Abstract
Background
Timely and efficient diagnostic workup of patients with head and neck cancer (HNC) is challenging. This observational study describes the implementation of an optimized multidisciplinary oncological diagnostic workup for patients with HNC and its impact on diagnostic and treatment intervals, survival, costs, and patient satisfaction.
Methods
All patients with newly diagnosed HNC who underwent staging and treatment at the Radboud University Medical Center were included. Conventional workup (CW) in 2009 was compared with the fast‐track, multidisciplinary, integrated care program, that is, optimized workup (OW), as implemented in 2014.
Results
The study included 486 patients with HNC (218 with CW and 268 with OW). The time‐to‐treatment interval was significantly lower in the OW cohort than the CW cohort (21 vs 34 days; P < .0001). The 3‐year overall survival rate was 12% higher after OW (72% in the CW cohort vs 84% in the OW cohort; P = .002). After correction for confounders, the 3‐year risk of death remained significantly lower in the OW cohort (hazard ratio, 1.73; 95% confidence interval, 1.14‐2.63; P = .010). Total diagnostic costs were comparable in the 2 cohorts. The general satisfaction score, as measured with the Consumer Quality Index for Oncological Care, was significantly better in a matched OW group than the CW group (9.1 vs 8.5; P = .007).
Conclusions
After the implementation of a fast‐track, multidisciplinary, integrated care program, the time‐to‐treatment interval was significantly reduced. Overall survival and patient satisfaction increased significantly, whereas costs did not change. This demonstrates the impact and improved quality of care achieved by efficiently organizing the diagnostic phase of HNC management.
Keywords: costs and cost analysis, delayed diagnosis, head and neck neoplasms, survival, time to treatment
Short abstract
After the implementation of a fast‐track, multidisciplinary, integrated care program for patients with head and neck cancer, this study shows a significant reduction in the time‐to‐diagnosis and time‐to‐treatment intervals, a significant increase in 3‐year overall survival, and an increase in patient satisfaction without increased diagnostic costs. This demonstrates the impact and improved quality of care achieved by efficiently organizing the diagnostic phase of head and neck cancer management.
Introduction
Head and neck cancer (HNC) represents a relatively rare and heterogeneous group of tumors. These tumors are characterized by relatively fast growth in a functionally important and vulnerable anatomic site of the human body. Therefore, these tumors and their treatment often have a substantial impact on important functions such as breathing, speech, and swallowing. Because of the complexity of diagnosis and treatment, a high degree of expertise and a multidisciplinary approach are required. 1 For this reason, care for patients with HNC has to be centralized. As a result, the number of patients referred to these specialized centers is rising. This increase challenges the resources of these high‐volume centers and may result in prolonged time‐to‐treatment intervals. 2 The organization and integration of the various aspects of health care for complex diseases such as HNC requiring a multidisciplinary approach have become increasingly important. 3 Simultaneously, the possibilities and complexity of diagnostic procedures and treatment options for patients with HNC are increasing, and this adds to the risk of increasing time‐to‐treatment intervals. 1 , 4 , 5 Time‐to‐treatment intervals are particularly important for patients with HNC because, as mentioned before, these tumors are relatively fast growing in an anatomically and functionally complex and delicate area. Although the effect of time‐to‐treatment intervals on overall survival has been variably reported, 6 , 7 , 8 , 9 recent large studies have demonstrated the unfavorable effects of long time‐to‐treatment intervals. 2 , 10 These 2 studies of large cohorts of patients with HNC found that overall survival was significantly lower with larger intervals between diagnosis and treatment. 2 , 10 In addition, studies comparing diagnostic and radiotherapy planning imaging have demonstrated a higher tumor volume with the latter, which indicates tumor progression during the diagnosis‐to‐treatment interval. 11 , 12 Increased tumor volume (and stage) may lead to more intensified therapy with increased associated morbidity and worse functional outcomes. This intensified treatment may compensate for the unfavorable effect of prolonged intervals on survival but comes at the price of additional costs and loss of function and quality of life.
In 2008, our Head and Neck Oncology Center (HNOC) started to redesign the diagnostic pathway for patients with HNC. We designed a fast‐track, multidisciplinary, integrated care program and implemented numerous interventions over a period of 4 years (2010‐2013) to enhance quality and diminish delays. This study was aimed at assessing the impact of this fast‐track, multidisciplinary, integrated care program on the quality of care and costs for patients with HNC.
Materials and Methods
Design
To assess the impact of the fast‐track, multidisciplinary, integrated care program on the quality of care for patients with HNC, we performed a before and after study by using medical record searches and questionnaires. The study was approved by the medical ethics committee.
Study Population
All consecutive patients with suspected HNC diagnosed in 2009 and 2014 and treated with curative intent at the Radboud University Medical Center in Nijmegen were included. Primary epithelial tumors of the lip, oral cavity, oropharynx, nasal vestibule, hypopharynx, larynx, and external auditory canal were included. In addition, all tumor types (except lymphomas) of the nasal cavity, paranasal sinuses, nasopharynx, and salivary glands and metastases in the neck from an unknown primary tumor were included. Patients with previous treatment for their tumor elsewhere, previous HNC, or previous (chemo)radiotherapy in the head and neck area were excluded. We identified patients by using a prospective patient registry.
Diagnostic Process
All patients with proven cancer or a suspicion of cancer were guaranteed access to a first consultation within 7 days of referral by the referring consultant.
All patients underwent a full oncological workup, including a medical history, a physical examination, and imaging, cytological, and/or histopathological examinations. Imaging consisted of computed tomography (CT) and/or magnetic resonance imaging of the head and neck, ultrasonography with fine‐needle aspiration cytology (FNAC) of lymph nodes suspected for metastases (in all cases except Tis/1 glottic carcinoma), chest radiography or CT (chest CT in case of suspicious low cervical lymph nodes or suspicious bilateral cervical lymph nodes), and positron emission tomography (PET)–CT for unknown primary tumors. All cases were discussed at the multidisciplinary tumor board meeting, which included consultant head and neck surgeons (ear, nose, and throat and maxillofacial surgeons), consultant radiation oncologists, consultant medical oncologists, consultant pathologists, consultant radiologists, consultant nuclear medicine physicians, geriatricians, physician assistants, and oncology nurses. Tumors were classified according to the TNM Classification of Malignant Tumors (the sixth or seventh edition depending on the date of diagnosis), 13 , 14 and a treatment recommendation was formulated.
The oncological workup for patients diagnosed in 2009 (conventional workup [CW]) and 2014 (optimized workup [OW]) is summarized in more detail in Table 1 and the supporting information.
Table 1.
Optimized Workup in the Fast‐Track, Multidisciplinary, Integrated Care Program
| Conventional Workup (2009) | Optimized Workup: Fast‐Track, Multidisciplinary, Integrated Care Program (2014) |
|---|---|
|
Day 1 (first consultation): b
|
|
Day 2:
| |
|
Day 3:
|
Abbreviations: CT, computed tomography; ENT, ear, nose, and throat; FDG, [18F]fluorodeoxyglucose; FNAC, fine‐needle aspiration cytology; HN, head and neck; MRI, magnetic resonance imaging; PET, positron emission tomography.
First consultation within 1 week. These visits did not routinely take place on the same day.
Guaranteed access to the multidisciplinary Head and Neck Oncology Center within 1 week.
Unless there was an indication for a procedure under general anesthesia for other reasons (eg, tonsillectomy, tracheotomy, or transoral laser microsurgery). Routine rigid laryngopharyngoscopy and biopsy under general anesthesia were no longer performed.
Generally, up to 2 nodes were punctured per side of the neck (1 suspected node in the area of primary drainage and the most caudal suspected node). The cytology specimen was immediately checked for cellular content, and when this was reported to be inadequate, repeat aspiration was performed in the same examination.
Data Collection
The medical records of the patients were reviewed during follow‐up to obtain information on the following: patient and tumor characteristics, American Society of Anesthesiologists score, type and date of imaging, biopsy, cytology and histopathology, date and outcome of the multidisciplinary tumor board meeting, type and starting date of treatment, date of last follow‐up, and outcome. In addition, because our tertiary HNOC mainly provides clinical care on referral from secondary medical care centers, information on imaging, biopsy, cytology, and histopathology previously performed by the referring clinics.
Costs for imaging and histopathology were based on national medical guidelines and data from the Dutch Health Authority. Costs for the initial multidisciplinary consultation and human papillomavirus analysis were not included in either group. More detailed information about the costs can be found in Supporting Table 1.
We defined the specialist‐to‐treatment interval as the time interval between the first consultation at the multidisciplinary HNOC and the start of treatment and then subcategorized these intervals. First, the specialist‐to‐diagnosis interval was defined as the time interval between the first consultation at the multidisciplinary HNOC and the final diagnosis, which was defined as the final Union for International Cancer Control classification based on the full and recent diagnostic workup at the multidisciplinary tumor board meeting. Second, the diagnosis‐to‐treatment interval was defined as the time interval between the final diagnosis and the start of treatment (ie, the date of surgery or the date of the first fraction of (chemo)radiotherapy).
Patient satisfaction was determined by means of the Consumer Quality (CQ) Index for Oncological Care developed by the Netherlands Institute for Health Services Research. 15 The CQ Index was measured prospectively in a random cohort of patients meeting our inclusion criteria for CW (2009‐2010) and OW (July 2013 to July 2016). The execution was performed by an independent third party. The questionnaire was sent in 2011 (CW) and in 2015 and 2017 (OW) to a random selection of patients at least 18 years old with stage I to III disease.
Outcome Measurements
The endpoints of the study were as follows: time intervals from the first consultation to diagnosis and start of treatment, number of medical imaging and pathological investigations, costs, oncological outcomes, effect of delays on oncological outcomes, and patient satisfaction.
For follow‐up, recurrence rates, and disease‐specific survival, we used the last outpatient checkup by the consultant head and neck surgeon or radiation oncologist. For overall survival, we used the date of last contact or the date of death.
Statistical Analysis
We used SPSS version 22 for statistical analyses. A Student t test was used to analyze differences in continuous variables between the 2 groups, and a Mann‐Whitney test was used in case of nonnormal distributions. A chi‐square test was used to analyze the difference in the distributions of the categorical variables between the 2 groups. Pearson's correlation coefficient was used to analyze the relationship between variables. A Spearman correlation test was performed to analyze the correlation of variables that were not normally distributed. Overall survival was analyzed with a Kaplan‐Meier curve and a log‐rank test. A multivariable Cox proportional hazards regression analysis was performed to correct for confounding factors between the studied groups for overall survival. Proportional hazards assumptions were tested. For the risk of recurrence and death due to disease, the crude cumulative incidence was calculated, and competing risk analyses were performed in Strata/SE 11.2. CW was set as 1, and OW was set as 0. A P value less than .05 was considered significant.
Results
Patients
During the study period, 394 and 457 patients presented at our HNOC in 2009 and 2014, respectively. A total of 218 patients were eligible for inclusion in the CW cohort (2009), and 268 were eligible for the OW cohort (2014). Figure 1 presents a flowchart of inclusion and reasons for exclusion. Table 2 presents the baseline characteristics and treatment details. A significant difference between the groups was found for age, with patients in the OW group being on average 2 years older (P = .012). Furthermore, there were significantly more patients at stage T1 (P = .028) and stage N0 (P = .037) in the OW cohort, and this resulted in significantly fewer patients with stage III to IV disease in this cohort (P = .033).
Figure 1.
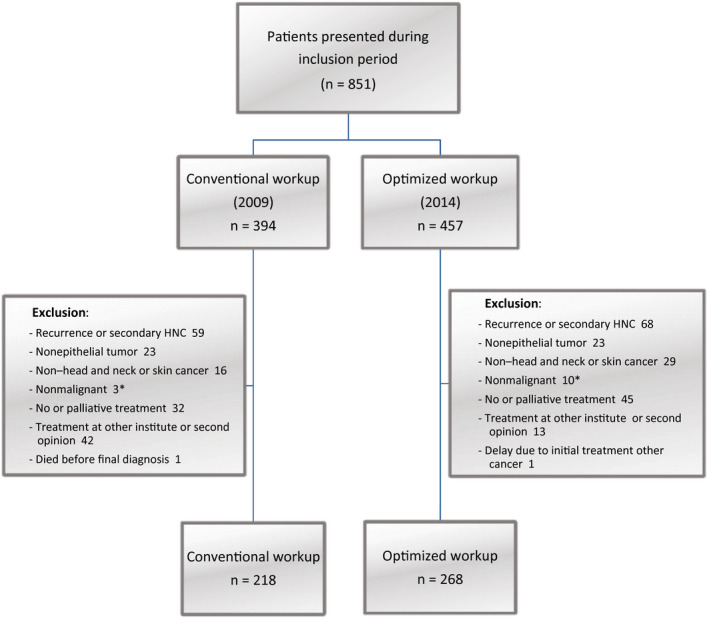
Flowchart. *Suspected malignant tumors with diagnostic and (surgical) therapeutic workup as malignant tumors were included. HNC indicates head and neck cancer.
Table 2.
Characteristics of the Patients at the Baseline
| Characteristic | Conventional Workup (n = 218) | Optimized Workup (n = 268) | P |
|---|---|---|---|
| Sex, No. (%) | .297 a | ||
| Male | 156 (71.6) | 180 (67.2) | |
| Female | 62 (28.4) | 88 (32.8) | |
| Age, mean (SD), y | 62.7 (10.6) | 64.8 (11.8) | .012 b |
| ASA score, No. (%) | .738 a | ||
| 1 | 41 (18.8) | 52 (19.4) | |
| 2 | 132 (60.6) | 161 (60.1) | |
| 3 | 44 (20.2) | 55 (20.5) | |
| 4 | 1 (0.5) | 0 | |
| Tumor site, No. (%) | .473 a | ||
| Lip | 7 (3.2) | 4 (1.5) | |
| Oral cavity | 53 (24.3) | 82 (30.6) | |
| Oropharynx c | 45 (22.6) | 39 (14.6) | |
| Hypopharynx | 13 (6.0) | 14 (5.2) | |
| Larynx | 60 (27.5) | 83 (31.0) | |
| Nose/paranasal sinuses | 14 (6.4) | 19 (7.1) | |
| Nasopharynx | 8 (3.7) | 5 (1.9) | |
| Salivary glands | 10 (4.6) | 13 (4.9) | |
| Ear canal | 2 (0.9) | 1 (0.4) | |
| Nodal metastasis | 6 (2.8) | 8 (3.0) | |
| T stage, No. (%) | |||
| T0 | 6 (2.8) | 7 (2.6) | .924 a |
| Tis | 8 (3.7) | 8 (3.0) | .674 a |
| T1 | 54 (24.8) | 91 (34.0) | .028 a |
| T2 | 72 (33.0) | 77 (28.7) | .307 a |
| T3 | 35 (16.1) | 43 (16.0) | .998 a |
| T4 | 40 (18.3) | 38 (14.2) | .213 a |
| Benign | 3 (1.4) | 4 (1.5) | .915 a |
| N stage, No. (%) | |||
| N0 | 132 (60.6) | 186 (69.4) | .037 a |
| N1 | 25 (11.5) | 18 (6.7) | .067 a |
| N2 | 55 (25.2) | 54 (20.1) | .183 a |
| N3 | 3 (1.4) | 6 (2.2) | .482 a |
| Benign | 3 (1.4) | 4 (1.5) | .915 a |
| UICC/AJCC stage, No. (%) d | .033 a | ||
| 0‐II | 93 (43.3) | 140 (53.0) | |
| III‐IV | 122 (56.7) | 124 (47.0) | |
| Treatment, No. (%) | .643 a | ||
| Surgery ± po(C)RT | 117 (53.7) | 147 (54.9) | |
| Radiotherapy | 68 (31.2) | 87 (32.5) | |
| Chemoradiation | 32 (14.7) | 34 (12.7) | |
| Chemotherapy | 1 (0.5) | 0 | |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; po(C)RT, postoperative (chemo)radiation; SD, standard deviation; UICC, Union for International Cancer Control.
Chi‐square test.
t test.
No difference in p16‐positive tumors (p = .174).
Excluding benign lesions.
Time to Diagnosis and Treatment Interval
The median specialist‐to‐diagnosis interval was 9.0 and 2.0 days for CW and OW, respectively. The median diagnosis‐to‐treatment interval was 25.0 and 18.0 days, respectively. The median specialist‐to‐treatment interval was 34 days for CW (range, 2‐83 days) and 21 days for OW (range, 2‐99 days). All intervals were significantly shorter in the OW group (see Table 3 and Supporting Fig. 1). We did not find a relevant correlation (r > 0.200) between tumor site and specialist‐to‐treatment interval. Overall, the specialist‐to‐treatment interval was within 30 days in 46% (CW) and in 80% (OW).
Table 3.
Medians, Means, SDs, and P Values for the Intervals in Days
| Interval | Conventional Workup (2009), Median/Mean (SD) | Optimized Workup (2014), Median/Mean (SD) | P a |
|---|---|---|---|
| Specialist to diagnosis | 9.0/10.0 (8.9) b | 2.0/6.2 (9.4) c | <.0001 |
| Diagnosis to treatment | 25.0/24.7 (11.8) b | 18.0/18.5 (8.1) c | <.0001 |
| Specialist to treatment | 34.0/33.4 (14.5) d | 21.0/24.4 (12.8) e | <.0001 |
Abbreviation: SD, standard deviation.
Mann‐Whitney test.
n = 195.
n = 259.
n = 218.
n = 268.
Number of Medical Examinations
Comparing 2009 with 2014, we found a significant increase in the number of patients who underwent chest radiography and ultrasonography with FNAC at the referring clinics, although the absolute numbers remained relatively low. Significantly fewer patients had histopathological examinations at the referring clinics. We found a significant increase in the number of PET‐CT scans in the OW group versus the CW group at our HNOC. The total number of ultrasonographs remained the same, but significantly more ultrasonographs with FNAC were performed in 2014. Rigid endoscopy was performed significantly less frequently and flexible endoscopic biopsy was performed significantly more frequently in the OW group than the CW group (P = .009 and P < .0001, respectively). The results are summarized in Supporting Tables 2 and 3.
Diagnostic Costs
The mean absolute costs for imaging, pathology, and endoscopy in the referring clinics were comparable in the 2 groups (Supporting Table 4). At our HNOC, there was a significant increase in pathology costs over time, but average costs per patient for endoscopy decreased from €703 in 2009 to €452 in 2014 (P < .0001). The total diagnostic costs of the referring clinic and our HNOC combined did not show a significant difference between CW and OW. The results are summarized in Supporting Table 4.
Oncological Outcomes
The mean follow‐up was 49 and 29 months in the CW and OW groups, respectively. The OW group showed significantly better overall survival. The 3‐year overall survival rate was 72% in the CW group and 84% in the OW group (P = .002; Fig. 2); 58 and 37 patients, respectively, died during this period. After correction for confounders (Table 4), the 3‐year risk of death was significantly higher for patients in 2009 than for patients in 2014 (hazard ratio [HR], 1.73; 95% confidence interval [CI], 1.14‐2.63; P = .010). The 3‐year disease‐specific survival rate was 82% in the CW group and 88% in the OW group (P = .056). After correction for confounders, a competing risk analysis showed that death due to disease was not different in the 2 groups (subdistribution hazard ratio [SHR], 1.33; 95% CI, 0.80‐2.22; P = .272). The 3‐year crude cumulative incidence for recurrent disease was not significantly different (SHR, 1.18; 95% CI, 0.83‐1.67; P = .361).
Figure 2.
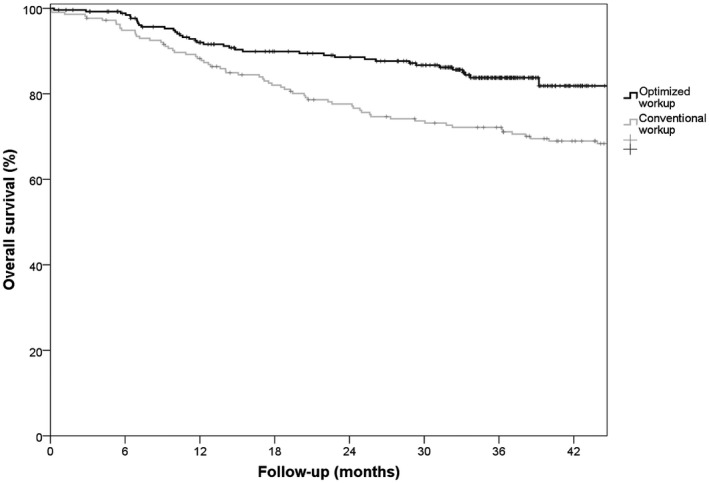
Kaplan‐Meier curves of overall survival. Overall survival was analyzed after the exclusion of 4 patients in the conventional workup group and 3 patients in the optimized workup group due to benign lesions instead of malignant lesions.
Table 4.
Multivariable Cox Regression Analysis for Overall Survival
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Year (1 = 2009; 0 = 2014) | 1.734 | 1.141‐2.634 | .010 a |
| Sex (1 = male; 0 = female) | 1.056 | 0.669‐1.666 | .815 |
| Age | 1.021 | 1.001‐1.043 | .043 a |
| ASA score (1 = 2‐4; 0 = 1) | 1.658 | 0.867‐3.172 | .127 |
| UICC/AJCC stage (1 = 3‐4; 0 = 0‐2) | 2.682 | 1.613‐4.460 | .0001 a |
| Synchronous malignant tumor at diagnosis | 1.150 | 0.457‐2.893 | .766 |
| Site: Tis/1 glottic | 0.166 | 0.022‐1.252 | .082 |
| Site: lip | 0.623 | 0.083‐4.698 | .646 |
| Site: oropharynx | 1.436 | 0.838‐2.462 | .188 |
| Site: ear | 5.125 | 1.211‐21.687 | .026 a |
| Site: hypopharynx | 1.777 | 0.896‐3.524 | .100 |
| HPV p16+ | 0.188 | 0.043‐0.817 | .026 a |
Abbreviations: AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; CI, confidence interval; HPV, human papillomavirus; UICC, Union for International Cancer Control.
P < .05.
The proportional hazards assumptions were met (P = .570).
Effect of an Increased Interval to the Start of Treatment on Oncological Outcomes
Overall, patients with a specialist‐to‐treatment interval longer than 30 days had a 5‐year overall survival rate of 58%, whereas patients with an interval of 30 days or less had a 5‐year overall survival rate of 78% (P = .003; Fig. 3). After correction for the covariates mentioned in Table 4 (excluding year), overall survival was still significantly better for patients with an interval of 30 days or less (HR, 1.52; 95% CI, 1.06‐2.17; P = .023). Disease‐specific survival was not statistically significantly influenced by delay.
Figure 3.
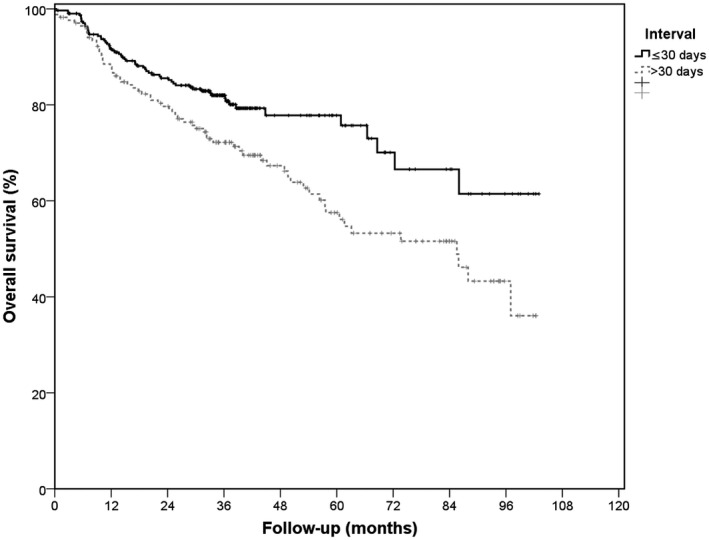
Kaplan‐Meier curves of specialist‐to‐treatment interval and overall survival. Overall survival was analyzed after the exclusion of 7 patients due to benign lesions instead of malignant lesions.
Patient‐Reported Experience
The CQ Index was determined for 55 patients in the CW group and for 84 patients in the OW group. The overall evaluation of satisfaction with care (on a scale of 0‐10, with 10 being the highest score) was 8.51 for CW patients and 9.05 for OW patients (P = .007).
Discussion
This study reports on outcomes before and after the implementation of a fast‐track, multidisciplinary, integrated care program at our HNOC. The main interventions included a 1‐day combined consultation with consultant head and neck surgeons, consultant radiation oncologists, and allied health professionals at 1 clinic; the introduction of transnasal digital video endoscopy with office‐based biopsy (OBB); fast and efficient planning of medical imaging; and swift cytological and histopathological assessment. This resulted in a significant decrease in the median specialist‐to‐treatment interval from 34 to 21 days. Three‐year overall survival and overall satisfaction with care were significantly higher in the OW group than the CW group. The diagnostic costs did not differ between the 2 cohorts.
One of the interventions introduced to reduce diagnostic intervals was digital video endoscopy with OBB, which obviates the need for endoscopy and biopsy as well as general anesthesia with its associated risks, consultations, and operating room planning issues. OBB is well tolerated and safe. 16 , 17 , 18 , 19 , 20 , 21 Studies have shown that when the histological outcome of OBB has been invasive squamous cell carcinoma, the diagnosis has been confirmed in 100% of the cases with a final histological diagnosis using operative biopsies. 21 , 22 , 23
Median specialist‐to‐diagnosis and specialist‐to‐treatment intervals were significantly decreased from 9 to 2 days and from 34 to 21 days, respectively. The 2018 annual report by the Dutch Head and Neck Audit describes the median time intervals of all Dutch HNC centers combined, including our center. It found a median specialist‐to‐diagnosis interval of 13 days and a specialist‐to‐treatment interval of 30 days for 2015‐2017. 24 The diagnosis‐to‐treatment interval at our HNC center was significantly decreased from 25 to 18 days. This interval from the final diagnosis to the start of treatment varies widely in the literature. Two large studies, including 13,140 and 51,655 patients with HNC in the Netherlands and the United States, found median diagnosis‐to‐treatment intervals of 37 and 26 days, respectively. 2 , 10 However, they defined the date of diagnosis as the date of the cytological or histological diagnosis and the date of the pathology report, respectively, 2 , 10 and not necessarily as the date of the final diagnosis and establishment of the treatment plan at a tumor board meeting, as in our study.
The introduction of our fast‐track, multidisciplinary, integrated care program with OW was cost‐effective and did not lead to an increase in total costs. The average cost per patient for all those who underwent endoscopy (rigid and/or flexible) at the HNOC decreased significantly because of the introduction of OBB. Naidu et al 23 and Fang et al 25 also reported significantly lowered costs with flexible endoscopic biopsy in comparison with biopsy under general anesthesia. Simons et al 26 redesigned their care processes by introducing OBB and performing PET‐CT in radiotherapy position and found that their redesign proved to be cost‐effective and led to reduced waiting times. Diagnostic costs are, however, just a fraction of the costs of treatment. 27 Although we did not study this in our cohorts, it is likely that disease progression caused by prolonged diagnostic and therapeutic intervals will result in a higher number of cases that need additional treatment or even revision of the treatment plan in some cases.
We found a 12% increase in 3‐year overall survival between 2009 and 2014, and it remained significantly higher after correction for confounders, especially including stage. Multivariable Cox proportional hazards analyses showed a 1.73 higher risk of death for the CW group compared with the OW group at the 3‐year follow‐up. Multivariable competing risk analyses of death due to HNC and recurrence rates also seemed in favor of OW, but the differences did not reach statistical significance. The nonsignificant adjusted HR of death due to HNC of 1.33 in the CW group versus the OW group may be explained by several changes. Tumor staging and subsequent treatment might be more adequate because of more up‐to‐date imaging and the constantly increasing accuracy of medical imaging, including the increased use of PET‐CT. Furthermore, there was an increased use of postoperative chemoradiation in patients in 2014, but this concerned only 0.9% and 4.9% of the patients in the CW and OW groups, respectively. These differences had only a minor impact on overall outcomes in the studied population. In addition to reduced time‐to‐treatment intervals, the systematic integration of upfront geriatric assessment for elderly patients and screening by dentists, dental hygienists, dieticians, and speech and swallow therapists also contributes to the improvement of quality of care and is also likely to have played a role in determining survival outcomes.
We found that a specialist‐to‐treatment interval longer than 30 days negatively influenced overall survival, even after adjustments for comorbidity, stage, and age, among others (HR, 1.52; 95% CI, 1.06‐2.17; P = .023). Two recent large studies by Murphy et al 2 and van Harten et al 10 studied the influence of the diagnosis‐to‐treatment interval on overall survival. Murphy et al found that the overall survival for patients treated with a curative intent was significantly lower if the interval between diagnosis and treatment was 61 days or longer in comparison with an interval of 30 days or less. Van Harten et al found significantly lower overall survival with longer intervals when the cutoff was set at 37 days (median) but not with a cutoff at 30 days. Van Harten et al, however, did not adjust for comorbidity.
We used the CQ Index to assess patients' experiences and found that overall satisfaction with care was high in both groups and significantly higher in the OW group. This may be due to improved quality of care and the faster diagnostic path. The literature provides insufficient data on patient‐reported outcome measures and delays in HNC. International comparisons would in any case be difficult to make because of societal or cultural differences and differences in the case mix.
An important limitation is the retrospective nature of our study. Complex cases requiring dedicated imaging, additional consultation, and diagnostic procedures for (incidental) findings (eg, pulmonary nodules on chest CT or nonphysiological intestinal [18F]fluorodeoxyglucose uptake on PET) will have prolonged time‐to‐treatment intervals. Although we did correct for stage and comorbidity, it remains unclear whether these factors still affected overall survival, especially in patients with a specialist‐to‐treatment delay longer than 30 days.
The most important difference in the patient characteristics between our studied cohorts was the higher number of patients with stage 0 to II disease in the OW group. In this group, we found more T1 carcinoma of the nose/paranasal sinuses (P = .006) and more N0 oral cavity cancer (P = .039). Analyzing our data separately for stage 0 to II and stage III to IV tumors, we found that specialist‐to‐diagnosis, diagnosis‐to‐treatment, and specialist‐to‐treatment intervals remained statistically significant in favor of the OW cohort, and total diagnostic costs were still comparable. The differences in baseline characteristics may be explained by increased awareness for HNC and earlier referral by general practitioners, dentists, and/or referring consultants. In addition to the implementation of a fast‐track, multidisciplinary, integrated care program, we actively encouraged referring specialists to refer patients with suspected HNC without imaging and histopathology to avoid unnecessary delays before oncological workup at our HNC center. There were no major alterations in treatment protocols between the investigated cohorts. As mentioned previously, there was increased use of postoperative chemoradiation in patients in 2014. Furthermore, peer review of radiotherapy target volume contouring has been introduced. In addition, reirradiation has become more accepted for recurrences and second primary tumors. 28 Lastly, the evolution of chemotherapy agents, targeted therapies, and immunotherapy after the development of an incurable recurrence or distant metastasis may increase overall survival. However, in the studied cohorts, there were no significant differences in these treatments or reirradiation for recurrent disease, distant metastasis, or second primary tumors.
In conclusion, after the implementation of a fast‐track, multidisciplinary, integrated care program, the interval from the first consultation to the start of treatment was considerably shortened, overall survival significantly improved, patient satisfaction increased, and costs remained the same. In addition, the detrimental effects on survival of progression of disease due to prolonged time‐to‐treatment intervals may be compensated by intensified or adjuvant treatment. Optimizing the diagnostic track and quality of care results in better oncological outcomes without increases in toxicity or costs.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Henrieke W. Schutte: Conceptualization, data curation, formal analysis, investigation, project administration, resources, visualization, writing–original draft, and writing–review and editing. Guido B. van den Broek: Conceptualization, supervision, and writing–review and editing. Stefan C. A. Steens: Conceptualization and writing–review and editing. Rosella P. M. G. Hermens: Methodology and writing–review and editing. Jimmie Honings: Conceptualization and writing–review and editing. Henri A. M. Marres: Conceptualization, visualization, and writing–review and editing. Matthias A. W. Merkx: Conceptualization and writing–review and editing. Willem L. J. Weijs: Conceptualization and writing–review and editing. Anne I. J. Arens: Conceptualization and writing–review and editing. Adriana C. H. van Engen–van Grunsven: Conceptualization and writing–review and editing. Carla M. L. van Herpen: Conceptualization and writing–review and editing. Johannes H. A. M. Kaanders: Conceptualization and writing–review and editing. Frank J. A. van den Hoogen: Conceptualization and writing–review and editing. Robert P. Takes: Conceptualization, supervision, visualization, writing–original draft, and writing–review and editing.
Supporting information
Supplementary Material
Schutte HW, van den Broek GB, Steens SCA, Hermens RPMG, Honings J, Marres HAM, Merkx MAW, Weijs WLJ, Arens AIJ, van Engen–van Grunsven ACH, van Herpen CML, Kaanders JHAM, van den Hoogen FJA, Takes RP. Impact of optimizing diagnostic workup and reducing the time to treatment in head and neck cancer. Cancer. 2020:126:3982‐3990. 10.1002/cncr.33037
References
- 1. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34:169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choudhury N, Hassen Y, Siddiqui J, Falzon A, Ghufoor K. A multidisciplinary audit of head and neck referrals: considerations for patients' timelines and outcomes. Eur Arch Otorhinolaryngol. 2013;270:3121‐3126. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein D, Jeremic G, Werger J, Irish J. Wait times in the diagnosis and treatment of head and neck cancer: comparison between wait times in 1995 and 2005—a prospective study. J Otolaryngol. 2007;36:336‐343. [PubMed] [Google Scholar]
- 5. Jones TM, Hargrove O, Lancaster J, Fenton J, Shenoy A, Roland NJ. Waiting times during the management of head and neck tumors. J Laryngol Otol. 2002;116:275‐279. [DOI] [PubMed] [Google Scholar]
- 6. Brinkerhoff BT, Choong NW, Massey BL, et al. Diagnosis to treatment interval and outcome in patients with locally‐advanced squamous cell carcinoma of the head and neck in a Veterans Affairs medical center. J Cancer Sci Ther. 2012;4:111‐115. [Google Scholar]
- 7. Caudell JJ, Locher JL, Bonner JA. Diagnosis‐to‐treatment interval and control of locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2011;137:282‐285. [DOI] [PubMed] [Google Scholar]
- 8. Fortin A, Bairati I, Albert M, Moore L, Allard J, Couture C. Effect of treatment delay on outcome of patients with early‐stage head‐and‐neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:929‐936. [DOI] [PubMed] [Google Scholar]
- 9. Pera E, Moreno A, Galindo L. Prognostic factors in laryngeal carcinoma. A multifactorial study of 416 cases. Cancer. 1986;58:928‐934. [DOI] [PubMed] [Google Scholar]
- 10. van Harten MC, Hoebers FJ, Kross KW, van Werkhoven ED, van den Brekel MW, van Dijk BA. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015;51:272‐278. [DOI] [PubMed] [Google Scholar]
- 11. Waaijer A, Terhaard CH, Dehnad H, et al. Waiting times for radiotherapy: consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66:271‐276. [DOI] [PubMed] [Google Scholar]
- 12. Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84:5‐10. [DOI] [PubMed] [Google Scholar]
- 13. Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumours. 6th ed Wiley‐Liss; 2002. [Google Scholar]
- 14. Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumors. 7th ed Wiley‐Blackwell; 2009. [Google Scholar]
- 15. NIVEL . De CQ‐index: het meten van klantervaringen in de zorg [in Dutch]. http://postprint.nivel.nl/ppPP3276.pdf
- 16. Wellenstein DJ, de Witt JK, Schutte HW, et al. Safety of flexible endoscopic biopsy of the pharynx and larynx under topical anesthesia. Eur Arch Otorhinolaryngol. 2017;274:3471‐3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen JT, Benyamini L. Transnasal flexible fiberoptic in‐office laryngeal biopsies—our experience with 117 patients with suspicious lesions. Rambam Maimonides Med J. 2014;5:e0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippert D, Hoffman MR, Dang P, McCulloch TM, Hartig GK, Dailey SH. In‐office biopsy of upper airway lesions: safety, tolerance, and effect on time to treatment. Laryngoscope. 2015;125:919‐923. [DOI] [PubMed] [Google Scholar]
- 19. Pan CT, Lee LA, Fang TJ, Li HY, Liao CT, Chen IH. NBI flexible laryngoscopy targeted tissue sampling in head and neck cancer patients with difficult airways. Eur Arch Otorhinolaryngol. 2013;270:263‐269. [DOI] [PubMed] [Google Scholar]
- 20. Richards AL, Sugumaran M, Aviv JE, Woo P, Altman KW. The utility of office‐based biopsy for laryngopharyngeal lesions: comparison with surgical evaluation. Laryngoscope. 2015;125:909‐912. [DOI] [PubMed] [Google Scholar]
- 21. Zalvan CH, Brown DJ, Oiseth SJ, Roark RM. Comparison of trans‐nasal laryngoscopic office based biopsy of laryngopharyngeal lesions with traditional operative biopsy. Eur Arch Otorhinolaryngol. 2013;270:2509‐2513. [DOI] [PubMed] [Google Scholar]
- 22. Cha W, Yoon BW, Jang JY, et al. Office‐based biopsies for laryngeal lesions: analysis of consecutive 581 cases. Laryngoscope. 2016;126:2513‐2519. [DOI] [PubMed] [Google Scholar]
- 23. Naidu H, Noordzij JP, Samim A, Jalisi S, Grillone GA. Comparison of efficacy, safety, and cost‐effectiveness of in‐office cup forcep biopsies versus operating room biopsies for laryngopharyngeal tumors. J Voice. 2012;26:604‐606. [DOI] [PubMed] [Google Scholar]
- 24. Dutch Institute for Clinical Auditing . DHNA Annual Report 2018 [in Dutch]. Accessed March 27, 2020. https://dica.nl/jaarrapportage‐2018/dhna
- 25. Fang TJ, Li HY, Liao CT, Chiang HC, Chen IH. Office‐based narrow band imaging–guided flexible laryngoscopy tissue sampling: a cost‐effectiveness analysis evaluating its impact on Taiwanese health insurance program. J Formos Med Assoc. 2015;114:633‐638. [DOI] [PubMed] [Google Scholar]
- 26. Simons PA, Ramaekers B, Hoebers F, et al. Cost‐effectiveness of reduced waiting time for head and neck cancer patients due to a lean process redesign. Value Health. 2015;18:587‐596. [DOI] [PubMed] [Google Scholar]
- 27. Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32:865‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bots WTC, van den Bosch S, Zwijnenburg EM, et al. Reirradiation of head and neck cancer: long‐term disease control and toxicity. Head Neck. 2017;39:1122‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


