Abstract
Oxylipins constitute a family of oxidized fatty acids, that are well known as tissue hormones in mammals. They contribute to inflammation and its resolution. The major classes of these lipid mediators are inflammatory prostaglandins (PGs) and leukotrienes (LTs) as well as pro‐resolving resolvins (Rvs). Understanding their biosynthetic pathways and modes of action is important for anti‐inflammatory interventions. Besides mammals, marine algae also biosynthesize mammalian‐like oxylipins and thus offer new opportunities for oxylipin research. They provide prolific sources for these compounds and offer unique opportunities to study alternative biosynthetic pathways to the well‐known lipid mediators. Herein, we discuss recent findings on the biosynthesis of oxylipins in mammals and algae including an alternative pathway to prostaglandin E2, a novel pathway to a precursor of leukotriene B4, and the production of resolvins in algae. We evaluate the pharmacological potential of the algal metabolites with implications in health and disease.
Keywords: biosynthesis, inflammation, natural products, oxylipins, pro-resolving
The uniqueness of oxylipin endowment in mammals is cracked by marine algae. They also form bioactive lipid mediators such as prostaglandins (PGs), leukotrienes (LTs), and resolvins (Rvs) in response to wounding as is observed in mammals. Contributions to the algal oxylipin chemistry kicked off the discovery of novel biosynthetic pathways in mammals with implications in health and disease.
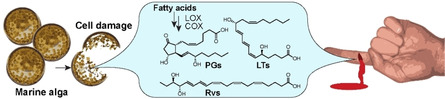
Introduction
The finding that certain bioactive natural products are produced in unrelated organisms opens opportunities for the investigation of their biosynthesis, for solving supply issues, and for the elucidation of their multifunctionality. But also mining for novel structures derived from similar biosynthetic pathways in different producers is a successful strategy for natural product research. In particular, the field of oxylipins was stimulated by the reciprocal investigation of mammalian, plant and algal pathways. Oxylipins are oxidized derivatives of unsaturated fatty acids that function as versatile tissue hormones in mammals but also as central hormones in plants. In algae they serve mainly as chemical defense.
Eicosanoids that are derived from unsaturated C20 fatty acids are termed lipid mediators since they regulate the initiation and resolution of inflammation in mammals.1, 2 The main families of these immunomodulatory compounds are the pro‐inflammatory prostaglandins (PGs), the leukotrienes (LTs), and the pro‐resolving resolvins (Rvs). They are biosynthesized by enzymes such as cyclooxygenases (COXs) and/or lipoxygenases (LOXs) and mediate biological effects via cognate G protein‐coupled receptors (GPCRs).1, 3 These potent molecules provide targets for the development of anti‐inflammatory drugs such as COX‐inhibitory coxibs and nonsteroidal anti‐inflammatory drugs (NSAIDs) to intervene in the pathogenesis of inflammation.4 However, side effects and inefficiency of these drugs are incentives to search for better alternatives. Resolvin derivatives have recently been recognized as promising anti‐inflammatory drug candidates due to high biological activity.5 Their medical use is yet constrained due to the limited supply caused by low yielding sources for the natural product and lack of economic, efficient syntheses.6
Marine algae produce structurally diverse bioactive oxylipins derived from C20 fatty acids as well and could thus be a valuable alternative source for lipid mediators. Algal oxylipins have mainly been examined in the context of their chemical ecology; their pharmaceutical potential has not yet been comprehensively evaluated.7 Herein, we discuss novel oxylipins from marine algae with structural similarities or identity to lipid mediators in mammals, comprising the classes of prostaglandins, leukotrienes, and resolvins. We focus on own recent contributions to algal oxylipin chemistry with special emphasis on biosynthetic aspects and implications in health and disease. The concept is strengthened that natural products occurring in different kingdoms of life provide unique opportunities for biosynthetic research and offer new avenues in the development of pharmaceutical applications (Figure 1).
Figure 1.
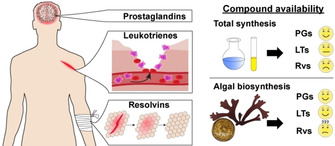
Inflammatory and pro‐resolving lipid mediators in humans as inspiration for the development of anti‐inflammatory drugs. Compound availability issues are complicating their pharmaceutical application. Algae could provide opportunities to overcome this hurdle.
Oxylipins in Algae
Oxygenated lipids are widespread in micro‐ and macroalgae with universal structural elements, but species‐specific structures.8 In particular, microalgae belonging to the group of diatoms as well as brown and red macroalgae are capable of producing high amounts of C20 polyunsaturated fatty acids (PUFAs) such as arachidonic acid and eicosapentaenoic acid. These fatty acids are the precursors for mammalian‐type oxylipins. PUFAs are processed by LOXs, COXs, and cytochrome P450 (CYPs) and subsequently transformed by hydroperoxide isomerases, hydroperoxide lyases, epoxide hydrolases, and peroxide reductases.9, 10, 11, 12 LOX activity with varied regioselectivity has been reported for marine diatoms, brown algae, and red algae.13, 14, 15, 16 The initially formed hydroperoxides give rise to a structurally diverse family of algal oxylipins.
In algae, a hormonal function of oxylipins is still under debate, but there is clear evidence that these compounds serve as pheromones that support sexual reproduction and act as chemical defense against pathogens and grazers.17, 18, 19 Often, the biosynthesis of algal oxylipins is induced upon wounding of the cells or tissue, leading to a rapid conversion of the stored precursor lipids to give the free fatty acids that function as substrates for further down‐stream metabolism.20 Regulation of oxylipin biosynthesis shares elements with that in mammals, where the enzymatic machinery (LOXs, COXs) increases fatty acid conversion to lipid mediators upon tissue damage‐induced rise of intracellular calcium.3
Algae are a rich source for hydroxylated eicosapentaenoic acids (HEPEs) or tetraenoic acids (HETEs), polyunsaturated aldehydes (PUAs), fatty acid hydroperoxides, and epoxy alcohols.7 Oxylipin formation has been particularly well studied in the red algae Gracilaria chilensis and Gracilaria vermiculophylla where a lipase‐, LOX‐ or COX‐mediated conversion of galacto‐ and phospholipids gives rise to eicosanoids.21 Among Gracilaria oxylipins, 7,8‐dihydroxy‐ETE (7,8‐diHETE) acts as a natural antifouling agent and deterrent against grazers.21, 22 In contrast to their prevalence in macroalgae, arachidonic acid‐derived HETEs are not the most abundant hydroxylated oxylipins in microalgae. In the diatom Skeletonema costatum, for example, eicosapentaenoic acid‐derived HEPEs prevail including 15‐hydroxy‐EPE (15‐HEPE) that inhibits the growth of the algicidal bacterium Kordia algicida.23 Diatoms are also well‐known producers of short‐chain α,β,γ,δ‐polyunsaturated aldehydes (PUAs). PUA mediated chemical defense is activated in response to cell damage and causes an increase in the offspring mortality of diatom‐grazing copepods.24 Fatty acid hydroperoxides, such as 15‐hydroperoxy‐eicosapentaenoic acid as well as epoxy alcohols formed by the diatoms Chaetoceros socialis and Thalassiosira rotula also contribute to this toxicity.8, 25
Prostaglandins
Most described eicosanoids from algae are LOX‐derived, but also COX‐cyclized lipid mediators with high structural resemblance to mammalian hormones are observed. We focus here on prostaglandins with activity in algae and mammals. PGF2α and PGE2 are the most common and best‐evaluated prostaglandins in algae. Both are derived from arachidonic acid and function primarily as defense molecules for macroalgae, while their function in microalgae is unknown.26, 27, 28, 29 PGF2α and PGE2 content can be associated with invasiveness of macroalgae as reported for the spreading of the red seaweed G. vermiculophylla in the Northern Atlantic.30 Algal PGE2 has also been linked to occasionally occurring food poisoning (e. g., nausea, vomiting, diarrhea) upon ingestion of raw red algae from the edible genus Gracilaria.31
Mining of G. vermiculophylla for arachidonic acid‐derived oxylipins with emphasis on prostaglandins revealed 15‐hydroperoxy‐PGE2. This hydroperoxide is the biosynthetic intermediate to PGE2 and 15‐keto‐PGE2 in algae (Figure 2).32 Interestingly, the hydroperoxide was previously not observed in mammals where the biosynthesis via PGH2 is well established. It becomes clear that mammals and algae employ different major routes to the same prostaglandin (Figure 2). The availability of the algal 15‐hydroperoxy‐PGE2 allowed screening the human lipidome for this metabolite. Indeed, human macrophages, key producers of lipid mediators during inflammation, contain this hydroperoxide.32, 33 15‐Hydroperoxy‐PGE2 is in fact a hitherto unidentified intermediate in an alternative route to PGE2 in mammals, where the formation of PGE2 proceeds along two independent pathways (Figure 2).31 The route via 15‐hydroperoxy‐PGE2 dominates in algae and renders a mixture of PGE2 and 15‐keto‐PGE2 whereas human macrophages preferentially rely on the canonical pathway via PGH2 with the 15‐hydroperoxy‐PGE2 route as a minor pathway (Figure 2).32
Figure 2.
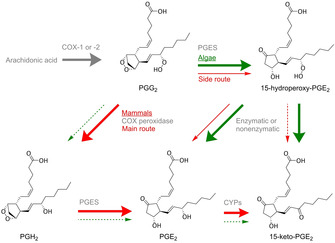
Biosynthesis of PGE2 in algae and in mammals. The canonical pathway in mammals follows the thick red arrows. Arachidonic acid is cyclized by COX‐1 or COX‐2 to PGG2, which is reduced by the peroxidase activity of the COX to PGH2 and subsequently converted by prostaglandin E synthase to PGE2. An alternative pathway was discovered in the red alga Gracilaria vermiculophylla (thick green arrows). There PGG2 is transformed into the novel intermediate 15‐hydroperoxy‐PGE2, which is enzymatically or nonenzymatically transformed to PGE2. Upon re‐investigation of the biosynthesis in human macrophages, the alternative route via 15‐hydroperoxy‐PGE2 was established in mammals as well (thin red arrows).32
The discovery of an independent route via the hydroperoxide might provide a new paradigm for treating inflammation‐related diseases. Selective COX peroxidase inhibitors may be developed to alleviate carcinogenic effects from side‐products formed by the COX peroxide reductase domain while still allowing PGE2 biosynthesis to occur via the hydroperoxide pathway.32 Regarding ingestion of raw alga, 15‐hydroperoxy‐PGE2 might be converted to PGE2 inside the human stomach promoting food poisoning.
Leukotrienes
Another class of mammalian‐like oxylipins in algae are arachidonic acid‐derived leukotrienes that function as immunomodulators in mammals. Leukotriene B4 (LTB4) is the most potent compound in mammals regulating inflammation and mediating chemotaxis of leukocytes for the host‐protective response.34 Elevated concentrations of LTB4 can lead to gastrointestinal toxicity.35 Only a few studies reported leukotrienes in marine algae. The two red macroalgae G. vermiculophylla and Murrayella periclados form LTB4 and derivatives with unknown biological function for the producers.36, 37
Upon re‐investigation of oxylipins formed upon wounding by G. vermiculophylla, we identified a novel oxylipin, (5R,8S)‐dihydroxy‐ETE (5R,8S‐diHETE).38 This acid‐labile molecule rearranges via an 1,8‐diol‐forming mechanism to inflammatory LTB4 isomers as an enantiomeric mixture (Figure 3).38 This step is likely to occur abiotically rather than being enzymatically catalyzed. The algal metabolites exhibit an enantio‐specific potency toward the LTB4 receptor 1.38 LTB4 is likely generated in algae via 7,8‐epoxy‐eicosatetraenoic acid as illustrated in Figure 3A. This process might be initiated by an 8‐LOX/hydroperoxide isomerase sequence followed by a nonselective acid‐catalyzed dehydration/hydration reaction.38 This route differs significantly from the biosynthesis in mammals that is initiated by a 5‐LOX leading to 5,6‐epoxy‐eicosatetraenoic acid and LTB4 (Figure 3B).38, 39
Figure 3.
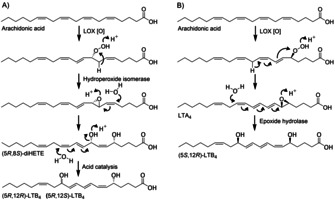
A) Proposed pathway for the formation of LTB4 isomers in algae. Arachidonic acid is oxygenated by an 8R‐LOX to yield a hydroperoxy‐fatty acid. This intermediate is then cyclized to the epoxide by a hydroperoxide isomerase followed by stereoselective enzymatic addition of water giving rise to (5R,8S)‐diHETE. Under acidic conditions, this precursor rearranges to (5R,12R/S)‐LTB4 (ee=95 %). B) Mammalian biosynthesis of (5S,12R)‐LTB4. Arachidonic acid is initially oxygenated by a 5S‐LOX forming 5‐HPETE that is subsequently dehydrated resulting in the precursor epoxide LTA4. This is then enzymatically hydrolyzed to LTB4.38, 39
The availability of LTB4 isomers enabled the investigation of structural requirements for receptor‐ligand interaction in mammals. The LTB4 receptor 1 is enantioselective with specificity for (5s,12r)‐LTB4.38 In addition to PGE2, algal leukotrienes and related hydroxylated fatty acids might also cause food poisoning.
Resolvins
Prostaglandins and leukotrienes have been well known for decades and contribute mainly to inflammatory processes. In contrast, the relatively newly discovered class of specialized pro‐resolving mediators is involved in the resolution of inflammation. The concentration of the specialized pro‐resolving mediators, resolvins, lipoxins, protectins, and maresins is elevated upon inflammation.40 Resolvins are mainly derived from ω‐3‐PUFAs such as eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid. Their formation is catalyzed by regioselective LOXs, COXs, and CYPs.40 These lipid mediators exhibit pro‐resolving effects including inhibition of pro‐inflammatory transcription factors, modification of the fatty acid composition of phospholipids, and activation of anti‐inflammatory transcription factors.41 Resolvins (Figure 4) are thus of interest for the development of pro‐resolving agents. Structures derived from those oxylipins are currently tested in pre‐clinical trials such as the RvE1‐analogue RX‐10045 for the treatment of keratoconjunctivitis sicca.42
Figure 4.
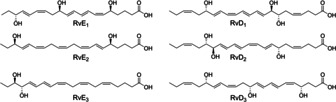
Selection of resolvins derived from eicosapentaenoic acid (E series) and docosahexaenoic acid (D series).
Microalgae produce high amounts ω‐3‐PUFAs and harbor a diverse family of eicosapentaenoic acid‐converting LOXs.43, 44 They are therefore promising candidates for the production of resolvins. In fact, an initial survey revealed the diatoms Coscinodiscus granii and Chaetoceros didymus producing RvE2 and RvE3.45 These findings may lay the foundation for an aquaculture‐based supply of resolvins and other SPMs.
Mechanisms of Enzymatic Transformations in Oxylipin Formation
While the central classes of enzymes involved in the initiation of the biosynthesis of prostaglandins, leukotrienes, and resolvins in algae and mammals are similar, pronounced differences are observed in the downstream transformation of intermediate hydroperoxides. In algae as well as in mammals the multistep enzymatic conversion of fatty acids is triggered by calcium‐dependent sphingomyelinases, galactolipases, or phospholipases that release precursor fatty acids such as arachidonic acid, eicosapentaenoic acid, docosapentanoic acid, and docosahexaenoic acid.3, 21, 46 Subsequent oxygenation is mediated by LOXs and COXs according to Figure 5.46, 47, 48 These iron‐containing enzymes are substrate‐specific and stereoselective. The resulting intermediates can be further metabolized by a second catalytic domain of a bi‐functional enzyme, such as a peroxide reductase activity of LOXs and COXs. Alternatively, other enzymes such as epoxide hydrolases that are prevalent in mammals or hydroperoxide isomerases that are algae‐specific can transform the reactive intermediates.47, 48, 49, 50
Figure 5.
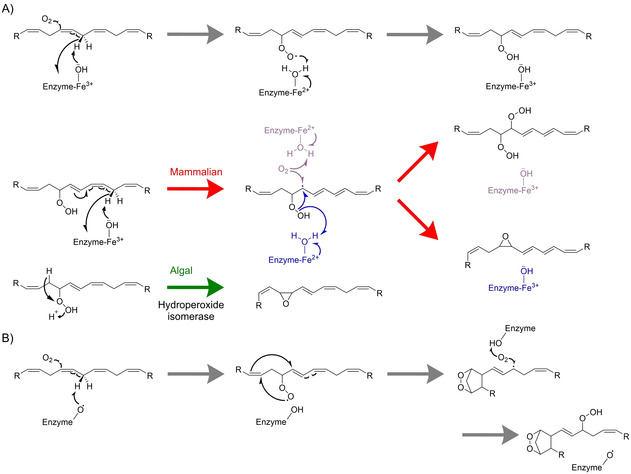
Central enzymatic steps in the biosynthesis of C20‐oxylipins in algae and mammals. A) LOX‐mediated oxidation with subsequent reaction steps catalyzed either by LOXs (mammals, red arrows) with different mechanisms (purple or blue) or by hydroperoxide isomerases (algae, green arrows). B) COX‐catalyzed cyclization in algae and mammals.
Phylogenetic analysis of LOXs from plants, mammals, moss, corals, algae and cyanobacteria suggests a common origin and also determinant amino acids and widely conserved reaction mechanisms.51 In a first, rate‐limiting step, LOXs abstract one hydrogen from a bisallylic methylene carbon forming a conjugated radical that is then oxygenated to a peroxy radical followed by a LOX‐mediated reduction to the corresponding anion and subsequent protonation yielding a hydroperoxide (Figure 5A).52 LOX‐derived hydroperoxy fatty acids are processed differently in algae and mammals (Figure 5A). In algae, the hydroperoxide is either reduced by the lipoxygenase itself to give a hydroxy‐fatty acid or cyclized to an epoxide via a hydroperoxide isomerase.49, 53 The epoxide can be opened by stereoselective addition of water yielding dihydroxy‐fatty acids such as (5R,8S)‐diHETE.38, 53 Micro‐ and macroalgae have also pronounced hydroperoxide lyase activities releasing polyunsaturated aldehydes as chemical defense.12 Here untypical transformations are observed including the action of halolyases introducing chlorine into the fatty acid metabolites.54
Mammalian LOXs can reduce the hydroperoxy intermediate forming a hydroxy‐fatty acid as also observed in algae.55 Alternatively, as it is the case in the LTB4 biosynthesis, LOXs can catalyze a second abstraction of a bisallylic hydrogen and mediate epoxide formation.55 Epoxides can be further hydrolyzed via epoxide hydrolases yielding dihydroxy‐fatty acids such as LTB4.50 1,2‐Diols are also accessible via the LOX‐mediated introduction of a second hydroperoxy group (Figure 5A) with subsequent double peroxide reduction assembling a 1,2‐diol.55
Alternatively, a COX‐mediated cyclization and second dioxygenation with protonation of the peroxy radical generating an endoperoxide/hydroperoxide intermediate is observed (Figure 5B).47, 56 How this intermediate is metabolized differs between algae and mammals. In mammals, the endoperoxide/hydroperoxide is reduced by a COX as reported for the transformation of PGG2 into PGH2.57 The endoperoxide motif can then be further processed to various end products such as PGE2 catalyzed by the prostaglandin E synthase.58 The peroxide reduction step mediated by a COX‐type activity in algae is not very prominent. The amino acid sequence of the algal enzyme shares just about 20 % of the mammalian counterpart, giving a possible explanation for this low activity.9, 32 Instead of being reduced, as it is the case in mammals the endoperoxide/hydroperoxide intermediate is likely processed by an enzyme opening the endoperoxide followed by reduction to PGE2.32
The general enzyme classes transforming unsaturated fatty acids in mammals and algae are thus very similar. Their organization in biosynthetic pathways are however fundamentally different, leading in diverse routes to identical products or to the formation of structural or enantiomeric isomers.
Conclusion
Oxylipins are not solely biosynthesized in mammals where they function as versatile inflammatory and pro‐resolving lipid mediators but are also generated in algae. Algae produce these metabolites in alternative pathways compared to mammals using different enzymes and intermediates but rely on similar transformation principles. Based on biosynthetic considerations derived from investigations of algae, novel mammalian pathways were discovered, and novel isomers of lipid mediators are accessible. Algae therefore provide important resources for pharmaceutical tools and guide novel strategies for intervention in oxylipin biosynthesis in mammals. Poor availability of mammalian lipid mediators has yet constrained potential pharmaceutical and research applications. This problem may be overcome by algae producing such mammalian‐like lipid mediators.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful for a Kekulé‐Stipend (H.J.) from the German chemical industry association Verband der Chemischen Industrie (VCI). This work was supported by the German Research foundation within the framework of the Collaborative Research Centre CRC1127 ChemBioSys.
H. Jagusch, T. U. H. Baumeister, G. Pohnert, ChemBioChem 2020, 21, 2419.
References
- 1. Funk C. D., Science 2001, 294, 1871–1875. [DOI] [PubMed] [Google Scholar]
- 2. Serhan C. N., Nature 2014, 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimizu T., Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [DOI] [PubMed] [Google Scholar]
- 4. Rainsford K. D., Subcell. Biochem. 2007, 42, 3–27. [DOI] [PubMed] [Google Scholar]
- 5. Serhan C. N., Chiang N., Dalli J., Semin. Immunol. 2015, 27, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newhouse T., Baran P. S., Hoffmann R. W., Chem. Soc. Rev. 2009, 38, 3010–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barbosa M., Valentão P., Andrade P. B., Mar. Drugs 2016, 14, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutignano A., Lamari N., d′Ippolito G., Manzo E., Cimino G., Fontana A., J. Phycol. 2011, 47, 233–243. [DOI] [PubMed] [Google Scholar]
- 9. Varvas K., Kasvandik S., Hansen K., Järving I., Morell I., Samel N., Biochim. Biophys. Acta 2013, 1831, 863–871. [DOI] [PubMed] [Google Scholar]
- 10. Andreou A., Brodhun F., Feussner I., Prog. Lipid Res. 2009, 48, 148–170. [DOI] [PubMed] [Google Scholar]
- 11. Gerwick W. H., Biochim. Biophys. Acta 1994, 1211, 243–255. [DOI] [PubMed] [Google Scholar]
- 12. Barofsky A., Pohnert G., Org. Lett. 2007, 9, 1017–1020. [DOI] [PubMed] [Google Scholar]
- 13. d′Ippolito G., Lamari N., Montresor M., Romano G., Cutignano A., Gerecht A., Cimino G., Fontana A., New Phytol. 2009, 183, 1064–1071. [DOI] [PubMed] [Google Scholar]
- 14. d′Ippolito G., Tucci S., Cutignano A., Romano G., Cimino G., Miralto A., Fontana A., Biochim. Biophys. Acta 2004, 1686, 100–107. [DOI] [PubMed] [Google Scholar]
- 15. Proteau P. J., Gerwick W. H., Lipids 1993, 28, 783–787. [DOI] [PubMed] [Google Scholar]
- 16. Bouarab K., Adas F., Gaquerel E., Kloareg B., Salaün J.-P., Potin P., Plant Physiol. 2004, 135, 1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowley A. F., Kühn H., Schewe T., Eicosanoids and Related Compounds in Plants and Animals, Princeton University Press, Princeton: 2015. [Google Scholar]
- 18. Gerwick W. H., Roberts M. A., Vulpanovici A., Ballantine D. L., Lipoxygenases and their metabolites, Springer, Berlin: 1999, 211–218. [Google Scholar]
- 19. Nylund G. M., Weinberger F., Rempt M., Pohnert G., PLoS One 2011, 6, 29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pohnert G., The Chemistry of Pheromones and Other Semiochemicals I, Springer, Berlin: 2004, 179–219. [Google Scholar]
- 21. Lion U., Wiesenmeier T., Weinberger F., Beltrán J., Flores V., Faugeron S., Correa J., Pohnert G., ChemBioChem. 2006, 7, 457–462. [DOI] [PubMed] [Google Scholar]
- 22. Rempt M., Weinberger F., Grosser K., Pohnert G., Beilstein J. Org. Chem. 2012, 8, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer N., Rettner J., Werner M., Werz O., Pohnert G., Mar. Drugs 2018, 16, pii: E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ianora A., Miralto A., Poulet S. A., Carotenuto Y., Buttino I., Romano G., Casotti R., Pohnert G., Wichard T., Colucci-D′Amato L., Terrazzano G., Smetacek V., Nature 2004, 429, 403–407. [DOI] [PubMed] [Google Scholar]
- 25. Fontana A., d′Ippolito G., Cutignano A., Romano G., Lamari N., Massa Gallucci A., Cimino G., Miralto A., Ianora A., ChemBioChem. 2007, 8, 1810–1818. [DOI] [PubMed] [Google Scholar]
- 26. Guschina I. A., Harwood J. L., Prog. Lipid Res. 2006, 45, 160–186. [DOI] [PubMed] [Google Scholar]
- 27. Pohnert G., Boland W., Nat. Prod. Rep. 2002, 19, 108–122. [DOI] [PubMed] [Google Scholar]
- 28. Gregson R. P., Marwood J. F., Quinn R. J., Tetrahedron Lett. 1979, 20, 4505. [Google Scholar]
- 29. Di Dato V., Orefice I., Amato A., Fontanarosa C., Amoresano A., Cutignano A., Ianora A., Romano G., ISME J. 2017, 11, 1722–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyberg C. D., Thomsen M. S., Wallentinus I., Eur. J. Phycol. 2009, 44, 395–403. [Google Scholar]
- 31. Fusetani N., Hashimoto K., Bull. Jpn. Soc. Sci. Fish. 1984, 50, 465–469. [Google Scholar]
- 32. Jagusch H., Werner M., Werz O., Pohnert G., Angew. Chem. Int. Ed. 2019, 58, 17641–17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., F. A. Kuehl Jr , Davies P., Nature 1977, 269, 149–151. [DOI] [PubMed] [Google Scholar]
- 34. Chen X.-S., Scheller J. R., Johnson E. N., Funk C. D., Nature 1994, 372, 179–182. [DOI] [PubMed] [Google Scholar]
- 35. Hudson N., Balsitis M., Everitt S., Hawkey C. J., Gut 1993, 34, 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sajiki J., Kakimi H., J. Chromatogr. A 1998, 795, 227–237. [DOI] [PubMed] [Google Scholar]
- 37. Bernart M. W., Gerwick W. H., Phytochemistry 1994, 36, 1233–1240. [Google Scholar]
- 38. Jagusch H., Werner M., Okuno T., Yokomizo T., Werz O., Pohnert G., Org. Lett. 2019, 21, 4667–4670. [DOI] [PubMed] [Google Scholar]
- 39. Radmark O., Werz O., Steinhilber D., Samuelsson B., Trends Biochem. Sci. 2007, 32, 332–341. [DOI] [PubMed] [Google Scholar]
- 40. Serhan C. N., Petasis N. A., Chem. Rev. 2011, 111, 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calder P. C., Biochim. Biophys. Acta 2015, 1851, 469–484. [DOI] [PubMed] [Google Scholar]
- 42. Serhan C. N., Chiang J Dalli N., Levy B. D., Cold Spring Harbor Perspect. Biol. 2014, 7, a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fontana A., d′Ippolito G., Cutignano A., Miralto A., Ianora A., Romano G., Cimino G., Appl. Chem. 2007, 79, 481–490. [Google Scholar]
- 44. Pohnert G., Angew. Chem. Int. Ed. 2000, 39, 4352–4354; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 4506–4508. [Google Scholar]
- 45. Meyer N., Rettner J., Werner M., Werz O., Pohnert G., Mar. Drugs 2018, 16, pii: E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolfe L. S., J. Neurochem. 1982, 38, 1–14. [DOI] [PubMed] [Google Scholar]
- 47. Mosblech A., Feussner I., Heilmann I., Plant Physiol. Biochem. 2009, 47, 511–517. [DOI] [PubMed] [Google Scholar]
- 48. Capra V., Rovati G. E., Mangano P., Buccellati C., Murphy R. C., Sala A., Biochim. Biophys. Acta 2015, 1851, 377–382. [DOI] [PubMed] [Google Scholar]
- 49. Hamberg M., Gerwick W. H., Arch. Biochem. Biophys. 1993, 15, 115–122. [DOI] [PubMed] [Google Scholar]
- 50. Newman J. W., Morisseau C., Hammock B. D., Prog. Lipid Res. 2005, 44, 1–51. [DOI] [PubMed] [Google Scholar]
- 51. Andreou A., Feussner I., Phytochemistry 2009, 70, 1504–1510. [DOI] [PubMed] [Google Scholar]
- 52. Borowski T., Broclawik E., J. Phys. Chem. B 2003, 107, 4639–4646. [Google Scholar]
- 53. Moghaddam M. F., Gerwick W. H., J. Nat. Prod. 1991, 54, 1619–1624. [DOI] [PubMed] [Google Scholar]
- 54. Wichard T., Pohnert G., J. Am. Chem. Soc. 2006, 128, 7114–7115. [DOI] [PubMed] [Google Scholar]
- 55. Maas R. L., Brash A. R., PNAS 1983, 80, 2884–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simmons D. L., Botting R. M., Hla T., Pharmacol. Rev. 2004, 56, 387–437. [DOI] [PubMed] [Google Scholar]
- 57. van der Donk W. A., Tsai A. L., Kulmacz R. J., Biochemistry 2002, 41, 15451–15458. [DOI] [PubMed] [Google Scholar]
- 58. Murakami M., Nakatani Y., Toniaka T., Kudo I., Prostaglandins Other Lipid Mediators 2002, 68, 383–399. [DOI] [PubMed] [Google Scholar]


