Abstract
A large percentage of the patients with keratinocyte carcinoma (KC, formerly known as non‐melanoma skin cancer) is of advanced age and often too frail for standard therapies. However, no specific treatment recommendations are given for this population. This review aimed to give an overview of the current literature on the best practice for the treatment of elderly patients with KC. A literature search was performed in MEDLINE, using ‘keratinocyte carcinoma’, ‘elderly’, ‘treatment’ and various synonyms. Case reports, reviews, comments, non‐English literature and studies with a sample size <15 were excluded. After selection, a total of 47 studies were reviewed. Two types of studies were identified, focusing on (I) the effect of age on treatment outcomes and (II) alternative treatment schedules for elderly patients. Studies on surgery, the gold standard, describe larger lesions and defect size in the elderly population. Recurrence rate, complication rate and disease‐specific survival were not affected by age. Depending on the expected morbidity of a suggested (re‐)excision and patient preferences, a conservative watchful waiting policy can be agreed upon as a shared decision. Other common treatment modalities, such as adjuvant radiotherapy, photodynamic therapy and systemic therapy for basal cell carcinoma (BCC), show comparable results in the elderly and younger population. Alternative treatment schedules for elderly patients include primary hypofractionated radiotherapy, which seems effective and well‐tolerated, although research is limited to case series. Additionally, localized and topical treatments seem safe and effective especially for low‐risk tumours. Data are lacking on the efficacy of systemic therapies of metastatic KC in elderly patients. Efficacy of most treatments (with the exception of photodynamic therapy) is not dependent on age. There is need for more research on the efficacy of adjusted treatment modalities, such as hypofractionated radiotherapy and palliative or curative systemic treatment.
Introduction
Keratinocyte carcinoma (KC, formerly known as non‐melanoma skin cancer) affects a significant number of patients with advanced age; the most diagnoses are made in patients aged 70 or above.1 In addition to cumulative ultraviolet (UV) radiation due to age, the most dominant factor contributing to the increased incidence is a change in UV exposure behaviour, such as longer vacations and more body exposure. Climate change and the use of artificial tanning devices may also contribute.1, 2, 3
The gold standard for the treatment of KC is surgery, which is often considered too aggressive in frail elderly patients. Lubeek et al.4 recently discussed that due to shorter life expectancy, patients may not live long enough to benefit from treatment, while treatment complications could result in a reduced quality of life. On the other hand, tumour progression can also deteriorate quality of life if patients live long enough. Prediction of treatment outcome in the elderly can be difficult. In a study on over 1200 cases, age itself was found not to be a predictor of postoperative outcome after major head and neck surgery.5 Since there is no consensus on ‘elderly’, the definition or threshold differs per study. The National Institute on Ageing produced the most accepted classification, with patients between 65 and 74 defined as ‘young old’, 75–84 as ‘older old’ and 85 or older as ‘oldest old’.6
The aim of the present review was to summarize the current literature on the best practice for the treatment of elderly patients with KC. We will discuss differences between curative treatment outcomes in elderly and younger patients, as well as non‐invasive treatment options and their applicability for the elderly patient.
Methods
To systematically identify the available literature in MEDLINE, a search term was created in October 2018 (Appendix S1, Supporting Information). A total of 3821 potentially relevant articles were found based on the title and abstract. After removal of non‐English literature, case reports, reviews, comments and application of inclusion criteria (KC in elderly or frail patients), 166 articles were subjected for full‐text review. After exclusion of papers without age‐ or frailty‐related outcomes, 47 articles were included for analysis (Fig. 1). These publications were reviewed by at least two authors and processed in the current paper. We found two types of original papers: papers analysing the effect of age on treatment outcomes and papers describing alternative treatment schedules for inoperable, frail elderly patients.
Figure 1.
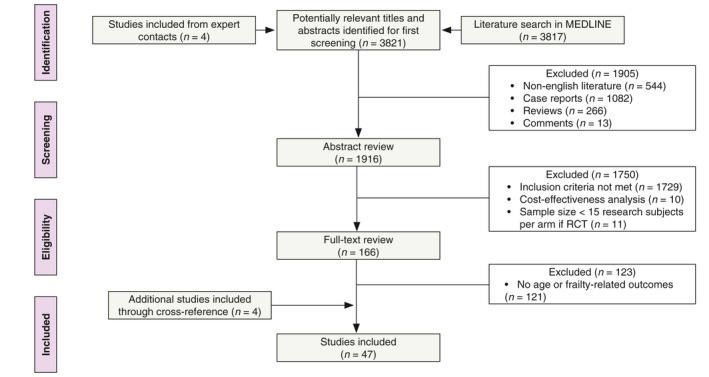
Flow chart depicting the search algorithm.
Results
Effect of age on treatment outcomes of common treatment modalities
Surgical treatment
Surgery is the gold standard treatment for KC. As opposed to simple excision, Mohs’ micrographic surgery (MMS) is characterized by fewer recurrences, and with the possibility of sparing healthy skin.7, 8, 9 Both treatments use local anaesthesia and are well‐tolerated by elderly patients.10, 11 Several studies analysed whether surgical treatment outcomes differ in elderly patients when compared to younger patients, as shown in Table 1.
Table 1.
Overview of literature on surgical treatment outcomes in elderly patients
| Study (year), type | Participants | Intervention and follow‐up | Primary outcome |
|---|---|---|---|
| Complexity | |||
| Batra et al.15 (2002), retrospective |
N = 1131 KCs. Age groups:<35, 35–44, 45–54, 55–64, 65–74, 75–84, ≥85 |
MMS, 3‐year inclusion, 3‐year FU | Age under 35 years protective for ≥3 stages MMS, multivariable OR: 0.12 (95% CI: 0.01–0.98) |
| Hoorens et al.17 (2016), retrospective |
N = 1062 HNBCCs. Age groups: <60, 60–69, 70–79, ≥80 |
MMS, 14‐year inclusion, FU not specified | Age ≥80 years predictive of >1 stage MMS, univariable OR: 1.9 (95% CI: 1.0–3.5) |
| Sahai et al.16 (2012), retrospective |
N = 231 KCs. Age complex cases (≥4 stages): 69.0 ± 14.6 years, age non‐complex cases: 66.0 ± 14 years |
MMS, 3‐year inclusion, FU not specified | No effect of age on complexity |
| Defect size | |||
| Camarero‐Mulas et al.12 (2017), prospective cohort |
N = 2575 pts with KC. Age groups: <80, ≥80 |
MMS, 3‐year inclusion, 1‐year FU | Larger tumours in elderly group |
| Dhiwakar et al.14 (2007), retrospective |
N = 463 pts with 638 HNKCs. Age groups: <80, ≥80 |
Conventional surgery, 10‐year inclusion, average FU: 26 months (range: 1–70) | Larger tumours and defect sizes in elderly group |
| Dinehart et al.13 (1992), retrospective |
N = 2728 BCCs. Age groups: 15–30, 56–70 |
MMS, 11‐year inclusion, median FU in young group: 4.2 years (range: 6 months–9.9 years), older group: 4.4 years (range: 5 months–10.7 years) | Larger tumours and defect sizes in elderly group |
| Eide et al.84 (2005), retrospective |
N = 860 pts with KC. Age groups: <40, 41–50, 51–60, 61–70, 71–80, 81–90, >90 |
MMS, 1‐year inclusion, FU not specified | Age is predictive for defect size, P < 0.014, partial R 2 = 0.019 |
| Recurrence rate | |||
| Camarero‐Mulas et al.12 (2017), prospective cohort |
N = 2575 pts with KC. Age groups: <80, ≥80 |
MMS, 3‐year inclusion, 1‐year FU | No effect of age on recurrence rate |
| Dzubow et al.18 (1982), retrospective |
N = 414 pts with primary cSCC. Age groups: 1–39, 40–59, ≥60 |
MMS, 15‐year inclusion, mean FU: 18.6 months (range: 1–136) | No effect of age on recurrence rate |
| Maghami et al.19 (2007), multicentre retrospective cohort |
N = 120 pts with KC. Age groups: ≤50, >50 |
Craniofacial surgery, 44‐year inclusion, median FU: 27 months (range: 1–279) | No effect of age on recurrence‐free survival |
| Mueller et al.20 (2010), retrospective case–control |
N = 101 pts with facial BCC. Age groups: ≤70, >70 |
Ablative surgery, 4‐year inclusion, 1‐year FU | No effect of age on recurrence rate, univariable OR: 1.4 (95% CI: 0.5–4.1) |
| Survival | |||
| Delaney et al.26 (2013), retrospective |
N = 214 pts with KC. All pts ≥90 years |
MMS, 9‐year inclusion, FU until 4 months after last inclusion | No difference in survival compared with general US population. Perioperative mortality not increased |
| Dhiwakar et al.14 (2006), retrospective |
N = 463 pts with 638 HNKCs. Age groups: <80, ≥80 |
Conventional surgery, 10‐year inclusion, average FU: 26 months (range: 1–70) | No effect of age on DFS |
| Maghami et al.19 (2007), multicentre retrospective cohort |
N = 120 pts with KC. Age groups: ≤50, >50 |
Craniofacial surgery, 44‐year inclusion, median FU: 27 months (range: 1–279) | No effect of age of DSS or OS |
| Pascual et al.27 (2013), prospective |
N = 130 pts with KC. All pts ≥80 years |
Surgery, minimum FU: 24 months | Age predictor for mortality, P = 0.002 (univariable) |
| Linos et al.28 (2016), cross‐sectional |
N = 2702 pts with 8064 KCs and 9653 distinct treatments. LLE vs. normal life expectancy |
Excision, MMS and C&ED, 20‐year inclusion, minimum FU: 1 year | No different treatment rates between groups |
| Postoperative complication rate | |||
| Bras et al.29 (2015), retrospective cohort |
N = 90 pts with head and neck cancer. Age groups: 65–74, ≥75 |
Conventional surgery, 15.5‐year inclusion, median FU: 12 months | No effect of age or frailty on complication rate |
| Bouhassira et al.30 (2016), retrospective |
N = 241 pts with cSCC, BCC or melanoma. Age groups: 75–85, >85 |
Conventional surgery, 3‐year inclusion, 2‐week FU | No effect of age on complication rate, P = 0.084 (univariable) |
| Camarero‐Mulas et al.12 (2017), prospective cohort |
N = 2575 pts with KC. Age groups: <80, ≥80 |
MMS, 3‐year inclusion, 1‐year FU | No effect of age on complication rate |
| Dhiwakar et al.14 (2006), retrospective |
N = 463 pts with 638 KNKCs. Age groups: <80, ≥80 |
Conventional surgery, 10‐year inclusion, average FU: 26 months (range: 1–70) | No effect of age on complication rate |
| Mueller et al.20 (2010), retrospective case–control |
N = 101 pts with facial BCC. Age groups: ≤70, >70 |
Ablative surgery, 4‐year inclusion, 1‐year FU | Older pts 2.7× more likely to develop wound healing disorders than younger pts, univariable OR: 2.7 (95% CI: 1.1–7.0) |
| Pascual et al.33 (2015), prospective |
N = 260 pts with 320 KCs Age groups: <80, ≥80 |
Surgery, inclusion or FU not specified | Haemorrhagic complications more common in older pts |
| Pascual et al.31 (2015), prospective |
N = 144 pts with 180 KCs. All pts ≥80 years |
Conventional surgery, 28‐month inclusion, FU 7 and 30 days after surgery | ADL not affected after surgery |
| Sclafani et al.32 (2012), retrospective |
N = 446 pts with skin tumours. Age groups: <60, ≥60 |
Repair of facial defects after MMS, 10‐year inclusion, average FU 7.74 months (SD: 11.97) | Age < 60 years associated with higher risk of overall complications |
| Postoperative radiotherapy | |||
| Terra et al.39 (2017), retrospective |
N = 90 pts with 99 HNcSCCs. Median age: 76 years (range: 39–106) |
PORT in different regimens, 14‐year inclusion, median FU: 24 months (95% CI: 24.9–35.8) | No effect of age > 50 (univariable, HR: 0.486; 95% CI: 0.048–4.513) or age > 70 (univariable, HR: 0.864; 95% CI: 0.113–6.628) on local recurrence rate |
| Ebrahimi et al.40 (2012), retrospective |
N = 229 pts with nodal metastatic HNcSCC. Age groups: ≤65, >65 |
Surgery in all pts, adjuvant RT in 88%, 30‐year inclusion, median FU: 3.8 years | Increased age associated with short DFI |
| Amoils et al.35 (2017), retrospective |
N = 80 pts with regional metastatic HNcSCC. Age groups: ≤70, >70 |
Surgery alone, surgery with adjuvant RT, or surgery with adjuvant CRT. 15‐year inclusion, FU not specified | No effect of age or treatment modality on survival, univariable HR: 1.2 (95% CI: 0.6–2.4) |
| Givi et al.36 (2011), retrospective |
N = 61 pts with nodal metastatic HNcSCC. Median age: 73 years (range: 43–90) |
Surgery in all pts, adjuvant RT in 40 pts. 14‐year inclusion, median FU: 15 months | No effect of age on OS. Adjuvant RT associated with longer OS, univariable HR: 0.24 (95% CI: 0.09–0.68) |
| Wang et al.37 (2011), retrospective |
N = 122 pts with nodal metastatic HNcSCC. Median age: 66 years (range: 18–95) |
Surgery in all pts, adjuvant RT in 102 pts. 28‐year inclusion, minimum FU: 24 months, median: 57 months | No effect of age on DFS. Adjuvant RT associated with longer OS |
ADL, activity of daily living; BCC, basal cell carcinoma; C&ED, curettage and electrodessication; CI, confidence interval; COD, cause of death; CRT, chemoradiotherapy; DFI, disease‐free interval; DFS, disease‐free survival; DSS, disease‐specific survival; FU, follow‐up; Gy, grey; HNBCC, head and neck basal cell carcinoma; HNcSCC, head and neck cutaneous squamous cell carcinoma; HNKC, head and neck keratinocyte carcinoma; HR, hazard ratio; KC, keratinocyte carcinoma; LLE, limited life expectancy; MMS, Mohs’ micrographic surgery; OR, odds ratio; OS, overall survival; PORT, postoperative radiotherapy; RT, radiotherapy.
All four studies on defect size conclude that higher age is associated with a larger lesion and defect size in MMS and conventional surgery.12, 13, 14 Studies on complexity show no association between age and the number of MMS stages necessary to achieve complete excision.15, 16 Only one study found that patients over 80 years old were almost twice as likely to need more than one MMS stage compared with patients under 80.17 Four studies focusing on recurrence rate concluded that age is not predictive for the recurrence of KC.12, 18, 19, 20 Nonetheless, the follow‐up might be too short to detect recurrence after MMS.7, 8 Codazzi et al.21 describe a cohort of 3957 excisions of BCC. Patients with complete excisions had a recurrence rate of 5.9%, whereas incomplete excisions recurred in 26.8%. However, only 50.7% of the patients complied with follow‐up for a year or longer, so longer‐term recurrences are underestimated in this study. Discussion of these data with patients might aid shared decision‐making on performing re‐excision vs. watchful waiting.
Data about recurrence rates in patients with cSCC and close or positive margins are lacking, probably because of the standard clinical practice to perform a re‐excision in those cases.22 The incomplete excision rate of cSCCs is about 6–16%; localization in the head and neck area, larger tumour diameter and deeper tumour invasion are risk factors for positive margins.23, 24 In the cohort described by Bovill et al.23, the patients with residual tumour cells in the re‐excision were slightly, but not significantly older compared to the patients with negative re‐excisions (78.9 years vs. 73.3 years). Residual tumour cells were found less in patients with longer delay between first and re‐excision. Nevertheless, since only a small percentage of the tissue is examined during histopathological examination of conventionally excised tumours, tumour cells could be present without being detected.25
Five papers on survival after surgery strongly indicate that skin surgery in the elderly population does not increase mortality.14, 19, 26, 27, 28 In the majority of these studies, age was not a predictive factor for disease‐specific survival (DSS). Furthermore, most of the studies involving postoperative complication rate show no difference in the occurrence of complications between age groups.12, 14, 29, 30, 31, 32 On the contrary, Mueller et al.20 found that patients >70 years of age were 2.7 times more likely to develop postoperative wound healing disorders compared with younger patients (P = 0.03) and Pascual et al.33 describe more haemorrhagic complications in the higher age group (P = 0.04). On the other hand, an age of <60 years was found to be associated with a higher risk of postoperative complications by Sclafani et al.32 (P = 0.01) (Table 1).
Surgery with adjuvant radiotherapy
According to the European guideline22, adjuvant or postoperative radiotherapy (RT) should be considered in the case of cSCC with substantial perineural involvement, and when surgical margins are not free and re‐intervention is not possible or unlikely to completely remove the tumour. Also, in the case of metastatic disease, adjuvant RT could improve survival compared with surgery or RT alone.22, 34 In most reviewed studies, advanced age was not associated with recurrence, poorer overall or disease‐free survival (DFS) after surgery with adjuvant RT (Table 1).35, 36, 37, 38, 39 Only one study found that patients with a shorter disease‐free interval (DFI) were significantly more likely to be aged >65 years.40 However, DFI was probably influenced by a subgroup of patients with delayed presentation of advanced nodal disease, which may bias the results.
Systemic treatment for BCC
For patients with locally advanced or metastatic BCC, treatment options are limited. Vismodegib is the first approved Hedgehog pathway inhibitor, which is indicated for these complicated cases of BCC. In the study of Chang et al.41, vismodegib showed similar efficacy and adverse effects in different age groups. Systemic therapy targeting cSCC will be addressed in part II. All studies concerning systemic therapy are summarized in Table 4.
Treatment modalities adjusted for the treatment of (frail) elderly patients
Alternative therapeutic strategies for elderly patients are used in several case series (Tables 2 and 3). Moreover, elderly patients with KC are more often not treated than younger patients.42
Table 2.
Overview of literature on alternative primary radiotherapy schedules and outcomes in elderly patients
| Study (year), type | Participants | Intervention and follow‐up | Reason for alternative treatment | Primary outcome |
|---|---|---|---|---|
| Cognetta et al.56 (2012), retrospective |
N = 1149 pts with 1715 non‐aggressive KCs. All pts >65 years |
Superficial X‐ray therapy (80 kV) with 35 Gy in 5 fractions, three times per week. 10‐year inclusion, average FU: 31.5 months (range: 1–120) | Patient preference | No effect of age on recurrence, multivariable HR: 0.99 (95% CI: 0.95–1.02) |
| Ferro et al.85 (2015), prospective phase II |
N = 31 pts with KC. All pts ≥70 years |
Electrons (6–9–12 MeV) or photons (6MV) with 30 Gy in 6 daily fractions. Median FU: 30 months (range: 8–72) | Unfit for other local treatments | Local control after 2 years 93.2% |
| Kouloulias et al.52 (2013), retrospective |
N = 38 pts with HNBCC. All pts ≥64 years |
Electrons (6 MeV) or photons (6MV) with 30 Gy in 5 weekly fractions. 7‐year inclusion, median FU: 48 months | Eligibility criteria based on location, tumour characteristics, immunosuppression and previous treatment | Three recurrences were found |
| Marriappan et al.53 (2014), retrospective cohort |
N = 25 pts with 37 BCCs. All pts ≥83 years |
42 Gy in 7 weekly fractions, no radiation technique mentioned. Median FU: 15 months (range: 1–89) | Unfit for daily treatment | Two recurrences were found |
| Pampena et al.38 (2016), retrospective cohort |
N = 385 pts with 436 KCs. Elderly disabled pts >60 years (I) vs. other pts (II) |
Orthovoltage, 37.75 Gy in 7 weekly fractions (I) or 45 Gy in 15 daily fractions (II). 6‐year inclusion, median FU: 31.8 months | Surgery was contraindicated or refused | No difference between treatment groups in OS (multivariable HR: 0.662, 95% CI: 0.387–1.131) DFS (multivariable HR: 0.483, 95% CI: 0.065–3.582), or cosmetic outcome (multivariable RR: 1.048, 95% CI: 0.170–6.473) |
| Pelissero et al.54 (2015), retrospective |
N = 117 pts with 141 facial BCCs. All pts frail and elderly, median age: 82 years (range: 75–103) |
Orthovoltage 55–150 kV or electrons 4–8 MeV with 25–30 Gy in 5–6 weekly fractions. 3‐year inclusion, median FU: 61 months (range: 42–85) | Unfit for daily radiation | Complete response in 98.3% of pts and 98.6% of tumours |
| Russi et al.55 (2015), retrospective |
N = 134 pts with 159 facial BCCs. All pts >75 years |
Orthovoltage 55‐150 kV or electrons 4–8 MeV with 25–30 Gy in 5–6 weekly fractions. Median FU: 64.5 months (range: 42–88) | Unfit for daily radiation | Complete response in 98.5% of pts and 98.7% of tumours. Recurrence in 3 pts |
| Terra et al.39 (2017), retrospective |
N = 48 pts with 52 HNcSCCs. Median age: 81 years (range: 50–100) |
Photons, electrons or orthovoltage (100–200 kV) with 55, 60 or 70 Gy in fractions of 2 Gy 5×/week. 14‐year inclusion, median FU: 23 months (95% CI: 20.9–29.9) | Not applicable, normal protocol | No effect of age on recurrence. Age > 50 univariable HR 0.486 (95% CI: 0.048–4.513), age >70 univariable HR: 0.864 (95% CI: 0.113–6.628) |
| Valeriani et al.86 (2017), prospective cohort |
N = 21 pts with 26 KCs. All pts >80 years |
Electrons (6–12 MeV) or photons (6MV) with 60 Gy in 10 or 12 weekly fractions. 4‐year inclusion, 2‐year FU | Unfit for surgery | Complete response in 92.4% of tumours. No effect of schedule on response or toxicity |
BCC, basal cell carcinoma; CI, confidence interval; DFS, disease‐free survival; FU, follow‐up; Gy, grey, HNBCC, head and neck basal cell carcinoma; HNcSCC, head and neck cutaneous squamous cell carcinoma; HR, hazard ratio; KC, keratinocyte carcinoma; kV, kilovolt; MeV, megaelectron volt; MV, megavolt; MMS, Mohs’ micrographic surgery; OR, odds ratio; OS, overall survival; pts, patients; RR, relative risk; RT, radiotherapy; vs, versus.
Table 3.
Overview of literature on alternative treatment schedules
| Study (year), type | Participants | Intervention and follow‐up | Reason for alternative treatment | Primary outcome |
|---|---|---|---|---|
| Local destructive therapy | ||||
| Kuflik et al.48 (2004), retrospective |
N = 2932 pts with 4406 KCs. Age of 3 pts ≤43, other pts 53–95 years |
(Curettage) cryosurgery, double freeze–thaw cycle. 30‐year inclusion, FU not specified | All tumours amenable for cryosurgery | Complete response in 98.6% of tumours. 62 recurrences |
| Lubeek et al.44 (2016), retrospective |
N = 102 pts with clinically suspected 109 nBCC. Mean age: 71 years (SD: 13) |
C&ED (20 Watt) in at least 2 cycles. 3‐year inclusion, median FU: 21 months (range: 1–66) | Every clinically suspected primary nBCC included | No effect of age or high‐risk tumour on recurrence risk |
| Samain et al.47 (2014), retrospective |
N = 138 pts with 144 facial BCCs. Mean age: 76.5 ± 11.1 years |
(Curettage) cryosurgery, single freeze–thaw cycle. 7‐year inclusion, median FU: 39.5 months (range: 18–74) | Decision made by multidisciplinary panel of experts. Cryosurgery contraindicated in recurrent and morpheiform BCCs | 5‐year recurrence‐free rate 94% |
| Stewart et al.49 (2015), retrospective |
N = 233 KCs. Average age BCC 78.3 years, cSCC 75.5 years |
Shave biopsy. 5‐year inclusion, FU not specified | Assessment of cost‐effectiveness of excision after shave biopsy | 42% of excisions of BCCs no residual tumour, 73% of cSCCs |
| Yakish et al.45 (2017), retrospective |
N = 80 pts with 89 cSCCs. Mean age 76 years (SD: 10, range: 45–95) |
Curettage. 1‐year inclusion, median FU: 6 years (range: 0–2502 days) | Assessment of effectiveness of curettage alone in cSCC | Complete response in 97% of tumours |
| Topical or intralesional therapy | ||||
| Anasagasti‐Angulo et al.58 (2009), prospective |
N = 16 pts with extensive, recurrent or resistant to other treatments KC. Median age: 70 years (range: 31–89) |
IFN formulation peri‐ and intralesionally, 3×/week for 3 weeks (4 pts also received chemotherapy). 4‐year inclusion, FU 1 year |
No other therapeutic options after surgery/RT/chemotherapy | Complete response in 46.7% of pts, partial response in 40.0%, 13.3% non‐responders |
| Ohson et al.57 (2006), prospective |
N = 11 pts with 14 BCCs. Age range: 59–92 years |
Imiquimod 5% cream, once daily for 5 consecutive days/week for 12 weeks. FU 12 weeks | Unfit for surgery or RT | Complete response in 50% of tumours |
| Borroni et al.64 (2013), prospective |
N = 36 pts with AK, BD or BCC. Age groups: 61–70, 71–80, 81–90 |
BP measurement before and after MAL‐PDT session. No further FU | Not specified | Highest prevalence of APH (33%) in pts aged 71–80 years |
| Choi et al.65 (2017), randomized clinical trial | N = 45 pts with microinvasive cSCC | MAL‐PDT or AFL‐PDT. FU at 1 week, 3, 12 and 24 months | Unfit for surgery | Complete response rate higher and recurrence rate lower in AFL‐PDT group |
| Fantini et al.62 (2011), retrospective |
N = 135 pts with 194 BCCs. Mean age: 71 ± 12.9 years |
MAL‐PDT. Median FU: 20 months | Not specified | No effect of age on treatment response |
| Lindberg‐Larsen et al.61 (2012), retrospective |
N = 90 pts with 157 BCCs (sBCC and thin nBCC). Age groups: ≤60, >60 |
MAL‐PDT. 3‐year inclusion, FU at 3, 6, 12 and 24 months | Other treatment modalities less favourable and good cosmetic outcome important | Higher recurrence rate in older pts |
| Nissen et al.60 (2015), 1: unblinded randomized controlled, 2: retrospective |
1: N = 30 healthy volunteers. Age groups: <55, ≥55. 2: N = 67 pts with 100 BCCs. Age groups: 2 of equal size based on median age (61 years) |
1: MAL and BF‐200 ALA for 24 h, 3‐month inclusion. 2: MAL‐PDT, minimum FU: 1 year |
Not specified |
1: declining PpIX formation with age, P < 0.001, R 2 = 0.42. 2: higher treatment efficacy in younger pts |
| Roozeboom et al.63 (2014), single‐blinded non‐inferiority randomized controlled trial |
N = 400 pts with sBCC, 385 pts analysed. Age groups: ≤60, >60 |
MAL‐PDT or imiquimod, 2.5‐year inclusion, FU at 3 and 12 months | Not specified | Higher probability of treatment success for imiquimod, except for tumours on lower extremities in older pts |
AFL‐PDT, ablative fractional laser–primed photodynamic therapy; AK, actinic keratosis; APH, acute postoperative hypertension; BCC, basal cell carcinoma; BD, Bowen's disease; BF‐200 ALA, BF‐200 aminolevulinic acid; BP, blood pressure; C&ED, curettage and electrodessication; cSCC, cutaneous squamous cell carcinoma; IFN, interferon; KC, keratinocyte carcinoma; MAL‐PDT, methyl aminolevulinate photodynamic therapy; nBCC, nodular basal cell carcinoma; PpIX, protoporphyrin IX; RT, radiotherapy; sBCC, superficial basal cell carcinoma; SD, standard deviation.
Local destructive therapy
Several localized treatments could be used as an alternative to treat elderly patients. Generally, it is only recommended for patients with low‐risk KC. Characteristics for high‐risk tumours are described in Samarasinghe et al.43 A practical and cheap therapeutic option is curettage with electrodessication (C&ED). A study by Lubeek et al.44 showed a 6% recurrence rate of BCCs treated with C&ED after a median follow‐up period of 21 months. Increasing age and high‐risk BCCs were not associated with a higher recurrence rate, indicating that C&ED may also be a good option for high‐risk BCCs. A study by Yakish et al.45 found a 97% local control rate of 89 cSCCs after treatment with curettage alone. Recurrence after a median follow‐up of 6 years was not associated with increasing age.
Two studies on the effectiveness of intralesional cryosurgery for nodular and superficial BCCs in elderly patients found low recurrence rates (0% and 5%, respectively).46, 47 In a study by Kuflik et al.48, 4406 new and recurrent KCs were treated with curettage and cryosurgery in a period of 30 years with an overall cure rate of 98.6%; however, follow‐up period (5 years) is only mentioned for a subgroup of patients.
Stewart et al.49 assessed elderly patients with KCs thought to be small enough for complete removal by shave biopsy. Examination of the surgical specimens taken after shave biopsy showed that 42% of the BCC specimens and 73% of the cSCC specimens were negative for residual tumour. Despite these high percentages, shave biopsy cannot be considered as a curative treatment for KC.
Primary radiotherapy
Primary RT is an alternative for surgery and is widely used for the treatment of KC with curative intent, also in elderly patients, although its efficacy is lower than that of surgery.50 In the case of inoperable tumours, when surgical defects would give functional or cosmetic problems, or patients with severe comorbidities, primary RT is even favoured over surgery. RT should also be taken into consideration when a patient refuses surgery.22 Older age has been identified as a risk factor for refusing surgery in breast cancer patients.51
Most studies on primary RT in elderly patients assessed the effectiveness of treatment schedules that were less intensive than traditional schemes, such as hypofractionated RT (weekly irradiation as opposed to daily fractions) and found that hypofractionated RT was effective and well‐tolerated in elderly patients38, 52, 53, 54, 55, as summarized in Table 2. However, these results should be handled with care as the studies suffer from the lack of control groups and short follow‐up. Studies that compared different age groups found no significant differences in recurrence rates.39, 56 Pampena et al.38 compared elderly disabled patients treated with weekly irradiation with other patients treated with daily irradiation. No differences were found in overall survival, DFS or cosmetic outcome.
Topical and intralesional treatment modalities
Topical therapy is generally used for the treatment of superficial BCC (sBCC) or cSCC in situ. However, it may also be used for nodular BCC (nBCC) when surgery or RT is unfeasible.
A study with imiquimod in 11 elderly patients who were unfit for surgery, with nBCC, superficial multicentric BCC and sclerosing BCC, reported a complete response rate around 50%.57 Intralesional treatment with interferon was applied in 16 elderly patients with extensive, recurrent or resistant to other procedures KC. The treatment was effective in 86.7% of the patients. No severe adverse events related to interferon were reported.58
Topical photodynamic therapy (PDT) with methyl aminolevulinate (MAL‐PDT) is approved for the treatment of actinic keratosis, Bowen's disease, primary sBCC and thin low‐risk nBCC.59 Studies are contradictory about the effectiveness of PDT in elderly patients. Nissen et al.60 reported that protoporphyrin IX formation decreases with age, leading to a decreased efficacy. Higher recurrence rates in patients older than 60 are also described.61 Fantini et al.62 did not find age to be predictive for the response rate of BCC. Interestingly, Roozeboom et al.63 found that sBCCs on the lower extremities of elderly patients were more effectively treated with MAL‐PDT compared to imiquimod. Acute postoperative hypertension (APH) is mentioned as a possible side‐effect of PDT, most prevalent in elderly patients, which could require immediate medical attention in the case of a hypertensive crisis.64 MAL‐PDT could also be combined with laser therapy, as demonstrated by Choi et al.65, which seemed to increase the response rate and decrease the recurrence rate. A summary of studies regarding topical and intralesional therapy in the elderly population is shown in Table 3.
Systemic treatment for cSCC
Systemic treatment may be applicable for elderly patients who are not eligible for conventional therapies. However, limited data are available on the effect of age on effectivity and safety. In a study with 14 elderly patients (mean age: 76 years) with aggressive, multiple or recurrent cSCC, treatment with oral 5‐fluorouracil (175 mg/m2 daily during 3 weeks which on average was repeated 4 times) was evaluated. Measurable improvement was seen in 64% of the patients, and the authors report grade I gastrointestinal symptoms as the only adverse effect.66
Recently, the immune checkpoint inhibitor cemiplimab has been approved in the United States of America and Europe for the systemic treatment of advanced cSCC in patients who are not eligible for surgery or radiation.67 A study by Migden et al.68 showed a response rate of cemiplimab around 50% in patients with advanced cSCC (median age: 73, range: 55–88), with durable disease control in around 65% of the responders. In patients with metastatic cSCC (median age: 71, range: 38–93), the response rate was 47%, with durable disease control in 61% of the patients. However, the effects of age or frailty were not mentioned in this study. Studies on immune checkpoint inhibitors for different indications show that old age is not a contraindication to immune checkpoint inhibition. Nonetheless, possible differences in efficacy between younger and elderly patients are described, but the patient numbers are small and specific clinical trials for elderly patients are needed.69, 70 All reviewed studies concerning systemic therapy are summarized in Table 4.
Table 4.
Overview of literature on systemic therapy
| Study (year), type | Participants | Intervention and follow‐up | Reason for alternative treatment | Primary outcome |
|---|---|---|---|---|
| Chang et al.41 (2016), 1: international multicentre non‐comparative phase 2 study, 2: multicentre open‐label non‐comparative expanded access study |
1: N = 104 pts, 71 with laBCC and 33 with mBCC. 2: 119 pts, 62 with laBCC, 57 with mBCC. Age groups: <65, ≥65 |
Oral vismodegib 150 mg/day. 1: median treatment 10.2 months (<65) and 9.2 months (≥65). 2: median treatment 5.4 months (<65) and 5.5 months (≥65) |
Unfit for surgery | No effect of age on treatment outcome or adverse events |
| Cartei et al.66 (2000), prospective |
N = 14 pts with pretreated cSCC Mean age: 74 years |
Mannitol‐coated 5‐FU tablets 175 mg/m2 daily for 3 weeks every 5 weeks. FU not specified | Resistant to current standard therapies, pts with high age | Tablets were well‐tolerated, response in 64% of pts |
| Espeli et al.87 (2016), prospective |
N = 26 pts with advanced or metastatic KC. Age groups: pts >80 vs. total group |
Carboplatin at area under the curve = 2 or cisplatin 40 mg, bleomycin 15 IU, MTX 40 mg total dose of methotrexate, 5‐fluorouracil 500 mg (CMFb) until best response, toxicity or progression of disease | Unfit for surgery |
Total group: response in 61.5% of pts, complete response in 26.9%. >80 years: response in 63.6% of pts, complete response in 18.2% |
5‐FU, 5‐fluorouracil; CMFb, cisplatin/carboplatin, methotrexate, fluorouracil, bleomycin; cSCC, cutaneous squamous cell carcinoma; IFN, interferon; KC, keratinocyte carcinoma; laBCC, locally advanced basal cell carcinoma; mBCC, metastatic basal cell carcinoma; MTX, methotrexate; nBCC, nodular basal cell carcinoma.
Discussion
Reviewing the literature on treatment of KC in elderly patients, we found that surgical treatment is well‐tolerated in elderly patients with a similar complication and cure rate as in younger patients. The majority of patients with KC have small tumours, which can be treated with radical excision under local anaesthesia. The burden of such a treatment is relatively low, with little chance of deterioration of the patient and a very good prognosis with a recurrence rate of 5 to 8% and cure rates up to 96% after 5 years.71 Older patients have larger tumours and consequently larger defect sizes after surgery. Whether this is due to a more aggressive disease in the elderly population or a patient's or doctor's delay is unclear. Although often patients do not report any symptoms from the tumour, it is likely that further growth – in the case of no treatment, or irradical treatment – will cause complaints at some point. Postponing treatment until symptoms arise probably leads to more extensive excision, with a higher risk of irradical resection and complications. Also, it is well known that cSCCs with a larger diameter are more likely to recur and metastasize, with the need for more aggressive treatment. It is also associated with strongly reduced survival rates.71 Discussion regarding treatment intention is more relevant for patients with locally or locoregionally advanced KCs, especially in cSCCs. As the immune response in ageing is generally worse due to immunosenescence,72 it could be hypothesized that this is also the case in the anticancer immune response after incomplete excision and that complete resection with adequate margins is even more important in the elderly KC patients.
MMS is the most common form of microscopically controlled surgery. Other forms such as ‘slow Mohs’ or the ‘Breuninger’ technique can also be considered, but generally take more time than MMS which can lead to a greater burden of treatment. Cure rates of MMS in BCC and cSCC are superior over conventional surgical excision.7, 8, 73 Previous studies report that MMS is well‐tolerated in elderly patients.26, 74 However, due to the retrospective nature of these studies, a selection bias is possible. Generally, only fitter octo‐ and nonagenarians are selected for MMS and their frail counterparts are not. MMS can still be considered in elderly frail patients, especially when the setting can be adjusted to suit the frail patient. Invasive surgery in the right setting could possibly give better outcomes than watchful waiting or radiotherapy, especially in the case of a high‐risk tumour. Regional networks where one centre can specialize in the treatment of elderly or frail patients could provide a better treatment setting.
Primary radiotherapy can be a good alternative for surgery, but standard daily irradiations for several weeks is not always feasible for the frail and elderly population. Hypofractionated schedules are applied in clinical practice to reduce the burden of treatment, and seem to achieve promising results. However, more evidence is necessary to determine whether hypofractionated schedules are non‐inferior to the daily schedules.
It is still debated whether elderly patients respond differently to treatment with PDT, and attention should be paid to the possibility of acute post‐treatment hypertension, especially in patients with hypertension in their medical history.64 Topical 5‐fluorouracil (5‐FU) is used on‐label in the treatment of actinic keratosis and sBCC, and off‐label for cSCC in situ. Cure rates between 60 and 90% are mentioned in literature, with higher cure rates in sBCCs. However, the application could be difficult for elderly patients with lesions on hard‐to‐reach locations.75, 76
The high number of studies describing adjusted treatment regimens in (frail) elderly patients demonstrates the challenging process of treatment decisions in this patient population. Unfortunately, most of these studies provide little or poor evidence, due to the lack of control groups, short follow‐up and poorly defined inclusion criteria. Decision‐making in elderly KC patients is very complex and is affected by many factors such as limited life expectancy, frailty, comorbidities and decreased mobility. Especially in older, more vulnerable patients, it is important to consider the potential benefits of a possible curative treatment against ‘wait‐and‐see’ or supportive care.77 Besides patient factors, the burden of treatment and the consequences of withholding (curative) treatment are important variables in the decision‐making process on treatment intention. Last but not least, patient preferences also play a role in this process. Preservation of functional independence and quality of life are often mentioned as important priorities for older patients, but their preferences in the treatment of KC are poorly studied and need further exploration in the form of wider use of patient‐reported outcome measures.78, 79
Palliative care or wait‐and‐see policy should not be confused with terminal care at the end of life. The main goal of palliative care is relief of symptoms caused by the disease, which possibly may but not directly will lead to the death of the patient. Supporting the patient in living life with as less limitations as possible is the main purpose.80 There is very limited literature focusing on palliative care in dermato‐oncology.81
If regional metastases are diagnosed, intensive surgical treatment and/or radiation therapy is advised as curative treatment. Pretreatment selection of patients eligible for this intensive treatment is challenging. Despite intensive treatment, 5‐year disease‐specific survival in these patients does not exceed 54–77%.82 A careful consideration of (individualized) treatment morbidity on the one hand and potential benefit of the treatment on the other hand should be made together with the patient. Clinical tools, such as frailty measurements and consultation of a geriatrician in elderly and vulnerable patients, could be helpful in the decision‐making process for both patient and clinician.29, 83
A limitation of this literature review is that due to the lack of consensus on the definition of ‘elderly’, the different statistical methods used in the reviewed studies and the many small sample sizes, treatment outcomes and age groups could not be systematically compared or re‐analysed.
Conclusion
When feasible, surgery is the gold standard for the treatment of elderly patients with KC. However, in elderly patients, tumours are typically larger at surgery which might lead to a greater defect size. Recurrence rate and disease‐specific survival do not differ between elderly and younger patients after surgical treatment. RT is a good alternative for surgery but can be a burden for frail elderly patients because of the frequency of treatments. Hypofractionated RT can be considered as a curative treatment since it was shown to be effective and well‐tolerated by most of the elderly patients, although controlled studies with better‐defined inclusion criteria and longer follow‐up are needed. Several alternative local destructive treatments can be considered in the case of elderly patients with low‐risk tumours or patients in which surgery or RT is contraindicated. In elderly patients with aggressive, advanced and/or unresectable KC, systemic treatment options are limited. When deciding on any form of treatment, it is important to consider the potential benefits against ‘wait‐and‐see’ or supportive care, especially in elderly or frail patients.
Supporting information
Appendix S1. Keywords used in the systematic search performed in MEDLINE
Funding sources
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Conflicts of interest
The authors declare that they have no conflict of interest.
Due to the nature of study (review article), no IRB approval is required.
References
- 1. Louwman W, Lemmens V, van Akkooi A, Nijsten TEC. Kankerzorg in beeld: de oudere patiënt. Integr Kankercent Ned 2016: 21–31. [Google Scholar]
- 2. Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080. [DOI] [PubMed] [Google Scholar]
- 3. Slaper H, van Dijk A, den Outer P, van Kranen H, Slobbe L. UV‐straling en gezondheid: Probleemveld en kennisbasis bij het RIVM, 2017.
- 4. Lubeek SF, van Vugt LJ, Aben KK, van de Kerkhof PC, Gerritsen MP. The epidemiology and clinicopathological features of basal cell carcinoma in patients 80 years and older: a systematic review. JAMA Dermatol 2017; 153: 71–78. [DOI] [PubMed] [Google Scholar]
- 5. Peters TT, van Dijk BA, Roodenburg JL, van der Laan BF, Halmos GB. Relation between age, comorbidity, and complications in patients undergoing major surgery for head and neck cancer. Ann Surg Oncol 2014; 21: 963–970. [DOI] [PubMed] [Google Scholar]
- 6. Website of the National Institute on Ageing [WWW document]. URL www.nia.nih.gov (last accessed: 19 December 2019).
- 7. van Loo E, Mosterd K, Krekels GA et al Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow‐up. Eur J Cancer 2014; 50: 3011–3020. [DOI] [PubMed] [Google Scholar]
- 8. Mosterd K, Krekels GA, Nieman FH et al Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal‐cell carcinoma of the face: a prospective randomised controlled trial with 5‐years’ follow‐up. Lancet Oncol 2008; 9: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 9. Stuart SE, Schoen P, Jin C et al Tumor recurrence of keratinocyte carcinomas judged appropriate for Mohs micrographic surgery using Appropriate Use Criteria. J Am Acad Dermatol 2017; 76: 1131–1138.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Limthongkul B, Samie F, Humphreys TR. Assessment of postoperative pain after Mohs micrographic surgery. Dermatol Surg 2013; 39: 857–863. [DOI] [PubMed] [Google Scholar]
- 11. Firoz BF, Goldberg LH, Arnon O, Mamelak AJ. An analysis of pain and analgesia after Mohs micrographic surgery. J Am Acad Dermatol 2010; 63: 79–86. [DOI] [PubMed] [Google Scholar]
- 12. Camarero‐Mulas C, Delgado Jimenez Y, Sanmartin‐Jimenez O et al Mohs micrographic surgery in the elderly: comparison of tumours, surgery and first‐year follow‐up in patients younger and older than 80 years old in REGESMOHS. J Eur Acad Dermatol Venereol 2018; 32: 108–112. [DOI] [PubMed] [Google Scholar]
- 13. Dinehart SM, Dodge R, Stanley WE, Franks HH, Pollack SV. Basal cell carcinoma treated with Mohs surgery. A comparison of 54 younger patients with 1050 older patients. J Dermatol Surg Oncol 1992; 18: 560–566. [DOI] [PubMed] [Google Scholar]
- 14. Dhiwakar M, Khan NA, McClymont LG. Surgery for head and neck skin tumors in the elderly. Head Neck 2007; 29: 851–856. [DOI] [PubMed] [Google Scholar]
- 15. Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol 2002; 138: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 16. Sahai S, Walling HW. Factors predictive of complex Mohs surgery cases. J Dermatolog Treat 2012; 23: 421–427. [DOI] [PubMed] [Google Scholar]
- 17. Hoorens I, Batteauw A, Van Maele G, Lapiere K, Boone B, Ongenae K. Mohs micrographic surgery for basal cell carcinoma: evaluation of the indication criteria and predictive factors for extensive subclinical spread. Br J Dermatol 2016; 174: 847–852. [DOI] [PubMed] [Google Scholar]
- 18. Dzubow LM, Rigel DS, Robins P. Risk factors for local recurrence of primary cutaneous squamous cell carcinomas. Treatment by microscopically controlled excision. Arch Dermatol 1982; 118: 900–902. [PubMed] [Google Scholar]
- 19. Maghami EG, Talbot SG, Patel SG et al Craniofacial surgery for nonmelanoma skin malignancy: report of an international collaborative study. Head Neck 2007; 29: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 20. Mueller CK, Nicolaus K, Thorwarth M, Schultze‐Mosgau S. Multivariate analysis of the influence of patient‐, tumor‐, and management‐related factors on the outcome of surgical therapy for facial basal‐cell carcinoma. Oral Maxillofac Surg 2010; 14: 163–168. [DOI] [PubMed] [Google Scholar]
- 21. Codazzi D, Van Der Velden J, Carminati M et al Positive compared with negative margins in a single‐centre retrospective study on 3957 consecutive excisions of basal cell carcinomas. Associated risk factors and preferred surgical management. J Plast Surg Hand Surg 2014; 48: 38–43. [DOI] [PubMed] [Google Scholar]
- 22. Stratigos A, Garbe C, Lebbe C et al Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus‐based interdisciplinary guideline. Eur J Cancer 2015; 51: 1989–2007. [DOI] [PubMed] [Google Scholar]
- 23. Bovill ES, Banwell PE. Re‐excision of incompletely excised cutaneous squamous cell carcinoma: histological findings influence prognosis. J Plast Reconstr Aesthet Surg 2012; 65: 1390–1395. [DOI] [PubMed] [Google Scholar]
- 24. Kosec A, Svetina L, Luksic I. Significance of clinical stage, extent of surgery and outcome in cutaneous squamous cell carcinoma of the head and neck. Int J Oral Maxillofac Surg 2013; 42: 82–88. [DOI] [PubMed] [Google Scholar]
- 25. Tolkachjov SN, Brodland DG, Coldiron BM et al Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc 2017; 92: 1261–1271. [DOI] [PubMed] [Google Scholar]
- 26. Delaney A, Shimizu I, Goldberg LH, MacFarlane DF. Life expectancy after Mohs micrographic surgery in patients aged 90 years and older. J Am Acad Dermatol 2013; 68: 296–300. [DOI] [PubMed] [Google Scholar]
- 27. Pascual JC, Belinchon I, Ramos JM. Mortality after dermatologic surgery for nonmelanoma skin cancer in patients aged 80 years and older. J Am Acad Dermatol 2013; 69: 1051–1052. [DOI] [PubMed] [Google Scholar]
- 28. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc 2016; 64: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bras L, Peters TT, Wedman J et al Predictive value of the Groningen Frailty Indicator for treatment outcomes in elderly patients after head and neck, or skin cancer surgery in a retrospective cohort. Clin Otolaryngol 2015; 40: 474–482. [DOI] [PubMed] [Google Scholar]
- 30. Bouhassira J, Bosc R, Greta L et al Factors associated with postoperative complications in elderly patients with skin cancer: a retrospective study of 241 patients. J Geriatr Oncol 2016; 7: 10–14. [DOI] [PubMed] [Google Scholar]
- 31. Pascual JC, Belinchon I, Ramos JM. Use of the Barthel index, activities of daily living, in dermatologic surgery in patients aged 80 years and older. Int J Dermatol 2015; 54: 222–226. [DOI] [PubMed] [Google Scholar]
- 32. Sclafani AP, Sclafani JA, Sclafani AM. Successes, revisions, and postoperative complications in 446 Mohs defect repairs. Facial Plast Surg 2012; 28: 358–366. [DOI] [PubMed] [Google Scholar]
- 33. Pascual JC, Belinchon I, Ramos JM. Cutaneous surgery complications in individuals aged 80 and older versus younger than 80 after excision of nonmelanoma skin cancer. J Am Geriatr Soc 2015; 63: 188–190. [DOI] [PubMed] [Google Scholar]
- 34. Canueto J, Jaka A, Toll A. The value of adjuvant radiotherapy in cutaneous squamous cell carcinoma: a review. Actas Dermosifiliogr 2018; 109: 476–484. [DOI] [PubMed] [Google Scholar]
- 35. Amoils M, Lee CS, Sunwoo J et al Node‐positive cutaneous squamous cell carcinoma of the head and neck: survival, high‐risk features, and adjuvant chemoradiotherapy outcomes. Head Neck 2017; 39: 881–885. [DOI] [PubMed] [Google Scholar]
- 36. Givi B, Andersen PE, Diggs BS, Wax MK, Gross ND. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck 2011; 33: 999–1004. [DOI] [PubMed] [Google Scholar]
- 37. Wang JT, Palme CE, Morgan GJ, Gebski V, Wang AY, Veness MJ. Predictors of outcome in patients with metastatic cutaneous head and neck squamous cell carcinoma involving cervical lymph nodes: improved survival with the addition of adjuvant radiotherapy. Head Neck 2012; 34: 1524–1528. [DOI] [PubMed] [Google Scholar]
- 38. Pampena R, Palmieri T, Kyrgidis A et al Orthovoltage radiotherapy for nonmelanoma skin cancer (NMSC): comparison between 2 different schedules. J Am Acad Dermatol 2016; 74: 341–347. [DOI] [PubMed] [Google Scholar]
- 39. Terra JB, Gaster MB, Halmos GB et al Local control of 151 head and neck cutaneous squamous cell carcinoma after radiotherapy: a retrospective study on efficacy and prognostic factors. Clin Otolaryngol 2017; 42: 851–855. [DOI] [PubMed] [Google Scholar]
- 40. Ebrahimi A, Clark JR, Ahmadi N, Palme CE, Morgan GJ, Veness MJ. Prognostic significance of disease‐free interval in head and neck cutaneous squamous cell carcinoma with nodal metastases. Head Neck 2013; 35: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 41. Chang AL, Lewis KD, Arron ST et al Safety and efficacy of vismodegib in patients aged >/=65 years with advanced basal cell carcinoma. Oncotarget 2016; 7: 76118–76124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basu P, Beckles A, Porter ML, Olbricht S. Non‐melanoma skin cancers are more likely to be untreated in elderly patients. J Am Acad Dermatol 2020; 82: 505–507. [DOI] [PubMed] [Google Scholar]
- 43. Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg 2012; 5: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lubeek SF, Arnold WP. A retrospective study on the effectiveness of curettage and electrodesiccation for clinically suspected primary nodular basal cell carcinoma. Br J Dermatol 2016; 175: 1097–1098. [DOI] [PubMed] [Google Scholar]
- 45. Yakish K, Graham J, Hossler EW. Efficacy of curettage alone for invasive cutaneous squamous cell carcinoma: a retrospective cohort study. J Am Acad Dermatol 2017; 77: 582–584. [DOI] [PubMed] [Google Scholar]
- 46. Har‐Shai Y, Sommer A, Gil T et al Intralesional cryosurgery for the treatment of basal cell carcinoma of the lower extremities in elderly subjects: a feasibility study. Int J Dermatol 2016; 55: 342–350. [DOI] [PubMed] [Google Scholar]
- 47. Samain A, Boullie MC, Duval‐Modeste AB, Joly P. Cryosurgery and curettage‐cryosurgery for basal cell carcinomas of the mid‐face. J Eur Acad Dermatol Venereol 2015; 29: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 48. Kuflik EG. Cryosurgery for skin cancer: 30‐year experience and cure rates. Dermatol Surg 2004; 30: 297–300. [DOI] [PubMed] [Google Scholar]
- 49. Stewart CM, Garlick J, Mcmullin J et al Surgical excision of non‐melanoma skin cancer in an elderly veteran's affairs population. Plast Reconstr Surg Glob Open 2015; 2: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alam M, Nanda S, Mittal BB, Kim NA, Yoo S. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol 2011; 65: 377–388. [DOI] [PubMed] [Google Scholar]
- 51. Gaitanidis A, Alevizakos M, Tsalikidis C, Tsaroucha A, Simopoulos C, Pitiakoudis M. Refusal of cancer‐directed surgery by breast cancer patients: risk factors and survival outcomes. Clin Breast Cancer 2018; 18: e469–e476. [DOI] [PubMed] [Google Scholar]
- 52. Kouloulias V, Papadavid E, Mosa E et al A new hypofractionated schedule of weekly irradiation for basal cell carcinoma of the head and neck skin area in elderly patients. Dermatol Ther 2014; 27: 127–130. [DOI] [PubMed] [Google Scholar]
- 53. Marriappan L, Ramasamy S, Robert F. Weekly radiotherapy for basal cell carcinoma in the frail and elderly. Br J Dermatol 2014; 171: 1237–1239. [DOI] [PubMed] [Google Scholar]
- 54. Pelissero A, Russi EG, Melano A et al Facial basal cell carcinomas treated with hypo‐fractionated radiotherapy: a retrospective analysis in 117 elderly patients. J Am Acad Dermatol 2015; 73: 166–168. [DOI] [PubMed] [Google Scholar]
- 55. Russi EG, Pelissero A, Melano A et al Facial basal cell carcinomas in elderly frail patients treated with low total‐dose radiotherapy. Anticancer Res 2015; 35: 4949–4953. [PubMed] [Google Scholar]
- 56. Cognetta AB, Howard BM, Heaton HP, Stoddard ER, Hong HG, Green WH. Superficial X‐ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol 2012; 67: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 57. Ohson K, DesGroseilliers JP, Weatherhead S, Weatherhead L. Imiquimod 5% cream use for the treatment of basal cell carcinomas in elderly patients, in long‐term care facilities, not amenable to surgical or radiation therapy. J Cutan Med Surg 2006; 10: 201–203. [DOI] [PubMed] [Google Scholar]
- 58. Anasagasti‐Angulo L, Garcia‐Vega Y, Barcelona‐Perez S, Lopez‐Saura P, Bello‐Rivero I. Treatment of advanced, recurrent, resistant to previous treatments basal and squamous cell skin carcinomas with a synergistic formulation of interferons. Open, prospective study. BMC Cancer 2009; 9: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morton CA, Szeimies R‐M, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications ‐ actinic keratoses, Bowen's disease, basal cell carcinoma. J Eur Acad Dermatol Venereol 2013; 27: 536–544. [DOI] [PubMed] [Google Scholar]
- 60. Nissen CV, Philipsen PA, Wulf HC. Protoporphyrin IX formation after topical application of methyl aminolaevulinate and BF‐200 aminolaevulinic acid declines with age. Br J Dermatol 2015; 173: 760–766. [DOI] [PubMed] [Google Scholar]
- 61. Lindberg‐Larsen R, Solvsten H, Kragballe K. Evaluation of recurrence after photodynamic therapy with topical methylaminolaevulinate for 157 basal cell carcinomas in 90 patients. Acta Derm Venereol 2012; 92: 144–147. [DOI] [PubMed] [Google Scholar]
- 62. Fantini F, Greco A, Del Giovane C et al Photodynamic therapy for basal cell carcinoma: clinical and pathological determinants of response. J Eur Acad Dermatol Venereol 2011; 25: 896–901. [DOI] [PubMed] [Google Scholar]
- 63. Roozeboom MH, Nelemans PJ, Mosterd K, Steijlen PM, Arits AH, Kelleners‐Smeets NW. Photodynamic therapy vs. topical imiquimod for treatment of superficial basal cell carcinoma: a subgroup analysis within a noninferiority randomized controlled trial. Br J Dermatol 2015; 172: 739–745. [DOI] [PubMed] [Google Scholar]
- 64. Borroni RG, Carugno A, Rivetti N, Arbustini E, Brazzelli V. Risk of acute postoperative hypertension after topical photodynamic therapy for non‐melanoma skin cancer. Photodermatol Photoimmunol Photomed 2013; 29: 73–77. [DOI] [PubMed] [Google Scholar]
- 65. Choi SH, Kim KH, Song KH. Effect of methyl aminolevulinate photodynamic therapy with and without ablative fractional laser treatment in patients with microinvasive squamous cell carcinoma: a randomized clinical trial. JAMA Dermatol 2017; 153: 289–295. [DOI] [PubMed] [Google Scholar]
- 66. Cartei G, Cartei F, Interlandi G et al Oral 5‐fluorouracil in squamous cell carcinoma of the skin in the aged. Am J Clin Oncol 2000; 23: 181–184. [DOI] [PubMed] [Google Scholar]
- 67. Cemiplimab approved for treatment of CSCC. Cancer Discov 2018; 8: OF2. [DOI] [PubMed] [Google Scholar]
- 68. Migden MR, Rischin D, Schmults CD et al PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018; 379: 341–351. [DOI] [PubMed] [Google Scholar]
- 69. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017; 123: 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Daste A, Domblides C, Gross‐goupil M et al Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer 2017; 82: 155–166. [DOI] [PubMed] [Google Scholar]
- 71. Alam M, Ratner D. Cutaneous squamous‐cell carcinoma. N Engl J Med 2001; 344: 975–983. [DOI] [PubMed] [Google Scholar]
- 72. Fuentes E, Fuentes M, Alarcon M, Palomo I. Immune system dysfunction in the elderly. An Acad Bras Cienc 2017; 89: 285–299. [DOI] [PubMed] [Google Scholar]
- 73. van Lee CB, Roorda BM, Wakkee M et al Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol 2018; 181: 338–343. [DOI] [PubMed] [Google Scholar]
- 74. Charles AJ Jr, Otley CC, Pond GR. Prognostic factors for life expectancy in nonagenarians with nonmelanoma skin cancer: implications for selecting surgical candidates. J Am Acad Dermatol 2002; 47: 419–422. [DOI] [PubMed] [Google Scholar]
- 75. Bahner JD, Bordeaux JS. Non‐melanoma skin cancers: photodynamic therapy, cryotherapy, 5‐fluorouracil, imiquimod, diclofenac, or what? Facts and controversies Clin Dermatol 2013; 31: 792–798. [DOI] [PubMed] [Google Scholar]
- 76. Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg 2013; 39: 1306–1316. [DOI] [PubMed] [Google Scholar]
- 77. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014; 5: 197–203. [DOI] [PubMed] [Google Scholar]
- 78. Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non‐small cell lung cancer: descriptive study based on scripted interviews. BMJ 1998; 317: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Di Maio M, Perrone F. Quality of Life in elderly patients with cancer. Health Qual Life Outcomes 2003; 1: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Balducci L, Dolan D. Palliative care of cancer in the older patient. Curr Oncol Rep 2016; 18: 70–72. [DOI] [PubMed] [Google Scholar]
- 81. Goto H, Kiyohara Y, Shindo M, Yamamoto O. Symptoms of and palliative treatment for unresectable skin cancer. Curr Treat Options Oncol 2019; 20: 34. [DOI] [PubMed] [Google Scholar]
- 82. Coombs AC, Butler A, Allison R. Metastatic cutaneous squamous cell carcinoma of the parotid gland: prognostic factors. J Laryngol Otol 2018; 132: 264–269. [DOI] [PubMed] [Google Scholar]
- 83. de Vries J, Heirman AN, Bras L et al Geriatric assessment of patients treated for cutaneous head and neck malignancies in a tertiary referral center: predictors of postoperative complications. Eur J Surg Oncol 2019; 46: 123–130. [DOI] [PubMed] [Google Scholar]
- 84. Eide MJ, Weinstock MA, Dufresne RG Jr et al Relationship of treatment delay with surgical defect size from keratinocyte carcinoma (basal cell carcinoma and squamous cell carcinoma of the skin). J Invest Dermatol 2005; 124: 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferro M, Deodato F, Macchia G et al Short‐course radiotherapy in elderly patients with early stage non‐melanoma skin cancer: a phase II study. Cancer Invest 2015; 33: 34–38. [DOI] [PubMed] [Google Scholar]
- 86. Valeriani M, Nicosia L, Agolli L et al Mono‐ and Bi‐weekly hypofractionated radiation therapy for the treatment of epithelial skin cancer in very elderly patients. Anticancer Res 2017; 37: 825–830. [DOI] [PubMed] [Google Scholar]
- 87. Espeli V, Ruegg E, Hottinger AF, Modarressi A, Dietrich PY. Weekly multi‐agent chemotherapy (CMF‐b) for advanced non‐melanoma skin cancer. Anticancer Res 2016; 36: 2359–2364. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Keywords used in the systematic search performed in MEDLINE


