variable regions of mouse heavy and light chains were performed on a human IgG4 backbone 12 , 13 and directed against the IL‐4 receptor α (IL‐4Rα) subunit of IL‐4 heterodimeric type I and type II receptors. These receptors mediate IL‐4 signaling (both type I and type II) and IL‐13 signaling (type II). As a dual antagonist of both IL‐4 and IL‐13 signaling through this pathway, dupilumab broadly suppresses type 2 inflammation. Dupilumab is approved for patients aged ≥12 years in the United States with moderate to severe AD inadequately controlled with topical prescription therapies or, when those therapies are not advisable, for the treatment of adult AD patients not adequately controlled with existing therapies in Japan and for use in patients aged ≥12 years with moderate to severe AD who are candidates for systemic therapy in the European Union. Dupilumab is approved for patients aged ≥12 years in the United States as an add‐on maintenance treatment for moderate to severe asthma with an eosinophilic phenotype or with oral corticosteroid‐dependent asthma; in Japan for severe or refractory asthma where symptoms are inadequately controlled with existing therapy; and in the European Union as add‐on maintenance treatment for severe asthma with type 2 inflammation characterized by raised blood eosinophils and/or raised fractional exhaled nitric oxide and inadequately controlled with high‐dose inhaled corticosteroids plus another medicinal product for maintenance treatment. Dupilumab is also approved in the United States as an add‐on treatment in patients aged ≥18 years with inadequately controlled chronic rhinosinusitis with nasal polyps. Clinical trials in adults 14 , 15 , 16 , 17 , 18 and adolescent populations 19 , 20 with moderate to severe AD, as well as in adult patients with asthma 21 , 22 , 23 , 24 and chronic rhinosinusitis with nasal polyps, 25 , 26 or eosinophilic esophagitis, 27 have demonstrated improvements in disease outcomes during dupilumab treatment, with an acceptable safety profile.
A population pharmacokinetics (PK) analysis in healthy volunteers and patients with moderate to severe AD showed that a target‐mediated 2‐compartment model with parallel linear and Michaelis‐Menten elimination from the central compartment adequately described the concentration‐time profile of dupilumab. 28 Dupilumab has a low volume of distribution and slow rate of elimination, which are characteristic of IgG antibodies in general. In addition to a nonspecific high‐capacity mechanism for pinocytosis and proteolytic degradation, monoclonal antibodies are subject to target‐mediated clearance involving specific interaction with the target receptor 29 (including, in the case of dupilumab, those on the surface of circulating mononuclear blood cells 30 ) and are characterized by nonlinear PK and acceleration of the elimination rate as antibody levels decline in the blood. 29 The metabolic pathway of dupilumab, on the other hand, has not been characterized. However, because dupilumab is a monoclonal IgG4 antibody, degradation into small peptides and amino acids via catabolic pathways similar to those of endogenous IgG would be expected. 31 Furthermore, drug‐drug interaction assessments between dupilumab and cytochrome P450 (CYP) substrates (including substrates of CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6) revealed no significant impact of dupilumab on enzyme activities in patients with moderate to severe AD. 32
This article describes 6 phase 1 studies of dupilumab in healthy adult subjects. The aims of these studies were to assess the safety, tolerability, and PK profiles of dupilumab in healthy adult subjects after single intravenous (IV) or subcutaneous (SC) doses; to examine the influence of body weight and race/ethnicity (Japanese versus non‐Japanese populations) on PK in the absence of disease; to confirm the consistency of systemic dupilumab exposure for products derived from various manufacturing and formulation processes; and to describe the pharmacodynamic (PD) responses of biomarkers known to be dependent on IL‐4 and IL‐13 signaling, including total immunoglobulin E (IgE) and thymus‐ and activation‐regulated chemokine (TARC).
Methods
Details of the clinical trials presented here are summarized in Table 1. All trials were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Investigational review boards (IRBs) and ethics committees at each site approved the study protocols and procedures (details of IRBs and ethics committees are reported in Table S1). All subjects provided written informed consent before their enrollment into the studies.
Table 1.
Dupilumab Single‐Dose Studies in Young Healthy Adults
| Study | Design and End Points | Dupilumab Product and Dose Groups |
|---|---|---|
| R668‐AS‐0907 (NCT01015027) |
|
|
| TDU12265 (NCT01537653) |
|
|
|
|
|
| PKM14161 |
|
|
| PKM14271 |
|
|
| R668‐HV‐1108 (NCT01484600) |
|
|
CxPy indicates cell line x, process y; IgE, immunoglobulin E; IV, intravenous; PK, pharmacokinetics; SC, subcutaneous; TARC, thymus‐ and activation‐regulated chemokine.
Study Populations
The subjects were men and women aged 18‐65 years, all of whom were healthy, as determined by medical history, physical examination, and clinical laboratory investigations. Use of effective contraception was mandatory in women of childbearing potential. Selected inclusion and exclusion criteria are listed in Table S2 in the online supplementary material.
Study Design, End Points, and Statistical Analyses
All studies were conducted according to the following scheme: prescreening of subjects from day −21 to day −2, enrollment of suitable subjects on day −1, administration of dupilumab on day 1, and monitoring of subjects for a period ranging from 57 to 85 days for the different studies. Dupilumab injections were usually given before 9 am on day 1, after subjects had fasted for ≥10 hours. Unless stated otherwise, all SC injections were delivered in periumbilical quadrants at injection rates comfortable to the subjects (20‐30 seconds for a 2‐mL injection); IV infusions were administered over a 2‐hour period.
In each study all randomized subjects were included in the safety population, and all randomized subjects without any major protocol deviations related to administration of the investigational medicinal product, for whom the primary PK data were considered sufficient and interpretable, were included in the PK population. Descriptive statistics were generated for all measurements and derived PK parameters. PD responses of biomarkers total IgE and TARC in serum were assessed in the R668‐AS‐0907 and TDU12265 studies only; these biomarker data were not adjusted for multiplicity, and thus, nominal P values are provided. Where applicable, statistical analyses were performed using SAS version 9 or higher (SAS Institute, Cary, North Carolina).
R668‐AS‐0907 and TDU12265 Studies
These were randomized, double‐blind, placebo‐controlled, sequential, ascending single‐dose studies to assess the safety, tolerability, and PK of single escalating doses of dupilumab in healthy subjects (of various racial backgrounds in R668‐AS‐0907 and exclusively Japanese in TDU12265). In R668‐AS‐0907, dupilumab was formulated as a lyophilized powder, which was then reconstituted to give a concentration of 50 mg/mL for IV or SC injection; in TDU12265, dupilumab was formulated as a 75 mg/mL or 150 mg/mL solution for SC injection. Dupilumab doses in R668‐AS‐0907 were 1, 3, 8, and 12 mg/kg IV and 150 mg and 300 mg SC; in TDU12265, the doses were 75, 150, 300, and 600 mg SC.
PKM12350, PKM14161, and PKM14271 Studies
The primary objectives of these 3 randomized, parallel‐group, single‐dose studies were to determine and compare the PK profiles of different dupilumab products after a single SC dose. The secondary objectives were to determine and/or compare the safety and tolerability of the dupilumab products. PKM12350 compared the SC PK of dupilumab from drug products derived from 2 cell lines and purification processes (designated C1P2 and C2P1; Table 1). The formulations for single SC injection contained dupilumab at a concentration of 150 mg/mL in vials. PKM14161 and PKM14271 compared the SC PK of dupilumab in 2 different formulations (formulation I in the vial and formulation II in the prefilled syringe) and presentations (vial and syringe). In PKM14161 the dose delivered was 300 mg SC (2 mL of 150 mg/mL dupilumab), and in PKM14271 the dose of each product was 200 mg.
Study R668‐HV‐1108
The primary objectives of this study were to assess the comparative safety and tolerability of dupilumab administered as fast or slow SC injections. In the fast‐injection group, subjects received 300 mg/2 mL over 30 seconds via manual injection, whereas in the slow‐injection group, subjects received the same dose over 10 minutes by syringe pump. The secondary objective of this study was to assess the comparative PK profiles of dupilumab after fast or slow injection.
PK Analysis
Blood samples were collected at various time points over 57 days (64 days in R668‐HV‐1108) to measure concentrations of functional dupilumab in serum. Serum concentrations of functional dupilumab (which represent antibody molecules with at least 1 available binding site, that is, the sum of free dupilumab [2 available binding sites] and dupilumab present in a 1:1 human IL‐4Rα/dupilumab complex) were determined using a validated ELISA method (Regeneron Pharmaceuticals, Inc., Tarrytown, New York). 28 , 32 , 33 The lower limit of quantification was 0.078 mg/L. The following PK parameters were determined using noncompartmental methods: arithmetic mean of peak concentration (Cmax), median time to Cmax (tmax), and arithmetic mean (±SD) of area under the concentration‐time curve to time of last measurable concentration (AUClast, calculated using actual times). Noncompartmental analyses were performed using WinNonLin software (WinNonLin Professional, version 5.2.1; Certara, Princeton, New Jersey) to derive PK parameters. Postdose samples were ascribed a 0 when concentrations were less than the lower limit of quantification.
PD Analysis
Serum samples were assayed (by Quest Diagnostics, Secaucus, New Jersey) using the ImmunoCAP (Phadia, Uppsala, Sweden) platform for the measurement of total IgE and a validated commercial ELISA (human CCL17/TARC Quantikine ELISA Kit #DDN00; R&D Systems Inc, Minneapolis, Minnesota) for the analysis of TARC according to manufacturer's instructions.
Safety and Tolerability
Safety was monitored through the recording of adverse events (AEs), physical examinations, clinical laboratory tests (hematology, biochemistry, and urinalysis), vital sign assessments, local injection‐site symptoms, and standard 12‐lead ECG recordings. Treatment‐emergent AEs (TEAEs) reported by either subjects or investigators were coded according to Medical Dictionary for Regulatory Authorities (MedDRA, version 12 and newer versions).
Injection‐site reactions (pain, erythema, edema) following injections of up to 2 mL of dupilumab were assessed in studies PKM12350, PKM14161, PKM14271, and R668‐HV‐1108. Pain was measured on a 100‐mm visual analog scale (VAS) ranging from 0 (no pain) to 100 (worst possible pain). 34 , 35 Erythema was measured as the maximum diameter in PKM12350 or on a 0‐4 scale (0, none; 4, severe erythema) in R668‐HV‐1108. Edema was measured as the maximum diameter in PKM12350 or on a 0‐4 scale (0, none; 4, severe edema) in R668‐HV‐1108. In the latter study local pruritus and tenderness were assessed on a 100‐mm VAS. In PKM14161 and PKM14271, injection‐site reactions (pain, erythema, edema, induration) were assessed on the 4‐point scale (mild, moderate, severe, potentially life‐threatening) recommended by the US Food and Drug Administration for the evaluation of vaccines. 36
Results
Subject Demographics
The 6 studies enrolled a total of 222 healthy adult subjects, of whom 134 (60.4%) were male; the male proportion varied from 33.3% in study R668‐HV‐1108 to 100% in study TDU12265 (Table S1). Baseline demographics and characteristics of the subjects are shown in Table S1. Within each study, baseline characteristics were comparable across treatment groups. Mean body weight, a factor that could potentially influence exposure to dupilumab, varied among the studies, ranging from slightly less than 65 kg in the Japanese study (TDU12265) to approximately 80 kg in the R668‐AS‐0907 study (enrolling subjects of various racial backgrounds) and in the 3 product comparison studies in primarily white subjects (PKM12350, PKM14161, and PKM14271). In addition, compared with the other studies that enrolled predominantly white subjects, most subjects in the R668‐AS‐0907 study were black (Table S1). Furthermore, there were no critical or major deviations from the study protocols.
PK of Dupilumab
Dupilumab PK After IV Administration
Mean concentration‐time profiles following single IV administration of dupilumab from the ascending‐dose first‐in‐human study (R668‐AS‐0907) are shown in Figure 1A. The concentration‐time profile of dupilumab was characterized by a short distribution phase, a linear β phase (indicating saturation of the target‐mediated pathway), and a terminal nonlinear target‐mediated elimination phase.
Figure 1.
Pharmacokinetics of single dupilumab doses. Mean concentration‐time profiles following single IV and SC administration of dupilumab in the ascending‐dose first‐in‐human study (R668‐AS‐0907). Left inset shows the AUClast/dose ratio following IV dupilumab administration, and right inset after SC dupilumab administration (A). Mean concentration‐time profiles following fast or slow injection of 300 mg SC dupilumab in the R668‐HV‐1108 study (B). Mean concentration‐time profiles following ascending SC dupilumab doses in the TDU12265 study in Japanese healthy subjects (C). The inset shows the AUClast/dose ratio following administration of the SC dupilumab doses. Horizontal dashed lines represent the lower limit of quantification (LLOQ). Mean values below the LLOQ were excluded. AUClast indicates area under the concentration‐time curve to time of last measurable concentration; IV, intravenous; SC, subcutaneous; SD, standard deviation.
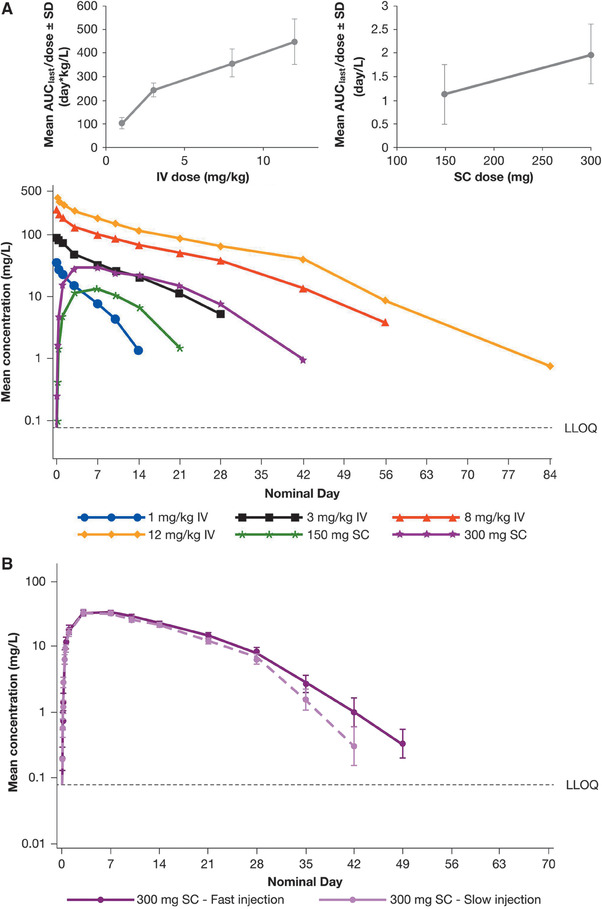
The PK parameters of functional dupilumab following IV infusion of different doses of dupilumab are summarized in Table 2. AUClast increased in a greater than dose‐proportional manner at lower doses, with a trend toward a dose‐proportional increase at higher doses. The mean AUClast/dose ratio increased from 104 day·kg/L at a dose of 1 mg/kg to 244, 358, and 447 day·kg/L at doses of 3, 8, and 12 mg/kg, respectively (Table 2; Figure 1A inset left 1). In contrast, Cmax increased in an approximately dose‐proportional manner, reflecting a similarly dose‐independent initial volume of distribution (Table 2).
Table 2.
Summary of the Pharmacokinetic Parameters of Functional Dupilumab From the Dose‐Escalation Studies
| R668‐AS‐0907 | TDU12265 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IV Administration (C1P1 as Lyophilized Powder) | SC Administration (C1P1 as Lyophilized Powder) | SC Administration (C2P1, Formulation I, Liquid in Vials) | |||||||||
| Parameters | Unit of Measurement | 1 mg/kg (n = 6) | 3 mg/kg (n = 6) | 8 mg/kg (n = 6) | 12 mg/kg (n = 6) | 150 mg (n = 6) | 300 mg (n = 6) | 75 mg (n = 6) | 150 mg (n = 6) | 300 mg (n = 6) | 600 mg (n = 6) |
| Cmax, mean ± SD | mg/L | 34.8 ± 6.6 | 92.0 ± 12.7 | 266 ± 48.6 | 421 ± 65.9 | 13.5 ± 6.9 | 32.4 ± 10.1 | 5.33 ± 1.50 | 10.4 ± 3.0 | 38.3 ± 15.3 | 70.1 ± 24.1 |
| Cmax/dose, mean ± SD | kg/L (IV) or 1/L (SC) | 25.8 ± 7.89 | 31.3 ± 4.29 | 33.7 ± 5.95 | 35.2 ± 4.94 | 0.0899 ± 0.0462 | 0.108 ± 0.0336 | 0.0711 ± 0.0200 | 0.0696 ± 0.0197 | 0.128 ± 0.0512 | 0.117 ± 0.0402 |
| tmax, median (min:max) | day | 0.105 (0.084:0.167) | 0.105 (0.083:0.250) | 0.167 (0.083:1.08) | 0.105 (0.0833:0.417) | 7.04 (3.03:7.20) | 3.01 (2.99:14.0) | 7.01 (3.00:7.03) | 7.01 (3.00:7.03) | 7.01 (6.99:10.00) | 7.00 (3.00:7.02) |
| AUClast, mean ± SD | day·mg/L | 140 ± 15 | 718 ± 82 | 2805 ± 347 | 5334 ± 1159 | 168 ± 95 | 594 ± 190 | 59 ± 21 | 150 ± 41 | 700 ± 234 | 1780 ± 699 |
| AUClast/dose, mean ± SD | day·kg/L (IV) or day/L (SC) | 104 ± 23.6 | 244 ± 27.9 | 358 ± 56.5 | 447 ± 94.5 | 1.12 ± 0.631 | 1.98 ± 0.634 | 0.789 ± 0.278 | 0.999 ± 0.275 | 2.33 ± 0.782 | 2.96 ± 1.17 |
AUClast indicates area under the concentration‐time curve to time of last measurable concentration; Cmax, maximum concentration in serum; C1P1, cell line 1, process 1 drug product; C1P2, cell line 1, process 2 drug product; C2P1, cell line 2, process 1 drug product; IV, intravenous; SC, subcutaneous; SD, standard deviation; tmax, time to Cmax.
Dupilumab PK After SC Administration
Concentration–time curves following administration of single SC dupilumab doses from the single ascending dose first‐in‐human study (R668‐AS‐0907) are shown in Figure 1A. After SC dosing, peak drug concentrations were achieved 3‐7 days after injection. AUClast increased in a greater‐than‐proportional manner with increasing dose (Table 2); thus the mean AUClast/dose ratio was 1.1 at 150 mg and 2.0 day/L at 300 mg (Table 2; Figure 1A inset 2). However, the Cmax/dose ratio following SC dosing was similar for the 150 mg and 300 mg dose groups (mean 0.09 and 0.11 1/L, respectively, Table 2), indicating approximate dose proportionality.
Studies PKM12350, PKM14161, and PKM14271 compared the PK profiles of different dupilumab preparations (formulation and presentation). Functional dupilumab exposure was similar for both the test and reference dupilumab preparations in all 3 studies (Table 3). For example, after a 300 mg SC dose in study PKM12350, mean Cmax was 28.9 mg/L and 27.2 mg/L, median tmax was 7.0 days and 7.0 days and AUClast was 487.5 day·mg/mL and 500 day·mg/mL for the test and reference dupilumab preparations, respectively (Table 3). Studies PKM14161 and PKM14271 investigated the PK of 300 mg and 200 mg dupilumab (the doses used in phase 3 trials with dupilumab), respectively, when reformulated to increase solubility and given SC via prefilled syringes, versus the previous product presented in a vial/syringe. At both dose levels, the PK parameters of the SC dupilumab product were similar for the 2 formulations in both studies. Furthermore, AUClast and Cmax for the 300‐mg dose were similar in the PKM14161 and R668‐AS‐0907 studies despite a change in manufacturing process and excipients in the formulation (Tables 2 and 3).
Table 3.
Summary of the Pharmacokinetic Parameters of Functional Dupilumab Present With Different Dupilumab Preparations and Rates of Injection
| PKM12350 | PKM14161 | PKM14271 | R668‐HV‐1108 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit of Measurement | C2P1 300 mg Test (Formulation I, Ready to Use Glass Vials) (n = 15) | C1P2 300 mg Reference (Ready to Use Glass Vials) (n = 13) | C2P1 300 mg Test (Formulation II in Prefilled Syringe) (n = 19) | C2P1 300 mg Reference (Formulation I in Glass Vial) (n = 19) | C2P1 200 mg Test (Formulation II in Prefilled Syringe) (n = 19) | C2P1 200 mg Reference (Formulation I in Glass Vial) (n = 19) | C1P2 300 mg Fast Injection (Vial for SC Use) (n = 18) | C1P2 300 mg Slow Injection (Vial for Delivery via Syringe Pump) (n = 18) |
| Cmax, mean ± SD | mg/L | 28.9 ± 9.1 | 27.2 ± 10.0 | 34.8 ± 17.5 | 34.3 ± 11.6 | 23.2 ± 7.8 | 22.8 ± 8.9 | 34.4 ± 10.3 | 35.0 ± 14.3 |
| tmax, median (min:max) | day | 7.0 (3.0:10.0) | 7.0 (2.0:10.0) | 7.0 (3.0:14.0) | 7.0 (3.0:10.0) | 3.1 (2.9:10.0) | 3.0 (1.0:7.2) | 5.0 (3.0:20.0) | 6.9 (2.9:7.2) |
| AUClast, mean ± SD | day·mg/L | 487.5 ± 199.6 | 500 ± 179.2 | 587 ± 302 | 575 ± 235 | 339 ± 128 | 323 ± 132 | 630 ± 223 | 530 ± 233 |
AUClast indicates area under the concentration‐time curve to time of last measurable concentration; Cmax, maximum concentration in serum; C1P2, cell line 1, process 2 drug product; C2P1, cell line 2, process 1 drug product; IV, intravenous; SC, subcutaneous; and SD, standard deviation; tmax, time to Cmax.
Dupilumab PK Following Fast or Slow SC Injections
Concentration‐time profiles following dosing with dupilumab 300 mg administered SC at fast (2 mL in 30 seconds) or slow (2 mL in 10 min) injection rates in study R668‐HV‐1108 are shown in Figure 1B. Functional dupilumab exposure was similar for dupilumab administered at both fast and slow injection rates, and the results were consistent with those observed in the studies described above. The mean values with fast versus slow dupilumab injection rates were 34.4 mg/L versus 35.0 mg/L, respectively, for Cmax and 630 day·mg/L versus 530 day·mg/L, respectively, for AUClast. Median tmax was 5.0 days (range 3.0‐20.0 days) after fast injection, and 6.9 days (range 2.9‐7.2 days) after slow injection (Table 3).
Dupilumab PK in Japanese Subjects
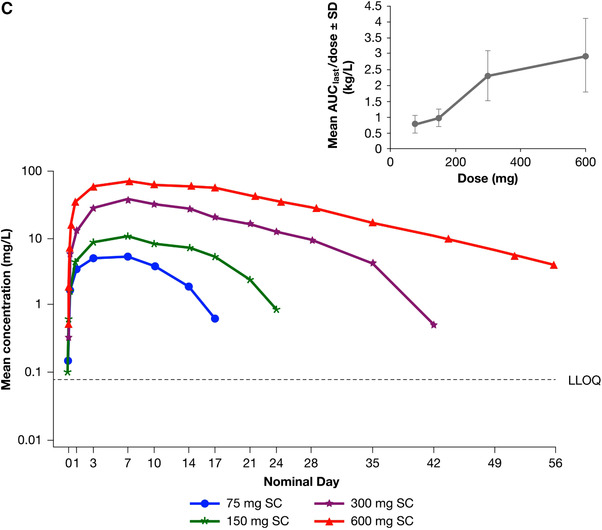
Study TDU12265 investigated the PK of SC dupilumab in Japanese subjects using an empirical power model. The concentration‐time profiles of the dupilumab 150‐ and 300‐mg SC doses (Figure 1C) were similar to those seen in study R668‐AS‐0907 (Figure 1A), with peak drug concentrations being achieved on average at 3‐7 days after dosing. Elimination was dose and concentration dependent. No functional dupilumab was detectable in serum after 2 months.
The PK properties of dupilumab in Japanese subjects following SC administration are consistent with those expected from studies conducted outside of Japan and are summarized in Table 2. Cmax and AUClast increased 3.6‐fold and 4.6‐fold, respectively, with a 2‐fold increase in dose from 150 mg to 300 mg, whereas for an 8‐fold increase in dose from 75 mg to 600 mg, Cmax and AUClast increased 13.1‐fold and 30.4‐fold, respectively (Table 2, Figure 1C inset).
Influence of Body Weight and Race/Ethnicity on the PK of Dupilumab
Body weight and race/ethnicity are factors that could potentially influence exposure to dupilumab. Among the healthy subjects receiving dupilumab, body weights ranged from 52.1 kg to 78.3 kg in the Japanese study (TDU12265) and from 58.0 kg to 95.1 kg in the R668‐AS‐0907 study, which enrolled subjects with various racial backgrounds. A plot of dupilumab exposure versus baseline body weight at 300 mg SC based on data from the 2 phase 1 studies shows a trend of decreasing exposure with increasing body weight (Figure 2A). However, when stratified between Japanese and non‐Japanese subjects, the exposure‐to–body weight relationship falls within the same continuum as that observed for non‐Japanese subjects. Plots of the mean concentration‐time profiles of functional dupilumab following administration of 150‐mg and 300‐mg SC doses also show similar mean exposure profiles in Japanese versus non‐Japanese subjects in the R668‐AS‐0907 and TDU12265 studies (Figure 2B).
Figure 2.
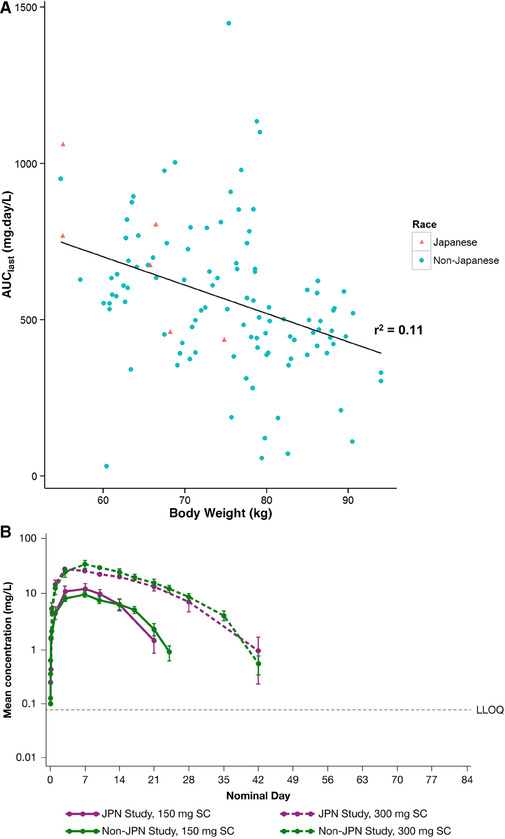
Influence of body weight and ethnicity on pharmacokinetics of dupilumab: AUClast vs baseline body weight after administration of 300 mg SC dupilumab (A), and concentration‐time profile of functional dupilumab after administration of 150 mg or 300 mg SC dupilumaba (B) in Japanese and non‐Japanese subjects in the TDU12265 and R668‐AS‐0907 studies. Horizontal dashed line represents the lower limit of quantification (LLOQ). Mean values below the lower limit of quantitation were excluded. AUClast indicates area under the concentration‐time curve to time of last measurable concentration; SC, subcutaneous.
Standard error around the mean is presented.
Dupilumab PD
Total IgE Concentrations in Serum
Concentrations of total IgE in serum were measured in studies R668‐AS‐0907 and TDU12265. In both studies baseline total IgE concentrations varied markedly between subjects and treatment groups (Table S1). In R668‐AS‐0907, median total IgE levels declined significantly in a dose‐dependent manner following dupilumab administration (nominal P = .005 for absolute change from baseline, nominal P = .001 for percentage change from baseline, Figure 3A). With the 2 highest IV doses, total IgE continued to decline after the measurement at the protocol‐defined end‐of‐treatment visit, day 29. In TDU12265, single SC doses of 75 mg or 150 mg had no effect on the percentage change in total IgE from baseline, although there was a trend toward decreased IgE levels following doses of 300 mg and 600 mg (Figure 3B).
Figure 3.
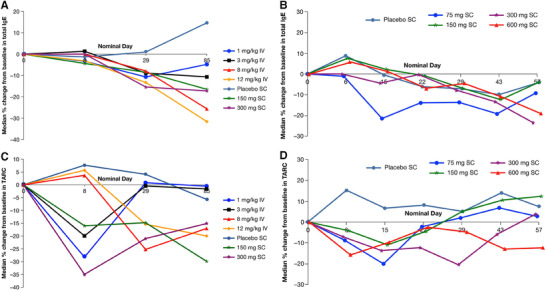
Total IgE and TARC levels in serum following dupilumab administration. Median percentage changes from baseline in total IgE in R668‐AS‐0907 (A) and TDU12265 (B) and median percentage changes from baseline in TARC in R668‐AS‐0907 (C) and TDU12265 (D). IgE indicates immunoglobulin E; IV, intravenously; SC, subcutaneous; TARC, thymus‐ and activation‐regulated chemokine.
Concentrations of TARC in Serum
Concentrations of TARC in serum were measured in studies R668‐AS‐0907 and TDU12665. In both studies decreases in TARC concentrations were observed following administration of dupilumab. In R668‐AS‐0907 both SC doses resulted in a significant reduction in median TARC concentrations, compared with placebo (nominal P = .044 for 150 mg and nominal P = .047 for 300 mg). There was a marked reduction in TARC with 300 mg dupilumab at day 8 (nominal P = .052) and day 29 (nominal P = .051) (Figure 3C). TARC modulation was significant, with 8 mg/kg at day 29 (nominal P = .029). In TDU12665 single SC doses between 75 mg and 600 mg were associated with dose‐dependent reductions in median TARC concentrations compared with placebo (Figure 3D).
Safety and Tolerability
In general, dupilumab was well tolerated and had a favorable safety profile across the wide range of doses administered in the studies with healthy subjects, and the majority of TEAEs were mild or moderate in severity. Overall, the most commonly reported TEAEs (reported as MedDRA preferred terms) were events of injection‐site reactions occurring in 0 to 66.7% of subjects receiving dupilumab across the studies and groups, as well as headache (5.3% to 33.3%) (Table 4 and Table S3). In the R668‐HV‐1108 study the incidence of injection‐site reactions was greater with slow injections than with fast injections: injection‐site induration in 66.7% versus 38.9% of subjects and erythema in 55.6% versus 16.7% of subjects, respectively (Table 4 and Table S3). VAS pain scores were low overall (a 20‐mm shift on a 100‐mm scale being clinically relevant) and generally similar between the 2 groups. Additional AEs reported in the studies are listed in Table S3.
Table 4.
Injection‐Site Reactions Reported in Phase 1 Studies
| HV1108 | PKM12350 | PKM14271 | ||||
|---|---|---|---|---|---|---|
| Eventsa | 300 mg SC Fast Injection (n = 18) | 300 mg SC Slow Injection (n = 18) | 300 mg SC C2P1 (n = 15) | 300 mg SC C1P2 (n = 15) | 200 mg SC C2P1 (Formulation I) (n = 19) | 200 mg SC C2P1 (Formulation II) (n = 19) |
| General disorders and administration site conditionsb | 8 (44.4) | 14 (77.8) | 8 (53.3) | 5 (33.3) | 3 (15.8) | 9 (47.4) |
| Injection‐site reactionc | … | … | … | … | 3 (15.8) | 8 (42.1) |
| Injection‐site erythemac | 3 (16.7) | 10 (55.6) | 4 (26.7) | 4 (26.7) | … | … |
| Injection‐site indurationc | 7 (38.9) | 12 (66.7) | … | … | … | … |
Data reported as proportion of patients, n (%). C1P2 indicates cell line 1, process 2 drug product; C2P1, cell line 2, process 1 drug product; MedDRA, Medical Dictionary for Regulatory Activities; SC, subcutaneous; TEAE, treatment‐emergent adverse event.
TEAEs reported occurred in ≥25% of patients in any treatment group in a study according to preferred term. TEAEs are summarized as MedDRA system organ class and preferred term.
System organ class.
Preferred term.
Across all studies, there were no consistent clinically relevant changes in physical examination findings, vital signs, or clinical laboratory investigations.
Discussion
The 6 phase 1 studies were performed to characterize the PK, PD, safety, and tolerability of dupilumab in healthy adult subjects after single‐dose IV and SC regimens and to assess the effects of manufacturing and product changes on the PK of dupilumab. Total IgE and TARC in serum, known to be dependent on IL‐4Rα–mediated signaling and to be associated with type 2 inflammation, 37 , 38 were measured as PD markers. We also preliminarily assessed the influence of body weight and race/ethnicity on drug exposure.
The first‐in‐human single‐dose and multiple‐ascending‐dose study R668‐AS‐0907 showed that the concentration‐time profile of functional dupilumab is characterized by a short distribution phase followed by a linear β phase and a terminal nonlinear elimination phase. The slope of the elimination phase of the dupilumab concentration‐time curves was steeper at the lower doses. Furthermore, as drug concentrations declined, the slope of the elimination curve became steeper; these changes in the slope indicated a continuous increase in dupilumab clearance as drug concentrations declined. This was reflected in the mean AUClast, which increased in a greater than dose‐proportional manner with increasing dose, indicating that the clearance is intrinsically greater at lower plasma concentrations of dupilumab. Notably, in the higher dose range, greater than dose‐proportional increases in AUClast were less evident, and the kinetics of dupilumab appeared to approach linearity. All of these observations are consistent with a saturable target‐mediated clearance pathway, which markedly affects clearance at lower plasma concentrations but not at concentrations sufficiently high to saturate that pathway. 39 It should also be noted that, based on the AUC, the PK of dupilumab in monkeys, unlike that in humans, is approximately dose proportional (data on file, at Regeneron Pharmaceuticals, Inc., not shown). Because dupilumab does not bind monkey IL‐R4α (data on file, Regeneron Pharmaceuticals, Inc.), dupilumab elimination in monkeys is not subject to target‐mediated clearance, which is a reasonable explanation for species differences in PK; this provides additional evidence that the nonlinear PK profiles observed in the healthy subjects (but not in monkeys) are a result of binding to IL‐4Rα.
With terminal target‐mediated clearance of dupilumab dominated by nonlinearity (ie, clearance being highly concentration dependent), estimation of the terminal elimination rate constant (and thus the terminal elimination half‐life) is dependent on the concentration range studied and is therefore not a constant value—except at concentrations high enough to saturate the IL‐4Rα, or extremely low concentrations at which there is no target binding. 29 Instead, such determination requires the application of a PK model that describes the full concentration‐time profile over a range of doses and should be used to predict these parameters of interest. 40 Overall, the PK profile for dupilumab resembles that of other therapeutic monoclonal antibodies that display nonlinear target‐mediated clearance, such as alirocumab 41 or sarilumab. 42 Our hypothesis before clinical studies in patients with moderate to severe AD 15 and patients with moderate to severe asthma 22 was that maximum efficacy would be observed at doses that achieved dupilumab concentrations in serum sufficient to achieve saturation of the IL‐4Rα receptor, as evidenced by linear/dose‐proportional PK profiles. In both diseases maximum efficacy was observed at doses that yielded dose‐proportional PK profiles, 28 , 43 consistent with this hypothesis.
Furthermore, the PK of SC‐administered dupilumab remained uniformly similar across several manufacturing‐process and product changes as the drug product evolved. This suggested that the dupilumab concentration in the product, injection volume, and formulation excipients had little or no effect on absorption.
Consistent with the known effect of body weight as a covariate influencing the exposure of monoclonal antibodies, the data presented here suggest a diminished exposure with increasing body weight on dupilumab PK. However, the effect of body weight on exposure was modest within the range of adult body weights in these studies. The enrolled Japanese subjects had a lower body weight distribution and a lower mean body weight of 10 kg to 20 kg compared with non‐Japanese subjects in the data set described here, consistent with body weight differences seen in population studies. 44 In consideration of possible PK differences based on ethnicity, there were no obvious differences in mean dupilumab concentrations over time when stratified by Japanese versus non‐Japanese ethnicity. The distribution of individual body weights versus exposure provides evidence that Japanese and non‐Japanese subjects of similar body weight have similar exposure and PK to dupilumab; thus, once corrected for body weight, no ethnicity‐related differences can be observed in the PK of dupilumab.
Dupilumab was generally well tolerated with an acceptable safety profile in healthy subjects; most common TEAEs across the studies were events of injection‐site reactions and headache. In the R668‐HV‐1108 study the slow injection (10 minutes) versus fast injection (30 seconds) of dupilumab was associated with higher rates of events of injection‐site reactions such as induration or erythema; however, there were no significant differences in pain scores (measured on a 100‐mm VAS) between the groups. This is consistent with findings reported by other studies, in which no significant or clinically meaningful differences in perceived pain of fast or slow SC injections of highly viscous solutions were observed. 45 , 46 This suggests that there is no advantage in using slow injections to avoid potential discomfort associated with SC administration.
Antagonism of IL‐4 and IL‐13 activity through the IL‐4Rα pathway with dupilumab resulted in decreases in total IgE and TARC in serum in healthy subjects. These data suggest that constitutive levels of these biomarkers of type 2 inflammation are at least partially dependent on IL‐4Rα signaling, even in apparently healthy populations. However, subjects were not screened extensively for allergen sensitization or other type 2‐mediated inflammation before enrollment. A standard reference range in healthy populations or a clear clinical cutoff for TARC has not yet been well established. In the R668‐AS‐0907 and TDU12265 studies, mean concentrations ranged from 206 pg/mL to 638 pg/mL for TARC and 177 kU/L to 230 kU/L for IgE, respectively.
Limitations of these studies include the fact that comparisons between the studies involved small study sizes and were not controlled for overall differences in investigation sites or subject demographics. In addition, the observed changes in biomarkers may not be entirely generalizable to patients with type 2 immune disorders because they were conducted in healthy subjects. However, the presence of unreported allergy in the healthy subjects cannot be excluded, as a mild allergic condition would not have precluded subjects from participating in these trials. Furthermore, the modest decreases in TARC and total IgE following dupilumab treatment in healthy subjects do not reflect the more robust suppression of these biomarkers observed in patients in asthma, 24 nasal polyposis, 25 , 26 and AD 14 , 47 trials, which have substantially higher baseline concentrations of these markers (7722‐11 360 pg/mL serum TARC; 3868‐5641 IU/mL serum total IgE).
Conclusions
Results from 6 phase 1 studies in normal, healthy, human subjects showed that dupilumab was generally well tolerated with an acceptable safety profile across a wide range of doses administered via the SC or IV route. Dupilumab exhibited target‐mediated PK consisting of parallel linear and nonlinear elimination with a target‐mediated phase highly dominated by nonlinearity. There were no apparent differences in PK and safety/tolerability profiles between Japanese and non‐Japanese populations. Dupilumab reduced concentrations of total IgE and TARC in serum, providing a proof‐of‐mechanism of its action in early clinical trials. Together, these results supported the continued development and use of dupilumab for the treatment of type 2 immune diseases such as asthma, AD, chronic sinusitis with nasal polyps, and eosinophilic esophagitis.
Funding
Research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing/editorial assistance were provided by Mihai Surducan, PhD and Jamie Lim, PhD of Excerpta Medica and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflicts of Interest
Z.L., M.L., and B.N.S. were employees of Sanofi at the time of the study and held stock and/or stock options in the company. M.K., Y.T., J.E.M., Y.L., Q.L., and C.O‐R. are employees of Sanofi and may hold stock and/or stock options in the company. A.R., J.D.H., J.D.D., A.T.D., P.K., and M.A. are employees and shareholders of Regeneron Pharmaceuticals, Inc. S.H. has nothing to disclose.
Supporting information
Supporting Information.
Acknowledgments
Research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers were NCT01015027 (R668‐AS‐0907), NCT01537653 (TDU12265), NCT01537640 (PKM12350), PKM14161 (not registered with clinicaltrials.gov), PKM14271 (not registered with clinicaltrials.gov), and NCT01484600 (R668‐HV‐1108). Medical writing/editorial assistance were provided by Mihai Surducan, PhD and Jamie Lim, PhD of Excerpta Medica and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
John Davis is a Fellow of the American College of Clinical Pharmacology.
References
- 1. Gandhi NA, Bennett BL, Graham NM, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35‐50. [DOI] [PubMed] [Google Scholar]
- 2. Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 4. Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483‐1494. [DOI] [PubMed] [Google Scholar]
- 5. Passali D, Cingi C, Cambi J, et al. A survey on chronic rhinosinusitis: opinions from experts of 50 countries. Eur Arch Otorhinolaryngol. 2016;273(8):2097‐2109. [DOI] [PubMed] [Google Scholar]
- 6. Lucendo AJ, Molina‐Infante J, Á Arias, et al. Guidelines on eosinophilic esophagitis: evidence‐based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26‐30. [DOI] [PubMed] [Google Scholar]
- 8. Simpson, EL , Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491‐498. [DOI] [PubMed] [Google Scholar]
- 9. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012;23(Suppl 23):1‐298. [PubMed] [Google Scholar]
- 10. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. J Allergy Clin Immunol. 2013;132(5):1132‐1138. [DOI] [PubMed] [Google Scholar]
- 11. Gandhi N, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425‐437. [DOI] [PubMed] [Google Scholar]
- 12. MacDonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy AJ, MacDonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153‐5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate‐to‐severe atopic dermatitis. N Engl J Med. 2014;371(2):130‐139. [DOI] [PubMed] [Google Scholar]
- 15. Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo‐controlled, dose‐ranging phase 2b trial. Lancet. 2016;387(10013):40‐52. [DOI] [PubMed] [Google Scholar]
- 16. Simpson EL, Bieber T, Guttman‐Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335‐2348. [DOI] [PubMed] [Google Scholar]
- 17. Blauvelt A, de Bruin‐Weller M, Gooderham M, et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet. 2017;389(10086):2287‐2303. [DOI] [PubMed] [Google Scholar]
- 18. de Bruin‐Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178(5):1083‐1101. [DOI] [PubMed] [Google Scholar]
- 19. Cork MJ, Thaçi D, Eichenfield L, et al. Dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: results from a phase IIa open‐label and subsequent phase III open‐label extension trial. Br J Dermatol. 2020;182(1):85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44‐56. 10.1001/jamadermatol.2019.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455‐2466. [DOI] [PubMed] [Google Scholar]
- 22. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet. 2016;388(10039):31‐44. [DOI] [PubMed] [Google Scholar]
- 23. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378(26):2475‐2485. [DOI] [PubMed] [Google Scholar]
- 24. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 25. Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469‐479. [DOI] [PubMed] [Google Scholar]
- 26. Bachert C, Han JK, Desrosiers M, et al. Dupilumab efficacy and safety in severe chronic rhinosinusitis with nasal polyps in the randomised phase 3 trials LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52. Lancet. 2019;394(10209):1638‐1650. [DOI] [PubMed] [Google Scholar]
- 27. Hirano I, Dellon ES, Hamilton JD, et al. Dupilumab efficacy in adults with active eosinophilic esophagitis: a randomized placebo‐controlled phase 2 trial. Gastroenterology. 2020;158(1):111‐122.e10. [DOI] [PubMed] [Google Scholar]
- 28. Kovalenko P, DiCioccio AT, Davis JD, et al. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL‐4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharmacometrics Syst Pharmacol. 2016;5(11):617‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dua P, Hawkins E, van der Graaf PH. A tutorial on target‐mediated drug disposition (TMDD) models. CPT Pharmacometrics Syst Pharmacol. 2015;4(6):324‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuber CE, Galizzi JP, Harada N, Durand I, Banchereau J. Interleukin‐4 receptors on human blood mononuclear cells. Cell Immunol. 1990;129(2):329‐340. [DOI] [PubMed] [Google Scholar]
- 31. D'Ippolito D, Dupilumab Pisano M. (Dupixent): an interleukin‐4 receptor antagonist for atopic dermatitis. P T. 2018;43(9):532‐535. [PMC free article] [PubMed] [Google Scholar]
- 32. Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease‐mediated drug‐drug interaction in patients with moderate‐to‐severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104(6):1146‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Food and Drug Administration (US FDA). Center for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review, dupilumab biologics license application number 761055Orig1s000. July 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761055Orig1s000ClinPharmR.pdf. Accessed January 23, 2020.
- 34. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1‐2):95‐97. [DOI] [PubMed] [Google Scholar]
- 35. Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227‐236. [DOI] [PubMed] [Google Scholar]
- 36. Food and Drug Administration (US FDA) . FDA guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. September 2007. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf. Accessed January 23, 2020.
- 37. Liddiard K, Welch JS, Lozach J, Heinz S, Glass CK, Greaves DR. Interleukin‐4 induction of the CC chemokine TARC (CCL17) in murine macrophages is mediated by multiple STAT6 sites in the TARC gene promoter. BMC Mol Biol. 2006;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He JS, Narayanan S, Subramaniam S, et al. Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Curr Top Microbiol Immunol. 2015;388:1‐19. [DOI] [PubMed] [Google Scholar]
- 39. Gabrielsson J, Peletier LA. Pharmacokinetic steady‐states highlight interesting target‐mediated disposition properties. AAPS J. 2017;19(3):772‐786. [DOI] [PubMed] [Google Scholar]
- 40. Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target‐mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507‐532. [DOI] [PubMed] [Google Scholar]
- 41. Martinez JM, Brunet A, Hurbin F, DiCioccio AT, Rauch C, Fabre D. Population pharmacokinetic analysis of alirocumab in healthy volunteers or hypercholesterolemic subjects using a Michaelis‐Menten approximation of a target‐mediated drug disposition model‐support for a biologics license application submission: Part I. Clin Pharmacokinet. 2019;58(1):101‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu C, Su Y, Paccaly A, Kanamaluru V. Population pharmacokinetics of sarilumab in patients with rheumatoid arthritis. Clin Pharmacokinet. 2019;58(11):1455‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meng Z, Ma L, Sheng T, et al. Dupilumab dose selection for a phase 3 study in asthma patients: pharmacokinetic/pharmacodynamic (PK/PD) modelling and clinical trial simulation. In PAGE . Abstracts of the Annual Meeting of the Population Approach Group in Europe. 2017;26:7161 www.page-meeting.org/?abstract=7161. Accessed January 23, 2020. [Google Scholar]
- 44. Walpole SC, Prieto‐Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bertau C, Filipe‐Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dias C, Abosaleem B, Crispino C, Gao B, Shaywitz A. Tolerability of high‐volume subcutaneous injections of a viscous placebo buffer: a randomized, crossover study in healthy subjects. AAPS PharmSciTech. 2015;16(5):1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guttman‐Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155‐172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


