Abstract
Patients with multiple myeloma (MM) inevitably relapse on initial treatment regimens, and novel combination therapies are needed. Ibrutinib is a first‐in‐class, once‐daily inhibitor of Bruton's tyrosine kinase, an enzyme implicated in growth and survival of MM cells. Preclinical data suggest supra‐additivity or synergy between ibrutinib and proteasome inhibitors (PIs) against MM. This phase 1/2b study evaluated the efficacy and safety of ibrutinib plus the PI carfilzomib and dexamethasone in patients with relapsed/refractory MM (RRMM). In this final analysis, we report results in patients who received the recommended phase 2 dose (RP2D; ibrutinib 840 mg and carfilzomib 36 mg/m2 with dexamethasone), which was determined in phase 1. The primary efficacy endpoint was overall response rate (ORR). Fifty‐nine patients with RRMM received the RP2D (18 in phase 1 and 41 in phase 2b). These patients had received a median of three prior lines of therapy; 69% were refractory to bortezomib, and 90% were refractory to their last treatment. ORR in the RP2D population was 71% (stringent complete response and complete response: 3% each). Median duration of clinical benefit and median duration of response were both 6.5 months. Median progression‐free survival (PFS) was 7.4 months, and median overall survival (OS) was 35.9 months. High‐risk patients had comparable ORR and median PFS (67% and 7.7 months, respectively) to non–high‐risk patients, whose ORR was 73% and median PFS was 6.9 months, whereas median OS in high‐risk patients was 13.9 months and not reached in non–high‐risk patients. The most common grade ≥3 hematologic treatment‐emergent adverse events (TEAEs) were anemia and thrombocytopenia (17% each); the most common grade ≥3 non‐hematologic TEAE was hypertension (19%). In patients with RRMM treated with multiple previous lines of therapy, ibrutinib plus carfilzomib demonstrated anticancer activity within the expected efficacy range. No new safety signals were identified and the combination was well‐tolerated.
Keywords: ibrutinib, carfilzomib, Bruton's tyrosine kinase inhibitor, multiple myeloma
1. INTRODUCTION
Multiple myeloma (MM) is characterized by clonal proliferation of plasma cells in the bone marrow, which leads to bone destruction and end‐organ damage.1 While stem cell transplant and novel drug combinations have contributed to improved outcomes, morbidity and mortality remain high. The availability of effective combination therapies including proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs) is critical, especially for high‐risk patients who historically respond well to combination therapies.2, 3
PIs target several anti‐apoptotic proteins that allow MM cells to survive and proliferate.4, 5 The PIs bortezomib (in combination with lenalidomide) and carfilzomib are approved for the treatment of relapsed/refractory MM (RRMM).6, 7, 8, 9, 10, 11 Bortezomib is associated with increased incidence of peripheral neuropathy and higher incidence of hematological adverse events (AEs) compared to other PIs used to treat patients with RRMM.12 Furthermore, patients who are refractory to bortezomib and IMIDs, such as lenalidomide, have poor outcomes compared to patients who are not refractory, with a median overall survival (OS) of 9 months vs ≥33 months, respectively.13 Carfilzomib is a next‐generation, selective, irreversible PI approved for the treatment of patients with MM who have received multiple previous therapies. Carfilzomib is structurally different from bortezomib and has demonstrated less off‐target activity (in particular neuropathy) than bortezomib toward a broad panel of proteases, and thus is an alternative PI to bortezomib in combination therapies.14
Ibrutinib is a first‐in‐class, once‐daily inhibitor of Bruton's tyrosine kinase (BTK) and is approved for the treatment of multiple B cell malignancies.15 BTK is overexpressed in 85% of MM cells, and BTK signaling may be required for MM cell growth and migration.16 Progression of MM leads to bone destruction via the interaction of MM cells with osteoclasts and factors that suppress bone formation and enhance bone resorption.17, 18 Preclinical data demonstrated that ibrutinib inhibited the growth of and reduced bone lysis in cultured BTK‐expressing human MM cells.16 Ibrutinib also reduced bone lysis, osteoclast number and activity, and tumor growth in a mouse MM model, and enhanced the activity of bortezomib and lenalidomide.16, 19 Ibrutinib plus dexamethasone provided a clinical benefit rate (CBR) of 28% and median progression‐free survival (PFS) of 4.6 months in patients with relapsed MM or RRMM who had a median of four prior therapies.20
Bortezomib and carfilzomib are known to stimulate osteoblasts; this observation, along with the observed osteoclastic inhibitory effects of ibrutinib, suggests that combining ibrutinib and carfilzomib may provide clinical benefit. Preclinical data support this synergy.16, 19, 21, 22, 23 This phase 1/2b, multicenter study evaluated the safety and efficacy of ibrutinib in combination with carfilzomib and dexamethasone in patients with RRMM who had multiple prior lines of therapy, all of whom had received bortezomib. Results from the phase 1 portion of the study, including determination of the recommended phase 2 dose (RP2D), have been previously reported.24 During phase 1, no dose‐limiting toxicities were observed, and the maximum tolerated dose of ibrutinib 840 mg once daily plus carfilzomib 20/36 mg/m2 and dexamethasone 20 mg was established as the RP2D.24 Here, we report the final efficacy and safety results in patients who received the RP2D in this phase 1/2b study. Outcomes in all patients who received treatment during the study, including subgroup analyses in high‐risk patients, are also presented.
2. PATIENTS AND METHODS
2.1. Study design
PCYC‐1119‐CA (NCT01962792) was a phase 1/2b, open‐label, multicenter study evaluating the safety and efficacy of ibrutinib in combination with carfilzomib plus dexamethasone in patients with RRMM who had received at least two previous lines of therapy. Details of the phase 1 study design were previously reported.24 For all patients, regardless of study phase, ibrutinib dosing began on day 8 of cycle 1 and was taken once daily continuously. Carfilzomib was administered intravenously for 30 (+10) min on days 1, 2, 8, 9, 15, and 16 of a 28‐day cycle from cycle 1 through cycle 12 and thereafter on days 1, 2, 15, and 16. The carfilzomib starting dose was 20 mg/m2 on days 1 and 2 of cycle 1, and if tolerated, the dose was increased to 36 mg/m2 on day 8 of cycle 1 and stayed at that level for subsequent cycles. Dexamethasone was administered (orally or intravenously) at a dose of 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of each treatment cycle, with patients ≥75 years of age receiving a reduced dose of 10 mg on these days.
2.2. Eligibility criteria
Key patient inclusion criteria were age ≥18 years; Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; measurable MM with at least two prior lines of therapy including bortezomib and an IMID and no response or disease progression to the most recent treatment regimen. Permitted laboratory parameters included adequate hematologic function without transfusion or growth factor support, including absolute neutrophil count ≥1000/mm3 and platelet counts ≥75 000/mm3, and adequate hepatic and renal function (including estimated creatinine clearance ≥30 mL/min). Key exclusion criteria included primary refractory disease defined as nonresponsive in patients who had never achieved a minimal response or better with any therapy, recent prior chemotherapy, prior treatment with ibrutinib or any other BTK inhibitor, prior carfilzomib treatment if patients were refractory or nonresponsive, requirement of strong CYP3A inhibitors or vitamin K antagonists (other anticoagulant and antiplatelet agent use was allowed on study), left ventricular ejection fraction <40%, and uncontrolled or symptomatic arrhythmias.
2.3. Endpoints and assessments
The primary efficacy endpoint for the phase 2b portion of the study was the overall response rate (ORR; defined as partial response [PR] or better) per investigator assessment according to 2011 International Myeloma Working Group response criteria.25 The analysis of ORR was conducted in the RP2D population, which included all patients from phase 1 and phase 2b who received the RP2D. Secondary efficacy endpoints were the rates of PFS, OS, and duration of response (DOR). The exploratory endpoint was the rate and duration of CBR, which included patients with minimal response [MR] or better according to IMWG response criteria.25 The safety and tolerability of ibrutinib in combination with carfilzomib and dexamethasone were also assessed.
Response evaluations were performed at the beginning of each cycle until disease progression or study closure, whichever occurred first. Stringent complete response (sCR) was defined as complete response (CR) plus normal serum free light chain ratio without bone marrow clonal cells. Very good partial response (VGPR) was defined as PR plus undetectable serum and urine M‐component or ≥90% reduction in serum M‐component plus urine M‐component <100 mg/24 h and ≥90% reduction in the difference between involved and uninvolved free light chain levels. Genomic high‐risk patients were defined as those who had the presence of at least a del17p or t(4;14) mutation. Cytogenetic variations were determined locally.
AEs were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03.26 Investigators assessed the occurrence of AEs and serious AEs at all evaluation timepoints during the study.
2.4. Statistical analyses
Efficacy and safety analyses were conducted in the RP2D population, which included patients enrolled during phase 1 or phase 2b who received at least one administration of the RP2D. Additional analyses were conducted in the all‐treated population, which included all patients who received at least one dose of study drug during the study. Efficacy was also analyzed by key subgroups within the RP2D and all‐treated patient populations.
2.5. Study oversight
This study was approved by the Institutional Review Board at each site, and all patients provided written consent. The study was sponsored by Pharmacyclics LLC, an AbbVie Company. Pharmacyclics led the study design, data collection, and statistical analysis. All authors had full access to all data.
2.6. Data sharing statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
3. RESULTS
3.1. Patients
Between January 15, 2014 and December 26, 2018, 84 patients with RRMM were enrolled (43 patients in phase 1 and 41 patients in phase 2b) across 18 centers. Fifty‐nine of these patients received treatment at the RP2D (18 patients in phase 1; 41 patients in phase 2b) (Table 1). In the RP2D population, median age was 63 years, 53% were male, and median time since initial diagnosis was 4.6 years (Table 2). Patients had received a median of three lines of prior therapy, and all patients were previously treated with bortezomib. Forty‐three patients (73%) had received a prior autologous stem cell transplant. All patients were refractory to or relapsed from previous therapies, including refractory to lenalidomide (n = 40, 68%) or bortezomib (n = 41, 69%), with 47% refractory to both lenalidomide and bortezomib; 90% were refractory to their last treatment (Table 3). Eighteen (31%) patients were considered high‐risk, and 33 (56%) were considered non–high‐risk (Table 2). The median time on study was 24.1 months (range: 0.9‐51.8). The median treatment duration for ibrutinib was 4.4 months (range: 0‐48.6); the most common reason for ibrutinib discontinuation was progressive disease (53%). There were no meaningful differences in baseline characteristics between the RP2D population and the all‐treated population (Tables 2 and 3).
Table 1.
Patient disposition
| Patients receiving RP2D n = 59; n (%) | |
|---|---|
| Patients treated | 59 (100) |
| Discontinued ibrutinib treatmenta | 57 (97) |
| Progressive disease | 31 (53) |
| AEs | 12 (20) |
| Investigator decision | 5 (8) |
| Withdrawal by patient | 5 (8) |
| Other | 4 (7) |
Abbreviations: AE, adverse event; RP2D, recommended phase 2 dose.
Two patients received carfilzomib but did not receive ibrutinib treatment.
Table 2.
Baseline demographics and disease characteristics
| Characteristic | Patients receiving RP2D n = 59 | All treated patients n = 84 |
|---|---|---|
| Median age, years (range) | 63 (34‐79) | 63.5 (34‐83) |
| Sex, n (%) | ||
| Female | 28 (47) | 41 (49) |
| Male | 31 (53) | 43 (51) |
| Median time since initial diagnosis, years (range) | 4.6 (0.5‐16.4) | 4.6 (0.5‐25.3) |
| ECOG performance status, n (%) | ||
| 0 | 21 (36) | 27 (32) |
| 1 | 32 (54) | 50 (60) |
| 2 | 6 (10) | 7 (8) |
| ISS stage at baseline, n (%) | ||
| I | 24 (41) | 39 (46) |
| II | 18 (31) | 23 (27) |
| III | 11 (19) | 16 (19) |
| Cytogenetic riska | ||
| High‐risk | 18 (31) | 22 (26) |
| Non–high‐risk | 33 (56) | 51 (61) |
| Unknown | 8 (14) | 11 (13) |
| Median hemoglobin, g/L (range) | 114 (69‐149) | 109 (69–149) |
| Median platelets, 109/L (range) | 164 (61‐443) | 167 (61‐443) |
| Median absolute neutrophil count, 109/L (range) | 2.3 (1.0‐9.3) | 2.3 (1.0–9.3) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System; RP2D, recommended phase 2 dose.
Cytogenetic risk assessment was performed locally. High cytogenetic risk was defined as the presence of t(4;14) and/or del17p.
Table 3.
Prior treatment exposure
| Treatment | Patients receiving RP2D n = 59 | All treated patients n = 84 |
|---|---|---|
| Median prior lines of therapy (range) | 3 (2‐10) | 3 (2‐10) |
| Prior stem cell transplant, n (%) | 59 (100) | 84 (100) |
| Prior lenalidomide, n (%) | 58 (98) | 82 (98) |
| Refractory to lenalidomide, n (%) | 40 (68) | 58 (69) |
| Prior bortezomib, n (%) | 59 (100) | 84 (100) |
| Refractory to bortezomib, n (%) | 41 (69) | 59 (70) |
| Refractory to lenalidomide and bortezomib, n (%) | 28 (47) | 42 (50) |
| Last treatment was a combination of a PI and an IMID, n (%) | 7 (12) | 15 (18) |
| Disease status to last treatment, n (%)a | ||
| Relapsed | 5 (8) | 9 (11) |
| Refractory | 53 (90) | 74 (88) |
Abbreviations: IMID, immunomodulatory drug; PI, proteasome inhibitor; RP2D, recommended phase 2 dose.
One patient had unknown disease status to their last treatment.
3.2. Efficacy
In the RP2D population (n = 59), 57 patients were considered response evaluable and two patients who did not receive ibrutinib were considered non‐evaluable. The ORR was 71%. Two patients (3%) each achieved a best response of sCR or CR, 12 patients (20%) achieved VGPR, and 26 patients (44%) achieved PR (Figure 1A). Three patients (5%) had progressive disease. The median DOR was 6.5 months (range: 0.5‐44.3). Median PFS was 7.4 months (95% CI: 4.6‐10.2), and the estimated 1‐year PFS rate was 33% (Figure 2A). Median OS was 35.9 months (95% CI: 23.1—not estimable [NE]), and the estimated 1‐year OS rate was 77% (Figure 3A). OS at 24 months was 60% (95% CI: 43.8%‐72.4%). The CBR was 78%, and the median duration of clinical benefit response was 6.5 months (95% CI: 3.7‐10.7). Similar efficacy results were observed in the all‐treated population (Figures 1A, 2A, and 3A).
Figure 1.
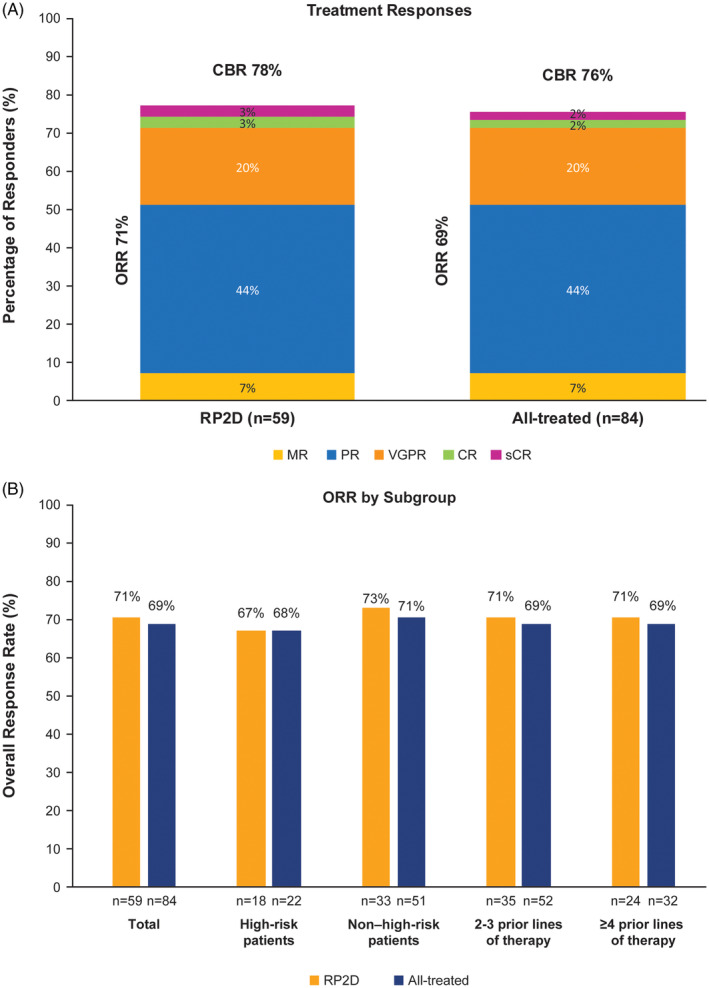
Best response and rate of clinical benefit response. Best response and CBR by response type for the RP2D population and the all‐treated population (A), and ORR by key subgroups for the RP2D population and the all‐treated population (B). Response was evaluated according to the International Myeloma Working Group (IMWG) response criteria, with ORR defined as a partial response or better. CBR, clinical benefit response; CR, complete response; MR, minimal response, ORR, overall response rate; PD, progressive disease; PR, partial response; RP2D, recommended phase 2 dose; sCR, stringent complete response; VGPR, very good partial response
Figure 2.
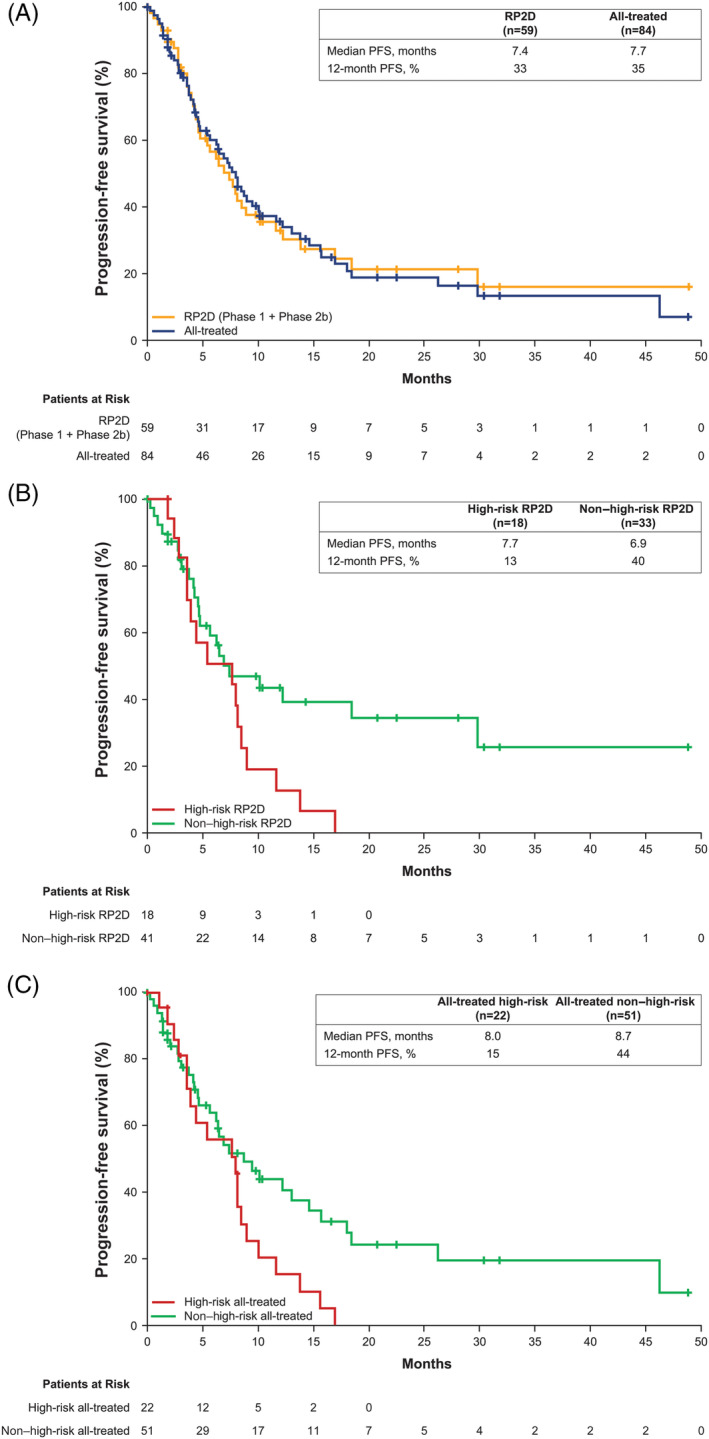
Progression‐free survival. Data shown are for patients receiving the RP2D and the all‐treated population (A). PFS data for high‐risk vs non–high‐risk patients in the RP2D population are shown in (B). PFS data for high‐risk vs non‐high‐risk patients in the all‐treated population are shown in (C). NE, not estimable; PFS, progression‐free survival. “+” indicates censored observation. NE, not estimable; PFS, progression‐free survival; RP2D, recommended phase 2 dose
Figure 3.
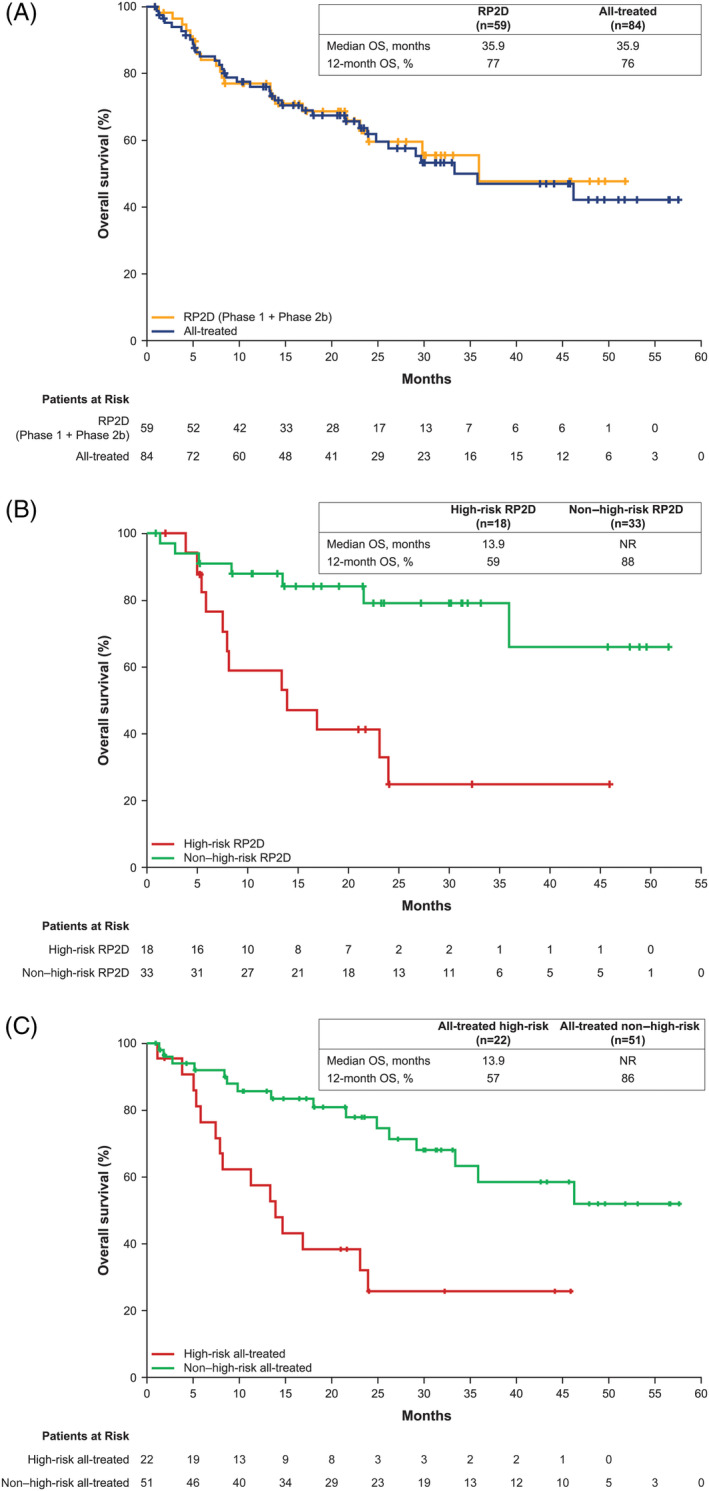
Overall survival. Data shown are for patients receiving the RP2D and the all‐treated population (A). OS data for high‐risk vs non–high‐risk patients in the RP2D population are shown in (B). OS data for high‐risk vs non–high‐risk patients in the all‐treated population are shown in (C). NE, not estimable; OS, overall survival. “+” indicates censored observation. NE, not estimable; OS, overall survival; RP2D, recommended phase 2 dose
ORR was also evaluated in key subgroups among patients receiving the RP2D. Patients with two or three prior lines of therapy (n = 35) and those with ≥4 prior lines of therapy (n = 24) each had an ORR of 71% (Figure 1B). In patients classified as high risk in the RP2D population (n = 18), ORR was 67% with four patients (22%) achieving VGPR and eight patients (44%) achieving partial response. The ORR among non–high‐risk patients in the RP2D population (n = 32) was 75% (Figure 1B). ORRs for high‐risk vs non‐high‐risk patients in the all‐treated population were similar to those reported for the RP2D population (Figure 1B).
High‐risk patients in the RP2D population had a median PFS of 7.7 months (95% CI: 3.6‐8.5) vs 6.9 months (95% CI: 4.6‐18.4) in non–high‐risk patients (Figure 2B), with a CBR of 78% in both subgroups. Median PFS rates for high‐risk vs non–high‐risk patients in the all‐treated population were 8.0 months (95% CI: 3.6‐8.9) vs 8.7 months (95% CI: 5.7‐14.6), respectively, with a CBR of 77% vs 76% (Figure 2C). Median OS for high‐risk patients in the RP2D population was 13.9 months (95% CI: 5.8‐24.0), with a 24‐month OS of 25% (95% CI: 6.8‐48.3) compared to not reached (95% CI: 35.9‐NE) and a 24‐month OS of 79% (95% CI: 58.6‐90.3) in non–high‐risk patients (Figure 3B). In the all‐treated population, OS for high‐risk patients was 13.9 months (95% CI: 7.5‐24) compared to not reached (95% CI: 33.4‐NE) in non–high‐risk patients (Figure 3C).
3.3. Safety
All 59 patients receiving the RP2D experienced a treatment‐emergent AE. Non‐hematologic AEs of any grade occurring in more than 20% of patients were diarrhea (n = 29, 49%), fatigue (n = 27, 46%), nausea (n = 26, 44%), cough (n = 21, 36%), and insomnia and upper respiratory tract infection (n = 18 each, 31%). Thirty‐eight patients (64%) experienced hematologic AEs of any grade, most frequently thrombocytopenia (n = 30, 51%), anemia (n = 25, 42%), and increased tendency to bruise (n = 7, 12%) (Table 4). Cardiac‐related AEs occurred in 15 patients (25%) and included palpitations in six patients (10%). Hypertension occurred in 16 patients (27%). Fifty patients (85%) experienced grade 3 or higher AEs, most frequently hypertension (n = 11, 19%), anemia and thrombocytopenia (n = 10 each, 17%), and pneumonia and fatigue (n = 7 each, 12%). At the RP2D, 15 patients (25%) experienced AEs leading to reduction of ibrutinib dose, including diarrhea and fatigue (n = 4 each, 7%) and thrombocytopenia (n = 3, 5%). AEs leading to discontinuation of ibrutinib occurred in 12 patients (20%) (Table 1). One death occurred within 30 days after the last treatment. This death was due to gastric hemorrhage 12 days after last dose and was considered possibly related to treatment with ibrutinib and dexamethasone. No patients died during study treatment.
Table 4.
Treatment‐emergent adverse events
| Event, n (%) | Patients receiving RP2D n = 59 | |
|---|---|---|
| Any grade | Grade ≥3 | |
| Patients experiencing an adverse event | 59 (100) | 50 (85) |
| Hematologic adverse events in ≥10% of patients | ||
| Thrombocytopenia | 30 (51) | 10 (17) |
| Anemia | 25 (42) | 10 (17) |
| Increased tendency to bruise | 7 (12) | 0 |
| Leukopenia | 7 (12) | 1 (2) |
| Decreased platelets | 7 (12) | 4 (7) |
| Contusion | 6 (10) | 0 |
| Non‐hematologic adverse events in ≥20% of patients | ||
| Diarrhea | 29 (49) | 6 (10) |
| Fatigue | 27 (46) | 7 (12) |
| Nausea | 26 (44) | 0 |
| ough | 21 (36) | 0 |
| Insomnia | 18 (31) | 5 (8) |
| Upper respiratory tract infection | 18 (31) | 1 (2) |
| Peripheral edema | 17 (29) | 0 |
| Dizziness | 16 (27) | 1 (2) |
| Hypertension | 16 (27) | 11 (19) |
| Muscle spasms | 16 (27) | 1 (2) |
| Dyspnea | 15 (25) | 4 (7) |
| Constipation | 14 (24) | 0 |
| Gastroesophageal reflux disease | 13 (22) | 1 (2) |
| Headache | 13 (22) | 1 (2) |
| Peripheral sensory neuropathy | 13 (22) | 2 (3) |
| Pyrexia | 13 (22) | 2 (3) |
4. DISCUSSION
Patients with MM inevitably relapse, and many are refractory to first‐line therapies.2, 13 Standard first‐line therapy options include bortezomib, as do several retreatment options for RRMM, but most patients will develop resistance or become refractory to bortezomib, which is a predictor of poor outcomes.27, 28 Development of effective alternative combination therapies is needed; we asked whether combining ibrutinib with a PI other than bortezomib could provide effective alternative treatment for patients with RRMM who have previously received bortezomib. Patients who have three or more prior lines of treatment, those who are refractory to both PIs and IMIDs, and patients with high‐risk genomic features such as deletion 17p (del17p) have especially poor outcomes, further underscoring the need for alternative treatment regimens.29, 30 Because ibrutinib has been shown to improve outcomes in patients with CLL with high‐risk genomic features,31 we asked whether ibrutinib might also benefit patients with MM with high‐risk genomic features.
This phase 1/2b study evaluated the efficacy and safety of the combination of ibrutinib plus carfilzomib/dexamethasone in patients with RRMM.24 Notably, 90% of patients receiving the RP2D had relapsed and were refractory to their last line of therapy, and most were refractory to bortezomib and/or an IMID. The ORR of 71% was comparable to existing regimens used to treat patients with RRMM containing bortezomib (63%‐84%).32, 33, 34
The ORR for patients in the RP2D population with high‐risk genomic features was 67%, which was comparable to non–high‐risk (75%); the same was true for the CBR, which was 78% in high‐risk and 81% in non–high‐risk patients. As expected, OS was shorter among high‐risk patients (median OS, 13.9 months) compared to non–high‐risk patients (median OS, not reached). Interestingly, the median PFS of 7.7 months in high‐risk patients in the RP2D population was slightly longer than the median PFS in non–high‐risk patients (6.9 months). However, these results should be interpreted with caution given the small number of high‐risk patients included in the subgroup analyses.
The safety profile of this combination in patients receiving the RP2D was consistent with those of the individual agents, and with the safety profile of patients from phase 1.24 Grade 3 or higher AEs were seen in most patients, similar to treatment with single‐agent ibrutinib and carfilzomib.20, 35 The only infection occurring in more than 20% of patients was upper respiratory tract infection, and most instances were grade 1 or 2. No patients died while on treatment.
5. CONCLUSIONS
The combination of ibrutinib plus carfilzomib and dexamethasone had an acceptable safety profile, consistent with those of the individual agents, and PFS and ORR were within the expected range in patients with RRMM previously treated with bortezomib, a population with limited retreatment options.
AUTHOR CONTRIBUTIONS
A.C. and Z.S. designed the study; A.C., R.F.C., C.G., C.K., J.V.M., R.N., M.L., S.Z.U., L.D.A., S.C., S.G., C.S., R.S., and J.V. contributed to data collection; Y.L. performed the data analyses; Y.L. and E.L. confirmed the accuracy of the data and compiled it for analysis; Z.S. confirmed the accuracy of the data; all authors had access to the data and were involved in the interpretation of data, contributed to the manuscript review and revisions, and approved the final version for submission.
CONFLICTS OF INTEREST
A.C.: Consulting/advisory role for Janssen, Celgene, Novartis, Amgen, Bristol‐Myers Squibb, Millennium/Takeda, Karyopharm, Sanofi, Seattle Genetics, and Oncopeptides; research funding from Pharmacyclics LLC, an AbbVie Company. R.F.C.: None. C.G.: Honoraria from Takeda, Celgene, Amgen, Janssen, Karyopharm, AbbVie, Adaptive, and Precision Bioscience; consulting/advisory role for Celgene, Takeda, Amgen, Karyopharm, Janssen, Precision‐Bioscience, Adaptive, and AbbVie; research funding from Celgene; speakers bureau fees from Celgene and Karyopharm; travel expenses from Karyopharm; member of Celgene Connect Registry. C.K.: Research funding from Pharmacyclics LLC, an AbbVie Company. J.V.M.: Consulting/advisory role for Celgene and Pharmacyclics LLC, an AbbVie Company; speakers bureau fees from Celgene. R.N.: Consulting/advisory role for and honoraria and travel expenses from Takeda, Celgene, Janssen, Amgen, and Bristol‐Myers Squibb; research funding from Takeda, Celgene, Janssen, Amgen, Bristol‐Myers Squibb, Seattle Genetics, Acetylon, and Pharmacyclics LLC, an AbbVie Company. M.L.: Consulting/advisory role for AbbVie, Bayer, Celgene, DAVA, Gilead Sciences, Janssen, Juno Therapeutics, Kite, Novartis, OncLive, Portola, Seattle Genetics, Spectrum, TG Therapeutics, Vaniam Group, and Verastem; research funding from Celgene, Curis, Janssen Scientific Affairs LLC, Juno Therapeutics, miRagen, and TG Therapeutics. S.Z.U.: Consulting/advisory role for Amgen, AbbVie, Celgene, Mundipharma, Sanofi, Seattle Genetics, Janssen, Takeda, and Skyline DX; research funding from Amgen, Bristol‐Myers Squibb, Celgene, Sanofi, Seattle Genetics, Janssen, Takeda, SkylineDx, and Pharmacyclics LLC, an AbbVie Company. L.D.A. Jr: Consulting/advisory role for Celgene, Janssen, and Amgen; research funding from Amgen, Janssen, Celgene, and Bristol‐Myers Squibb; speakers bureau fees from Takeda, Celgene, and Amgen. S.C.: Honoraria from Cardinal Health; consulting/advisory role for Takeda. S.G.: Dr. Girnius' current affiliation is TriHealth Cancer Institute, Cincinnati, OH. He reports honoraria from Takeda, Celgene, and Akcea; consulting/advisory role for Takeda and Akcea; speakers bureau fees and travel expenses from Takeda and Celgene; honoraria from Takeda, Celgene, and Akcea. C.S.: Consulting/advisory role for Amgen, Takeda, and Celgene. R.S.: Consulting/advisory role for and honoraria and travel expenses from Amgen; research funding from Ono Pharmaceuticals. Y.L., Z.S., and E.L.: Employment with Pharmacyclics LLC, an AbbVie Company; stock ownership in AbbVie. J.V.: Speakers bureau fees from Celgene, Takeda, and Amgen.
ACKNOWLEDGMENTS
We thank the patients for participating in the PCYC‐1119‐CA phase 1/2b study and their families. Medical writing support was provided by Jo Bairzin, PhD, and funded by Pharmacyclics LLC, an AbbVie Company.
Chari A, Cornell RF, Gasparetto C, et al. Final analysis of a phase 1/2b study of ibrutinib combined with carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma. Hematological Oncology. 2020;38:353–362. 10.1002/hon.2723
Funding information Pharmacyclics LLC, an AbbVie Company, Grant/Award Number: No grant or award number
REFERENCES
- 1. Manier S, Kawano Y, Bianchi G, Roccaro AM, Ghobrial IM. Cell autonomous and microenvironmental regulation of tumor progression in precursor states of multiple myeloma. Curr Opin Hematol. 2016;23(4):426‐433. [DOI] [PubMed] [Google Scholar]
- 2. Ziogas DC, Dimopoulos MA, Kastritis E. Prognostic factors for multiple myeloma in the era of novel therapies. Expert Rev Hematol. 2018;11(11):863‐879. [DOI] [PubMed] [Google Scholar]
- 3. Chan HSH, Chen CI, Reece DE. Current review on high‐risk multiple myeloma. Curr Hematol Malig Rep. 2017;12(2):96‐108. [DOI] [PubMed] [Google Scholar]
- 4. Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36(4):561‐584. [DOI] [PubMed] [Google Scholar]
- 5. Okazuka K, Ishida T. Proteasome inhibitors for multiple myeloma. Jpn J Clin Oncol. 2018;48(9):785‐793. [DOI] [PubMed] [Google Scholar]
- 6. Velcade (bortezomib) for injection, for subcutaneous or intravenous use [package insert]. Cambridge, MA: Millennium Pharmaceuticals Inc; 2019. [Google Scholar]
- 7. European Medicines Agency . Velcade (bortezomib) EPAR Summary to the public. https://www.ema.europa.eu/en/documents/overview/velcade-epar-summary-public_en.pdf. Accessed August 1, 2019.
- 8. Revlimid (lenalidomide) capsules, for oral use [package insert]. Summit, NJ: Celgene Corporation; 2019. [Google Scholar]
- 9. European Medicines Agency . Revlimid (lenalidomide). Summary of opinion (post authorisation). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-revlimid-ii-89g_en.pdf. Published January 26, 2017. Accessed November 14, 2019.
- 10. Kyprolis (carfilzomib) for injection, for intravenous use [package insert]. South San Francisco, CA: Onyx Pharmaceuticals Inc.; 2019. [Google Scholar]
- 11. European Medicines Agency . Kyprolis (carfilzomib): an overview of Kyprolis and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/kyprolis-epar-medicine-overview_en.pdf. Accessed November 14, 2019.
- 12. Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol. 2014;21(4):e573‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arastu‐Kapur S, Anderl JL, Kraus M, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17(9):2734‐2743. [DOI] [PubMed] [Google Scholar]
- 15. Imbruvica (Ibrutinib) capsules, for oral use [package insert]. Sunnyvale, CA: Pharmacyclics LLC; 2019. [Google Scholar]
- 16. Tai YT, Chang BY, Kong SY, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood. 2012;120(9):1877‐1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsumoto T, Abe M. Bone destruction in multiple myeloma. Ann N Y Acad Sci. 2006;1068:319‐326. [DOI] [PubMed] [Google Scholar]
- 18. Terpos E, Christoulas D, Gavriatopoulou M, Dimopoulos MA. Mechanisms of bone destruction in multiple myeloma. Eur J Cancer Care (Engl). 2017;26(6). 10.1111/ecc.12761. [DOI] [PubMed] [Google Scholar]
- 19. Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF‐kappaB. Cell Signal. 2013;25(1):106‐112. [DOI] [PubMed] [Google Scholar]
- 20. Richardson PG, Bensinger WI, Huff CA, et al. Ibrutinib alone or with dexamethasone for relapsed or relapsed and refractory multiple myeloma: phase 2 trial results. Br J Haematol. 2018;180(6):821‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zangari M, Terpos E, Zhan F, Tricot G. Impact of bortezomib on bone health in myeloma: a review of current evidence. Cancer Treat Rev. 2012;38(8):968‐980. [DOI] [PubMed] [Google Scholar]
- 22. Zangari M, Yaccoby S, Pappas L, et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96(2):333‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu B, Chen Y, Usmani SZ, et al. Characterization of the molecular mechanism of the bone‐anabolic activity of carfilzomib in multiple myeloma. PLoS One. 2013;8(9):e74191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chari A, Larson S, Holkova B, et al. Phase 1 trial of ibrutinib and carfilzomib combination therapy for relapsed or relapsed and refractory multiple myeloma. Leuk Lymphoma. 2018;59(11):2588‐2594. [DOI] [PubMed] [Google Scholar]
- 25. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Updated June 14, 2010. Accessed November 14, 2019.
- 27. Robak P, Drozdz I, Szemraj J, Robak T. Drug resistance in multiple myeloma. Cancer Treat Rev. 2018;70:199‐208. [DOI] [PubMed] [Google Scholar]
- 28. Robak P, Robak T. Bortezomib for the treatment of hematologic malignancies: 15 years later. Drugs R D. 2019;19(2):73‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443‐2448. [DOI] [PubMed] [Google Scholar]
- 30. Lakshman A, Painuly U, Rajkumar SV, et al. Natural history of multiple myeloma with de novo del(17p). Blood Cancer J. 2019;9(3):32 10.1038/s41408-019-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones J, Mato A, Coutre S, et al. Evaluation of 230 patients with relapsed/refractory deletion 17p chronic lymphocytic leukaemia treated with ibrutinib from 3 clinical trials. Br J Haematol. 2018;182(4):504‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palumbo A, Chanan‐Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754‐766. [DOI] [PubMed] [Google Scholar]
- 33. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open‐label, multicentre study. Lancet Oncol. 2016;17(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 34. Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vij R, Siegel DS, Jagannath S, et al. An open‐label, single‐arm, phase 2 study of single‐agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158(6):739‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]


