Abstract
Background
Origin of human adult Leydig cells (ALCs) is not well understood. This might be partly due to limited data available on the identification and location of human precursor and stem Leydig cells (SLCs) which hampers the study on the development of ALCs.
Objectives
The aim of the present study was to investigate whether described human (PDGFRα, NGFR) and rodent (NES, PDGFRα, THY1, NR2F2) SLC markers are expressed by a common cell population within human adult testicular interstitial cells in vivo and before and after in vitro propagation.
Materials and methods
Immunohistochemical analyses were used to identify localization of human adult testicular interstitial cells expressing described SLC markers. Next, interstitial cells were isolated and cultured. The percentage of cells expressing one or more SLC markers was determined before and after culture using flow cytometry.
Results
NR2F2 and PDGFRα were present in peritubular, perivascular, and Leydig cells, while THY1 was expressed in peritubular and perivascular cells. Although NES and NGFR were expressed in endothelial cells, co‐localization with PDGFRα was found for both in vitro, although for NGFR only after culture. All marker positive cells were able to undergo propagation in vitro.
Discussion
The partly overlap in localization and overlap in expression in human testicular cells indicate that PDGFRα, NR2F2, and THY1 are expressed within the same ALC developmental lineage from SLCs. Based on the in vitro results, this is also true for NES and after in vitro propagation for NGFR.
Conclusion
Our results that earlier described SLC markers are expressed in overlapping human interstitial cell population opens up further research strategies aiming for a better insight in the Leydig cell lineage and will be helpful for development of strategies to cure ALC dysfunction.
Keywords: markers, human testis, stem Leydig cells, propagation
1. INTRODUCTION
Human stem Leydig cells (SLCs) might be an interesting cell population for possible use in future cell therapy to restore testosterone levels in adult men with primary hypogonadism. Although the current therapy for hypogonadism, testosterone replacement therapy (TRT), is successful in restoring serum testosterone levels, bone density, and muscle mass, it increases risks of prostate cancer, cardiovascular disease, and infertility. 1 , 2 Future SLC cell therapy to restore physiological testosterone production in the testis will circumvent these adverse effects as well as the burden of lifelong TRT. To achieve this, proper identification of human adult SLCs is essential. Limited data are available on the identity and origin of these cells as well as there is uncertainty regarding the location of the SLCs in the adult human testis.
The development of human Leydig cells can be divided in three phases, based on morphological characteristics and a triphasic pattern in hormone release. The first Leydig cell population appears during fetal life and is responsible for the high testosterone production between 8 and 16 weeks of gestation. 3 A second peak in testosterone levels, and concomitantly in Leydig cell number, is found during the neonatal period 2‐3 months after birth. 4 , 5 , 6 , 7 Although during childhood a period of quiescence in steroidogenesis is seen, an infantile Leydig cell population is identified, which is thought to have developed from regressed neonatal Leydig cells (NNLC). 4 , 8 Just before puberty, the hypothalamic‐pituitary‐testicular axis is reactivated and adult Leydig cells (ALCs) develop that are responsible for the production of testosterone during puberty and adult life 5 (reviewed in 9 ). These ALCs are thought to develop through differentiation of stem/precursor cells, and/or originate from regressed NNLCs and infantile Leydig cells. 6 , 9
The majority of studies targeting the identification of SLCs in the adult testis have been performed in rodents. These studies show that SLCs in the rodent testis are mainly located in peritubular and perivascular regions. 10 , 11 , 12 A number of studies in rodents have identified markers for SLCs such as platelet‐derived growth factor receptor alpha (PDGFRα, also called CD140a), 13 , 14 , 15 nestin (NES), 16 , 17 integrin subunit alpha V (ITGAV, also called CD51), 16 , 18 nuclear receptor subfamily 2, group F, member 2 (NR2F2, also called COUP‐TFII), 19 and Thy‐1 cell surface antigen (THY1, also called CD90). 20
Morphological changes during ALC development show similarities in humans and rodents and in both, rodents and primates, this developmental process is dependent on LH (reviewed in 21 ). However, there are also differences in Leydig cell development between rodent and human. In contrast to the triphasic development of the Leydig cell population in human, there is a biphasic pattern in Leydig cell development in rodent with fetal Leydig cells (FLCs) and ALCs. A neonatal Leydig cell population is lacking in rodents. In addition, whereas in human the development of the FLC population is dependent on hCG, this is not the case for rodent FLCs which develop in the absence of functional luteinizing hormone chorionic gonadotropin receptors (LHCGRs). 22 , 23
This raises the question if markers described for rodent SLCs are applicable for the identification and isolation of human SLCs from the adult testis. Using the rodent SLC marker PDGFRα, cells in the human testis located around the seminiferous tubules in the peritubular cell layer were identified. 24 These cells were able to differentiate in vitro into cells with Leydig cell characteristics. 24 , 25 Another SLC marker, nerve growth factor receptor (NGFR, also called CD271 or p75) is expressed in NES+ cells in both a peritubular and vascular cell location in the adult human testis. 26
Since several origins for ALCs are suggested in the adult human testis, namely undifferentiated multipotent stem cells, regressed NNLC, infantile LCs or regressed FLCs, 6 , 9 , 27 it is interesting to investigate whether the SLC markers identify one or various SLC/progenitor cell populations. Therefore, the aim of this study was to determine whether previously identified human and rodent SLC markers are expressed by a common cell population within the adult human testicular interstitium. We investigated in which cell population the reported SLC markers are expressed in the interstitial compartment of the adult human testis. In addition, we investigated whether isolated interstitial cells (co‐)express the SLC markers before and after in vitro propagation.
2. MATERIALS AND METHODS
2.1. Human testicular tissue
Adult spare testicular tissue was obtained from 9 prostate cancer patients who had undergone bilateral orchiectomy. This surgery was part of their treatment procedure, and spare tissue was donated for research after informed consent. In accordance with Dutch law, spare tissues can be used for research with permission of the patients without further permission of an ethical committee since no additional interventions were needed to obtain these samples. In all cases, full spermatogenesis was confirmed by histological analysis. Immediately after receiving the tissue, part of the biopsies were fixed overnight in paraformaldehyde (4% (w/v) PFA (Merck, Germany) in phosphate‐buffered saline (PBS, pH = 7.4)) or modified methacarn (88.8% (v/v) methanol (Merck Millipore, USA) + 11.2% (v/v) glacial acetic acid) fixatives, or first fixed for 6 hours in 4% phosphate‐buffered formaldehyde solution (Roti‐Histofix, Carl Roth GmbH, Germany) followed by overnight fixation in diluted bouin (23.8% (v/v) 37% formaldehyde (Merck, Germany) + 71.4% (v/v) 0.9% picric acid (Sigma‐Aldrich, USA) + 4.8% (v/v) glacial acid (Merck Millipore, USA)). The fixed biopsies were then dehydrated and embedded in paraffin and stored at 4°C for later use. In addition, remaining small biopsy pieces of tissue were cryopreserved in Minimal Essential Medium (MEM, Gibco, Thermo Fisher Scientific, USA) supplemented with 20% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, USA) and 8% dimethylsulfoxide (DMSO, EuroClone S.p.A., Italy) using a Coolcell freezing container (BioCision, USA) and stored at −196°C until cell isolation.
2.2. Selection of markers for rodent SLCs
Five markers, namely NR2F2, PDGFRα, THY1, NES, and NGFR, were selected since these markers were proposed in the literature to identify SLCs in rodents and/or humans (Table 1). CD51 was excluded from this selection, because this marker was shown to be expressed by ALCs in the human testis and therefore not suitable for the identification of SLCs exclusively. 26
Table 1.
Overview of described markers identifying stem Leydig cells
| Marker | Studies | Species | Evidence that marker identifies stem cells | Described location in testis |
|---|---|---|---|---|
| NR2F2 | Kilcoyne et al (2014) 19 | Fetal and adult rats |
After EDS treatment, these cells start expressing 3β‐HSD Transgenic lineage tracing of NR2F2+ cells from fetal testis into ALCs in adult testis |
Peritubular layer and other cells in interstitium |
| PDGFRα | Ge et al (2006) 15 | Neonatal rats |
In vitro differentiation into testosterone‐producing cells, expressing Leydig cell markers Cells express 3β‐HSD after transplantation in testis of EDS‐treated rats |
Not described |
| Stanley et al (2012) 13 | EDS‐treated adult rats |
In vitro differentiation into testosterone‐producing cells In vitro recovery of cell population and testosterone production after EDS treatment |
Peritubular layer | |
| Landreh et al (2013) 14 | Neonatal rats | Increase in steroidogenic gene expression and testosterone production after (Bu)2 cAMP stimulation in vitro | Peritubular layer | |
| Landreh et al (2014) 24 | Adult human |
Expression of pluripotent markers Increase in steroidogenic gene expression and progesterone production after forskolin stimulation in vitro |
Peritubular layer | |
| Eliveld et al (2019) 25 | Adult human |
Cells are able to differentiate into adipogenic, osteogenic, and chondrogenic lineage in vitro Increase in steroidogenic gene expression and progesteron production after in vitro differentiation |
Not described | |
| THY1 | Li et al (2016) 20 | Adult rats | After culture of cells with LH and SAG, cells express 3β‐HSD and produce testosterone | Peritubular layer |
| NES | Davidoff et al (2004) 17 | Adult rats |
After EDS treatment, expression of NES in the testis increased After EDS treatment, NES expressing cells become positive for CytP450 |
Vascular smooth muscle cells and pericytes |
| Jiang et al (2014) 16 | Nes‐GFP transgenic mouse |
Cells have self‐renewal capacity and clonogenic potential in vitro Cells are able to differentiate into neural, osteogenic, adipogenic, and chondrogenic lineage in vitro After In vitro differentiation, cells produce testosterone and express Leydig cell markers |
Peritubular layer and other cells in interstitium | |
| NGFR | Zhang et al (2017) 26 | Adult human |
Cells have self‐renewal capacity in vitro Cells are able to differentiate into adipogenic, osteogenic, and chondrogenic lineage in vitro Increase in steroidogenic gene expression and testosterone production after in vitro differentiation Increase in serum testosterone levels after transplantation in testis of EDS‐treated rats |
Peritubular cells and blood vessels |
Abbreviations: EDS, ethane dimethane sulfonate; 3β‐HSD, 3‐beta‐hydroxysteroid dehydrogenase; ALC, Adult Leydig cell; (Bu)2cAMP, dibutyryl cyclic adenosine monophosphate; LH, Luteinizing hormone; SAG, Smoothened agonist; CytP450, Cytochrome P450 side chain cleavage enzyme; GFP, green fluorescent protein.
2.3. Immunohistochemical and immunofluorescence staining
For immunohistochemical and fluorescence staining, 5‐µm paraffin sections were used. Before staining, the sections were deparaffinized and rehydrated. When necessary, antigen retrieval was performed using a heat‐induced method in a microwave for 10 min in 0.01 M citrate buffer (pH = 6). To inactivate endogenous peroxidase, a 10 min blocking step with 3% hydrogen peroxide (H2O2, Millipore, USA) in tris‐buffered saline (TBS, pH = 7.6) was performed. Non‐specific binding of the antibodies was blocked for 1 hour at room temperature using superblock (ScyTek Laboratories, USA). Then, slides were incubated with the primary antibody diluted in bright diluent (Immunologic, The Netherlands) overnight at 4°C (for antibody details optimized for immune staining see Table S1). The second day, the sections were incubated with matched ready‐to‐use poly‐HRP secondary antibody (Immunologic, The Netherlands) for 1 hour at room temperature. For visualization, DAB bright (Immunologic, The Netherlands) was used and counterstaining was performed with hematoxylin. After dehydration, slides were covered with Entellan (Millipore, USA) and coverslips. TBS was used as a wash buffer. The staining was analyzed using an Olympus BX41 light microscope, and pictures were taken using the Leica Application Suite V4 program. For all antigens, immunohistochemical staining was performed on at least three biological replicates.
For double staining using immunofluorescence, autofluorescence was blocked with True black lipofuscin autofluorescence quencher (Biotium, USA). After overnight incubation with the primary antibody (Table S1), slides were incubated with 2 µg/ml secondary antibodies goat‐anti‐mouse 488 or 555 and donkey‐anti‐rabbit 488 or 555 (Invitrogen, Thermo Fisher Scientific, USA). Sections were analyzed using a Leica DM5000B fluorescence microscope, and pictures were taken using the Leica Application Suite Advanced Fluorescence program. Double staining with DAB and Fast Red was performed using the Polink DS‐MR‐Hu D1 Kit (GBI Labs, USA) according to the protocol of the manufacturer.
Details regarding the primary and secondary antibodies and incubation protocols used for immunohistochemistry and immunofluorescence can be found in Table S1. All experiments were performed on at least three donors. Matched IgG isotypes were used as a negative control.
2.4. Cell isolation and culture
The interstitial cell fraction (ICF) was isolated from cryopreserved‐thawed human testicular tissue fragments according to methods previously described. 28 , 29 Briefly, a single enzymatic digestion step was used with 500 U/ml trypsin TRL3 (Worthington biochemical corporation, USA), 900 U/ml hyaluronidase type II (Sigma‐Aldrich, USA), and 450 U/ml collagenase type I (Worthington Biochemical Corporation, USA). As a result, an enriched cell population containing interstitial cells, endothelial, perivascular, and peritubular cells will be present in the ICF cell isolate. For culture, cells were plated at a density of 4000 cells/cm2. Cells were cultured for a period of about 3.5 to 4 weeks up to passage 3 or 4, respectively, using a propagation medium as described for rat SLCs with some modifications 15 : DMEM/F12 with glutamax (Gibco, Thermo Fisher Scientific, USA) 2% FBS, 1% penicillin/streptomycin (pen/strep, Gibco, Thermo Fisher Scientific, USA), 1% Insulin/Transferrin/Sodium selenite (ITS, Gibco, Thermo Fisher Scientific, USA), 1nM dexamethasone (Sigma‐Aldrich, USA), 10 ng/ml platelet‐derived growth factor‐BB (PDGF‐BB, Peprotech, USA), 10 ng/ml epidermal growth factor (EGF, Peprotech, USA), 5 ng/ml human basic fibroblast growth factor (FGF2, Sigma‐Aldrich, USA), and 1 ng/ml leukemia inhibitory factor (LIF, Prospec, Israel) at 37°C in a humidified 5% CO2 water‐jacketed incubator. This medium was refreshed twice a week. The cells were passaged when a maximum of 80% confluence was reached by detaching the cells using accutase (Sigma‐Aldrich, USA) after which the cells were plated again at a density of 4000 cells/cm2.
2.5. Flow cytometry analysis
For the flow cytometry analyses on non‐cultured cells, we let the cells recover after enzymatic cell isolation for 24 hours before continuing with the FACS procedure because the enzymes used for cell isolation affects the presence of the cell surface markers (data not shown). For FACS analyses on cultured cells, the cells directly after detaching with accutase at passage 3 or 4 were used. The cells were fixed in BD Cytofix/Cytoperm (BD biosciences, USA) after staining with the Zombie violet fixable viability kit (BioLegend, USA) to identify dead cells and select for viable cells during analyses. Fixed cells were stained with fluorochrome‐conjugated antibodies to detect the presence of the various SLC markers. We titrated all antibodies first to determine the correct antibody concentration for FACS analyses. Details of the used antibodies for FACS analyses are described in Table S2. FACS analyses were performed on the BD FACSCanto II Flowcytometer or the BD LSRFortessa cell analyzer flow cytometer. FlowJo 10.2 software was used to analyze the data and GraphPad Prism 5 program to make the graphs. All experiments were reproduced on at least three donors. Matched isotype controls were used to determine background fluorescence. The flow cytometry data were expressed as the mean percentage of positive cells ± SEM.
3. RESULTS
3.1. Localization of putative SLC markers in the adult human testis
NR2F2 immunostaining was detected in peritubular cells, perivascular cells, and in Leydig cells (Figure 1). Co‐localization of NR2F2 with Cytochrome P450 Family 17, Subfamily A, Member 1 (CYP17A1), a specific marker for Leydig cells, confirmed that human Leydig cells express NR2F2 (Figure S1). Cells positive for THY1 were found surrounding the seminiferous tubules and the blood vessels. THY1 immunostaining was not observed in Leydig cells (Figure 1). Expression of NES in the adult human testis was limited to the endothelial cells of the blood vessels (Figure 1).
Figure 1.
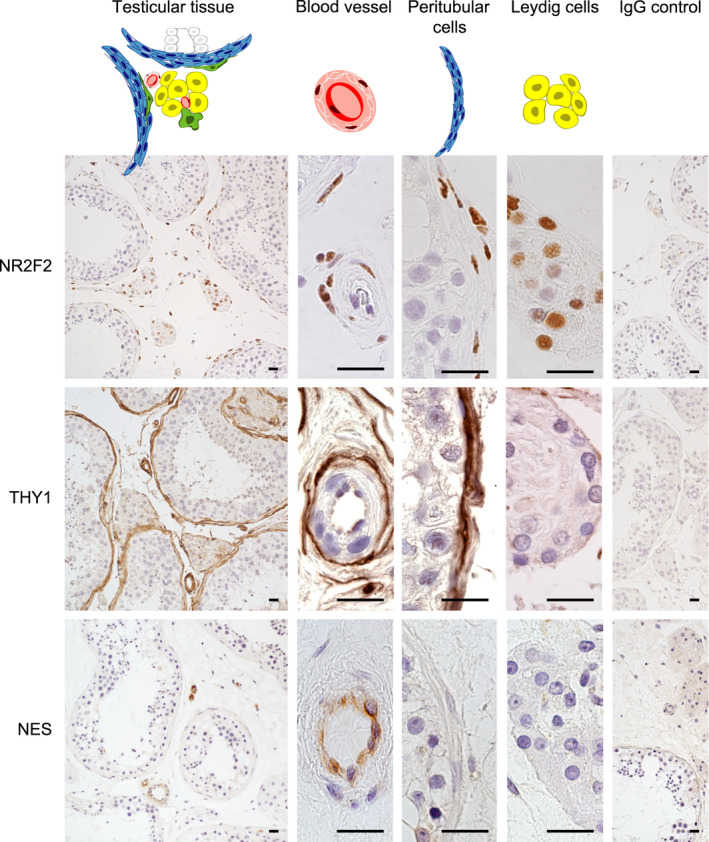
Location of rodent stem Leydig cell markers in human testicular tissue. Representative pictures of the immunohistochemical staining. Left column is showing an overview of the immunohistochemical staining in the testis, and middle columns in higher magnifications are showing blood vessels, cells of the peritubular myoid cell layer and Leydig cells, respectively. In the right column, the IgG control is shown. Scale bar is 20 µm
3.2. In vitro propagation of the human interstitial cell fraction containing cells with putative SLC markers
The isolated, uncultured, interstitial cell fraction (ICF) included cells that were positive for the markers NR2F2 (6 ± 2.5%), THY1 (17 ± 5.4%), and NES (6 ± 1.7%) (Figure 2A and C). After 23‐25 days of culture, ICF cell number increased 100‐ to 400‐fold with a high variability per cell culture and per patient. Within this increased cell population, the percentage of the cells positive for SLC markers increased to 89% (±5.2), 100% (±0.05), and 54% (±5.9) for NR2F2, THY1, and NES, respectively (Figure 2B and C).
Figure 2.
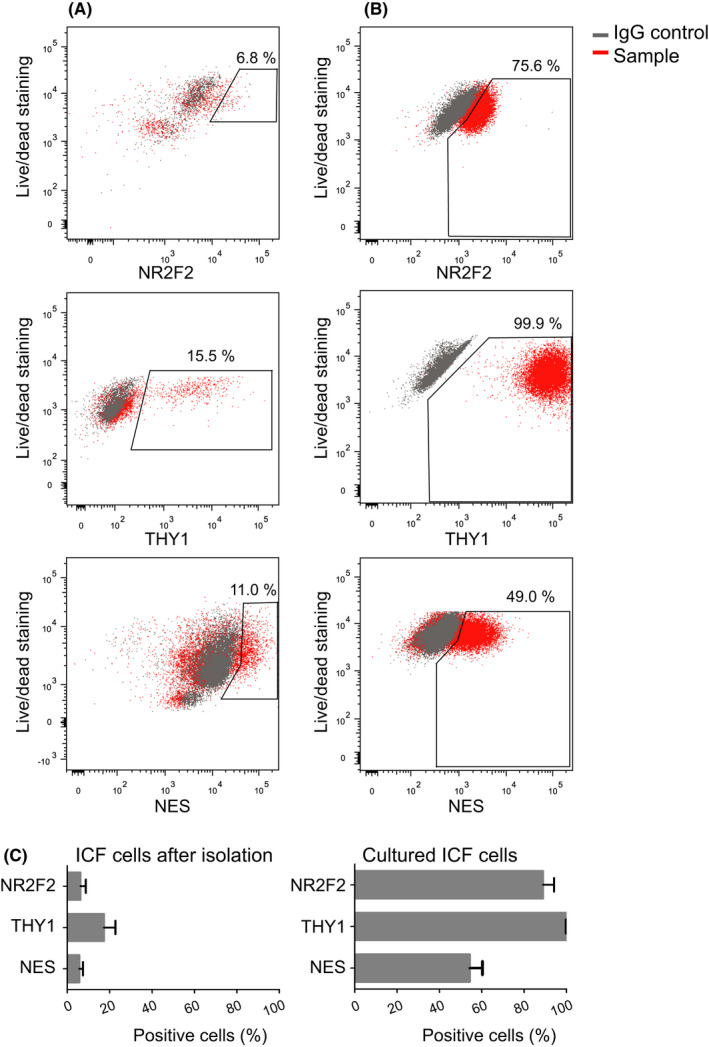
In vitro propagation of human interstitial cells expressing putative stem Leydig cell markers NR2F2, THY1, or NES. Representative fluorescence‐activated cell sorting (FACS) analyses of the markers on isolated primary interstitial cells before (A) and after culture (B). (C) Percentage of cells within the interstitial cell fraction (ICF) expressing the indicated markers before (n = 3) and after culture (n = 4). Before culture, the percentage of cells expressing each marker was below 25%, while after culture almost all cells expressed NR2F2 and THY1 and around half of the cells were positive for NES. Data are represented as mean percentage ± SEM
3.3. Putative SLC marker expression in relation to the reported human SLC marker PDGFRα
PDGFRα immunostaining was, like NR2F2, found in both peritubular and perivascular cells, as well as in Leydig cells (Figure 3A). The isolated uncultured ICF cells consisted of 15% (±3.4) PDGFRα+ cells (Figure 3B and C). Within this PDGFRα+ cell population, the majority of the cells were positive for THY1 and around half of the PDGFRα+ cells expressed NES (Figure 3C). Due to high cell loss during staining of the intranuclear cell marker NR2F2 for FACS analyses, we could not collect sufficient cell numbers of the uncultured cell fraction to analyze NR2F2 expression within the PDGFRα+ cell population. Since we were also not able to obtain reliable results for co‐localization of NR2F2 with PDGFRα by immunofluorescence staining on sections of fixed testes, we studied the co‐expression of NR2F2 and PDGFRα on propagated ICF cells using FACS. After 3 weeks of culture, 96% (± 2.3) of the ICF cells were positive for PDGFRα+ (Figure 3B and D). Almost all PDGFRα+ cells expressed NR2F2 and THY1, while about half of the PDGFRα+ cells expressed NES (Figure 3D), indicating that the percentages of at least the THY1+ and NES+ populations within the PDGFRα+ population remain constant during culture. There were THY1+/PDGFRα‐ and NES+/PDGFRα− cells present both immediately after isolation and following culture (Figure S2).
Figure 3.
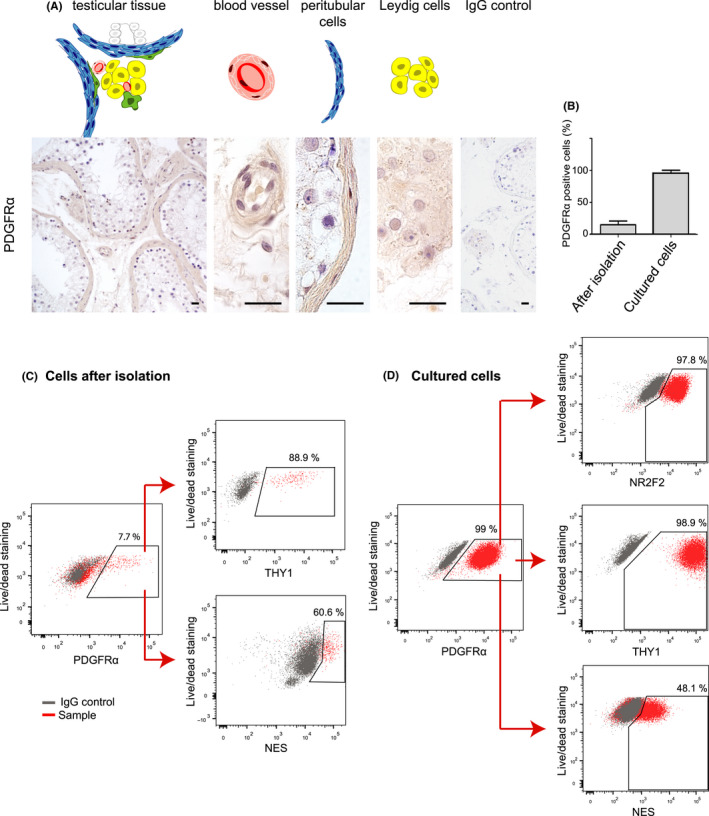
Rodent stem Leydig cell markers in relation to PDGFRα in human testis. The expression of PDGFRα was determined in human testicular tissue and cells. (A) Representative pictures of the immunohistochemical staining of PDGFRα on perivascular, peritubular and adult Leydig cells in human adult testis. (B) Percentage of PDGFRα+ cells within the interstitial cell fraction before (n = 3) and after culture (n = 4). Representative FACS plots of uncultured (C) and cultured (D) ICF cells. Directly after isolation, almost all PDGFRα+ cells co‐expressed also THY1 (89%), while NES is co‐expressed in around 60% of the PDGFRα+ cells. After 3.5 weeks of culture, almost all PDGFRα+ cells co‐expressed NR2F2 and THY1 (98% and 99%, respectively), and half of the PDGFRα+ cells co‐expressed NES. Scale bar is 20 µm. Data are represented as mean percentage ± SEM
3.4. Putative SLC marker expression in relation to the reported human SLC marker NGFR
Immunohistochemical staining revealed expression of NGFR in the endothelial cells (Figure 4A). Co‐localization with an endothelial marker CD31 confirmed localization of NGFR in the endothelial cell layer (Figure S3). Co‐localization of NGFR with NES was confirmed (Figure 4B). No co‐localization was found for NGFR (arrowhead) with the perivascular expression of NR2F2 (arrow) (Figure 4C) and NGFR with THY1 (Figure 4D). Immediately after isolation, a low percentage of cells expressing NGFR could be identified (3 ± 1.3%) within the ICF (Figure 4E and G). After propagation of the ICF cells, the NGFR+ cells were present in a lower percentage (1 ± 0.5%) (Figure 4F‐G). Since the total ICF increased 100‐ to 400‐fold in cell number, this indicates an increase in number of NGFR+ cells by 30‐ to 130‐fold during 3.5 weeks of culture. Hardly any of the NGFR+ cells co‐expressed PDGFRα immediately after isolation (Figure 4E), but after culture a clear NGFR+/PDGFRα+ cell population could be identified (Figure 4F).
Figure 4.
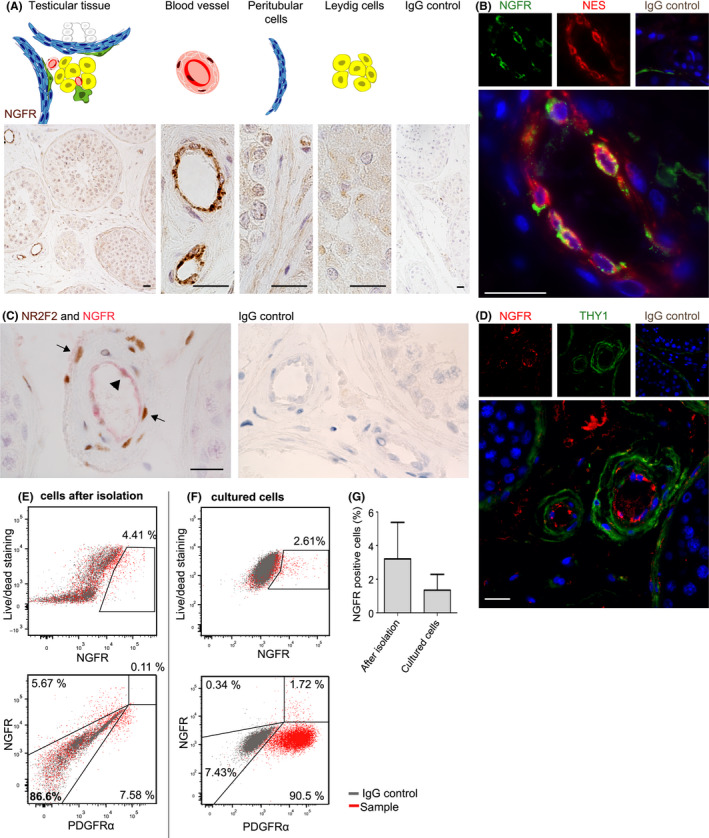
Rodent stem Leydig cell markers in relation to NGFR in human testis. The expression of NGFR was investigated in human testicular tissue, in isolated and cultured cells. (A) NGFR expression was limited in cells of the inner layer of the blood vessels. (B) These NGFR+ cells of the inner layer of the blood vessels were co‐localizing NES. NR2F2 and THY1 are expressed by outer cell layers of the blood vessels, and no co‐localization was found with NGFR in sections of the human testis (C and D). (E) Representative FACS analyses of interstitial cells after isolation. The small percentage of NGFR‐positive cells was negative for PDGFRα. (F) Representative FACS analyses of interstitial cells after culture. Still a small percentage of NGFR+ cells could be found, of which the majority co‐expressed PDGFRα. (G) Average of percentage of NGFR‐positive cells of uncultured (3 ± 1.3%) and cultured cell population (1 ± 0.5%) (n = 3). Scale bar is 20 µm. Data are represented as mean percentage ± SEM
4. DISCUSSION
All investigated markers were detected in cells located in or around the blood vessels in adult human testicular tissue. While expression of NES and NGFR seemed to be limited to the vascular endothelial cells, NR2F2, PDGFRα, and THY1 were expressed in the outer perivascular layer of the blood vessels. NR2F2, PDGFRα, and THY1 were also expressed in the peritubular cells, while on top of that NR2F2 and PDGFRα were also detected in ALCs. In addition to the expression of these putative SLC markers in interstitial cells in vivo, we were able to identify cells expressing these markers in isolated and in vitro propagated ICF cells. After this phase of proliferation, almost all cells expressed PDGFRα, NR2F2, and THY1 and around half of these cells expressed NES. Only a small proportion of these cells expressed NGFR. The interstitial localization and (partly) co‐expression patterns in vitro of the various SLC markers suggest that these markers identify partly a common cell population of interstitial cells.
The strength of the present study is that we, to our knowledge, for the first time simultaneously investigated the expression of several putative SLC markers in human testicular tissue both in vivo and in vitro. This combination allows us to investigate co‐localization and co‐expression of these SLC markers.
A limitation of the present study is that due to the low number of cells in the isolated ICF fractions we were not able to investigate the differentiation potential of the various cell populations positive for these markers into functional Leydig cells. We and others have previously shown that propagated human testicular PDGFRα+ and NGFR+ cells are able to differentiate into cells with Leydig cell characteristics. 24 , 25 , 26 Therefore, we focussed in the present study on the co‐localization of these markers with other SLC markers.
To our knowledge, our results show for the first time, the localization of NR2F2 in adult human testis. Peritubular cells, perivascular cells, and ALCs express NR2F2. For PDGFRα, of which the expression was observed at the same locations as NR2F2, our results differ partly from other studies where PDGFRα expression in the adult human testis is described for peritubular cells, 24 , 30 spermatids, 24 or Sertoli and Leydig cells. 31 This suggests that PDGFRα immunostaining in the human testis is highly variable, presumably depending on the antibody source and staining protocol used. Our present immunohistochemical staining pattern of PDGFRα was confirmed by our FACS analyses identifying co‐expression of PDGFRα and NR2F2 in almost 100% of cultured ICF cells, while in this analysis a different antibody was used for PDGFRα because of the suitability for FACS. The current study further shows that THY1, one of the markers to identify MSCs according to the minimal criteria, 32 is expressed by peritubular cells and perivascular cells in the adult human testis, and not by ALCs. This is in line with studies on human peritubular cells where expression of THY1 was found. 30 , 33 The co‐expression of PDGFRα and THY1 immediately after isolation and following propagation of the cells in vitro confirmed the immunohistochemical localization results that the THY1+ cells might be a subpopulation of the PDGFRα+ cells. Furthermore, since after culture almost 100% of the cells were positive for NR2F2, PDGFRα, and THY1, the current culture conditions are suitable for the propagation of this NR2F2+/PDGFRα+/THY1+ cell population.
Based on the presence of NR2F2 and PDGFRα in ALCs, these markers are not suitable to identify solely SLCs in the human testis. THY1 might therefore be a more specific marker for enrichment of human SLCs since the expression is limited to perivascular and peritubular cells, regions described as SLC niche. 10 , 11 , 12 , 20 , 34 A common progenitor for ALCs and myoid cells has been suggested before puberty explaining the possible close relation of SLCs to myoid cells. 35
Our finding that in the adult human testis the expression of the previously described SLC markers NES and NGFR is limited to the endothelial cells is unexpected as endothelial cells are committed differentiated cells in the inner wall of blood vessels. This suggests NES and NGFR might not be expressed by human SLCs and are expressed by a distinct cell population compared to the NR2F2+/PDGFRα+/THY1+ cell population. This is different from previous studies where expression of NES in the human testis has been described for peritubular cells and Leydig cells in addition to endothelial cells, 36 or be present in peritubular and vascular cells together with NGFR (Zhang et al, 2017). A possible explanation is that the expression in peritubular and Leydig cells of NES and NGFR may be very low and therefore difficult to detect by immunohistochemistry. Another explanation for the discrepancy in NES staining may be differences in antibody source and staining protocols.
Our in vitro observations showed that ICF contained NES+/PDGFRα+ cells showing a common cell population of these two markers that might contain SLCs. Since we already find this NES+/PDGFRα+ population in ICF cells before culture, this implies there is a NES+/PDGFRα+ cell population present in the testicular tissue, which we were not able to detect by immunohistochemistry. Half of the NR2F2+/PDGFRα+/THY1+ cell population expressed NES. This subpopulation might be interesting in the enrichment of SLCs.
We did not detect a NGFR+/PDGFRα+ cell population before culture. Whether NGFR might also select for at least a subpopulation of SLCs in parallel to endothelial cells in vivo could not be confirmed by immunohistochemistry and FACS in the present study. In contrast to ICF cells immediately after isolation, we did observe a NGFR+/PDGFRα+ cell population following propagation of the isolated ICF cells, similar to what has been described by Zhang and colleagues for cultured NGFR+ spheres. 26 Furthermore, in that study it was shown that NGFR+ cells isolated from adult human testis can differentiate in vitro into testosterone‐producing cells. The appearance of this NGFR+/PDGFRα+ cell population in vitro in our study could be explained by a low undetectable expression of NGFR immediately after isolation that increases during culture. Another explanation might be that, although we tried to avoid this as much as possible, the enzymes used in the isolation protocol might have caused damage to some membrane markers and not intracellular markers. This might explain the discrepancy in expression of NES (intracellular marker) and NGFR (membrane marker) immediately after cell isolation. Another possibility is that NGFR+/PDGFRα+ cells might appear during culture due to the phenomenon of endothelial to mesenchymal transition (EndMT). This is an important developmental mechanism where endothelial cells transdifferentiate into MSCs in various tissues, (reviewed in 37 ), including the testis, 38 , 39 , 40 but can also occur in endothelial cells in vitro. 41 Whether EndMT also plays a role in Leydig cell development in vitro and/or in vivo needs further investigation.
We investigated the propagation potential of the ICF cells in vitro, as this will be pivotal to obtain enough cells to allow further analyses on the characteristics and functional properties of the cells. The presence of proliferative activity is one of the hallmarks of stem cells, indicating that the current cell culture condition stimulates proliferation of interstitial cells, including putative SLCs. The total ICF cells increased 100‐ to 400‐fold in cell number in vitro. Since we cultured a mixed cell population of testicular ICF cells, we cannot rule out that the increase in number of cells expressing SLC markers could be due to cells that gain putative SLC marker expression during culture rather than propagation of a cell population expressing the SLC markers from the start of the culture. This should be kept in mind when further investigating these putative human cultured SLCs in more detail.
Based on our results, we show the studied markers are expressed within the same LC developmental lineage. Our results cannot assign the markers to any of the specific lineage of stem Leydig cells, regressed NNLCs, infantile LCs or regressed FLCs. When interpreting the results, we propose a model for human ALC development based on localization in vivo and co‐expression before and after culture of the investigated markers. When using the ALC developmental scheme as introduced by Prince 5 and extended by Svechnikov et al, 42 we hypothesize that the markers PDGFRα, NR2F2, and THY1 belong to the developmental lineage of SLCs to ALCs since all three markers are detected in perivascular and peritubular cells, the previously identified SLC niche. Since THY1 is not expressed by ALCs, in contrast to PDGFRα and NR2F2, THY1+ cells are presumably less differentiated in the direction of ALCs (Figure 5). Although SLCs might be present within the PDGFRα+/NR2F2+/THY1+ cell population, none of these markers identifies exclusively SLCs. Although the immuno‐localization of NES and NGFR within endothelial cells would suggest no involvement in human ALC development, the presence of NES+/PDGFRα+ cells before and after culture indicates part of the NES expressing cells also belong to the same cell lineage as PDGFRα, NR2F2, and THY1 expressing cells. These cells might represent a cell type earlier in the process toward ALC development since it is a subpopulation of the PDGFRα+ cells (Figure 5). The in vitro expression after culture of NGFR+/PDGFRα+ in a small cell population in the current study and differentiation potential toward ALC of NGFR+ human testicular cells in previous study 26 might imply involvement of NGFR in human ALC development as well.
Figure 5.
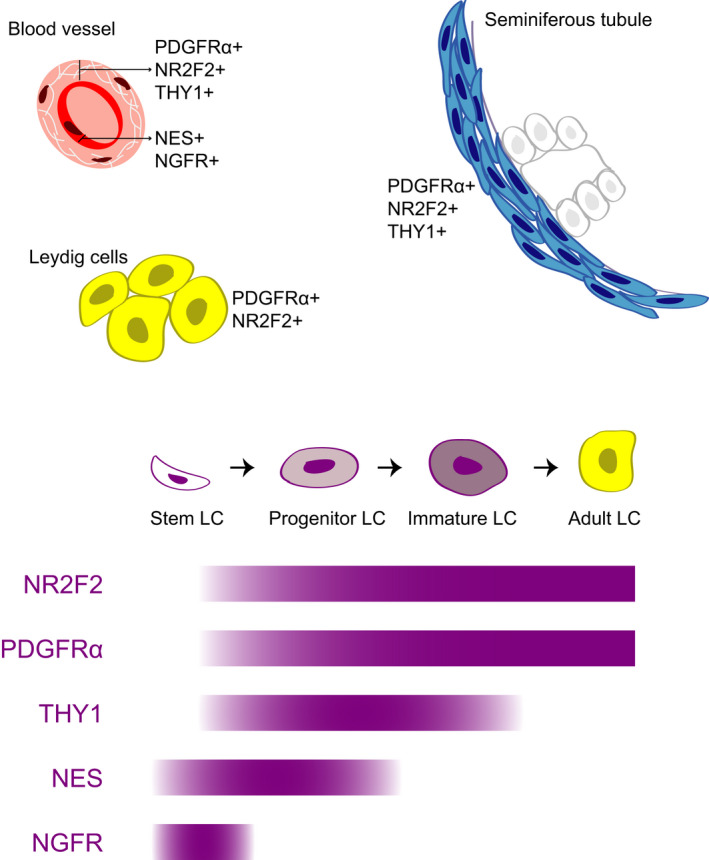
Proposed scheme on the development of the human SLC toward ALC. Proposed scheme of human SLC marker expression during ALC development based on the data from the current study. Upper part shows the localization of cells expressing the markers within the adult testis by immunohistochemistry. The bars in the lower part show the expression patterns of the SLC markers within the human ALC development based on immunohistochemistry and FACS co‐expression. LC, Leydig cell
Identification of human SLCs is an important step in the development of possible future cell therapies for Leydig cell dysfunction. In the present study, expression of several putative SLC markers is evaluated in human testicular tissue and in isolated and cultured ICF cells. The combined study of the putative SLC markers allowed us to investigate co‐localization and co‐expression and showed that the putative markers overlap at least partly. Although these markers might be useful in the enrichment of human SLCs, none of these markers seemed to be expressed in one specific cell type in vivo. Our results and proposed hypothesis of Leydig cell development in humans opens up further research strategies aiming for a better insight in the development of Leydig cells and toward novel therapies to rescue Leydig cell dysfunction.
5. CONCLUSION
We show, based on in vivo locations and co‐expression in vitro, that NR2F2, PDGFRα, and THY1 are located in the perivascular and peritubular compartment and are expressed within the same ALC developmental lineage, containing SLCs. Cells expressing NES and NGFR are mostly located within the blood vessels, but based on partly overlap with the PDGFRα+ population in vitro, we hypothesized that these cells represent a subpopulation of NR2F2+/PDGFRα+/THY1+ cells in an early phase in the same LC development lineage.
DISCLOSURES
The authors declare no competing or financial interests.
AUTHORS' CONTRIBUTION
All authors contributed in the conception and design of the study and revising the manuscript. JE took the lead in writing the manuscript and took part in the execution of the experiments and analyses of the data and the interpretation of this data. S.v.D. and C.d.W. took part in the collection and analyses of the data. F.v.d.V, SR, KT, and A.v.P. contributed in the interpretation of the data.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
ACKNOWLEDGEMENTS
This study was funded by Amsterdam UMC, location Academic Medical Center.
We thank EA van den Berg (Center for Reproductive Medicine, Amsterdam UMC (AMC)) for her help in optimizing the antibodies used for FACS analyses.
Eliveld J, van Daalen SKM, de Winter‐Korver CM, et al. A comparative analysis of human adult testicular cells expressing stem Leydig cell markers in the interstitium, vasculature, and peritubular layer. Andrology. 2020;8:1265–1276. 10.1111/andr.12817
REFERENCES
- 1. Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: A review. Ther Clin Risk Manag. 2009;5(1):427‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: A systematic review and meta‐analysis of placebo‐controlled randomized trials. BMC Med. 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reyes FI, Borodits RS, Winter JSD, Faiman C. Studies on human sexual development. 2. Fetal and maternal serum gonadotropin and sex steroid concentrations. J Clin Endocrinol Metab. 1974;38(4):612‐617. [DOI] [PubMed] [Google Scholar]
- 4. Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat Rec. 1990;228(4):405‐417. [DOI] [PubMed] [Google Scholar]
- 5. Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol. 2001;168(2):213‐216. [DOI] [PubMed] [Google Scholar]
- 6. Nistal M, Paniagua R, Regadera J, Santamaria L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res. 1986;246(2):229‐236. [DOI] [PubMed] [Google Scholar]
- 7. Codesal J, Regadera J, Nistal M, Regadera‐Sejas J, Paniagua R. Involution of human fetal Leydig cells. An immunohistochemical, ultrastructural and quantitative study. J Anat. 1990;172:103‐114. [PMC free article] [PubMed] [Google Scholar]
- 8. Prince FP. Ultrastructure of immature leydig cells in the human prepubertal testis. Anat Rec. 1984;209(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 9. Prince FP. The human Leydig cell In: Payne AH, Hardy MP, eds. The Leydig Cell in Health and Disease. Humana Press; 2007:71‐89. [Google Scholar]
- 10. Kerr JB, Donachie K, Rommerts FFG. Selective destruction and regeneration of rat Leydig cells in vivo. Cell Tissue Res. 1985;242(1):145‐156. [DOI] [PubMed] [Google Scholar]
- 11. Teerds KJ, de Rooij DG, Rommerts FFG, Wensing CJG. The regulation of the proliferation and differentiation of rat leydig cell precursor cells after EDS administration or daily HCG treatment. J Androl. 1988;9(5):343‐351. [DOI] [PubMed] [Google Scholar]
- 12. Kerr JB, Bartlett JMS, Donachie K, Sharpe RM. Origin of regenerating Leydig cells in the testis of the adult rat – An ultrastructural, morphometric and hormonal assay study. Cell Tissue Res. 1987;249(2):367‐377. [DOI] [PubMed] [Google Scholar]
- 13. Stanley E, Lin C‐Y, Jin S, et al. Identification, proliferation, and differentiation of adult leydig stem cells. Endocrinology. 2012;153(10):5002‐5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα‐positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372(1–2):96‐104. [DOI] [PubMed] [Google Scholar]
- 15. Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: Identification, isolation, and lineage‐specific development. Proc Natl Acad Sci. 2006;103(8):2719‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang MH, Cai B, Tuo Y, et al. Characterization of Nestin‐positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24(12):1466‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone‐producing Leydig cells revealed. J Cell Biol. 2004;167(5):935‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zang ZJ, Wang J, Chen Z, et al. Transplantation of CD51+ stem Leydig cells: a new strategy for the treatment of testosterone deficiency. Stem Cells. 2017;35(5):1222‐1232. [DOI] [PubMed] [Google Scholar]
- 19. Kilcoyne KR, Smith LB, Atanassova N, et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci. 2014;111(18):E1924‐E1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Wang Z, Jiang Z, et al. Regulation of seminiferous tubule‐associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci. 2016;113(10):2666‐2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum Reprod Update. 2015;21(3):310‐328. [DOI] [PubMed] [Google Scholar]
- 22. Zhang F‐P, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15(1):172‐183. [DOI] [PubMed] [Google Scholar]
- 23. Majdic G, Saunders PTK, Teerds KJ. Immunoexpression of the Steroidogenic Enzymes 3‐Beta Hydroxysteroid Dehydrogenase and 17 α‐hydroxylase, C17,20 Lyase and the Receptor for Luteinizing Hormone (LH) in the Fetal Rat Testis Suggests That the Onset of Leydig Cell Steroid Production Is Indepen. Biol Reprod. 1998;58(2):520‐525. [DOI] [PubMed] [Google Scholar]
- 24. Landreh L, Spinnler K, Schubert K, et al. Human testicular peritubular cells host putative stem leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99(7):1227‐1235. [DOI] [PubMed] [Google Scholar]
- 25. Eliveld J, Van Den BEA, Chikhovskaya JV, et al. Primary human testicular PDGFRα+ cells are multipotent and can be differentiated into cells with Leydig cell characteristics in vitro. Hum Reprod. 2019;34(9):1621‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang M, Wang J, Deng C, et al. Transplanted human p75‐positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis. 2017;34(10):e3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shima Y, Miyabayashi K, Sato T, et al. Fetal leydig cells dedifferentiate and serve as adult leydig stem cells. Dev. 2018;145(23). [DOI] [PubMed] [Google Scholar]
- 28. van Pelt AMM, Morena AR, van Dissel‐Emiliani FMF, et al. Isolation of the synchronized A spermatogonia from adult vitamin a‐deficient rat testes1. Biol Reprod. 1996;55(2):439‐444. [DOI] [PubMed] [Google Scholar]
- 29. Chikhovskaya JV, van Daalen SKM, Korver CM, Repping S, van Pelt AMM. Mesenchymal origin of multipotent human testis‐derived stem cells in human testicular cell cultures. Mol Hum Reprod. 2014;20(2):155‐167. [DOI] [PubMed] [Google Scholar]
- 30. Schmid N, Flenkenthaler F, Stöckl JB, et al. Insights into replicative senescence of human testicular peritubular cells. Sci Rep. 2019;9(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basciani S, Mariani S, Arizzi M, et al. Expression of platelet‐derived growth factor‐A (PDGF‐A), PDGF‐B, and PDGF receptor‐α and ‐β during human testicular development and disease. J Clin Endocrinol Metab. 2002;87(5):2310‐2319. [DOI] [PubMed] [Google Scholar]
- 32. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 33. Albrecht M, Rämsch R, Köhn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: A new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab. 2006;91(5):1956‐1960. [DOI] [PubMed] [Google Scholar]
- 34. Schulze C, Holstein AF. Leydig cells within the Lamina Propria of seminiferous tubules in four patients with azoospermia. Andrologia. 1978;10(6):444‐452. [DOI] [PubMed] [Google Scholar]
- 35. Guo J, Nie X, Giebler M, et al. The dynamic transcriptional cell atlas of testis development during human puberty. Cell Stem Cell. 2020;26(2):262‐276.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lobo MVT, Arenas MI, Alonso FJM, et al. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res. 2004;316(3):369‐376. [DOI] [PubMed] [Google Scholar]
- 37. Piera‐Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99(2):1281‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romereim SM, Cupp A. Mesonephric cell migration into the gonads and vascularization are processes crucial for testis development. In: Piprek RP. ed. Molecular Mechanisms of Cell Differentiation in Gonad Development. Results and Problems in Cell Differentiation, Vol 58 Springer; 2018:67‐100. [DOI] [PubMed] [Google Scholar]
- 39. Coveney D, Cool J, Oliver T, Capel B. Four‐dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci USA. 2008;105(20):7212‐7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Combes AN, Wilhelm D, Davidson T, et al. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326(1):112‐120. [DOI] [PubMed] [Google Scholar]
- 41. Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle‐like cells in vitro. J Cell Sci. 1992;103(2):521‐529. [DOI] [PubMed] [Google Scholar]
- 42. Svechnikov K, Landreh L, Weisser J, et al. Origin, development and regulation of human leydig cells. Horm Res Paediatr. 2010;73(2):93‐101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2


