Abstract
Mixed hematopoietic chimerism induction as a way to foster tolerance to donor organs in recipients who have been sensitized to donor antigens is challenging. Donor‐specific antibodies (DSA) are a dominant barrier toward successful donor bone marrow engraftment. Although desensitization methods are routinely used in recipients with allosensitization for allogeneic bone marrow transplantation, engraftment is frequently unsuccessful. To overcome the barrier of prior sensitization we tested enzymatic desensitization of donor‐specific IgG using imlifidase and endoglycosidase of Streptococcus pyogenes (EndoS), which both partially block the function of DSA in mice, as a novel approach to improve murine bone marrow engraftment in primed hosts. We found that EndoS was capable of inhibiting antibody‐mediated killing of donor cells in vivo. Furthermore, the effect of EndoS depended on the titer of DSA and the genetic background of the recipients. In combination with imlifidase, EndoS improved the survival of donor bone marrow cells. Together with cyclophosphamide, bortezomib, T cell depletion, and nonlethal irradiation, imlifidase in combination with EndoS allowed allogeneic bone marrow engraftment in sensitized recipients. We conclude that enzymatic inactivation of DSA, using the combination of imlifidase and EndoS, can be used for inducing donor hematopoietic chimerism in allosensitized recipient mice in combination with other desensitization strategies.
Keywords: alloantibody, animal models: murine, basic (laboratory) research/science, bone marrow/hematopoietic stem cell transplantation, desensitization, immunosuppression/immune modulation, tolerance: chimerism, translational research/science
Short abstract
Imlifidase and EndoS overcome the barrier of donor‐specific antibody, facilitating the achievement of hematopoietic chimerism in a mouse model of bone marrow transplantation.
Abbreviations
- BMC
bone marrow cells
- BMT
bone marrow transplantation
- CFSE
carboxyfluorescein succinimidyl ester
- CTV
cell trace violet dye
- CyBor
bortezomib and cyclophosphamide
- DSA
donor specific antibodies
- EndoS
endoglycosidase of Streptococcus pyogenes
- FcγR
Fcγ receptor
- GVHD
graft‐versus‐host disease
- IdeS
IgG degrading enzyme of Streptococcus pyogenes
- IVIG
intravenous immune globulins
- TBI
total body irradiation
1. INTRODUCTION
Hematopoietic chimerism via allogeneic bone marrow transplantation (BMT) is a robust method for inducing tolerance to organs from the same donor. 1 However, sensitization to donor antigens prior to transplantation is a significant barrier to both successful bone marrow and solid organ transplantation. 2 , 3 Although challenging in these sensitized recipients, if successful, achieving hematopoietic chimerism could reverse such allosensitization in theory through the generation of specific tolerance in T and B cells, resulting in a reduction of donor‐specific antibodies (DSA) and cytotoxic T cells. 4
Humoral immunity against donor antigens is believed to be the dominant barrier for primary bone marrow (BM) engraftment in sensitized hosts. 5 , 6 Various combinations of DSA desensitization methods, including plasmapheresis that removes DSA, mismatched platelet transfusion that adsorbs DSA, as well as rituximab and bortezomib, which inhibit antibody production, have the capacity to improve BM engraftment. Human intravenous immune globulins (IVIG) have also been shown to have some beneficial immune modulatory effects in DSA‐positive recipients who underwent transplantation. 2 , 7 However, these empirical treatments were not always effective in reducing the titer of DSA to the cut‐off levels that permit successful engraftment. 2 Furthermore, a rapid rebound of DSA may occur even after successful desensitization and thus additional interventions are required. 3 Novel approaches that can either further reduce the titer or inhibit the effector functions of residual DSA would, therefore, be helpful in improving the BM engraftment.
Imlifidase, previously known as IgG degrading enzyme of Streptococcus pyogenes (IdeS), enables kidney transplantation in human leukocyte antigen (HLA)‐incompatible highly sensitized recipients through cleavage of donor‐specific IgG into Fc‐ and F(ab′)2 fragments, abrogating complement activation and Fcγ receptor (FcγR)‐mediated mechanisms. 8 Imlifidase is a highly interesting candidate for desensitization to enable bone marrow engraftment and chimerism induction in sensitized hosts. 9 Endoglycosidase of Streptococcus pyogenes (EndoS), which deglycosylates the Fc portion of all subclasses of human IgG and thus reduces the affinity of IgG to FcγRs, is also an interesting candidate. 10 The inhibition of complement‐ and FcγR‐mediated effector functions has been shown to inhibit red blood cell lysis and to alleviate antibody‐mediated arthritis in animal models. 11 , 12 We, therefore, hypothesized that imlifidase and/or EndoS could be used for allogeneic BMT in sensitized recipients. Imlifidase cleaves all human IgG subclasses; however, it cleaves only mouse IgG2c and IgG3 but not IgG1 and IgG2b. 13 Interestingly, EndoS has been shown to reduce complement‐ and FcγR‐mediated functions of murine IgG1 and IgG2b. However, EndoS‐treated IgG2a and IgG2c have been shown to maintain cytolytic activity via FcγR but IgG2c has also been shown to lose some binding affinity depending on the conditions. 11 , 12 , 14 We, therefore, studied imlifidase and EndoS in combination to achieve the greatest effect on DSA‐inactivation in mice to establish a model of enzymatic desensitization prior to BMT.
Whereas extensive information exists for imlifidase, no data for EndoS on DSA‐inactivation have previously been reported. 8 , 9 We show here that EndoS inhibits DSA‐mediated killing of donor bone marrow cells (BMC) in a DSA titer‐dependent manner. The combination of imlifidase and EndoS improves the survival of donor hematopoietic cells in allosensitized mice. Using a stringent model of sensitized NOD recipients that are resistant to irradiation and tolerance induction, 15 , 16 we show that a combined approach that includes both imlifidase and EndoS permits the generation of mixed hematopoietic chimerism in mice.
2. MATERIALS AND METHODS
2.1. Animals
Adult NOD/ShiLtJ (H‐2g7; termed NOD), FVB/NJ (H‐2q; termed FVB), C57BL/6J (H‐2b; termed B6.CD45.2), B6.SJL‐Ptprc a Pepcb./Boy (H‐2b, termed B6.CD45.1), B6.NOD‐(D17Mit21‐D17Mit10) (H‐2g7; termed B6.H‐2g7), NOD.B10Sn‐H2b/J (H‐2b; termed NOD.H‐2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME), bred and housed in a specific pathogen‐free facility at the University of Alberta. All care and handling of animals were conducted in accordance with the guidelines of the Canadian Council on Animal Care. All NOD mice used for chimerism induction were females at 8‐10 weeks of age.
2.2. Reagents for in vivo experiments
Imlifidase and EndoS were provided by Hansa Biopharma AB (Lund, Sweden) and used with permission. Anti‐CD4 (clone Gk1.5, rat IgG2b), anti‐CD90 (clone YTS154, rat IgG2b), anti‐CD8α (clone YTS169.4, rat IgG2b), and anti‐MHC‐I H‐2Kb (clone B8.24.3, mouse IgG2b) mAbs were generated in house. The YTS 169.4 anti‐mouse CD8α mAb producing cells were developed by Professor H Waldmann and Dr SP Cobbold (Department of Pathology, University of Cambridge) and obtained via Cambridge Enterprise Limited (Cambridge, UK). Cyclophosphamide (29875) and bortezomib (A2614) were purchased from Sigma (St. Louis, MO) and ApexBio (Boston, MA), respectively.
2.3. In vivo EndoS mediated monoclonal DSA inhibition assay
NOD or B6.H‐2g7 mice were intravenously (iv) injected with vehicle, anti‐MHC‐I H‐2Kb (10 μg) alone, or a mixture of EndoS (30 μg) and anti‐MHC‐I H‐2Kb (10 or 100 μg) as a pretreatment. EndoS and anti‐H‐2Kb were mixed right before injection. Four hours after this pretreatment, 5 million cells of a 1:1 mixture of carboxyfluorescein succinimidyl ester (CFSE) labeled NOD and cell trace violet dye (CTV) labeled B6 BMC were iv injected into the pretreated NOD mice. Similarly, a 1:1 mixture of CFSE labeled B6.H‐2g7 and CTV labeled NOD.H‐2b BMC were injected into pretreated B6.H‐2g7 mice. Blood was collected at 1, 2, and 3 hours post‐cell administration and analyzed by flow cytometry. Splenocytes and BMC from one hind limb were collected from each mouse and analyzed at 4 hours post‐BMC injection.
2.4. Serum DSA detection assay
NOD mice were sensitized by i.p. administration of 20 × 106 FVB splenocytes. Sera were collected prior to and at 4‐6 weeks post‐sensitization as well as at 4 hours post imlifidase and EndoS treatment. FVB splenocytes (2 × 105) were treated with FcR blockade (anti‐mouse CD16/CD32 rat IgG2b antibodies, clone 2.4G2, BE0307, Bio X cell) for 5 minutes, followed by incubation with a titrated amount of sera in 100 μL for 30 minutes. Cells were washed twice and incubated with fluorochrome conjugated secondary antibodies in 100 μL for 30 minutes. The following secondary antibodies were used: FITC conjugated F(ab′)₂ fragment from rabbit anti‐mouse IgG Fc antibody (1:200, 315‐096‐046, Jackson ImmunoResearch, West Grove, PA), APC conjugated goat anti‐mouse IgG1 Fc antibody (1:100, 115‐135‐205, Jackson ImmunoResearch), and FITC conjugated goat anti‐mouse IgG3 Fc antibody (1:100, 115‐095‐209, Jackson ImmunoResearch). Cells were washed twice and analyzed by flow cytometry. Hanks' Balanced Salt Solution with 2% fetal bovine serum was used for cell washes and reconstitution.
2.5. BMT protocol and definition of chimerism
To determine the short‐term survival of donor BMC in sensitized recipients, NOD mice that had been sensitized to B6.CD45.1 splenocytes were T cell depleted (anti‐CD4, 0.25 mg, anti‐CD8, 0.25 mg, anti‐CD90 0.3 mg, i.p.) 2 days prior to BMT and iv injected with EndoS and imlifidase 4 hours prior to BMT (80 × 106 B6.CD45.2 BMC via iv injection). Splenocytes and BMC were analyzed at 4 hours post‐BMC injection.
For long‐term chimerism induction, NOD mice that had been sensitized to FVB splenocytes were treated with imlifidase and EndoS iv on day ‐6 with respect to the date of BMT. Cyclophosphamide (150 mg/kg, i.p. or iv) and bortezomib (1 mg/kg, iv) were given on day ‐4. T cell‐depleting antibodies were administered i.p. on day ‐2, 2, 6, 11, and 16. A repeated dose of imlifidase and EndoS and 6 Gy total body irradiation (TBI, Gammacell 1000 Elite; Theratronics, Ottawa, Ontario, Canada) was given at 4 hours prior to BMT on day 0. FVB BMC (80 × 106) were given iv via the lateral tail vein on day 0. In experiments determining the effects of cyclophosphamide and bortezomib on sensitized recipients prior to BMT, a lower dose (20 × 106) of BMC was given to limit potential adsorption of DSA on donor BMC. Peripheral blood was collected for flow cytometry analysis at the indicated time points. For long‐term chimerism, recipients were considered chimeric when at least 5% of MHC‐I+ cells in the lymphocyte gate were donor derived at day 28 post‐BMT.
2.6. Antibodies and flow cytometry
Fluorochrome‐labeled antibodies against mouse H‐2Kd (SF1‐1.1.1), H‐2Kq (KH114), H‐2Kb (AF6‐88.5), CD45.2 (104), CD19 (6D5), CD138 (281‐2), B220 (RA3‐6B2), TCRβ (H57‐597), CD4 (RM4‐5 or RM4‐4), CD8β (H35‐17.2), CD11b (M1/70), CD11c (N418), CD49b (DX5), CD122 (TM‐β1), were purchased from BD Pharmingen (San Diego, CA), BioLegend (San Diego, CA), or Thermo Fisher Scientific (Waltham, MA). An LSR II flow cytometer was used for data acquisition, and data analysis was performed using FlowJo (TreeStar software; Ashland, OR).
2.7. Statistical analysis
Mann‐Whitney U test, Ratio paired t test, one‐way analysis of variannce with Holm‐Sidak's multiple comparison test, and Fisher's exact test were used where appropriate, as indicated. All statistical analyses were done using Prism (GraphPad Software, La Jolla, CA).
3. RESULTS
3.1. EndoS inhibits the monoclonal DSA mediated killing of donor BMC
To evaluate the effect of EndoS on inhibiting the antibody‐mediated killing of donor BMC, DSA passive transfer experiments were performed. Of all DSA, anti‐donor major histocompatibility complex (MHC) or HLA antibodies are of most importance in the clinic. 2 Therefore, naïve NOD mice were injected with mouse IgG2b antibodies targeting MHC‐I Kb expressing cells, treated with EndoS or left untreated, and thereafter subjected to bone marrow transfer from B6 mice.
As shown in Figure 1A,B in NOD recipients given a single dose of 10 μg anti‐Kb mAb, the ratios of B6 to NOD cells in blood at 1 hour post‐BMT were significantly increased in mice treated with EndoS as compared to those that did not receive enzyme treatment. This difference in ratio of B6 to NOD cells in blood between the two groups remained stable at 2 and 3 hours post‐BMT. Similarly, mice given 100 μg anti‐Kb mAb with EndoS led to an increased ratio of B6 to NOD cells in the blood at 1 and 2 hours compared with treatment with 100 μg anti‐Kb mAb alone. However, the increased ratio did not last to 3 hours, suggesting that residual mAb effector function accumulated over time. At 4 hours post‐BMT, a significant increase in the ratio of B6 to NOD cells in both BM and spleen was also observed in mice treated with EndoS and 10 μg anti‐Kb mAb as compared to those that received 10 μg anti‐Kb mAb only.
FIGURE 1.
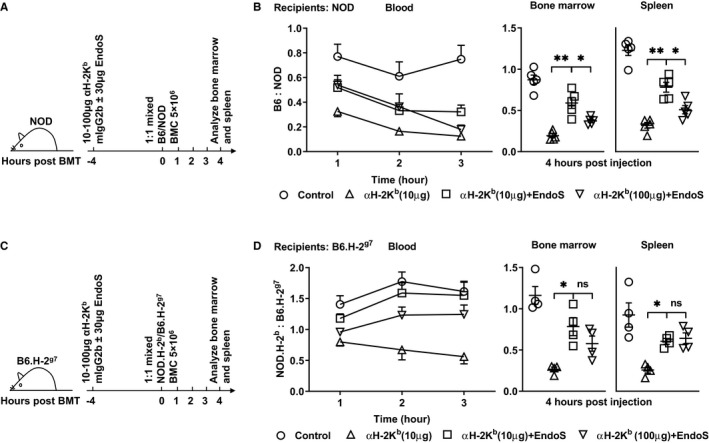
Endoglycosidase of Streptococcus pyogenes (EndoS) inhibits monoclonal donor‐specific antibodies mediated killing of donor bone marrow cells (BMC). Naive NOD (panel A,B) or B6.H‐2g7 (panel C,D) were given 30 μg EndoS and/or anti‐H‐2Kb mAb (10 or 100 μg) intravenously 4 h prior to the infusion of a mixture of carboxyfluorescein succinimidyl ester (CFSE) labeled NOD/ cell trace violet dye (CTV) labeled B6 (BMC; panel A,B) or CFSE labeled B6.H‐2g7/CTV labeled NOD.H‐2b BMC (panel C,D). Shown are experiment protocols for NOD (panel A) and B6.H‐2g7 (panel C). Shown are the ratios of dye labeled B6 to NOD cells (panel B) or NOD.H‐2b to B6.H‐2g7 cells (panel D) in the blood (left panels) collected 1‐3 h after bone marrow transplantation (BMT), in host spleens (middle panels) and bone marrow (BM, right panels) collected at 4 h after BMT. Mean ± SEM are shown. Data were pooled from five (panel B) and four (panel D) independent experiments. Mann‐Whitney U test (middle and right panels) was used for the comparisons shown; *P < .05 and **P < .01
Of note, NOD mice lack hemolytic complement C5, which is essential for complement dependent cytotoxicity and is not genetically linked with MHC genes. 17 Thus, the effect of DSA in NOD mice may be decreased compared with complement sufficient hosts. We, therefore, also examined the role of EndoS on DSA in complement sufficient hosts. NOD MHC congenic B6.H‐2g7 mice were used as recipients. EndoS improved the ratios of donor to recipient cells to a similar extent in B6.H‐2g7 mice as compared to NOD hosts (Figure 1C,D).
In brief, EndoS improved survival of donor cells in the presence of anti‐MHC antibodies whether or not the recipients were complement sufficient, suggesting an effect on other mechanisms of depletion (eg, FcγR‐mediated).
3.2. EndoS improves survival of donor BMC in presensitized recipients
Next, we investigated if EndoS could improve donor BMC survival in allosensitized recipients that had a diversified antibody repertoire against donor antigens. In order to test this, we combined EndoS with imlifidase. Imlifidase cleaves murine IgG2c and IgG3 but is not able to cut murine IgG1 and IgG2b. 13 Therefore, EndoS was coadministered to attenuate the effector function of the murine IgG isotypes that are not cleaved by imlifidase. 11 , 13 , 14 As shown in Figure 2A, imlifidase and EndoS together led to a significant reduction of DSA‐IgG in NOD mice that had been sensitized to FVB splenocytes. This decline in IgG targeting donor cells was likely due to imlifidase, and not EndoS, because deglycosylation still allows the Fc‐specific detection antibody to bind. The differential sensitivity for murine IgG isotypes is also illustrated by the approximately 80% reduction of DSA‐IgG3 (Figure 2C), a subclass that is cleaved by imlifidase, whereas no change in the level of DSA‐IgG1 (Figure 2B) was seen. Although the degradation of IgG3 by imlifidase caused only a moderate reduction of intact IgG, EndoS could further contribute to the reduction of DSA‐IgG effector functions through the deglycosylation of imlifidase resistant IgG molecules. The combination of both enzymes allowed us to analyze donor cell survival in sensitized recipients with polyclonal DSA.
FIGURE 2.
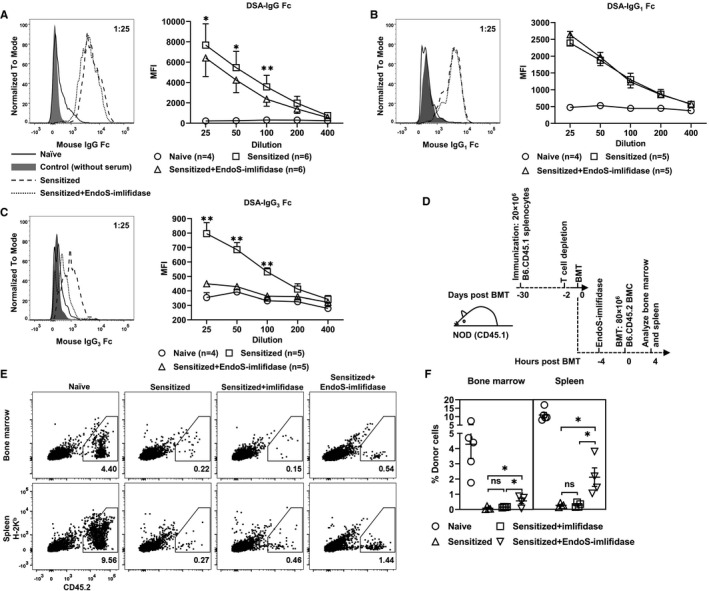
Endoglycosidase of Streptococcus pyogenes (EndoS)‐imlifidase reduces donor‐specific antibodies (DSA)‐mediated killing of donor bone marrow cells (BMC) in sensitized recipients. A‐C, Naive NOD mice were immunized with FVB splenocytes 4 weeks prior to the administration of EndoS‐imlifidase. Sera were harvested prior to immunization, prior to and 4 h after enzyme treatment. Representative histograms on the left are for DSA‐IgG Fc (panel A), DSA‐IgG1 Fc (panel B), DSA‐IgG3 Fc (panel C), and DSA‐IgG3 heavy chain (panel D) with sera at a 1:25 dilution. Mean fluorescence intensity (MFI) of DSA in the titrated sera is shown on the right. Mean ± SEM are shown. Ratio paired t test was used to compare MFI of DSA before and after enzyme treatment at each serum dilution with *P < .05, **P < .01. D, Schematic of the experiment shown in E,F. Naive NOD mice were immunized with B6.CD45.1 splenocytes 4 weeks prior to injection of T cell–depleting mAbs. EndoS‐imlifidase was administrated 2 d post‐T cell depletion. Four hours after enzyme treatment, NOD mice were injected with 80 million B6.CD45.2 BMC intravenously. Splenocytes and BMC were analyzed for the expression of MHC‐I H‐2Kb and CD45.2. E,F, Shown are representative dot plots of the four different treatment groups (on the left) and the percentage of donor cells (on the right, mean ± SEM). One‐way analysis of variance with Holm‐Sidak's multiple comparisons were used to compare values between the three sensitized groups with *P < .05
In addition to DSA, primed donor antigen‐specific cytotoxic T cells may contribute to the rapid killing of donor BMC. Therefore, CD45.1 NOD recipients that had been sensitized with B6.CD45.1 splenocytes were T cell‐depleted 2 days before imlifidase and EndoS treatment in order to avoid the acute cytotoxic effect mediated by sensitized T cells (Figure 2D). Over 95% of T cells in the peripheral blood were depleted in the recipients at 2 days after giving T cell‐depleting mAbs (data not shown). Here, the CD45.1/2 system was used to assist the identification of surviving donor BMC, the MHC staining on which may be interfered with by DSA. As shown in Figure 2E‐F, B6.CD45.2 donor cells were almost completely eliminated at 4 hours post‐BMT in sensitized NOD mice when given vehicle control (BM 0.22% and spleen 0.27%) or only imlifidase (BM 0.15% and spleen 0.46%). In contrast, close to 0.5% of BMC and around 1.5% of splenocytes in primed NOD mice treated with EndoS and imlifidase were from the B6.CD45.2 donor. Thus, administration of imlifidase and EndoS 4 hours prior to BMT rescued a significant proportion of donor BMC in allosensitized recipients as compared to sensitized recipients treated with vehicle or imlifidase alone (Figure 2F). Interestingly, the majority of residual donor cells in recipients treated with imlifidase and EndoS demonstrated low MHC‐I Kb staining, suggesting donor MHC epitopes were blocked by either deglycosylated DSA or F(ab′)2 of DSA (Figure 2E). Alternatively, the surviving donor cells may have been those that expressed less MHC class I to begin with.
Taken together, these data indicated that imlifidase and EndoS together improved the donor BMC survival in allosensitized recipients.
3.3. Bortezomib and cyclophosphamide treatment prior to BMT reduced B cells in BM
In order to use imlifidase and/or EndoS for BMT in sensitized recipients, methods that reduce DSA‐producing cells are necessary to provide a longer window of the low DSA environment for the continuous survival and further development of donor cells post‐BMT. In an attempt to reduce existing plasma cells and B cells that can differentiate into plasma cells after BMT, we employed bortezomib to deplete antibody‐producing cells and cyclophosphamide to reduce B cells prior to BMT in sensitized mice (Figure 3A). 18 , 19 The combination of bortezomib and cyclophosphamide (CyBor) has been used in patients with nontransplant eligible multiple myeloma and for prevention of graft‐versus‐host disease (GVHD) post‐allogeneic BMT but rarely used for the purpose of DSA desensitization. 20 , 21 , 22
FIGURE 3.
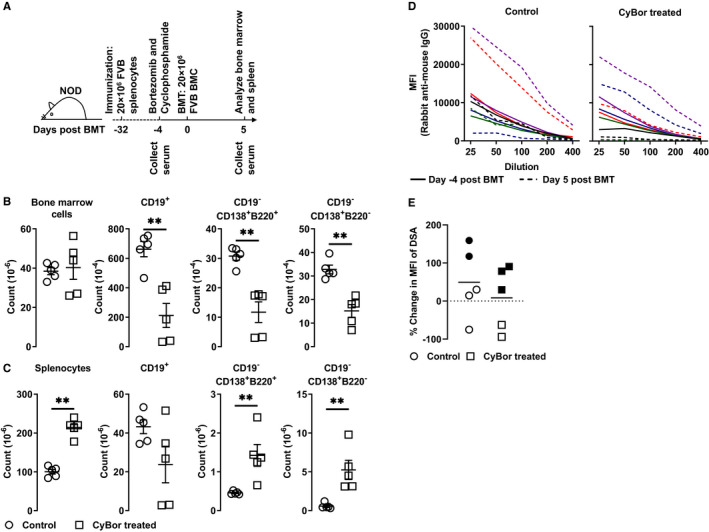
Bortezomib/cyclophosphamide prior to bone marrow transplantation (BMT) reduces bone marrow B cells in sensitized recipients. A, Schematic of the experiment shown in B‐E. Four weeks after immunization with FVB splenocytes, NOD mice were treated with cyclophosphamide and bortezomib (CyBor) intravenously. Four days after CyBor treatment, bone marrow transplantation with 20 million FVB bone marrow cells (BMC) was done. Splenocytes and BMC were collected 5 d after BMT for analysis. Sera were collected before CyBor treatment and 5 d post‐BMT. Shown are cell counts of B cells and plasma cells in the bone marrow (panel B) and spleens (panel C) in mice given CyBor or vehicle. D, Sera were collected prior to immunization and 5 d post‐BMT, ie 9 d after CyBor treatment. Shown are mean fluorescence intensity (MFI) of donor‐specific antibodies (DSA)‐IgG Fc in the titrated sera from individual control (on the left) or CyBor treated mice (on the right). E, Shown are percentile changes at day 9 in MFI of DSA at the 1:25 dilution compared to pretreatment. Filled and empty symbols represent data collected in two separate experiments
At 5 days after BMT, the cellularity of BMC in the BM did not differ between groups. Interestingly, the overall number of splenocytes increased in the group of mice pretreated with CyBor. However, compared to the vehicle group, BM CD19+ B cells, CD19−CD138+B220+ plasmablasts, and CD19−CD138+B220− plasma cells were significantly reduced in mice treated with CyBor (Figure 3B). In contrast to the reduction of B cells in the BM, the reduction of splenic CD19+ B cells was not significant at the time examined in the CyBor treated group. Moreover, there were significant increases of CD19−CD138+B220+ plasmablasts and CD19−CD138+B220− plasma cells in the spleens from CyBor treated mice (Figure 3C).
We then examined whether the CyBor treatment prevented increased DSA formation stimulated by the BMC injection. As shown in Figure 3D, DSA levels increased substantially in two of five mice in the control group and two of five mice in the CyBor treated group, suggesting that CyBor was not able to decrease DSA levels. However, when percentile changes of DSA levels 5 days after BMT (9 days post‐CyBor) were compared, the increases of DSA tended to be less in mice treated with CyBor, suggesting that CyBor treatment prior to BMT may inhibit the increase of DSA stimulated by BMC injection (Figure 3E).
In summary, these data showed the effect of CyBor in reducing B cell numbers was pronounced in BM and CyBor may limit the increase in DSA caused by the BMC injection.
3.4. Engraftment is achievable in presensitized recipients with combination of Imlifidase, EndoS, T cell depletion, and CyBor
With these data, we hypothesized that imlifidase and EndoS in combination with T cell depletion antibodies and bone marrow plasma cell depletion by CyBor, together with a nonlethal dose of irradiation, and a large dose of BMC would allow engraftment of donor cells in presensitized recipients. We explored if such protocol would induce chimerism in NOD mice as well as in B6.H‐2g7 mice, which are MHC matched with NOD but are not resistant to chimerism induction. 23 Recipient mice were sensitized with FVB cells 4 weeks prior to the chimerism induction. Naive and primed recipients were given the same conditioning protocol, as indicated in the methods section and Figure 4A.
FIGURE 4.
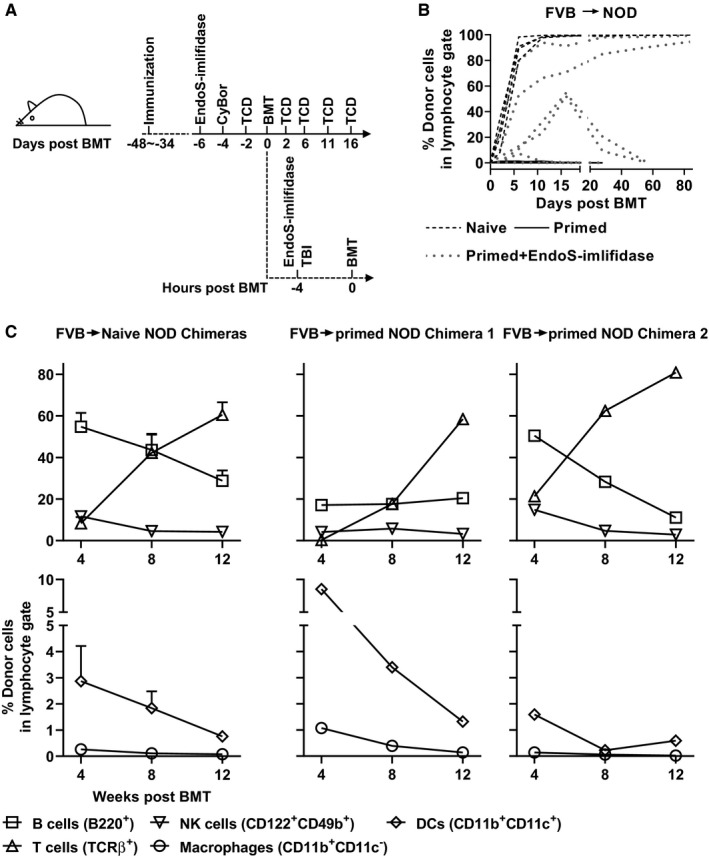
Endoglycosidase of Streptococcus pyogenes (EndoS)‐imlifidase allows hematopoietic chimerism in presensitized recipients. A, Schematic of the chimerism induction protocol; naive B6.H‐2g7 or NOD mice were immunized with FVB splenocytes 4‐6 wk prior to chimerism induction. For chimerism induction, bortezomib and cyclophosphamide was given on day ‐4 with respect to the date of bone marrow transplantation (BMT). T cell–depleting (TCD) antibodies were administered i.p. on day ‐2, 2, 6, 11, and 16. Some recipients that had been sensitized to FVB splenocytes were treated with EndoS‐imlifidase iv on day ‐6 and a repeated dose on day 0 at 4 h before BMT. Six Gy total body irradiation was given at 4 h prior to BMT on day 0. FVB bone marrow cells (80 × 106) were given on day 0. B, Shown are the proportions of donor cells in lymphocyte gate in peripheral blood over time. C, Shown are percentages of different lineages of donor cells in lymphocyte gate in peripheral blood from naïve NOD chimeras (n = 4, on the left, mean ± SEM) and primed NOD chimeras (n = 2, on the right). Data were pooled from six independent experiments
As expected, while all naive mice became nearly fully chimeric with FVB cells at 4 weeks post‐BMT, donor cells were rejected in primed mice that were not treated with imlifidase and EndoS. As shown in Figure 4B, donor cells were not detectable even at 2 days post‐BMT in sensitized NOD mice that did not receive enzymes. In contrast, donor cells were more than 5% on day 4 or 9 after BMT in five of seven sensitized NOD recipients given enzyme treatment. Furthermore, in four enzyme‐treated sensitized NOD mice, chimerism levels increased steadily to over 50% on day 16 post‐BMT. Eventually, five of the eight presensitized NOD and B6.H‐2g7 mice were chimeric with donor cells at 4 weeks post‐BMT, with two primed NOD mice being stable mixed chimeras with multiple lineages of donor cells in the periphery (Table 1 and Figure 4C). No sign of GVHD was observed in any chimeras. In an attempt to simplify this protocol by eliminating either cyclophosphamide or bortezomib, it appeared that both of them were essential for the success of the current protocol for inducing chimerism in sensitized recipients (Table 1).
TABLE 1.
Endoglycosidase of Streptococcus pyogenes (EndoS)‐imlifidase allows hematopoietic chimerism in presensitized recipients
| Treatment group | Engraftment a | Chimerism levels e |
|---|---|---|
| Not primed | ||
| Bortezomib and cyclophosphamide (CyBor) | 6/6 a | >90% |
| Primed | ||
| CyBor | 0/7 b | |
| CyBor‐EndoS‐imlifidase* | 5/8 c | 98%, 85%, 57%, 20%, 9% |
| Cy‐EndoS‐imlifidase | 0/2 d | |
| Bor‐EndoS‐imlifidase | 0/3 d | |
See figure legend of Figure 4 for details of chimerism induction protocol.
Represents B6.H‐2g7 (n = 2) and NOD recipients (n = 4).
Represents B6.H‐2g7 (n = 2) and NOD recipients (n = 5).
Represents one B6.H‐2g7 (n = 1) and NOD recipients (n = 7).
Represents NOD recipients.
Shown are chimerism levels at 4 wk post bone marrow transplantation.
P < .05 by two‐sided Fisher's exact test when compared to “CyBor” primed group.
In summary, imlifidase and EndoS together enable donor BMC engraftment in presensitized recipient mice when combined with CyBor and standard conditioning agents.
4. DISCUSSION
DSA is a main obstacle for allogeneic BMT in sensitized recipients. 5 , 6 Previous work showed that imlifidase can be used for eliminating DSA and EndoS can inhibit IgG mediated cytotoxicity in different models. 10 , 11 , 12 However, neither of the two enzymes have yet been tested for the purpose of facilitating BMT, where the high expression of MHC on bone marrow derived cells may increase sensitivity to remaining functional DSA. With the promising results from recent clinical trials for kidney transplantation in sensitized recipients, together with our preclinical BMT results, there is now little doubt that imlifidase could be used in BMT for desensitization in humans. 8 , 9
In the current study, we also addressed the potential use of EndoS in BMT. We found that EndoS alone improved survival of donor cells in the presence of DSA in vivo. Considering EndoS‐treated IgG reduces ability to fix complement, as reported by Maria Allhorn and Mattias Collin, our finding that EndoS improved the survival of donor cells to a similar extent in B6.H‐2g7 and NOD suggested that additional mechanisms, such as FcγRs, were a major mediator of the pathogenicity of DSA in this BMT‐model. 12 The differences between NOD and B6.H‐2g7 mice given a low or high dose of monoclonal DSA and EndoS indicate that the non‐MHC genes may have an impact on the efficacy of EndoS in different individuals. This difference between NOD and B6.H‐2g7 may be attributable to the different binding capacities of IgG2b with various Fc receptors in mice on the NOD and B6 background. 24 , 25 The FcR polymorphisms may be important as well. 26 Our results also suggest that the effects of EndoS are more potent on lower titer DSA.
We found that the combination of imlifidase and EndoS improved the survival of donor BMC and allowed donor chimerism in sensitized mice that had been conditioned with T cell depletion, CyBor, and sublethal irradiation. In our protocol, the effect of T cell depletion in the periphery was not affected by EndoS. This suggests that with appropriately designed timing, EndoS can be used together with antibody‐based products like IVIG and B cell depletion antibodies such as rituximab.
With regard to the use of cyclophosphamide and bortezomib, both have immune modulatory effects other than targeting B cells or plasma cells. 27 For example, cyclophosphamide can facilitate the chimerism induction in sensitized recipients by reducing memory T cells as shown in our previous study. 28 As for bortezomib, our finding is consistent with the published data showing the compensatory increase of splenic B cells after bortezomib treatment, which in turn resulted in humoral compensation. 29 However, whether or not this increase of splenic B cells after BMT is accompanied by a rebound of DSA in the current study remains unknown. Importantly, T cell depletion employed in our protocol may potentially inhibit the recovery and maturation of both naïve and memory B cells, and the generation of de novo DSA.
Lastly, the findings of this study have to be considered in light of some limitations. Although imlifidase cleaves all the human IgG subclasses, it cleaves only two subclasses of mouse IgG, and IgM is not affected. Although IgM DSA levels are low compared to IgG, 6 they may have reduced the levels of chimerism we observed. In the clinic, IgM DSA could be removed by plasmapheresis. In order to achieve maximum effect on DSA in mice, we had to combine EndoS and imlifidase. It has been shown that imlifidase temporally inhibits the activation of memory B cells by cleavage of membrane‐bound BCR in vitro, but it is unclear if this effect of imlifidase is important in the success of chimerism induction in sensitized recipients. 30 On the other hand, imlifidase only cleaves mouse IgG2c and IgG3 and the effect of imlifidase on mouse IgG was not complete in this model (Figure 2A). A protocol with imlifidase as only the desensitizing agent is likely to be more efficient in humans where imlifidase completely inactivates the IgG DSA pool. 8 , 9 Thus, our findings may underestimate the potential for these enzymes in the clinical setting. The second limitation concerns the toxicity of the chimerism induction protocol. However, the current study is a proof of principle study showing that modulating IgG Fc can be strategically useful for BMT in sensitized recipients. Furthermore, EndoS or imlifidase can be used in combination with other desensitization methods. Currently, it is not known whether the enzyme‐mediated blocking of DSA prevents a rebound in antibody. Perhaps maintaining a certain level of DSA while blocking DSA function, that is, deglycosylation of IgG Fc, may have less potential to trigger a rebound than complete removal of the DSA. We employed a short time frame for repeated enzyme injection (6 days between injections) in order to avoid reduced activity as a result of host anti‐enzyme antibody production. 31 The greater efficacy of imlifidase in the human setting may allow the enzymes to be given separately, imlifidase followed by EndoS, alleviating the concern of anti‐enzyme antibodies.
Finally, we conclude that the combination of imlifidase and EndoS can be used for inducing donor chimerism in allosensitized recipient mice in combination with other desensitization strategies.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. JL, LB, and CCA declare no competing financial interests. A‐KR, RB, and CK are employed by Hansa Biopharma and are holders of shares or share warrants in Hansa Biopharma AB.
AUTHOR CONTRIBUTIONS
JL designed and performed experiments, analyzed data, and wrote the manuscript; CCA designed experiments, analyzed data, and wrote the manuscript; LB generated reagents and edited the manuscript. RB designed and performed experiments, interpreted data and revised the manuscript. A‐KR and CK designed experiments, interpreted data, and revised the manuscript.
ACKNOWLEDGMENTS
We thank Perveen Anwar and HSLAS staff for assistance with animal care and technical assistance. This work was supported by grants to CCA from the Canadian Diabetes Association and the Canadian Institutes of Health Research (PS148588) and to CCA and JL from the Edmonton Civic Employees Charitable Assistance Fund; JL is supported by a doctoral studentship from Li Ka Shing Foundation (Sino‐Canadian Exchange program).
Lin J, Boon L, Bockermann R, Robertson A‐K, Kjellman C, Anderson CC. Desensitization using imlifidase and EndoS enables chimerism induction in allosensitized recipient mice. Am J Transplant. 2020;20:2356–2365. 10.1111/ajt.15851
Contributor Information
Jiaxin Lin, Email: colinand@ualberta.ca, Email: jlin9@ualberta.ca.
Colin C. Anderson, Email: colinand@ualberta.ca.
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available from the corresponding author CCA on request.
REFERENCES
- 1. Oura T, Cosimi AB, Kawai T. Chimerism‐based tolerance in organ transplantation: preclinical and clinical studies. Clin Exp Immunol. 2017;189(2):190‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciurea SO, Cao K, Fernandez‐Vina M, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor‐specific anti‐HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(5):521‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sethi S, Choi J, Toyoda M, Vo A, Peng A, Jordan SC. Desensitization: overcoming the immunologic barriers to transplantation. J Immunol Res. 2017;2017:6804678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colson YL, Schuchert MJ, Ildstad ST. The abrogation of allosensitization following the induction of mixed allogeneic chimerism. J Immunol. 2000;165(2):637‐644. [DOI] [PubMed] [Google Scholar]
- 5. Taylor PA, Ehrhardt MJ, Roforth MM, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu H, Chilton PM, Tanner MK, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108(10):3611‐3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA‐sensitized patients. Am J Transplant. 2006;6(3):459‐466. [DOI] [PubMed] [Google Scholar]
- 8. Lorant T, Bengtsson M, Eich T, et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti‐HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am J Transplant. 2018;18(11):2752‐2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med. 2017;377(5):442‐453. [DOI] [PubMed] [Google Scholar]
- 10. Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20(12):3046‐3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass‐dependent manner. Proc Natl Acad Sci USA. 2008;105(39):15005‐15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allhorn M, Briceño JG, Baudino L, et al. The IgG‐specific endoglycosidase EndoS inhibits both cellular and complement‐mediated autoimmune hemolysis. Blood. 2010;115(24):5080‐5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nandakumar KS, Johansson BP, Björck L, Holmdahl R. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo. Arthritis Rheum. 2007;56(10):3253‐3260. [DOI] [PubMed] [Google Scholar]
- 14. Kao D, Danzer H, Collin M, et al. A monosaccharide residue is sufficient to maintain mouse and human IgG subclass activity and directs IgG effector functions to cellular Fc receptors. Cell Rep. 2015;13(11):2376‐2385. [DOI] [PubMed] [Google Scholar]
- 15. Li H, Kaufman CL, Boggs SS, et al. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in nonobese diabetic (NOD) mice. J Immunol. 1996;156(1):380‐388. [PubMed] [Google Scholar]
- 16. Pearson T, Markees TG, Serreze DV, et al. Genetic disassociation of autoimmunity and resistance to costimulation blockade‐induced transplantation tolerance in nonobese diabetic mice. J Immunol. 2003;171(1):185‐195. [DOI] [PubMed] [Google Scholar]
- 17. Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993;42(11):1574‐1578. [DOI] [PubMed] [Google Scholar]
- 18. Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus‐like disease from nephritis. Nat Med. 2008;14(7):748‐755. [DOI] [PubMed] [Google Scholar]
- 19. Turk JL, Poulter LW. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972;10(2):285‐296. [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Homsi A‐S, Cole K, Bogema M, Duffner U, Williams S, Mageed A. Short course of post‐transplantation cyclophosphamide and bortezomib for graft‐versus‐host disease prevention after allogeneic peripheral blood stem cell transplantation is feasible and yields favorable results: a phase I study. Biol Blood Marrow Transplant. 2015;21(7):1315‐1320. [DOI] [PubMed] [Google Scholar]
- 21. Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshihara S, Maruya E, Taniguchi K, et al. Risk and prevention of graft failure in patients with preexisting donor‐specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508‐515. [DOI] [PubMed] [Google Scholar]
- 23. Chan WFN, Razavy H, Luo B, Shapiro AMJ, Anderson CC. Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third‐party alloantigens in nonmyeloablative mixed chimeras. J Immunol. 2008;180(8):5177‐5186. [DOI] [PubMed] [Google Scholar]
- 24. Gavin AL, Tan PS, Hogarth PM. Gain‐of‐function mutations in FcγRI of NOD mice: implications for the evolution of the Ig superfamily. EMBO J. 1998;17(14):3850‐3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luan JJ, Monteiro RC, Sautes C, et al. Defective Fc gamma RII gene expression in macrophages of NOD mice: genetic linkage with up‐regulation of IgG1 and IgG2b in serum. J Immunol. 1996;157(10):4707‐4716. [PubMed] [Google Scholar]
- 26. van Sorge NM, van der Pol WL, van de Winkel JG. FcγR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61(3):189‐202. [DOI] [PubMed] [Google Scholar]
- 27. Al‐Homsi AS, Feng Y, Duffner U, et al. Bortezomib for the prevention and treatment of graft‐versus‐host disease after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2016;44(9):771‐777. [DOI] [PubMed] [Google Scholar]
- 28. Hong S‐H, Yoon I‐H, Kim Y‐H, et al. High‐dose cyclophosphamide‐mediated anti‐tumor effects by the superior expansion of CD44(high) cells after their selective depletion. Immunobiology. 2010;215(3):182‐193. [DOI] [PubMed] [Google Scholar]
- 29. Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28(7):1991‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Järnum S, Bockermann R, Runström A, Winstedt L, Kjellman C. The bacterial enzyme IdeS cleaves the IgG‐type of B cell receptor (BCR), abolishes BCR‐mediated cell signaling, and inhibits memory B cell activation. J Immunol. 2015;195(12):5592‐5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winstedt L, Järnum S, Nordahl EA, et al. Complete removal of extracellular IgG antibodies in a randomized dose‐escalation phase I study with the bacterial enzyme IdeS‐a novel therapeutic opportunity. PLoS One. 2015;10(7):e0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author CCA on request.


