Abstract
Objective
The RNS System is a direct brain‐responsive neurostimulation system that is US Food and Drug Administration–approved for adults with medically intractable focal onset seizures based on safety and effectiveness data from controlled clinical trials. The purpose of this study was to retrospectively evaluate the real‐world safety and effectiveness of the RNS System.
Methods
Eight comprehensive epilepsy centers conducted a chart review of patients treated with the RNS System for at least 1 year, in accordance with the indication for use. Data included device‐related serious adverse events and the median percent change in disabling seizure frequency from baseline at years 1, 2, and 3 of treatment and at the most recent follow‐up.
Results
One hundred fifty patients met the criteria for analysis. The median reduction in seizures was 67% (interquartile range [IQR] = 33%‐93%, n = 149) at 1 year, 75% (IQR = 50%‐94%, n = 93) at 2 years, 82% (IQR = 50%‐96%, n = 38) at ≥3 years, and 74% (IQR = 50%‐96%, n = 150) at last follow‐up (mean = 2.3 years). Thirty‐five percent of patients had a ≥90% seizure frequency reduction, and 18% of patients reported being clinically seizure‐free at last follow‐up. Seizure frequency reductions were similar regardless of patient age, age at epilepsy onset, duration of epilepsy, seizure onset in mesial temporal or neocortical foci, magnetic resonance imaging findings, prior intracranial monitoring, prior epilepsy surgery, or prior vagus nerve stimulation treatment. The infection rate per procedure was 2.9% (6/150 patients); five of the six patients had an implant site infection, and one had osteomyelitis. Lead revisions were required in 2.7% (4/150), and 2.0% (3/150) of patients had a subdural hemorrhage, none of which had long‐lasting neurological consequences.
Significance
In this real‐world experience, safety was similar and clinical seizure outcomes exceeded those of the prospective clinical trials, corroborating effectiveness of this therapy and suggesting that clinical experience has informed more effective programming.
Keywords: brain‐responsive neurostimulation, drug‐resistant, medically intractable epilepsy, RNS System
Key Points.
Retrospective chart review was made of 150 patients treated with the RNS System at eight epilepsy centers
Safety experience with the RNS System in this real‐world study was comparable to clinical trials
Median seizure frequency reductions were 67% at 1 year, 75% at 2 years, and 82% at ≥3 years
Effectiveness outcomes with the RNS System in this real‐world study exceeded those in clinical trials
1. INTRODUCTION
Neuromodulation is an important treatment option for patients with medically intractable focal onset seizures who are not optimal candidates for surgical resection or ablation due to an unfavorable risk‐benefit profile or patient preference. The RNS System (NeuroPace) is the only direct brain‐responsive neurostimulator for the treatment of adults with medically intractable disabling focal onset seizures with one or two seizure foci. Safety and effectiveness were demonstrated in a randomized controlled clinical trial 1 and in a prospective open‐label clinical trial over 9 years of treatment. Median percent seizure frequency reductions were 44% at 1 year, 53% at 2 years, 2 and 75% at 9 years of treatment. 3 Outcomes with any epilepsy therapy outside of clinical trials may differ from the preapproval experience. 4 , 5 , 6 Moreover, experience in the RNS System clinical trials 1 , 2 , 3 has greatly informed real‐world use. The purpose of this multicenter, retrospective study was to describe the safety and efficacy of direct brain‐responsive neurostimulation for adults with medically intractable focal onset seizures in the real‐world setting.
2. MATERIALS AND METHODS
Eight comprehensive epilepsy centers performed a retrospective chart review of their patients treated with the RNS System between 2013 and 2018. All study protocols were approved by the institutional review boards (IRBs) of participating centers; all IRBs waived the requirement for obtaining informed consent. All data collected before August 15, 2018 were included. Patients included in the analysis were all treated according to the US Food and Drug Administration (FDA)‐approved indication.
The RNS System includes a cranially implanted neurostimulator that is connected to one or two depth or cortical strip leads, each containing four electrode contacts. The leads are placed at the seizure focus or foci. The neurostimulator continuously monitors intracranial electroencephalogram (ICEEG) and delivers stimulation in response to detections of patient‐specific abnormal patterns. Continuous counts of detections, with time and location, and brief ICEEG recordings are stored for physician review. Stimulation can be delivered to eloquent areas of the brain without acute or chronic stimulation‐related side effects. 7 , 8 , 9 Detection and stimulation settings are adjusted intermittently to optimize treatment. A suggested protocol for initial stimulation programming and subsequent adjustments based on clinical trial experience is provided to all centers by the device manufacturer.
Patient demographics, epilepsy history, seizure characteristics, presurgical localization results, RNS System lead placement, and postimplant safety and efficacy outcomes were collected retrospectively from medical records.
Surgical procedures were categorized into four groups: (1) RNS System neurostimulator and leads implanted as the sole procedure, (2) neurostimulator and leads implanted concurrently with the removal of intracranial EEG diagnostic monitoring (ICM) electrodes, (3) neurostimulator and leads implanted concurrently with resective surgery, and (4) neurostimulator and leads implanted concurrently with removal of ICM electrodes and a resective procedure.
The primary outcomes were safety, defined as serious adverse events, and efficacy, defined as the median percent change in clinically reported disabling seizures (focal aware motor, focal unaware, and generalized seizures) during RNS System treatment at 1 year, 2 years, ≥3 years, and the most recent follow‐up period. Seizure frequencies for the preceding 3 months at each timepoint were compared to a 3 months pretreatment baseline. The responder rate (percent of patients with ≥50% reduction in seizures) was calculated at the same timepoints.
A secondary outcome measure was overall impression of response to treatment using the Clinical Global Impressions Scale (CGIS), a clinician‐determined summary measure of the patient's ability to function 10 that has been used in other epilepsy therapy trials. 11 Antiepileptic drugs (AEDs) were also documented at each time point.
Statistical differences in outcome between the following groups were calculated using the Kruskal‐Wallis test: patients with mesial temporal or neocortical seizure foci; and patients with or without magnetic resonance imaging (MRI) abnormalities, prior intracranial monitoring, prior epilepsy surgery, or prior vagus nerve stimulation (VNS) treatment. Relationships between clinical outcomes and patient age, age at epilepsy onset, or duration of epilepsy were evaluated using linear regression. The relationship between clinical outcomes and CGIS scores was also assessed. Finally, the median percent reduction in clinical seizure frequency was assessed for those who added, discontinued, or both added and discontinued AEDs. These were compared to the median percent reduction for patients who had no changes in AEDs using a Mann‐Whitney U test. For all analyses, α was set to .05.
3. RESULTS
One hundred fifty patients were treated with the RNS System and followed for a minimum of 1 year, with mean follow‐up of 2.3 years. Patient demographics are provided in Table 1. The mean age at time of implant was 39 years, and the mean duration of epilepsy was 20 years. There was a wide range in baseline seizure frequency, with a median of 7.7 disabling seizures per month (mean = 52, range = 0.1‐3000 per month, SD ± 257). Patients had a variety of epilepsy etiologies, with the most common being “unknown.” MRI of the brain was abnormal for 60% of patients; the type of MRI abnormality was not captured in this study.
TABLE 1.
Patient demographics
| Total N | 150 |
| Gender, female, n (%) | 77 (51%) |
| Mean age, y (range) | 39 (18‐69) |
| Mean duration of epilepsy, y (range) | 20 (2‐60) |
| Median baseline disabling seizures per mo, n (mean; range; SD) | 7.7 (52; 0.1‐3000; ±257) |
| Etiology, n (%) a | |
| Unknown | 86 (52.1%) |
| Structural | 31 (18.8%) |
| Infection | 13 (7.9%) |
| TBI | 8 (4.8%) |
| Genetic | 6 (3.6%) |
| Head trauma | 4 (2.4%) |
| Hypoxic injury | 4 (2.4%) |
| Stroke | 5 (3.0%) |
| Pregnancy‐related | 2 (1.2%) |
| Febrile convulsions | 2 (1.2%) |
| Metabolic | 2 (1.2%) |
| Immune | 2 (1.2%) |
| MRI of the brain, n (%) | |
| Abnormal | 90 (60%) |
| Normal | 60 (40%) |
| Prior intracranial monitoring, n (%) | 123 (82%) |
| Prior VNS, n (%) | 48 (32%) |
| Prior epilepsy surgery, n (%) | 49 (33%) |
Abbreviations: MRI, magnetic resonance imaging; SD, standard deviation; TBI, traumatic brain injury; VNS, vagus nerve stimulation.
Some patients had more than one etiology.
Sixty‐seven percent of patients had two leads implanted. However, additional leads could be implanted that were not connected at the time of the initial procedure; the mean number of leads per patient was 2.6 (range = 2‐5). There were seven instances (4.7%) in which there was a procedure to connect a previously unconnected lead. Of the total of 371 leads implanted, 54% were cortical strip leads and 46% were depth leads. Common lead locations and types of leads with respect to the region of seizure onset are provided in Table 2.
TABLE 2.
RNS System lead locations and lead types
| Lead location | Patients, % | Leads, mean n | Lead type | |
|---|---|---|---|---|
| Depth leads, % | Cortical strip leads, % | |||
| Mesial temporal | 44 | 2.3 | 76 | 24 |
| Unilateral | 29 | 2.4 | 47 | 53 |
| Bilateral | 71 | 2.2 | 89 | 11 |
| Neocortical | 41 | 2.7 | 78 | 22 |
| Frontal | 40 | 2.9 | 17 | 83 |
| Temporal | 31 | 2.3 | 10 | 90 |
| Parietal | 12 | 2.9 | 30 | 70 |
| Occipital | 14 | 3.0 | 0 | 100 |
| Insula | 10 | 3.0 | 72 | 28 |
| Multilobar | 9 | 2.3 | 78 | 22 |
| Mesial temporal + neocortical | 6 | 2.7 | 42 | 58 |
Sixty‐five percent (n = 98) of patients had the neurostimulator and leads implanted without ICM immediately prior. However, 49 patients (33%) had ICM electrodes explanted at the same time as the neurostimulator and leads were placed. Of these 49 patients, three patients (6%) had ICM depth electrodes only, 20 had ICM grids/strips (41%), and 26 patients (53%) had a combination of grids, strips, and depth electrodes for ICM. The mean time for ICM prior to placement of the neurostimulator and leads was 11 days (range = 3‐26 days). Ten patients (7%) had resections concurrent with the neurostimulator and lead placement; seven of these 10 also had ICM electrode removal at the same time.
Once brain‐responsive neurostimulation was enabled, clinic visits occurred about every 3‐4 months. Stimulation was enabled postimplantation (mean = 41 days, median = 27 days, range = 2‐245 days), typically after the first seizure was recorded (Figure S1). Neurostimulator settings were programmed by the physician and generally followed a manufacturer‐recommended therapy protocol, with initial stimulation settings of 200 Hz, 160‐microsecond pulse width, 100‐millisecond burst duration, and current sufficient to achieve a charge density of 0.5 µC/cm2. The total duration of stimulation was a median of 4.5 min/d (interquartile range [IQR] = 1.4‐9.3 min/d). Depending on the clinical response, charge density was incrementally increased by 0.5 µC/cm2 at each visit. Changes in charge density and current amplitude over time are shown in Figure 1 and are compared to similar data for patients in the treatment group of the randomized controlled Pivotal study. 1
FIGURE 1.
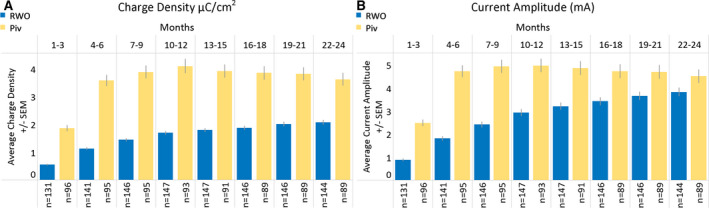
Stimulation charge density and current amplitude over time in real‐world and clinical trial populations of patients treated with the RNS System. Changes in charge density (A) and current amplitude (B) are shown as programmed for patients in this real‐world outcome (RWO) study and in patients randomized to the active stimulation arm of the randomized controlled Pivotal (Piv) trial. Time periods represent months since initial placement of the brain‐responsive neurostimulator and leads. The mean values are represented with standard error of the mean (SEM; light gray bars). Although charge density and current amplitude increase over time for both cohorts, the overall values are lower for the patients treated in the RWO study and the initial rate of increase is lower than for the patients in the Piv trial
3.1. Efficacy outcomes
The median seizure frequency reduction was 67% (IQR = 33%‐93%, n = 149) at 1‐year follow‐up, 75% (IQR = 50%‐94%, n = 93) at 2‐year follow‐up, and 82% at 3 or more years of follow‐up (IQR = 50%‐96%, n = 38; Figure 2). At the most recent follow‐up (mean = 2.3 years), the median percent seizure frequency reduction was 74% (IQR = 50%‐96%, n = 150); 35% of patients had ≥90% seizure reduction, and 18% of patients reported being clinically seizure‐free at the most recent follow‐up. Responder rates (≥50% reduction in seizure frequency) were 66% at 1 year, 77% at 2 years, and 84% at 3 or more years. Seizure frequency reductions at 1 year were similar regardless of patient age, age at epilepsy onset, or duration of epilepsy (P > .05), location of seizure foci (mesial temporal or neocortical; P = .13), brain MRI abnormalities (P = .60), prior intracranial monitoring (P = .88), prior epilepsy surgery (P = .20), or prior VNS treatment (P = .62). Results were similar when patients who had a resection concurrent with placement of the RNS System neurostimulator and leads were excluded; median percent seizure reductions were 63% at 1 year (mean = 45%, IQR = 26%‐93%), 74% at 2 years (mean = 63%, IQR = 50%‐94%), and 81% at 3 or more years of follow‐up (mean = 74%, IQR = 64%‐98%). Reductions in seizure frequency at 1 year of follow‐up were not significantly related to postimplantation days to initiate therapy (r = −.14, P = .19; Figure S1). There was no statistically significant difference in any of the outcome results between the eight centers (P > .05). In a post hoc comparison of 2‐year responder rates from this study and the open‐label period of the Pivotal trial, seizure outcomes in the real‐world cohort were significantly better (chi‐squared proportion test, P < .05).
FIGURE 2.
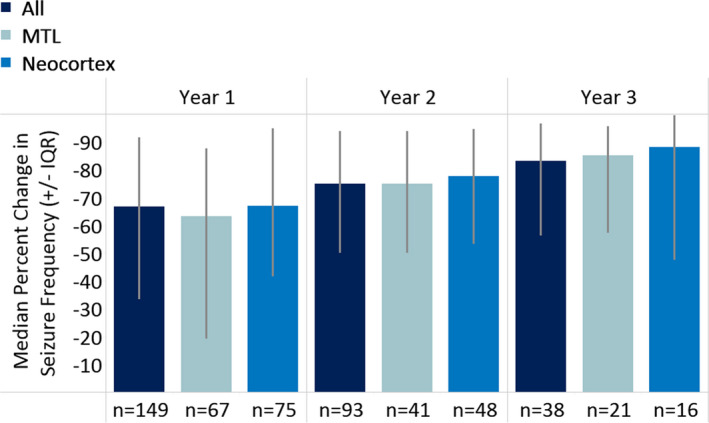
Seizure frequency outcomes with RNS System treatment. Outcomes are provided for all patients combined and for patients with seizures of mesial temporal lobe (MTL) and of neocortical onsets. The median percent change in seizure frequency at 1, 2, and 3 or more years is presented with the interquartile ranges (IQRs; light gray lines) for each year of treatment compared to a pretreatment baseline
3.2. Safety outcomes
Seventeen of the 150 patients (11.3%) had a total of 22 device‐related serious adverse events (SAEs), as summarized in Table 3.
TABLE 3.
Device‐related SAEs
| SAE | Patients, n (%) a |
|---|---|
| Infection | 6 (4.0%) |
| Implant site soft tissue | 5 (3.3%) |
| Osteomyelitis | 1 (0.7%) |
| Lead revision | 4 (2.7%) |
| Subdural hematoma | 3 (2.0%) |
| Scalp erosion | 2 (1.3%) |
| Worsening of seizures | 2 (1.3%) |
| Pulmonary embolism | 1 (0.7%) |
| Third nerve palsy | 1 (0.7%) |
| Hemiparesis | 1 (0.7%) |
| Incision site pain | 1 (0.7%) |
| Pseudomeningocele | 1 (0.7%) |
Abbreviation: SAE, serious adverse event.
Some patients had more than one SAE.
Six patients (4%) had infections. Of these, five patients (3.3%) had implant site soft‐tissue infections; two of these five patients had the neurostimulator and leads removed, and the other three were treated with debridement, washout, and antibiotics. One patient (0.7%) had osteomyelitis that was diagnosed 18 months after the neurostimulator and lead implantation. The neurostimulator and leads were removed, and the patient was treated with intravenous antibiotics. A subsequent cranioplasty was performed, and the infection resolved. Three of the six patients with infections had a neurostimulator and lead implanted as the sole procedure, and the other three patients had the neurostimulator and leads implanted at the time that ICM electrodes were explanted. The durations of ICM for these three patients were 11, 12, and 20 days. Two of these patients had ICM with depth electrodes only and one had a combination of subdural grid and strip electrodes. One of the six patients also had a resection concurrent with the neurostimulator and lead placement and explantation of ICM electrodes. There was no significant difference in the rate of infection between the group that had the neurostimulator and leads implanted as the sole procedure (3.0%) and those who had ICM electrodes removed in the same procedure as the neurostimulator and leads were placed (6.1%, P = .38).
Three patients had subdural hematomas. One of the three was being treated with anticoagulants because of a pulmonary embolism following placement of the RNS System neurostimulator and leads. A subsequent fall led to a subdural hematoma that required evacuation and also caused damage to a lead, which required lead revision. A second patient had a postimplant fall resulting in a subdural hematoma that was considered minor; however, the patient had an acute worsening of seizures. The third patient had a postoperative subdural hematoma and then had a procedure to remove a cortical strip lead, after which there was intraparenchymal bleeding. There were no long‐lasting neurological consequences for any of these patients.
Other SAEs included one patient with a third nerve palsy noted 2 days after the RNS System neurostimulator and leads were implanted; this resolved when the subtemporal strip was retracted. Scalp erosion occurred in one patient who also had an infection at the implant site, and in a second patient near a depth lead incision. Two patients were seen in emergency departments because of changes in seizures, one with an acute worsening of seizures and a new seizure type, and the other for an increase in seizure frequency that resolved. One patient had an acute hemiparesis that immediately recovered when a cortical strip lead was removed. Because of pain, one patient had a procedure to reseat a bone screw used to secure the cranially mounted tray into which the neurostimulator is seated; a cortical strip lead was added at the time of the revision, because the patient only had one lead placed at the original implant. Another patient had a ventriculoperitoneal shunt placed because of a persistent pseudomeningocele that resolved; the shunt was subsequently weaned and removed.
There were two patient deaths, neither of which was considered to be related to treatment with the RNS System. One patient with a history of depression and suicidality committed suicide. Another patient died as a result of renal cancer.
3.3. Secondary outcomes
The CGIS was completed for 140 of 150 (93%) patients. At the most recent follow‐up, 75% of patients were rated by their physician as “very much improved” or “much improved,” 14 patients (10%) as having “no change,” and three patients (2%) as minimally or much worse (Figure 3). There was a significant positive correlation between CGIS and percent change in seizure frequency at last follow‐up (r = .39, n = 140, P = .00001).
FIGURE 3.
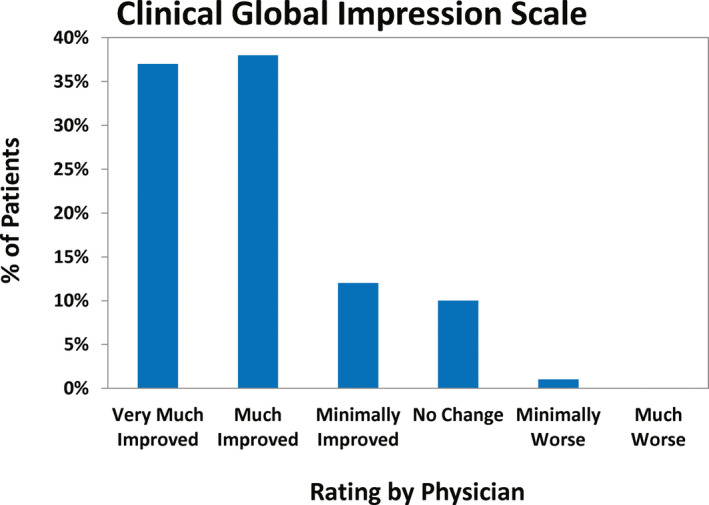
Clinician‐determined assessment of change in the patients’ ability to function using the Clinical Global Impressions Scale 10 at most recent follow‐up
One of the two patients considered to be “minimally worse” had no improvement in seizure frequency and a decrease in cognitive function; it was not known whether this was related to continued seizures or to the RNS System. The other had an increase in seizures and an implant site infection that required device explantation. The patient described as “much worse” had no seizure reduction with the RNS System, developed osteomyelitis 18 months postoperatively, and eventually required explantation of the neurostimulator and leads.
AEDs were not changed in 45% (67/150) of patients during the study. Sixteen percent (24/150) had at least one AED discontinued, and 12% (18/150) had at least one added. An additional 27% (41/150) had at least one AED added and discontinued during the study. The changes in seizure frequency at last follow‐up were not different between these groups (P > .05).
4. DISCUSSION
This multicenter, real‐world, retrospective review provides the first report on effectiveness of direct brain‐responsive neurostimulation outside of the clinical trials that supported FDA approval of the RNS System. Seizure frequency reductions of 67% at 1 year and 75% at 2 years of treatment in the real‐world cohort are similar to the seizure reductions achieved at 6 and 9 years of treatment in the clinical trials. 2 , 3 Other neuromodulation therapies for epilepsy also show progressive reductions in seizure frequency over time in both clinical trial and real‐world environments. 12 , 13 , 14
Our findings support the use of direct brain‐responsive neurostimulation therapy for medically refractory patients with focal onset seizures. Despite the introduction of many AEDs, the likelihood of achieving seizure freedom with additional trials of AEDs after failing the first two AEDs is <5%, 15 and the patient remains at risk for AED‐related adverse events. 16 Furthermore, if a seizure onset zone can be localized, surgical resection or ablation of the tissue can result in seizure remission for 52%‐65% of patients, depending on the location. 17 , 18 , 19 , 20 However, if two areas of seizure onset are identified, the onset zone overlaps with eloquent cortex, the tissue cannot be safely or completely resected or ablated, or the patient declines resection or ablation, direct brain‐responsive neurostimulation may be considered. 21 Moreover, patients may prefer to attempt a reversible neuromodulatory treatment approach prior to considering irreversible resection or ablation.
Infection rates were 2.9% per procedure, similar to those observed in the RNS System clinical trials (4.1% per procedure), and are consistent with infection rates reported with other implanted devices for epilepsy 13 and movement disorders. 22 , 23 , 24 , 25 The rate of infection in patients who had ICM electrodes explanted at the same time as the neurostimulator and leads were placed (6.1%) compares favorably to those in patients who have undergone only intracranial monitoring (5.7%) 26 or resection for epilepsy (2.1%‐8.5%). 27 Because the risks and types of infection are not significantly higher when the RNS System implant procedure occurs in association with intracranial monitoring or concurrent with a resection, potential benefits of these combined approaches can be weighed against the potential additive infection risks associated with multiple surgeries.
The incidence and types of intracranial hemorrhage in this real‐world cohort were also similar to the clinical trials of the RNS System (2.1% in the 2‐year randomized controlled trial 2 and 2.7% over long‐term follow‐up 3 ) and to published experience with deep brain stimulation for epilepsy and movement disorders. 22 , 25 Procedure‐related hemorrhages did not result in long‐lasting medical or neurological consequences.
There are several possible explanations for the rapid and robust real‐world response to treatment with direct brain‐responsive neurostimulation relative to the initial clinical trials. Greater efficacy in real‐world applications compared to clinical trial experience is also described with AEDs. 4 , 5 , 6 This has been postulated to be because patients in the real world may have less severe epilepsy than patients who choose to participate in clinical trials. This is not the likely explanation in this case, because demographics and disease‐related characteristics of the real‐world patients were very similar to the patients in the RNS System clinical trials, including age, duration of epilepsy, baseline seizure frequency, region of seizure onset, types and locations of leads, and the percent of patients previously treated with VNS or resective surgery. 2 , 3 In the randomized controlled Pivotal trial, 59% of patients had prior intracranial monitoring, 2 compared to 82% in this real‐world cohort. Although it is possible that this contributed to improved outcome, there was no difference in outcomes in the Pivotal and Long‐Term Treatment trials between patients who had or had not undergone intracranial monitoring.
Another potential explanation is that accumulated experience in the clinical trials has informed more efficient and effective detection and stimulation programming strategies, which have evolved with clinical experience. In the initial clinical trials, study protocols required at least 10 visits over the first 24 months after implantation, and programming was often changed at each of these visits. In current clinical practice, patients are usually seen 6‐8 times over the first 24 months and stimulation is not generally adjusted at every visit. Contemporary practice recognizes that a change in clinical seizure frequency may not be evident for 2‐4 months after a reprogramming. Thus, more frequent programming may obscure a favorable seizure response. Charge density, which is a function of the current amplitude and the stimulation pathway configuration, was also different, with more gradual increase in current and lower overall charge density in the real‐world patients than in the early clinical trials. Another difference between the clinical trials and present‐day practice is that detection is programmed so that stimulation is delivered in response to activity typically preceding seizures, such as a run of epileptiform spikes or changes in amplitude or rhythmicity, rather than clearly developed electrographic seizures. Stimulating into well‐developed electrographic seizures may not always disrupt electrographic seizure activity, perhaps because the seizure has already propagated beyond the region that can be impacted by spatially limited stimulation. It is possible that delivering acute disruptive stimulation into abnormal (interictal) electrographic activity (not only seizures) is effective because of providing longer‐term neuromodulation. 19 , 28 This hypothesis is supported by the observation that the total duration of stimulation received was a median of 4.5 min/d (IQR = 1.4‐9.3 min/d) in the real‐world patients, which is significantly greater than in the Pivotal trial population (median = 3.0 minutes of stimulation per day, IQR = 1.5‐5.1 min/d) at 2 years postoperatively (P = .024), although substantially less than with open‐loop scheduled stimulation approaches.
This study has limitations, including the retrospective nature of data collection and that patient‐reported seizure counts—although still a gold standard outcome measure for epilepsy clinical trials—are unreliable. 29 , 30 , 31 , 32 , 33 Seizures may be undercounted in the real world compared to clinical trials in which daily entries into a seizure diary are required, but within‐patient comparison to preimplant seizure frequency may mitigate this concern. Another limitation is that seizure severity was not captured due to inconsistent assessment across centers. However, the CGIS is an accepted instrument for measuring overall response to an intervention and represents the physician's perception of changes in the total burden of seizures. 10 The significant positive correlation between CGIS and changes in patient‐reported seizure frequency lends credence to this metric.
The expectation of benefit may be higher in patients who know they will be receiving active treatment compared to patients in randomized controlled trials in which some fraction will receive placebo. In the RNS System randomized controlled Pivotal trial, 50% were randomized to sham stimulation and did not receive active stimulation until 6 months after implant. However, at 6 months, all patients entered an open‐label period and knew that they were receiving neurostimulation 1 . Therefore, patients in the open‐label periods of the prospective clinical trials also knew that they were receiving stimulation, so the expectation of benefit can be reasonably assumed to be similar to these patients treated outside of clinical trials. Thus, the improved efficacy in the real world is likely independent of differences in patient expectation.
Real‐world patients with long‐standing epilepsy and disabling focal onset seizures resistant to multiple different treatments experienced substantial and sustained reduction in seizure frequency with direct brain‐responsive neurostimulation. The safety experience was similar to the clinical trials of the RNS System and of other brain stimulation devices. In current practice with brain‐responsive neurostimulation, clinical seizure outcomes exceeding those of the prospective clinical trials are achieved years earlier, corroborating effectiveness of this therapy and suggesting improvement in treatment efficiency.
CONFLICT OF INTEREST
B.R. has received research support from NeuroPace. V.R.R. has received support from and/or has served as a paid consultant for NeuroPace. S.E.P. has received support from and/or has served as a paid consultant for NeuroPace and Boston Scientific Corporation. D.E.B. has received support from and/or has served as a paid consultant for Greenwich Pharmaceuticals, Eisai Co, Sunovion Pharmaceuticals, NeuroPace, SK Biopharmaceuticals, UCB, and Zogenix. E.B.G. has received support from and/or has served as a paid consultant for NeuroPace, LivaNova, and Xenon Pharmaceuticals. J.E.G. is a prior employee of NeuroPace and has equity ownership/stock options. C.N.H. has received support from and/or has served as a paid consultant for NeuroPace in the form of research sponsorship of the long‐term postmarket outcomes. B.C.J. has received support from and/or has served as a paid consultant for NeuroPace, Medtronic, Eisai Co, Harvard Pilgrim Health Care, National Institutes of Health, Centers for Disease Control and Prevention, National Science Foundation, Defense Advanced Research Projects Agency, and the journals Neurology and Epilepsia. R.P.G. has received support from and/or has served as a paid consultant for NeuroPace's Scientific Advisory Council. C.H.H. has received support from and/or has served as a paid consultant for Boston Scientific Corporation. The remaining authors have no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1
ACKNOWLEDGMENTS
We would like to acknowledge Martha Morrell, MD, Tara Skarpaas, PhD, and Emily Mirro from NeuroPace for providing the Pivotal trial data and for facilitating the data collection, analysis, and manuscript review.
Razavi B, Rao VR, Lin C, et al. Real‐world experience with direct brain‐responsive neurostimulation for focal onset seizures. Epilepsia. 2020;61:1749–1757. 10.1111/epi.16593
Babak Razavi and Vikram R. Rao contributed equally.
Funding information
This was an investigator‐initiated study. The study design, data collection, data analysis, and manuscript writing were completed by the listed authors. IRB submission and data entry fees were supported by NeuroPace.
The copyright line for this article was changed on July 30, 2020 after original online publication
Contributor Information
Babak Razavi, Email: brazavi@stanford.edu.
Casey H. Halpern, Email: chalpern@stanford.edu.
REFERENCES
- 1. Morrell MJ, RNS System in Epilepsy Study Group . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. [DOI] [PubMed] [Google Scholar]
- 2. Heck CN, King‐Stephens D, Massey AD, et al. Two‐year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nair DR, RNS System Investigators , Morrell MJ, et al. Nine‐Year Prospective Safety and Effectiveness Outcomes From the Long‐Term Treatment Trial of the RNS® System. Poster session presented at: 72nd Annual Meeting of the American Epilepsy Society; New Orleans, LA.
- 4. Lhatoo SD, Wong IC, Polizzi G, Sander JW. Long‐term retention rates of lamotrigine, gabapentin, and topiramate in chronic epilepsy. Epilepsia. 2000;41:1592–6. [DOI] [PubMed] [Google Scholar]
- 5. Morrell MJ, Leppik I, French J, Ferrendelli J, Han J, Magnus L. The KEEPER trial: levetiracetam adjunctive treatment of partial‐onset seizures in an open‐label community‐based study. Epilepsy Res. 2003;54:153–61. [DOI] [PubMed] [Google Scholar]
- 6. Sander JW. New antiepileptic drugs in practice—how do they perform in the real world? Acta Neurol Scand Suppl. 2005;181:26–9. [DOI] [PubMed] [Google Scholar]
- 7. Geller EB, Skarpaas TL, Gross RE, et al. Brain‐responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58:994–1004. [DOI] [PubMed] [Google Scholar]
- 8. Jobst BC, Kapur R, Barkley GL, et al. Brain‐responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58:1005–14. [DOI] [PubMed] [Google Scholar]
- 9. Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial‐onset epilepsy. Epilepsia. 2015;56:1836–44. [DOI] [PubMed] [Google Scholar]
- 10. Busner J, Targum SD. The Clinical Global Impressions Scale. Psychiatry (Edgmont). 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 11. Chaves J, Breia P, Pimentel J, et al. Eslicarbazepine acetate as adjunctive therapy in clinical practice: ESLADOBA study. Acta Neurol Scand. 2017;136:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta‐analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–55. [DOI] [PubMed] [Google Scholar]
- 13. Salanova V, Witt T, Worth R, et al. Long‐term efficacy and safety of thalamic stimulation for drug‐resistant partial epilepsy. Neurology. 2015;84:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KJ, Shon YM, Cho CB. Long‐term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact Funct Neurosurg. 2012;90:379–85. [DOI] [PubMed] [Google Scholar]
- 15. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahajan UV, Parker JJ, Williams NR, et al. Adjunctive repetitive transcranial magnetic stimulation delivers superior quality of life for focal epilepsy compared to anti‐epileptic drugs: a meta‐analytic utility prediction study. Brain Stimul. 2020;13:430–2. [DOI] [PubMed] [Google Scholar]
- 17. Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. [DOI] [PubMed] [Google Scholar]
- 18. de Tisi J, Bell GS, Peacock JL, et al. The long‐term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378:1388–95. [DOI] [PubMed] [Google Scholar]
- 19. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and efficiency of surgery for temporal lobe epilepsy study group. A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med. 2001;345:311–8. [DOI] [PubMed] [Google Scholar]
- 20. Wu C, Jermakowicz WJ, Chakravorti S, et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: a multicenter study of 234 patients. Epilepsia. 2019;60:1171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma BB, Rao VR. Responsive neurostimulation: candidates and considerations. Epilepsy Behav. 2018;88:388–95. [DOI] [PubMed] [Google Scholar]
- 22. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghosh PS, Machado AG, Deogaonkar M, Ghosh D. Deep brain stimulation in children with dystonia: experience from a tertiary care center. Pediatr Neurosurg. 2012;48:146–51. [DOI] [PubMed] [Google Scholar]
- 24. Air EL, Ostrem JL, Sanger TD, Starr PA. Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr. 2011;8:566–74. [DOI] [PubMed] [Google Scholar]
- 25. Deep‐Brain Stimulation for Parkinson's Disease Study Group , Obeso JA, Olanow CW, et al. Deep‐brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–63. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt RF, Wu C, Lang MJ, et al. Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia. 2016;57:1697–708. [DOI] [PubMed] [Google Scholar]
- 27. Georgiadis I, Kapsalaki EZ, Fountas KN. Temporal lobe resective surgery for medically intractable epilepsy: a review of complications and side effects. Epilepsy Res Treat. 2013;2013:752195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed‐loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 2019;76:800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260–4. [DOI] [PubMed] [Google Scholar]
- 30. Fisher RS, Blum DE, DiVentura B, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24:304–10. [DOI] [PubMed] [Google Scholar]
- 31. Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64:1595–9. [DOI] [PubMed] [Google Scholar]
- 32. Kerling F, Mueller S, Pauli E, Stefan H. When do patients forget their seizures? An electroclinical study. Epilepsy Behav. 2006;9:281–5. [DOI] [PubMed] [Google Scholar]
- 33. Tatum WO, Winters L, Gieron M, et al. Outpatient seizure identification: results of 502 patients using computer‐assisted ambulatory EEG. J Clin Neurophysiol. 2001;18:14–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


