Abstract
Dendritic cells (DCs) orchestrate innate inflammatory responses and adaptive immunity through T‐cell activation via direct cell–cell interactions and/or cytokine production. Tolerogenic DCs (tolDCs) help maintain immunological tolerance through the induction of T‐cell unresponsiveness or apoptosis, and generation of regulatory T cells. Mesenchymal stromal cells (MSCs) are adult multipotent cells located within the stroma of bone marrow (BM), but they can be isolated from virtually all organs. Extracellular vesicles and exosomes are released from inflammatory cells and act as messengers enabling communication between cells. To investigate the effects of MSC‐derived exosomes on the induction of mouse tolDCs, murine adipose‐derived MSCs were isolated from C57BL/6 mice and exosomes isolated by ExoQuick‐TC kits. BM‐derived DCs (BMDCs) were prepared and cocultured with MSCs‐derived exosomes (100 μg/ml) for 72 hr. Mature BMDCs were derived by adding lipopolysaccharide (LPS; 0.1μg/ml) at Day 8 for 24 hr. The study groups were divided into (a) immature DC (iDC, Ctrl), (b) iDC + exosome (Exo), (c) iDC + LPS (LPS), and (d) iDC + exosome + LPS (EXO + LPS). Expression of CD11c, CD83, CD86, CD40, and MHCII on DCs was analyzed at Day 9. DC proliferation was assessed by coculture with carboxyfluorescein succinimidyl ester‐labeled BALB/C‐derived splenocytes p. Interleukin‐6 (IL‐6), IL‐10, and transforming growth factor‐β (TGF‐β) release were measured by enzyme‐linked immunosorbent assay. MSC‐derived exosomes decrease DC surface marker expression in cells treated with LPS, compared with control cells ( ≤ .05). MSC‐derived exosomes decrease IL‐6 release but augment IL‐10 and TGF‐β release (p ≤ .05). Lymphocyte proliferation was decreased (p ≤ .05) in the presence of DCs treated with MSC‐derived exosomes. CMSC‐derived exosomes suppress the maturation of BMDCs, suggesting that they may be important modulators of DC‐induced immune responses.
Keywords: exosomes, in vitro, mesenchymal stem cell, tolerogenic dendritic cell
Mesenchymal stromal cell‐derived exosomes suppress bone marrow‐derived dendritic cell maturation, implicating them as important modulators of dendritic cell‐induced immune responses.

1. INTRODUCTION
Stem cells are classified into embryonic and adult stem cells (Körbling & Estrov, 2003). Mesenchymal stromal cells (MSCs) are adult multipotent cells located within the stroma of bone marrow (BM), but they can also be isolated from virtually all organs including adipose tissue. These cells are able to self‐renew under controlled conditions and may differentiate into various cells other than mesodermal cell lineages such as osteocytes and adipocytes (Spaggiari, Abdelrazik, Becchetti, & Moretta, 2009). MSCs do not express specific markers, but they are negative for hematopoietic cell markers (CD45) and positive for CD90, CD73, and CD105 (Barry, Boynton, Haynesworth, Murphy, & Zaia, 1999). Moreover, MSCs have low immunogenicity and immunomodulation characteristics (B. Zhang et al., 2009). These cells are able to secrete several cytokines, growth factors, and extracellular matrix molecules (Majumdar, Thiede, Haynesworth, Bruder, & Gerson, 2000).
MSCs have been used in regeneration and transplantation (Le Blanc et al., 2004), and particularly in the prevention and treatment of graft versus host disease (Le Blanc et al., 2004). MSCs have a strong inhibitory effect on immune cells, such as dendritic cells (DCs; Beyth et al., 2005; B. Zhang et al., 2009), natural killer (NK) cells (Spaggiari, Capobianco, Becchetti, Mingari, & Moretta, 2006), B cells (Corcione et al., 2006), and unconventional T cells (NKT and Tγδ cells; Prigione et al., 2009).
Exosomes are vesicles with a diameter of 40–100 nm (Cocucci, Racchetti, & Meldolesi, 2009). Exosomes were first described as being released from sheep reticulocytes (Trajkovic et al., 2008). Exosomes do not contain cellular organelles, but they contain proteins and nucleic acids originating from the mother cells (van den Boorn, Dassler, Coch, Schlee, & Hartmann, 2013). For example, they may possess fusion and transport proteins (annexins and flotillin), heat shock proteins (HSP70), cell‐surface markers (CD9, CD81, and CD63), endosomal sorting complexes required for transport (ESCRT complex; TSG101, ALIX; Vickers & Remaley, 2012), phospholipases, and other lipid‐related proteins (Théry, Ostrowski, & Segura, 2009). Almost all cells produce and release exosomes in vivo and in vitro (De Toro, Herschlik, Waldner, & Mongini, 2015), including many inflammatory cells such as fibroblasts, intestinal epithelial cells, neuronal cells, adipocytes, and tumor cells (Zwicker et al., 2009). Furthermore, they are detectable in many biological fluids including blood, breast milk, urine, saliva, amniotic liquid, synovial fluid, and malignant effusions of ascites (Zwicker et al., 2009).
MSC‐derived exosomes play critical roles in tissue damage and repair through repressing inflammatory responses, inhibiting apoptosis, inducing proliferation, and enhancing angiogenesis (Yin, Ji, Wu, Jin, & Qian, 2019). MSC‐derived exosomes participate in tissue repair by the transfer of proteins, nucleotides, and lipids (Biancone, Bruno, Deregibus, Tetta, & Camussi, 2012). For example, human umbilical cord MSC‐derived exosomes promote angiogenesis during skin repair following burn injury (B. Zhang et al., 2015), and MSC‐derived exosomes have therapeutic effects in liver disease (Fouraschen et al., 2012), kidney disease (G. Zhang et al., 2016), cardiovascular disease (L. Liu, Jin, Hu, Li, & Shen, 2017), and neurological disease (Cui et al., 2017). Recently, human MSC‐derived exosomes were shown to impair antigen uptake by immature monocyte‐derived DCs and halted DC maturation. In addition, these MSC‐derived exosomes reduced the expression of DC maturation and activation markers (CD83, CD38, and CD80), decreased the release of interleukin‐6 (IL‐6) and IL‐12p70, and increased transforming growth factor‐β (TGF‐β) production (Reis et al., 2018).
DCs are the most potent antigen‐presenting cells (APCs) and are derived from hematopoietic progenitor cells in the BM (Guilliams & van de Laar, 2015). They are essential for initiating a primary adaptive immune response via the capture, processing, and presentation of antigen to naive CD4+ T cells (Steimle & Frick, 2016). Activated DCs have expressed MHC molecules, costimulatory receptors (CD80, CD86), and proinflammatory cytokines to induce T‐cell proliferation (J. Liu & Cao, 2015).
In contrast to classical immunogenic DCs, tolerogenic DCs (tolDCs) play an important role in the maintenance of immunological tolerance via the induction of T‐cell unresponsiveness or apoptosis, and generation of regulatory T (Treg) cells (Yoo & Ha, 2016). tolDCs usually display low levels of surface costimulatory molecules, which become upregulated on DC maturation, and high levels of anti‐inflammatory cytokines such as IL‐10 and TGF‐β (Thomson & Robbins, 2008). The mechanism(s) by which TolDCs induce anergy in T cells and cause Treg activation are unknown (Yoo & Ha, 2016). Thus, the aim of the present study is to investigate whether MSC‐derived exosomes can drive mouse BM‐derived DCs (BMDCs) to become tolDCs.
2. MATERIALS AND METHODS
2.1. Reagents
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and IL‐4 were purchased from PeproTech (NY). Penicillin/streptomycin and Trypsin‐EDTA 10X were obtained from BioSera Company (Nuaille, France). Lipopolysaccharide (LPS) was purchased from Sigma‐Aldrich (MO). Phosphate‐buffered saline (PBS) was purchased from Biotech (Bio Basic, Canada). Fetal bovine serum (FBS), phytohemagglutinin (PHA), Dulbecco's modified Eagle's medium (DMEM), and Roswell Park Memorial Institute (RPMI) 1640 were obtained from Gibco (NY). BCA protein quantification kit was purchased from Thermo Scientific Pierce (Rockford, IL). Carboxyfluorescein succinimidyl ester (CFSE) dye was obtained from Thermo Fisher Scientific (CA). ELISA kits for IL‐10, IL‐6, and TGF‐β were obtained from Invitrogen (Camarillo, CA), BioLegend (San Diego, CA), and R&D Systems (MN), respectively. Monoclonal antibodies against mouse CD105‐APC (Cat number: 17‐1051‐82), CD73‐PE (Cat number: 12‐0731‐82), CD90‐PE Cyanine (Cat number: 15‐0909‐42), CD45‐FITC (Cat number: 11‐0451‐81), MHCII‐FITC (Cat number: 11‐5321‐82), PE‐CD83 (Cat number: 12‐0831‐82) and CD86 (Cat number: 12‐0861‐82) were obtained from eBioscience (San Diego, CA). Monoclonal antibodies against mouse CD11c‐APC (Cat number: 550261) and CD40‐FITC (Cat number: 553790) were purchased from BD Bioscience (Franklin Lakes, NJ). Fc blocking reagents were obtained from BioLegend (San Diego, CA). Mitomycin‐C, PKH kit, and ExoQuick‐TC kit were purchased from Kyowa (Tokyo, Japan), Sigma‐Aldrich, and System Biosciences (Palo Alto, CA), respectively.
2.2. Animals and protocols
Female C57BL/6 mice (6–8‐week old) were purchased from the Pasteur Institute, Tehran, Iran. The animals were housed in pathogen‐free and standard laboratory conditions. All experiments in these studies were approved by the Shahid Beheshti University guidelines for animal care (number of approval ethic committee: IR.SBMU.MSP.REC.1395535).
2.3. Culture of adipose‐derived MSCs
Murine adipose‐derived MSCs (AD‐MSCs) were isolated from abdominal fat tissue C57BL/6 mice as previously described (Baghaei et al., 2017). Briefly, C57BL/6 mice were euthanized with CO2; after cutting the midline of the abdominal region and isolation the adipose tissue, it was washed in the PBS and the extracellular matrix of tissue was digested with type I collagenase. Then, homogenous tissue was centrifuged and the sediment was cultured in DMEM (Gibco) supplied with 10% FBS and penicillin–streptomycin 0.01% in 75 cm2 flask, at 37°C in 95% humidity and 5% CO2. Nonadherent cells were removed by changing the culture medium twice a week. After 2–3 weeks when the cells reached 80% confluency, they were trypsinized and used for experiments.
2.4. Adipogenic differentiation potential of MSC
To assay the differentiation of MSCs derived from adipose tissue, cultured cells from the second passage were cultured in complete media supplemented with indomethacin (100 mM), 3‐isobutyl‐methylxanthine (0.5 mM), dexamethasone (250 mM), and insulin (5 mM) for 21 days and then characterized with staining of Oil Red O.
2.5. Immunophenotyping of MSCs
After 2–3 weeks when MSCs reached 80% confluency, they were passaged. Passage 2 MSCs were characterized by cell‐surface expression using CD90‐PE Cyanine, CD45‐FITC, CD73‐PE, CD105‐APC, and isotypes controls according to the manufacturer's instructions (all antibodies were purchased from eBioscience). Cells were analyzed using a FACSCalibur flow cytometer (FACSCalibur; BD Bioscience), and data was analyzed by FlowJo 7.6.1.
2.6. Isolation and purification of MSCs‐derived exosomes
After reaching 80–90% confluency, MSCs at passage 2 were adapted to serum‐free culture by gradual reduction of serum concentrations over a 2‐week period. After 48 hr, cell supernatants were collected and, after filtering by 0.22 μm filters, the supernatants were stored at −70°C and exosomes were isolated using an ExoQuick‐TC kit according to the manufacturer's instructions. After 48 hr, cell supernatants were collected and exosomes were isolated using an ExoQuick‐TC kit according to the manufacturer's instructions. Briefly, the cells were centrifuged at 3,000g for 15 min to remove cells and cell debris. The supernatant was transferred to a sterile vessel, and an appropriate volume of ExoQuick‐TC (1:5) was added. The samples were mixed before incubation overnight at +4°C for 24 hr. Exosomes were isolated by centrifugation for 30 min at 1,500g.
2.7. Exosome protein quantification
The protein content of purified exosomes was measured using a BCA protein assay kit (Thermo Scientific Pierce) according to the manufacturer's instructions. BSA standards or samples (25 µl) were transferred to a 96‐well plate, to which 200 µl working reagent (working reagent 50:1 ratio of assay reagents A and B) was added. The plate was incubated at 37°C for 30 min, and then it was analyzed with a spectrophotometer at 562 nm (MPR4+; Hiperion, Roedermark, Germany).
2.8. Dynamic light scattering of exosomes
The size of isolated exosomes was determined using dynamic light scattering (DLS) using a Zetasizer Nano ZS DLS instrument (Malvern Instruments, UK) as previously described (Martínez‐Gutierrez et al., 2012). All experiments were performed in triplicate.
2.9. Scanning and transmission electron microscopy of exosomes
The shape and size of the isolated exosomes was assessed by scanning electron microscopy (SEM; KYKY‐EM 3200). Briefly, 1 μg/ml of exosome solution was left to dry on a glass slide for 24 hr, before the surface was covered with a thin layer of gold and analyzed by SEM. Experiments were carried out in triplicate.
For transmission electron microscopy (TEM), MSCs‐derived exosomes were fixed in paraformaldehyde and glutaraldehyde. Then, exosomes were loaded onto formvar/carbon‐coated grids, contrasted with 2% uranyl acetate and examined by TEM (Zeiss‐EM10C‐100 kV).
2.10. Induction of mouse BMDCs
The method for generating BMDCs was adapted from that described by Lutz et al. (1999) with slight modifications. BM cells were isolated from C57BL/6 mice femur and tibia and cultured in six‐well Petri dishes (1 × 106 cells/well) in RPMI 1640 (Gibco) with 10% FBS (Gibco), supplemented with recombinant mouse GM‐CSF and IL‐4 (both 20 ng/ml; PeproTech). On Day 6, nonadherent and loosely adherent cells were harvested for analysis by flow cytometry (surface marker expression). To prime DCs with exosomes, DCs were treated with 100 μg/ml exosomes on Day 6 of culture for 72 hr. On Day 8 of culture, 0.1 μg/ml LPS (Sigma‐Aldrich) was added to induce DC maturation.
The shape of DCs was examined using phase‐contrast microscopy. The expression of DC surface marker was assessed by flow cytometry using cells stained with CD11c‐APC, FITC‐MHCII, FITC‐CD40, PE‐CD83, and PE‐CD86 with Fc blocking reagents.
2.11. Uptake of labeled exosomes
To assay transfer of exosome into DCs, MSCs‐derived exosomes were labeled with PKH67 dye (Sigma‐Aldrich) according to the manufacturer's instructions. Briefly, the isolated exosomes were incubated with the dye for 5 min at room temperature, and the labeling reaction was stopped by the addition of cold FBS (1X). 1 × 105 DCs (harvested at Day 6) were treated with 50 μl of PKH67‐labeled exosomes. After 24 hr, PKH‐positive cells were detected by a fluorescent microscope (Nikon, ECLIPSE80i).
2.12. Evaluation of DC surface markers
To assess the effect of MSC‐derived exosomes on DCs, immature DCs (iDCs) were treated with MSC‐derived exosomes for 72 hr. Flow cytometry evaluation of DCs' surface markers, including CD11C, MHCII, CD83, CD86, and CD40, was performed in the following groups: iDCs (Ctrl), iDCs treated with 100 μg/ml MSC‐derived exosome for 72 hr (Exo), mature DCs (LPS) treated for 24 hr with 0.1 μg/ml LPS, and iDCs treated with 100 μg/ml MSC‐derived exosomes and 0.1 μg/ml LPS (Exo + LPS).
BMDCs were harvested from culture plates, centrifuged at 300g for 5 min, and resuspended in FACS buffer. Cells were incubated in the dark for 30 min at 4°C with antibodies. Thereafter, they were washed with washing buffer twice and determined in 10,000 cells by Flow cytometry (FACSCalibur; BD Bioscience). The data were analyzed by FlowJo software Version 7.2.2.
2.13. Lymphocyte proliferation assay
To evaluate the ability of DCs in different groups to activate lymphocytes, a lymphocyte proliferation assay was performed, as described previously (Mortaz et al., 2009). Lymphocytes from the spleen of female BALB/C mice were labeled with CFSE (Thermo Fisher Scientific) as before (Quah & Parish, 2010). Briefly, lymphocytes were resuspended to 20 × 106/ml in RPMI 1640 medium enriched with 10% FBS at 20°C. A final concentration of 5 μM of dye was added to 1 ml aliquots of lymphocytes and then mixed rapidly to ensure homogeneous labeling of cells. Cells were incubated at 37°C for 15 min and then washed three times with PBS supplemented with 5% FBS. DCs from the different groups detailed above (Ctrl, Exo, LPS, and Exo + LPS) were treated with 10 μg/ml Mitomycin‐C for 40 min and then cocultured with CFSE‐labeled lymphocytes at 1:3, 1:10, and 1:30 ratios. The mixed cultures were incubated for 72 hr in a 96‐well plate at 37°C and 5% CO2. Lymphocyte proliferation was evaluated by flow cytometry for CFSE density.
CFSE‐labeled lymphocytes without any treatment and CFSE‐labeled lymphocytes treated with 2% PHA (Gibco) were used as negative and positive controls, respectively.
2.14. Cytokine assay
Levels of mouse IL‐6 (BioLegend), IL‐10 (Invitrogen), and TGF‐β (R&D Systems, UK) in DCs culture supernatants were quantified, on Day 9 of culture, by enzyme‐linked immunosorbent assay according to the manufacturer's instructions.
2.15. Statistical analysis
Experimental results are presented as mean ± standard error of the mean. Results were tested statistically using an unpaired two‐tailed Student's t test or one‐way analysis of variance, followed by the Newman–Keuls test for comparing all pairs of groups. Analyses were performed in Graph Pad Prism (Graph Pad Prism 4.0, CA). Results were considered statistically significant when p < .05.
3. RESULTS
3.1. Characterization and differentiation potential of AD‐MSCS
The shape of AD‐MSCs was examined after 7 days (Figure 1a) and after 21 days in a supplemented adipogenic induction medium (Figure 1b). MSCs became differentiated over this time frame as confirmed by Oil Red O staining for lipid vacuoles (Figure 1b). AD‐MSCs obtained at Passage 2 were positive for CD73, CD90, and CD105 markers, and relatively negative for the hematopoietic stem cell marker CD45 (Figure 1c).
Figure 1.
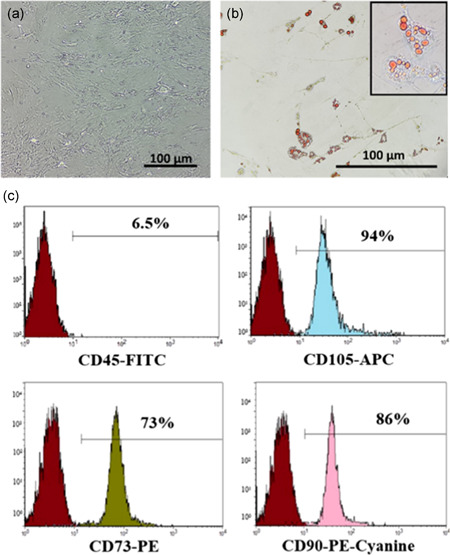
Characterization of adipose tissue‐derived mesenchymal stem cells (AD‐MSCs). Representative images and flow plots of (a) spindle shape of AD‐MSCs after 7 days as determined using phase‐contrast microscopy (×200). (b) Adipogenic differentiation after 21 days was determined by Oil Red O staining (×200). (c) Immunophenotyping of Passage 2 AD‐MSCs using flow cytometric analysis of CD45, CD105, CD73, and CD90 staining
3.2. Developing mouse DCs from BM nuclear cells
The morphology of iDCs was assessed by phase‐contrast microscopy, and cells demonstrated projections on Day 6 (Figure 2a). DC surface markers were detected by flow cytometry with iDC having low cell‐surface expression of MHC class II, CD86, CD83, and CD40 (Figure 2b).
Figure 2.
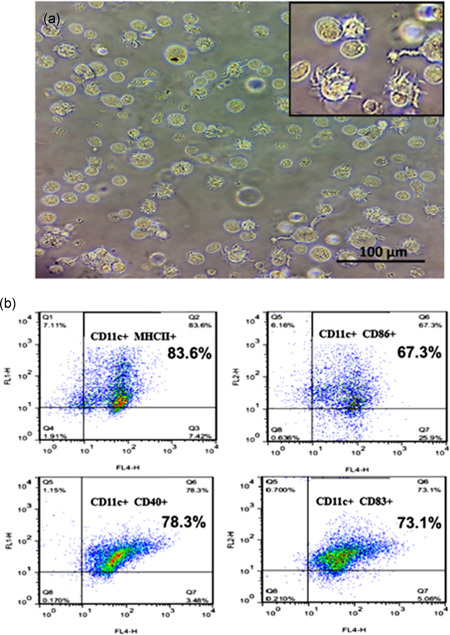
Morphology and phenotypic characterization of DCs. Representative images and flow plots of (a) morphology of immature dendritic cells assessed by phase‐contrast microscopy. The figure indicates immature dendritic cells with cytoplasmic veils appearing on their surfaces. (b) Flow cytometric immunophenotyping of DC using antibodies against CD11, MHC11, CD86, CD40, and CD83. DCs, dendritic cells
3.3. Characterization of exosomes
DLS analysis indicated that the average exosome size was 90 nm (Figure 3a). The spherical shape and size of isolated exosomes (<100 nm) was confirmed by SEM (Figure 3b).
Figure 3.
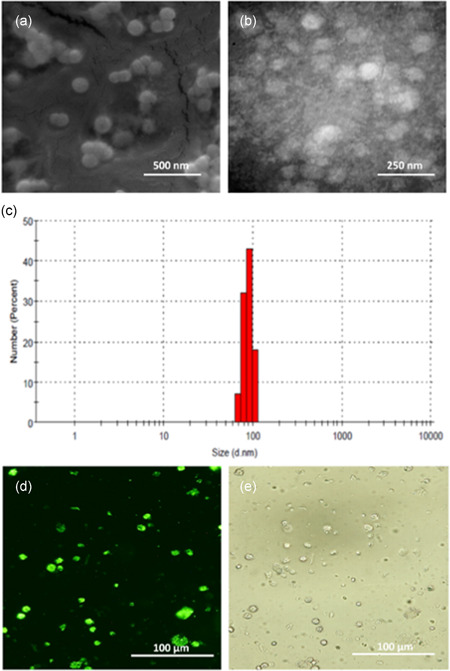
Characterization of stem cell‐derived exosomes and their uptake by dendritic cells (DCs). The spherical shape and size of isolated exosomes was confirmed by (a) scanning and (b) transmission electron microscopy. (c) The average size of exosomes was measured by dynamic light scattering to show a single peak at 90 nm. Mesenchymal stromal cell‐derived exosomes were labeled with green PKH67 dye, and their uptake by bone marrow‐derived DCs determined by intracellular localization was detected by (d) fluorescent microscopy and (e) phase‐contrast microscopy. Results are representative of n = 3 independent experiments
3.4. Internalization of exosomes by DCs
To visualize the internalization of exosomes by DCs, exosomes were labeled with a green fluorescent lipid dye (PKH67). DCs were treated with PKH‐labeled exosomes, and they became fluorescent after 24 hr. Phase contrast (Figure 3c) and fluorescent microscopy (Figure 3d) revealed that exosomes were internalized by DCs.
3.5. Microscopic morphology of DCs in various study groups
Phase‐contrast microscopy was used to characterize the morphology of DCs on Day 9. The iDC left as a control group (Figure 4a) or stimulated with LPS for full maturation (Figure 4b), as demonstrated by the presence of macropinosomes and cytoplasmic vacuoles. MSC‐derived exosome‐treated iDCs showed the appearance of cytoplasmic veils on the surface of cells and some cells displayed dendrite formation (Figure 4c). Combined LPS and exosome‐exposed iDCs (Figure 4d) had characteristics of both types of individually exposed cells.
Figure 4.

The effect of exposure to mesenchymal stromal cell (MSC)‐derived exosomes (72 hr) and/or lipopolysaccharide (LPS; 24 hr) on dendritic cell (DC) morphology. Phase‐contrast microscopy was used to characterize the morphology of DCs following treatment of control immature DCs (Ctrl) (a), LPS‐treated DCs (b), MSC‐derived exosome‐treated DCs (Exo) (c), and Exo + LPS‐treated DCs (d). Results are representative of n = 3 independent experiments
3.6. Effects of exosomes on DC phenotype and maturation
Phenotype analysis examined the cell‐surface expression of CD11c, MHCII (Figures 5a and 5e), the costimulatory molecules CD86 (Figures 5b and 5f), CD40 (Figures 5c and 5g), and the maturation marker CD83 (Figures 5d and 5h). LPS treatment significantly enhanced the expression of MHC11 (111.2 ± 11.3 vs. 67.1 ± 9.1 MFI units; p < .05; Figures 5a and 5e), CD86 (85.5 ± 5.8 vs. 52.9 ± 5.2 MFI units; p < .05; Figure 5b and 5f), and CD40 (62.6 ± 8.9 vs. 28.4 ± 3.7 MFI units; p < .01; Figures 5c and 5g) but not of CD83 (56.2 ± 9.5 vs. 35.6 ± 5 MFI units; p = ns; Figures 5d and 5h), compared with iDCs. Exosomes did not significantly affect the expression of these markers (Figure 5). Although combined exposure of cells to exosomes and to LPS resulted in a significant induction of MHCII (63 ± 7.1 vs. 111.2 ± 11.3 MFI units; p < .01; Figures 5a and 5e), there was no significant induction of CD86 (Figures 5b and 5f), CD40 (Figures 5c and 5g), or of CD83 (Figures 5d and 5h), compared with control cells. However, the effect of combined exosome/LPS‐stimulation did not differ significantly from the effect of LPS alone (Figure 5). Furthermore, when exosomes were added together with LPS to iDCs on Day 6, their differentiation and maturation in response to LPS did not significantly differ.
Figure 5.
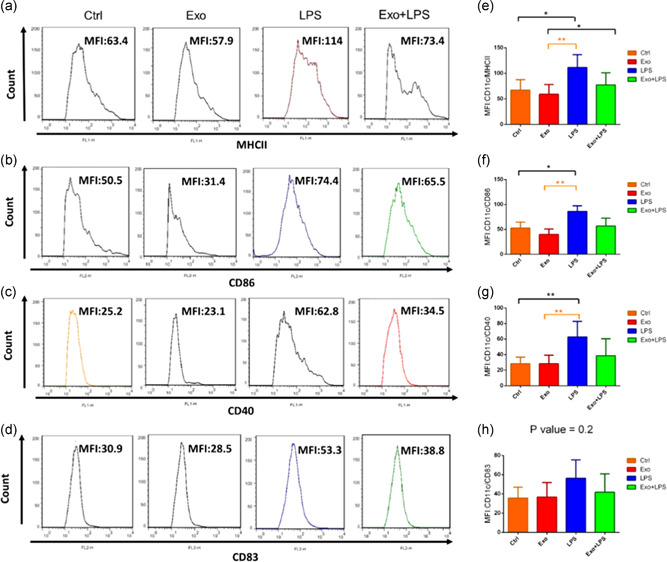
The effect of mesenchymal stromal cell‐derived exosomes and/or lipopolysaccharide (LPS) on dendritic cell (DC) expression of surface markers. The phenotype of immature DCs (Ctrl), MDC‐derived exosome (Exo)‐treated DCs, LPS‐treated DCs and Exo + LPS‐treated DCs were assessed by flow cytometry. DC cells were gated by CD11c+ MHCII+, and then based on this gate, a histogram was drawn up. Representative histogram shows the expression of MHCII (a). DC cells were gated by CD11c+CD86+, and then based on this gate, a histogram was drawn up. Representative histogram shows the expression of CD86 (b). DC cells were gated by CD11c+CD40+, and then based on this gate, a histogram was drawn up. Representative histogram shows the expression of CD40 (c). DC cells were gated by CD11c+CD83+, and then based on this gate, a histogram was drawn up. Representative histogram shows the expression of CD83 (d). Bar graphs represent mean fluorescence intensity (MFI; arbitrary units [AU] of fluorescence) ± SEM percentage expression of CD11c‐MHCII (e) and costimulatory molecules CD86 (f), CD40 (g), and CD83 (h) in the various groups from n = 3 independent experiments. *p < .05, **p < .01. SEM, standard error of the mean
3.7. Exosome‐treated DCs stimulate lymphocyte proliferation
CFSE‐labeled splenic lymphocytes were cocultured with allogenic BMDCs at DC:T cell ratios of 1:3 (Figure 6a), 1:10 (Figure 6b), and 1:30 (Figure 6c) for 72 hr. The MFI of CFSE indicates the opposite of the lymphocyte proliferation rate. Exosome‐treated DCs significantly attenuated lymphocyte proliferation at 3:1 (243.2 ± 7.4 vs. 41.2 ± 6 MFI units; p < .001), 10:1 (188.8 ± 17.8 vs. 36.9 ± 3.7 MFI units; p < .001), and 30:1 (157 ± 18.6 vs. 31.4 ± 5.6 MFI units; p < .001) ratios, with no difference in effect size, compared to LPS‐stimulated DCs (Figure 6a–c). The presence of exosomes resulted in a reduced effect of LPS on lymphocyte proliferation, which no longer reached significance compared with control levels with exosome + LPS‐exposed DCs at 3:1 (91.3 ± 5.9 vs. 41.2 ± 6 MFI units), 10:1 (88.5 ± 2.6 vs. 36.9 ± 3.7 MFI units), and 30:1 (86.7 ± 3.4 vs. 31.4 ± 5.6 MFI units) ratios, compared with LPS‐stimulated cells (Figure 6a–c).
Figure 6.
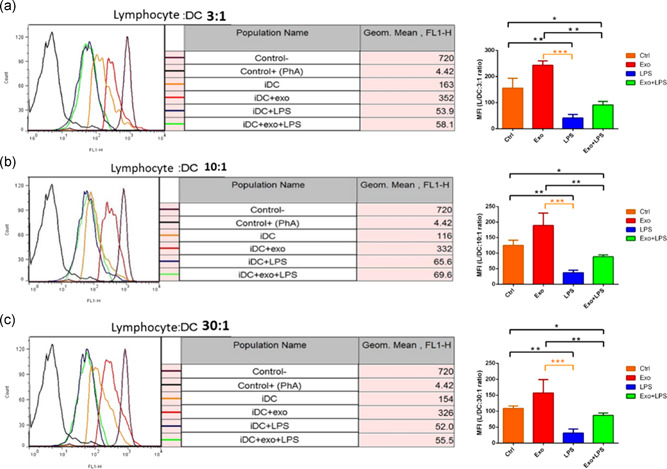
The effect of mesenchymal stromal cell‐derived exosomes (Exo) and/or lipopolysaccharide (LPS) on dendritic cell (DC)‐induced lymphocyte proliferation. Immature DCs (Ctrl), Exo (100 μg/ml)‐treated DCs, LPS (0.1 μg/ml)‐treated DCs, and Exo + LPS‐treated DCs were treated with mitomycin‐C. Cells were then cocultured with carboxyfluorescein succinimidyl ester (CFSE)‐labeled splenic lymphocytes at ratios of 1:3 (a), 1:10 (b), and 1:30 (c) for 72 hr. Data represent the mean fluorescence intensity (MFI; arbitrary units [AU] of fluorescence) ± SEM triplicate value of CFSE intensity. *p < .05, **p < .01, and ***p < .001. SEM, standard error of the mean
3.8. Cytokine production altered in exosome‐treated DCs
To further characterize MSC‐derived exosome‐treated DCs, cell culture supernatants from the different groups (Ctrl, Exo, LPS, and Exo + LPS) were collected after 72 hr, and the levels of IL‐6 (Figure 7a), TGF‐β (Figure 7b), and IL‐10 (Figure 7c) were analyzed. Compared with iDCs (Ctrl), exosome‐exposed DCs had no effect on IL‐6 release (64 ± 1.5 vs. 61.4 ± 1.1; Figure 7a), but they enhanced the release of TGF‐β (48.1 ± 5.6 vs. 392.9 ± 7.9; p < .001; Figure 7b) and IL‐10 (75 ± 0.3 vs. 682.7 ± 31; p < .001; Figure 7c).
Figure 7.
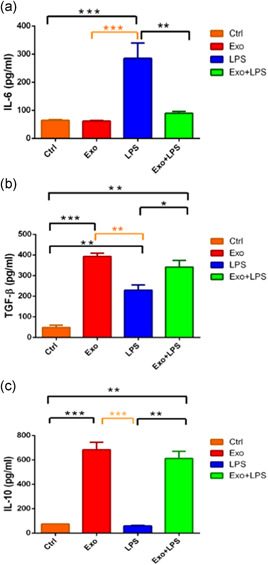
The effect of mesenchymal stromal cell‐derived exosomes (Exo) and/or lipopolysaccharide (LPS) on mediator release. This measure is the opposite of the proliferation rate. The mean ± SEM of IL‐6 (a), TGF‐β (b), and IL‐10 (c) levels in dendritic cell (DC) culture supernatants after 72 hr were measured by an enzyme‐linked immunosorbent assay in n = 3 independent experiments. *p < .05, **p < .01, and ***p < .001. IL, interleukin; SEM, standard error of the mean; TGF‐β, transforming growth factor‐β
In contrast, LPS‐stimulated cells had enhanced release of IL‐6 (284.7 ± 27.6 vs. 64 ± 1.5 pg/ml; p < .001; Figure 7a) and TGF‐β (229 ± 12.8 vs. 48.1 ± 5.6 pg/ml; p < .01; Figure 7b) but decreased production of IL‐10 (59.3 ± 2.2 vs. 75 ± 0.3 pg/ml; p < .001; Figure 7c), compared with immature (Ctrl) DCs. Exosome + LPS‐exposed cells had decreased release of IL‐6 (89.5 ± 3.4 vs. 284.7 ± 27.6 pg/ml; p < .01; Figure 7a) but increased secretion of TGF‐β (341.2 ± 16.4 vs. 229 ± 12.8 pg/ml; p < .05; Figure 7b) and IL‐10 (611 ± 29.3 vs. 51.3 ± 2.2 pg/ml; p < .01; Figure 7c), compared with LPS‐stimulated cells.
4. DISCUSSION
In this study, we investigated the ability of MSC‐derived exosomes to modulate CD11c+ DC function. We show that MSC‐derived exosomes affect the release of TGF‐β, IL‐10, and IL‐6 from DCs, with an effect on the expression of DC costimulatory markers or on the ability of DCs to modulate lymphocyte proliferation. In contrast, LPS‐induced DC maturity markedly enhanced expression of MHCII and the costimulatory markers CD86 and CD40 but not of CD83. LPS‐treated DCs also enhance lymphocyte proliferation and the release of IL‐6 and TGF‐β. Exosomes, in the presence of LPS, prevented LPS‐mediated induction of DC cell‐surface markers and of IL‐6 expression but did not affect lymphocyte proliferation.
DCs have a critical role in the pathogenesis of most autoimmune diseases, and both MSCs and MSC‐derived extracellular vesicles (EVs) exert immunosuppressive effects on immune cells such as T cells by different mechanisms (Favaro et al., 2016). EVs are cellular products that carry specific paracrine signals that may reflect the local cellular environment and may present therapeutic opportunities (Del Fattore et al., 2015).
Our results confirm several previous studies where MSCs or their supernatants have been shown to affect monocyte‐derived DC differentiation and maturity. A murine study, which examined the direct effects of MSCs on DCs, revealed that mature DCs cocultured with MSCs had a significantly reduced expression of CD83, suggesting a reversal towards a more immature phenotype. Meanwhile, decreased expression of presentation molecules (HLA‐DR and CD1a) and costimulatory molecules (CD80 and CD86), and downregulated IL‐12 secretion were also observed. These results were confirmed by Jung et al. (2007). The human study showed that after activation of DCs with GM‐CSF and IL‐4, direct coculture with MSCs strongly inhibited the early differentiation of monocytes into DCs, but this effect was reversible (Jiang et al., 2005). Finally, the allostimulatory ability of MSC‐treated mature DCs on T cells was impaired (Jiang et al., 2005).
Park, Kim, Ryu, Woo, and Ryu (2015) investigated the effects of murine tonsil‐derived MSCs (T‐MSCs) on the DCs and found that the suppressive effect of T‐MSCs on DC function and on the downstream induction of CD4+ lymphocyte proliferation and differentiation was mediated by both direct contact or via soluble mediators such as GM‐CSF, RANTES, IL‐6, and MCP‐1. Further evidence using mouse cells indicated that cell–cell contact was not necessary for these effects as coculture of mature DCs with BMSCs in a Transwell system also reduced the expression of the CD83 maturation marker and that of the CD80 and CD86 stimulatory molecules on DCs (Wang et al., 2008). W. Zhang et al. (2004) studied the effects of human MSCs and supernatants of their cells on the monocyte‐derived DCs. They indicated that MSCs inhibited the expression of CD40, CD80, CD86, and HLA‐DR during differentiation and increased expression of CD40, CD83, and CD86 during maturation of DCs. MSC supernatants have no effect on DC differentiation; however, they inhibit the expression of CD83 in DC (W. Zhang et al., 2004). Several human studies showed that MSCs and MSCs supernatants could suppress the differentiation and maturation of DCs, and T‐cell proliferation was inhibited in the presence of treated DCs (Deng et al., 2016).
Several studies indicate that MSC‐EVs are cell‐derived products with potential therapeutic applications for the treatment of immunological disorders (Bruno, Deregibus, & Camussi, 2015). A human study suggested that membrane vesicles derived from MSCs have an immunosuppressive effect on B lymphocytes. These particles, due to their advantages in terms of standardization, safety, and feasibility, should be further evaluated as immunosuppressive agents in place of the parent cells (Budoni et al., 2013). An additional study investigated the ex vivo immunomodulatory effects of MSC‐derived MVs towards antigen‐activated EAE mice lymphocytes. This showed that MSC‐derived MVs significantly can inhibit autoreactive lymphocyte activation, proliferation and induce lymphocyte‐derived anti‐inflammatory cytokine secretion (Mokarizadeh et al., 2012).
A study using mouse and human samples revealed that MSC exosomes are immunologically active and have the potential to reduce an activated immune system through the induction of Tregs and anti‐inflammatory cytokines (B. Zhang et al., 2013). Finally, in vitro experiments characterizing the immunomodulatory effect of human adipose MSCs‐derived exosomes (exo‐hASCs) on in vitro stimulated T cells indicate that these exosomes may be beneficial therapeutically in the treatment of inflammation‐related diseases (Blazquez et al., 2014).
High concentrations of human MSC‐derived EVs can drive immunosuppression of DCs (Favaro et al., 2016). These MSC‐derived EVs penetrated into the DC to deliver surface receptors and other proteins as well as functional mRNAs and miRNAs. These factors could mediate the cell proliferation and immunomodulatory effects of EVs (Favaro et al., 2016). A more recent study investigating the immunosuppressive effects of human MSC‐EVs on T lymphocytes (Del Fattore et al., 2015) determined that EVs derived from MSCs express galectin‐1 and PD‐L1, both of which are expressed on the surface of mesenchymal cells (Del Fattore et al., 2015). Whereas galectin‐1 induces apoptosis of active T cells, PD‐L1 acts as an inhibitory molecule and induces the proliferation and function of Tregs (Del Fattore et al., 2015).
These data suggest that investigating the composition of MSC‐derived EVs may provide evidence for EV function. A recent proteomic analysis of human MSC‐EVs revealed the presence of 938 proteins, including those associated with extracellular matrix–receptor interaction, focal adhesion, and disease‐specific pathways (Mardpour et al., 2019). Specific immune‐related proteins, such as IL‐10, HGF, LIF, CCL2, VEGFC, CCL20, CXCL2, CXCL8, CXCL16, DEFA1, HERC5, and IFITM2, were present, which may help drive MSC‐EVs migration, regulate immune cell interactions, and control inflammatory responses. A comparison between the MSC‐EV transcriptome and proteome identified nonoverlapping mediators, suggesting that greater information about possible downstream effects of EV contents would be obtained using multiomic integration methods (Fathi et al., 2009). However, several studies suggest that EVs derived from MSCs are less effective than the cells themselves, and the biological effect of EVs may vary, depending on extracellular microenvironment (Burrello et al., 2016).
Although the present study indicates that MSC‐derived exosomes induce tolDCs, the exact protein or miRNA constituent that mediates this effect needs to be resolved. Future studies using RNA sequencing and proteomic analysis along with CRISPR/Cas9 deletion screens or antibody blocking studies may delineate the critical factors involved in the induction of tolDCs by MSC‐derived exosomes. It is important to investigate how an altered microbiota, exemplified by exposure to LPS and to cytokines, affects exosomal content and release and subsequent DC function.
In conclusion, the present in vitro study confirms that MSC‐derived exosomes induce immature and mature DCs into a tolerogenic DC population that has low expression levels of costimulatory markers. They are capable of suppressing lymphocyte activities, increasing secretion of IL‐10 and TGF‐β and decreasing secretion of IL‐6.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
M. S., S. M. H., and E. M. conceived the project and developed the experimental design. A. A., M. V., M. K.‐D., G. F., and J. G. contributed data. M. S., S. M. H., and I. A. analyzed data and revised the manuscript, and E. M. developed the experimental design of corresponding studies. M. S., I. A., and E. M. wrote the paper. S. M. H., M. V., M. K.‐D., G. F., J. G., and I. A. edited the manuscript. All authors reviewed the manuscript.
Shahir M, Mahmoud Hashemi S, Asadirad A, et al. Effect of mesenchymal stem cell‐derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol. 2020;235:7043–7055. 10.1002/jcp.29601
Contributor Information
Seyed Mahmoud Hashemi, Email: smmhashemi@sbmu.ac.ir.
Esmaeil Mortaz, Email: emortaz@gmail.com.
DATA AVAILABILITY STATEMENT
We confirming absence of shared data in this paper.
REFERENCES
- Baghaei, K. , Hashemi, S. M. , Tokhanbigli, S. , Rad, A. A. , Assadzadeh‐Aghdaei, H. , Sharifian, A. , & Zali, M. R. (2017). Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterology and Hepatology From Bed to Bench, 10(3), 208 10.22037/ghfbb.v0i0.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, F. P. , Boynton, R. E. , Haynesworth, S. , Murphy, J. M. , & Zaia, J. (1999). The monoclonal antibody SH‐2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochemical and Biophysical Research Communications, 265(1), 134–139. 10.1006/bbrc.1999.1620 [DOI] [PubMed] [Google Scholar]
- Beyth, S. , Borovsky, Z. , Mevorach, D. , Liebergall, M. , Gazit, Z. , Aslan, H. , … Rachmilewitz, J. (2005). Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood, 105(5), 2214–2219. 10.1182/blood-2004-07-2921 [DOI] [PubMed] [Google Scholar]
- Biancone, L. , Bruno, S. , Deregibus, M. C. , Tetta, C. , & Camussi, G. (2012). Therapeutic potential of mesenchymal stem cell‐derived microvesicles. Nephrology Dialysis Transplantation, 27(8), 3037–3042. 10.1093/ndt/gfs168 [DOI] [PubMed] [Google Scholar]
- Blazquez, R. , Sanchez‐Margallo, F. M. , de la Rosa, O. , Dalemans, W. , Álvarez, V. , Tarazona, R. , & Casado, J. G. (2014). Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Frontiers in Immunology, 5, 556 10.3389/fimmu.2014.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boorn, J. G. , Dassler, J. , Coch, C. , Schlee, M. , & Hartmann, G. (2013). Exosomes as nucleic acid nanocarriers. Advanced Drug Delivery Reviews, 65(3), 331–335. 10.1016/j.addr.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Bruno, S. , Deregibus, M. C. , & Camussi, G. (2015). The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunology Letters, 168(2), 154–158. 10.1016/j.imlet.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Budoni, M. , Fierabracci, A. , Luciano, R. , Petrini, S. , Di Ciommo, V. , & Muraca, M. (2013). The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplantation, 22(2), 369–379. 10.3727/096368911X582769 [DOI] [PubMed] [Google Scholar]
- Burrello, J. , Monticone, S. , Gai, C. , Gomez, Y. , Kholia, S. , & Camussi, G. (2016). Stem cell‐derived extracellular vesicles and immune‐modulation. Frontiers in Cell and Developmental Biology, 4, 83 10.3389/fcell.2016.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci, E. , Racchetti, G. , & Meldolesi, J. (2009). Shedding microvesicles: Artefacts no more. Trends in Cell Biology, 19(2), 43–51. 10.1016/j.tcb.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Corcione, A. , Benvenuto, F. , Ferretti, E. , Giunti, D. , Cappiello, V. , Cazzanti, F. , … Pistoia, V. (2006). Human mesenchymal stem cells modulate B‐cell functions. Blood, 107(1), 367–372. 10.1182/blood-2005-07-2657 [DOI] [PubMed] [Google Scholar]
- Cui, G.‐H. , Wu, J. , Mou, F.‐F. , Xie, W.‐H. , Wang, F.‐B. , Wang, Q.‐L. , … Liu, J.‐R. (2017). Exosomes derived from hypoxia‐preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. The FASEB Journal, 32(2), 654–668. 10.1096/fj.201700600R [DOI] [PubMed] [Google Scholar]
- De Toro, J. , Herschlik, L. , Waldner, C. , & Mongini, C. (2015). Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Frontiers in Immunology, 6, 203 10.3389/fimmu.2015.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore, A. , Luciano, R. , Pascucci, L. , Goffredo, B. M. , Giorda, E. , Scapaticci, M. , … Muraca, M. (2015). Immunoregulatory effects of mesenchymal stem cell‐derived extracellular vesicles on T lymphocytes. Cell Transplantation, 24(12), 2615–2627. 10.3727/096368915X687543 [DOI] [PubMed] [Google Scholar]
- Deng, Y. , Zhang, Y. , Ye, L. , Zhang, T. , Cheng, J. , Chen, G. , … Yang, Y. (2016). Umbilical cord‐derived mesenchymal stem cells instruct monocytes towards an IL10‐producing phenotype by secreting IL6 and HGF. Scientific Reports, 6, 37566 10.1089/scd.2013.0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi, A. , Pakzad, M. , Taei, A. , Brink, T. C. , Pirhaji, L. , Ruiz, G. , … Baharvand, H. (2009). Comparative proteome and transcriptome analyses of embryonic stem cells during embryoid body‐based differentiation. Proteomics, 9(21), 4859–4870. 10.1002/pmic.200900003 [DOI] [PubMed] [Google Scholar]
- Favaro, E. , Carpanetto, A. , Caorsi, C. , Giovarelli, M. , Angelini, C. , Cavallo‐Perin, P. , … Zanone, M. M. (2016). Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia, 59(2), 325–333. 10.1007/s00125-015-3808-0 [DOI] [PubMed] [Google Scholar]
- Fouraschen, S. M. G. , Pan, Q. , de Ruiter, P. E. , Farid, W. R. R. , Kazemier, G. , Kwekkeboom, J. , … de Jonge, J. (2012). Secreted factors of human liver‐derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells and Development, 21(13), 2410–2419. 10.1089/scd.2011.0560 [DOI] [PubMed] [Google Scholar]
- Guilliams, M. , & van de Laar, L. (2015). A Hitchhiker's guide to myeloid cell subsets: Practical implementation of a novel mononuclear phagocyte classification system. Frontiers in Immunology, 6, 406 10.3389/fimmu.2015.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X.‐X. , Zhang, Y. I. , Liu, B. , Zhang, S.‐X. , Wu, Y. , Yu, X.‐D. , & Mao, N. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood, 105(10), 4120–4126. 10.1182/blood-2004-02-0586 [DOI] [PubMed] [Google Scholar]
- Jung, Y. J. , Ju, S. Y. , Yoo, E. S. , Cho, S. J. , Cho, K. A. , Woo, S. Y. , … Ryu, K. H. (2007). MSC–DC interactions: MSC inhibit maturation and migration of BM‐derived DC. Cytotherapy, 9(5), 451–458. 10.1080/14653240701452057 [DOI] [PubMed] [Google Scholar]
- Körbling, M. , & Estrov, Z. (2003). Adult stem cells for tissue repair—A new therapeutic concept? New England Journal of Medicine, 349(6), 570–582. 10.1056/NEJMra022361 [DOI] [PubMed] [Google Scholar]
- Le Blanc, K. , Rasmusson, I. , Sundberg, B. , Götherström, C. , Hassan, M. , Uzunel, M. , & Ringdén, O. (2004). Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. The Lancet, 363(9419), 1439–1441. 10.1016/S0140-6736(04)16104-7 [DOI] [PubMed] [Google Scholar]
- Liu, J. , & Cao, X. (2015). Regulatory dendritic cells in autoimmunity: A comprehensive review. Journal of Autoimmunity, 63, 1–12. 10.1016/j.jaut.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Jin, X. , Hu, C.‐F. , Li, R. , & Shen, C.‐X. (2017). Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cellular Physiology and Biochemistry, 43(1), 52–68. 10.1159/000480317 [DOI] [PubMed] [Google Scholar]
- Lutz, M. B. , Kukutsch, N. , Ogilvie, A. L. J. , Rößner, S. , Koch, F. , Romani, N. , & Schuler, G. (1999). An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods, 223(1), 77–92. 10.1016/S0022-1759(98)00204-X [DOI] [PubMed] [Google Scholar]
- Majumdar, M. K. , Thiede, M. A. , Haynesworth, S. E. , Bruder, S. P. , & Gerson, S. L. (2000). Human marrow‐derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long‐term hematopoiesis when differentiated toward stromal and osteogenic lineages. Journal of Hematotherapy & Stem Cell Research, 9(6), 841–848. 10.1089/152581600750062264 [DOI] [PubMed] [Google Scholar]
- Mardpour, S. , Hamidieh, A. A. , Taleahmad, S. , Sharifzad, F. , Taghikhani, A. , & Baharvand, H. (2019). Interaction between mesenchymal stromal cell‐derived extracellular vesicles and immune cells by distinct protein content. Journal of Cellular Physiology, 234(6), 8249–8258. 10.1002/jcp.27669 [DOI] [PubMed] [Google Scholar]
- Martínez‐Gutierrez, F. , Thi, E. P. , Silverman, J. M. , de Oliveira, C. C. , Svensson, S. L. , Hoek, A. V. , … Pryzdial, E. L. G. (2012). Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 8(3), 328–336. 10.1016/j.nano.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Mokarizadeh, A. , Delirezh, N. , Morshedi, A. , Mosayebi, G. , Farshid, A.‐A. , & Mardani, K. (2012). Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunology Letters, 147(1‐2), 47–54. 10.1016/j.imlet.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Mortaz, E. , Kraneveld, A. D. , Smit, J. J. , Kool, M. , Lambrecht, B. N. , Kunkel, S. L. , … Folkerts, G. (2009). Effect of cigarette smoke extract on dendritic cells and their impact on T‐cell proliferation. PLoS one, 4(3):e4946 10.1371/journal.pone.0004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. , Kim, Y.‐H. , Ryu, J.‐H. , Woo, S.‐Y. , & Ryu, K.‐H. (2015). Immune suppressive effects of tonsil‐derived mesenchymal stem cells on mouse bone‐marrow‐derived dendritic cells. Stem Cells International, 2015, 106540 10.1155/2015/106540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione, I. , Benvenuto, F. , Bocca, P. , Battistini, L. , Uccelli, A. , & Pistoia, V. (2009). Reciprocal interactions between human mesenchymal stem cells and γδ T cells or invariant natural killer T cells. Stem Cells, 27(3), 693–702. 10.1634/stemcells.2008-0687 [DOI] [PubMed] [Google Scholar]
- Quah, B. J. C. , & Parish, C. R. (2010). The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. Journal of Visualized Experiments, 44, e2259 10.3791/2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, M. , Mavin, E. , Nicholson, L. , Green, K. , Dickinson, A. M. , & Wang, X.‐N. (2018). Mesenchymal stromal cell‐derived extracellular vesicles attenuate dendritic cell maturation and function. Frontiers in Immunology, 9, 2538 10.3389/fimmu.2018.02538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari, G. M. , Abdelrazik, H. , Becchetti, F. , & Moretta, L. (2009). MSCs inhibit monocyte‐derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC‐derived prostaglandin E2. Blood, 113(26), 6576–6583. 10.1182/blood-2009-02-203943 [DOI] [PubMed] [Google Scholar]
- Spaggiari, G. M. , Capobianco, A. , Becchetti, S. , Mingari, M. C. , & Moretta, L. (2006). Mesenchymal stem cell‐natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL‐2‐induced NK‐cell proliferation. Blood, 107(4), 1484–1490. 10.1182/blood-2005-07-2775 [DOI] [PubMed] [Google Scholar]
- Steimle, A. , & Frick, J.‐S. (2016). Molecular mechanisms of induction of tolerant and tolerogenic intestinal dendritic cells in mice. Journal of Immunology Research, 2016, 1958650 10.1155/2016/1958650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Ostrowski, M. , & Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology, 9(8), 581–593. 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- Thomson, A. W. , & Robbins, P. D. (2008). Tolerogenic dendritic cells for autoimmune disease and transplantation. Annals of the Rheumatic Diseases, 67(Suppl 3), iii90–iii96. 10.1136/ard.2008.099176 [DOI] [PubMed] [Google Scholar]
- Trajkovic, K. , Hsu, C. , Chiantia, S. , Rajendran, L. , Wenzel, D. , Wieland, F. , … Simons, M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science, 319(5867), 1244–1247. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- Vickers, K. C. , & Remaley, A. T. (2012). Lipid‐based carriers of microRNAs and intercellular communication. Current Opinion in Lipidology, 23(2), 91–97. 10.1097/MOL.0b013e328350a425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Sun, B. , Wang, D. , Ji, Y. , Kong, Q. , Wang, G. , … Li, H. (2008). Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T‐cell tolerance. Scandinavian Journal of Immunology, 68(6), 607–615. 10.1111/j.1365-3083.2008.02180.x [DOI] [PubMed] [Google Scholar]
- Yin, S. , Ji, C. , Wu, P. , Jin, C. , & Qian, H. (2019). Human umbilical cord mesenchymal stem cells and exosomes: Bioactive ways of tissue injury repair. American Journal of Translational Research, 11(3), 1230. [PMC free article] [PubMed] [Google Scholar]
- Yoo, S. , & Ha, S.‐J. (2016). Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Network, 16(1), 52–60. 10.4110/in.2016.16.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Ge, W. , Li, C. , You, S. , Liao, L. , Han, Q. , … Zhao, R. C. H. (2004). Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte‐derived dendritic cells. Stem Cells and Development, 13(3), 263–271. 10.1089/154732804323099190 [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Liu, R. , Shi, D. , Liu, X. , Chen, Y. , Dou, X. , … Liao, L. (2009). Mesenchymal stem cells induce mature dendritic cells into a novel Jagged‐2–dependent regulatory dendritic cell population. Blood, 113(1), 46–57. 10.1182/blood-2008-04-154138 [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Wang, M. , Gong, A. , Zhang, X. , Wu, X. , Zhu, Y. , … Qian, H. (2015). HucMSC‐exosome mediated‐Wnt4 signaling is required for cutaneous wound healing. Stem Cells, 33(7), 2158–2168. 10.1002/stem.1771 [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Yin, Y. , Lai, R. C. , Tan, S. S. , Choo, A. B. H. , & Lim, S. K. (2013). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells and Development, 23(11), 1233–1244. 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Zou, X. , Huang, Y. , Wang, F. , Miao, S. , Liu, G. , … Zhu, Y. (2016). Mesenchymal stromal cell‐derived extracellular vesicles protect against acute kidney injury through anti‐oxidation by enhancing Nrf2/ARE activation in rats. Kidney and Blood Pressure Research, 41(2), 119–128. 10.1159/000443413 [DOI] [PubMed] [Google Scholar]
- Zwicker, J. I. , Liebman, H. A. , Neuberg, D. , Lacroix, R. , Bauer, K. A. , Furie, B. C. , & Furie, B. (2009). Tumor‐derived tissue factor–bearing microparticles are associated with venous thromboembolic events in malignancy. Clinical Cancer Research, 15(22), 6830–6840. 10.1158/1078-0432.CCR-09-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We confirming absence of shared data in this paper.


