Abstract
Introduction
In the PROMISE study, a multinational randomized controlled trial (RCT) of the effectiveness of spinal cord stimulation (SCS) with multicolumn surgical leads as a treatment of low back pain, clinicians followed their usual practice. An early, unplanned safety analysis revealed that the infection rate in Belgium (5/23), where trial duration was a median 21.5 days, was significantly higher than the 1/64 rate observed in the other study countries (median 5.8 days, p < 0.01). This report reviews infections observed in the PROMISE study after study completion.
Materials and Methods
For all infections related to SCS, we used descriptive statistics and tests of independent variables to analyze potentially contributing factors (age, sex, coexisting medical conditions, tobacco use, lead type, and trial duration) between subjects with infections versus those without. Cumulative incidence curves were created using the Kaplan–Meier method and compared between the two strata using a log‐rank test.
Results
Among nine (5.2%) infections in 174 subjects trialed, the only significant contributing factor to infection was trial duration: median 21 days (range 3–56) for those with infection vs. six days (1–41) for those without (p = 0.001; Wilcoxon rank‐sum test). The cumulative incidence of infection for subjects trialed >10 days was 24.1% vs. 1.4% for subjects trialed ≤10 days (p < 0.001). After the protocol was amended to limit trial duration to 10 days, 14 infection‐free trials were performed in Belgium.
Conclusions
Although not part of the preplanned analysis, our observation supports the hypothesis of a cause‐effect relationship between trial duration and the risk of infection and the conclusion that prolonged SCS trials should be avoided.
Keywords: Infection, pain, PROMISE study, SCS, trial duration
INTRODUCTION
Early in the 50‐year history of spinal cord stimulation (SCS), screening trials were introduced as a way of identifying responders before implantation of a complete system 1, 2, 3. This practice continues today, with most screening trials requiring temporary connections from the internal lead(s) to an external pulse generator. These connections heighten the risk of infection, and many clinicians have reported SCS infections related to the screening trial 4. Nevertheless, guidelines propose that “… under appropriate infection control conditions, the staged trial and completion implant pathway can be utilized in select patients without a significant increase in infection rates” 4. This guideline, despite its apparent goal of reassuring clinicians conducting screening trials, does not define “significant increase.” For any patient, developing an infection during SCS treatment is a significant event that can forever obviate any real or potential therapeutic benefit provided by SCS, and an infection that becomes manifest only after implantation of a complete system, precipitating its removal and possible replacement, also substantially increases the cost of the therapy.
Investigators have yet to define the role that the length of a screening trial with externalized components plays in supporting or undermining “appropriate infection control 4.”
In the PROMISE study, a multinational randomized controlled trial (RCT) that assessed the effectiveness of SCS with surgical leads (Specify™ 5‐6‐5, Medtronic, Inc.) as a treatment of low back pain, randomized 218 predominant back pain subjects to optimal medical management (OMM) or SCS plus optimal medical management. At six months, pain relief, health‐related quality of life, and function improved more in the SCS group than the OMM group 5. The protocol initially asked experienced clinicians to follow their usual practice for trialing and system implant, which included generally accepted safeguards to minimize the risk of infection. The study enrolled subjects starting in January 2013. By the end of September 2014, an early safety analysis revealed that the device‐related infection rate in Belgium (5/23, 21.7%) was significantly higher than the rate observed in the other eight study countries (1/64, 1.6%, Fisher's exact test, p < 0.01).
Belgium has a reimbursement requirement that, before being deemed successful, SCS trials must last at least 28 days (as opposed to the norm of approximately a week in other settings); thus, the Belgium trial duration was a median 21.5 days (standard deviation [SD] 11.9) vs. a median 5.8 days (SD 3.4, Wilcoxon rank‐sum test, p < 0.01) elsewhere. Based on that interim analysis, the protocol was modified to restrict trial duration to ≤10 days, which aligned with U.S. labeling for trialing leads.
This report provides details about the infections observed in the PROMISE study 5 along with analysis of all infections after study close.
MATERIALS AND METHODS
All procedures were done following contemporary, generally accepted practices to minimize the risk of infection, including but not limited to sterile techniques and antibiotic prophylaxis. We defined trial duration as the number of days from placement of the trial lead to the trial completion date, when all system components were internalized or removed permanently, and the postoperative period as 90 days following the subject's first day of trialing. If a subject had a second trial, the time to infection would begin with the first day of the first trial.
We quantified all the postoperative infections that the PROMISE study's independent Clinical Events Committee deemed related to SCS and analyzed potentially contributing factors including age, sex, coexisting medical conditions, tobacco use, study country, lead type, and trial duration.
Statistical Analysis
We used descriptive statistics to summarize the demographic information. A two‐sample t‐test (or Wilcoxon rank sum test depending on the data normality) was used to compare the continuous variables, a Fisher's exact test was used to compare categorical variables in patients with and without infection. Cumulative incidence curves were created using the Kaplan–Meier method and compared between the two strata using a log‐rank test. A p‐value of <0.05 was considered statistically significant. The validated statistical software package SAS (version 9.4) was used for the analyses.
RESULTS
Of the 174 study subjects who had screening trials after randomization or after the end of the randomized phase at six months, the mean age was 53.4 years, 59.8% were female, mean body mass index (BMI) was 30, 36.4% were currently using tobacco, 11.0% had diabetes mellitus, and 2.3% had other risk factors for poor wound healing or infection 4 (Table 1).
Table 1.
Demographic and Trial Factors Relative to Postoperative Infection.
| Infection n = 9 | No infection n = 165 | P‐Value | |
|---|---|---|---|
| Age in years, mean | 53.4 (SD 8.8) | 53.4 (SD 11.2) | 0.999 |
| Sex, n (%) | |||
| Female | 6 (66.7) | 98 (59.4) | 0.742 |
| Male | 3 (33.3) | 67 (40.6) | |
| BMI, n (%) | |||
| BMI <= 30 | 6 (66.7) | 91 (55.2) | 0.733 |
| BMI > 30 | 3 (33.3) | 74 (44.9) | |
| Immunodeficiency, n (%) | |||
| No | 9 (100) | 161 (97.6) | 1.000 |
| Yes | 0 | 4 (2.4) | |
| Diabetes mellitus, n (%) | |||
| No | 8 (88.9) | 147 (89.1) | 1.000 |
| Yes | 1 (11.1) | 18 (10.9) | |
| Tobacco user, n (%) | |||
| No | 4 (44.4) | 107 (64.9) | 0.288 |
| Yes | 5 (55.6) | 58 (35.1) | |
| Lead type, n (%) | |||
| Percutaneous | 1 (11.1) | 55 (33.3) | 0.275 |
| Surgical | 8 (88.9) | 110 (66.7) | |
| Trial duration in days | |||
| Median | 21 (range 3–56) | 6 (range 1–41) | 0.001 |
| Mean | 22.6 (SD 16.6) | 7.4 (SD 5.4) | |
| Trial ≤ 10 days, n (%) | 2 (22.2) | 143 (86.7) | < 0.001 |
| Trial > 10 days, n (%) | 7 (77.8) | 22 (13.3) |
In the 180 trials (six patients had two trials), percutaneous leads intended for replacement with a surgical lead after a successful trial were used in 58 patients (32.2%), and 122 patients (67.8%) received surgical leads intended to become permanent if the trial was successful. The mean trial duration was 8.1 days (SD 7.1, median 6, range 0–56 days). Of the 180 trials, 29 lasted >10 days (see Table 1).
Among the 174 subjects trialed, nine (5.2%) experienced a postoperative infection: seven overt implant site infections with wound breakdown, one implant site cellulitis, and one extradural abscess. In four patients, the infection was identified after implantation of the complete SCS system (see Table 2). Among these nine subjects, eight (8/118, 6.8%) had surgical leads for the trial and one (1/56, 1.8%) received a percutaneous lead(s).
Table 2.
Infection Timing, With Rows in Chronological Order by Date That First Trial Commenced (With Top Row Earliest).
| Screening Trial Duration (Days) | Time From Trial Start to Full Implant | Time From Trial Start to Infection | |
|---|---|---|---|
| Belgium | 13 | 34 | 53 |
| Belgium | 21 | N/A | 18 |
| Belgium | 29 | N/A | 29 |
| Canada | 27 | 27 | 52 |
| Belgium | 56 | N/A | 10 |
| Belgium | 36 | N/A | 58 |
| USA* | 3 | 16 | 66 |
| France | 7 | N/A | 7 |
| Spain | 11 | 12 | 21 |
Percutaneous electrodes; the rest were surgical leads.
When comparing contributing factors between those subjects with infection vs. without infection, the only significant factor was trial duration: median 21 days (range 3–56) for those with infection vs. 6 days (1–41) for those without (p = 0.001, Wilcoxon rank‐sum test). As presented in Table 1, the rate of infection was somewhat lower for percutaneous than paddle lead trials (1/55 vs. 8/110), but this was not statistically significant (p = 0.275). Trial duration overlapped in the groups of subjects with and without postoperative infection, with some subjects with longer than average trial duration (up to 41 days) remaining infection free. As shown in Fig. 1, the cumulative incidence of postoperative infection for subjects trialed >10 days was 24.1% (95% CI: 12.3–44.1%) at 90 days vs. 1.4% (95% CI: 0.3–5.4%) for subjects trialed ≤10 days (p < 0.001, log‐rank test).
Figure 1.
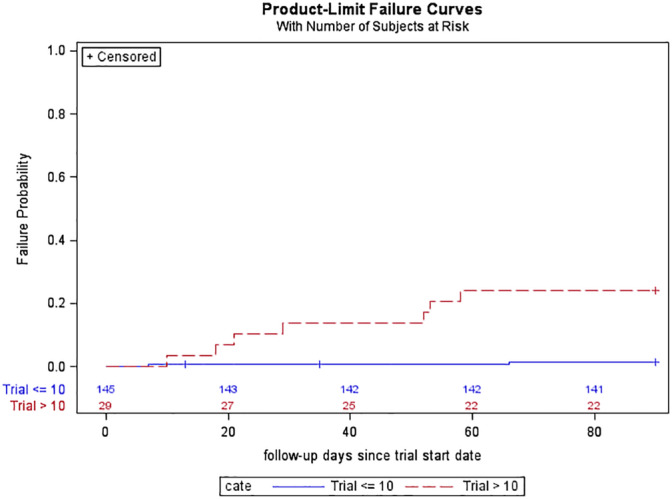
Cumulative incidence of postoperative infection for subjects with trial duration ≤10 days versus >10 days. [Color figure can be viewed at wileyonlinelibrary.com]
As indicated in Table 2, postoperative infection onset fell into three categories:
1. Infection was symptomatic at or prior to the end of the trial, and the neurostimulator was not implanted (four subjects, three from Belgium and one from France). One of these patients had an apparent infection beginning on day 10 and was treated with antibiotics for the remainder of an ultimately unsuccessful trial lasting 56 days.
2. Infection was symptomatic after completion of the trial, and the neurostimulator was not implanted (one subject).
3. Infection was symptomatic after the trial and complete system implant; thus, the infection might have been related to the trial or to the implantation (four subjects).
Before the protocol change, five infections were reported in Belgium and one in Canada (trial duration of 27 days). After the change, one infection each occurred in the United States, France, and Spain (see Table 2), with none occurring among the last 14 trials performed in Belgium. None of the six patients with two trials in the PROMISE study developed an infection.
DISCUSSION
Infection is an inherent risk with any invasive procedure, particularly when a foreign body is implanted; it occurred in 2.45–5% of cases in large SCS series and meta‐analyses and in up to 14% in small series 6, 7, 8, 9, 10, 11. Infection is the most common acute postsurgical SCS complication leading to removal of SCS equipment 12 and, as removal is generally followed by replacement of the entire system, is one of the more costly complications.
SCS infection risks might be affected by patient characteristics (e.g., diabetes, obesity, immunodeficiency, and smoking) and by choice of perioperative techniques (e.g., antisepsis, prophylactic antibiotics, and trial protocols), and risk mitigation has been the subject of many publications 4, 13, 14, 15, 16, 17, 18. Trial duration has been identified as an important risk factor for infection 6, 19. In a report on infection by Rudiger and Thomson, Deer is quoted as saying “Obviously, the length of trial with an exposed external lead may lead to an increase in infection.” 20 The shortest trials, of course, are “on‐table” trials culminating in pulse generator implantation in a single stage, and one case series involving such trials reported no infections among the 80 patients 21.
Until now, screening trials and their duration have not been examined as a risk factor in a prospective study even though a large retrospective case series reported that trials of >5 days resulted in an average infection rate of 3.70% compared with a rate of 1.58% for those trialed <5 days (p = 0.02) 8. Previous studies from Belgium provide conflicting evidence, with one clinician suggesting that the standard practice of 28–30 day trials “likely contributed to the incidence rate of infection” of 8.8% 22, whereas the infection rate in another, larger series, was 4.8%, only slightly higher than what is considered normal 23. In 2018, surgical lead labeling was clarified to say that the surgical leads used in this study are not indicated for use in test stimulation outside of the operating room.
Experience with indwelling percutaneous devices, for example, central venous lines and ventriculostomy catheters, teaches that with time, the cumulative risk of infection for an individual patient can only increase 24, as the pooled risk per catheter day does not disappear over duration of treatment 25, 26.
Even in cases when the patient did not become clinically infected, investigators have found that explanted SCS trial leads are frequently colonized with bacteria 27, 28. Thus, discarding a trial lead that had been connected to external equipment and implanting a new one for chronic use might mitigate the risk of infection; indeed, a retrospective series by Simopoulos et al. found that the infection rate was significantly lower with this approach than after implanting a temporary external extension and retaining the lead in successful trials (1.35 after discarding the trial lead versus 6.52% after retaining it, p = 0.02) 29. We observed similar rates of infection (1.8% with temporary percutaneous leads vs. 6.8% with retained surgical leads), but with our smaller sample of 174 vs. 286 in the study by Simopoulos, our difference was not statistically significant.
Limitations
Our finding of increased infection risk with prolonged trials was based on real‐world variations in trialing time, mainly driven by reimbursement requirements. Put in other words, while this was an unplanned, additional analysis of RCT data, SCS trial time was not dictated as a part of the study protocol. Thus, while our observation supports the existence of an association, and even a hypothesis of cause and effect, it is important to acknowledge potential confirmation bias.
Likewise, the fact that no further infection occurred in Belgium after we proscribed prolonged trials should not be over‐interpreted. It is possible that, having observed an unusually high infection rate early in the trial, we simply observed regression to the mean after we changed the protocol.
The protocol change that we undertook (and were obliged to undertake, based on safety monitoring) alerted all centers not only to our concern about trial duration but also to our concern about infection in general. Awareness of this might have led to changes in infection control practices at study centers, and these changes (as opposed to the elimination of prolonged trials) might have played a role in the observed improvement. Indeed, improvement in SCS infection rates has been reported in nonrandomized case series after additional infection control measures were implemented 15, 30. Perhaps this also reflects regression to the mean.
That said, we believe that the present report is a significant addition to the literature and suggests that trial duration should be minimized. A dedicated prospective study would be the best way to address this question, but given sample size and funding requirements, it seems unlikely that such a study will be undertaken.
CONCLUSIONS
The PROMISE study incorporated the usual measures to mitigate infection, but trial duration was initially dictated by investigator discretion and local practice. As the study proceeded, we noted that in one country with a 28‐day trial requirement, the rate of infection was 21.7%, exceeding the highest value in the literature and exceeding by an order of magnitude the 1.6% rate in the rest of the study population. After limiting trial duration to 10 days, we observed a much lower rate for the remainder of the study. Ultimately, the overall rate of 5.2% was only slightly higher than what is considered normal.
This analysis of SCS trialing patterns supports the hypothesis of a cause‐effect relationship between trial duration and the risk of infection and the practical conclusion that prolonged SCS trials should be avoided to reduce this risk.
Authorship Statement
The manuscript was drafted by Dr. North with assistance from Ms. Shipley and reviewed by Dr. Rigoard, Dr. Desai, Dr. Vangeneugden, Dr. Ratopoulos, Dr. Van Havenburgh, Dr. Deruytter, and Dr. Remacle, all of whom provided data. Statistical analyses were performed by Dr. Tan. Technical review was provided by Ms. Shipley, Ms. Johnson, and Ms. Van den Abeele. All authors approved the final version of the manuscript.
COMMENTS
In addition to the manuscript, these observations are well known to the community and it's the merit of the authors to publish the interim results of the infection rate of one partner of the study, the change of the protocol and the diminution of their infection rate. It's a dilemma that longer trials have a better reliable responder rate. It's the strategy of many others to have a clinical trial as short as possible but as reliable as possible. Considering the results of the authors, a trial period of about 10 days seems appropriate but we will have patients with a sudden and sustained effect where we will implant earlier after 5‐6 days. The authors should have emphasized more the role of the exchange of the trial electrodes. I miss the discussion of usage of antibiotics and the rigorous use of an antiseptic regimen as some centers e.g. Cologne/Germany dropped their infection rate by this practice.
Werner Braunsdorf, MD
Magdeburg, Germany
***
It is a very interesting article about SCS infections. Although it is already suspected that longer trial duration may be closely related to infection incidence, this article shows us that relation in a clearer way. However, there is a limitation because in four infected patients of the study, infection may have happened during the definitive SCS device implantation. It would be interesting to carry out a multicentric trial comparing results and complications with “on‐table” trial (in the operating room) versus classic ambulatory trial.
Damian Bendersky, MD
Buenos Aires, Argentina
***
I think the paper is well written. I would believe that most practitioners of SCS would not perform long trials because of concerns for infection. I do not think this observation is significant.
Nestor Tomycz, MD
Pittsburgh, PA USA
***
This manuscript provides important insight into the link between the length of spinal cord stimulator trial and risk of infection. Clinicians will benefit from having data to help guide their trial planning. In addition, as new devices are developed and reimbursement policies are updated, this information will help give structure when considering trial length.
Bryan Hoezler, MD
Provo, UT USA
***
Implant infection is a serious adverse event. It can render future SCS unsuccessful, cause long‐term harm and increase health costs. Mitigating this risk should take priority over perceived ideas about overall cost effectiveness being improved by prolonged trial periods with or without use of temporary or definitive leads. However, changes in practice have to be supported by the available data and consensus. It is important that this data gets into the public domain so it can be used in consensus forming and guidelines for best practice. Reimbursement committees that insist on a 3‐week trial period will soon be recognized as harmful. Furthermore, since prolonged trial periods have little to support their use in predicting long‐term outcome, we really need to reflect and change.
Simon Thomson, MBBS
Basildon Essex, UK
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of Financial Support: Medtronic.
Conflict of Interest: The nonprofit Neuromodulation Foundation, which employs Ms. Shipley, and of which Dr. North is an unpaid officer, has received grants and/or consulting income from Abbott (formerly St. Jude), Boston Scientific, Medtronic, Nevro, Nuvectra, and Stimwave. Dr. North has received royalties from Abbott and Nuvectra and consulting income from Nuvectra and Stimwave; his spouse has equity in Stimwave. Dr. Desai has received personal fees from Medtronic and Halyard Health as well as stock options from dorsaVi, Smart Implant Systems, and Medical Wearables Solutions. Dr. Remacle has received scientific support from Medtronic and personal fees from Depuy. Dr. Van Havenbergh has received grants from Medtronic. Ms. Johnson, Ms. Van den Abeele, and Dr. Tan are employees of Medtronic. Dr. Rigoard has received grants, personal fees, and nonfinancial support from Medtronic, Abbott, and Boston Scientific. Dr. Deruytter, Dr. Raftopoulos, and Dr. Vangeneugden declare no competing interests.
REFERENCES
- 1. Erickson DL. Percutaneous trial of stimulation for patient selection for implantable stimulating devices. J Neurosurg 1975;43:440–444. [DOI] [PubMed] [Google Scholar]
- 2. Hosobuchi Y, Adams JE, Weinstein PR. Preliminary percutaneous dorsal column stimulation prior to permanent implantation. Technical note. J Neurosurg 1972;37:242–245. [DOI] [PubMed] [Google Scholar]
- 3. Sweet WH, Wepsic JG. Stimulation of the posterior columns of the spinal cord for pain control: indications, technique, and results. Clin Neurosurg 1974;21:278–310. [DOI] [PubMed] [Google Scholar]
- 4. Deer TR, Provenzano DA, Hanes M, Pope JE, Thomson SD et al. The neurostimulation appropriateness consensus committee (NACC) recommendations for infection prevention and management. Neuromodulation 2017;20:31–50. [DOI] [PubMed] [Google Scholar]
- 5. Rigoard P, Basu S, Desai M et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain 2019;160:1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg 2014;82:1359–1368. [DOI] [PubMed] [Google Scholar]
- 7. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20‐year literature review. J Neurosurg 2004;100:254–267. [DOI] [PubMed] [Google Scholar]
- 8. Hoelzer BC, Bendel MA, Deer TR et al. Spinal cord stimulator infection rates and risk factors: a multicenter retrospective study. Neuromodulation 2017;20:558–562. [DOI] [PubMed] [Google Scholar]
- 9. Kumar K, Wilson J, Taylor R, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine 2006;5:191–203. [DOI] [PubMed] [Google Scholar]
- 10. Mekhail N, Mathews M, Nageeb F, Guirguis M, Mekhail M et al. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract 2011;11:148–153. [DOI] [PubMed] [Google Scholar]
- 11. Turner JA, Loeser JD, Deyo RA, Sander SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systemic review of effectiveness and complications. Pain 2004;108:137–147. [DOI] [PubMed] [Google Scholar]
- 12. Patel SK, Gozal YM, Saleh MS, Gibson JL, Karsy M, Mandybur GT. Spinal cord stimulation failure: evaluation of factors underlying hardware explantation. J Neurosurg Spine 2020;32:133–138. [DOI] [PubMed] [Google Scholar]
- 13. Arocho‐Quinones EV, Huang CC, Ward BD, Pahapill PA. Care bundle approach to minimizing infection rates after neurosurgical implants for neuromodulation: a single surgeon experience. World Neurosurg 2019;128:e87–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bendel MA, O'Brien T, Hoelzer BC, Deer TR, Pittelkow TP et al. Spinal cord stimulator related infections: findings from a multicenter retrospective analysis of 2737 implants. Neuromodulation 2017;20:553–557. [DOI] [PubMed] [Google Scholar]
- 15. Burgher AH, Barnett CF, Obray JB, Mauck WD. Introduction of infection control measures to reduce infection associated with implantable pain therapy devices. Pain Pract 2007;7:279–284. [DOI] [PubMed] [Google Scholar]
- 16. Falowski SM, Provenzano DA, Xia Y, Doth AH. Spinal cord stimulation infection rate and risk factors: results from a United States payer database. Neuromodulation 2019;22:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Provenzano DA, Deer T, Phelps AL, Drennen ZC, Thomson S et al. An international survey to understand infection control practices for spinal cord stimulation. Neuromodulation 2016;19:71–84. [DOI] [PubMed] [Google Scholar]
- 18. Provenzano DA, Falowski SM, Xia Y, Doth AH. Spinal cord stimulation infection rate and incremental annual expenditures: results from a United States payer database. Neuromodulation 2019;22:302–310. [DOI] [PubMed] [Google Scholar]
- 19. Weinand ME, Madhusudan H, Davis B, Melgar M. Acute vs prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation 2003;6:15–19. [DOI] [PubMed] [Google Scholar]
- 20. Rudiger J, Thomson S. Infection rate of spinal cord stimulators after a screening trial period. A 53‐month third party follow‐up. Neuromodulation 2011;14:136–141. [DOI] [PubMed] [Google Scholar]
- 21. Gopal H, Fitzgerald J, McCrory C. Spinal cord stimulation for FBSS and CRPS: a review of 80 cases with on‐table trial of stimulation. J Back Musculoskelet Rehabil 2016;29:7–13. [DOI] [PubMed] [Google Scholar]
- 22. Logé D, De Coster O, Washburn S. Technological innovation in spinal cord stimulation: use of a newly developed delivery device for introduction of spinal cord stimulation leads. Neuromodulation 2012;15:392–401. [DOI] [PubMed] [Google Scholar]
- 23. Van Buyten J‐P, Al‐Kaisy A, Smet I, Palmisani S, Smith T. High‐frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 2013;16:59–65. [DOI] [PubMed] [Google Scholar]
- 24. Arabi Y, Memish ZA, Balkhy HH et al. Ventriculostomy‐associated infections: incidence and risk factors. Am J Infect Control 2005;33:137–143. [DOI] [PubMed] [Google Scholar]
- 25. Maki DG1, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159–1171. [DOI] [PubMed] [Google Scholar]
- 26. Ramanan M, Lipman J, Shorr A, Shankar A. A meta‐analysis of ventriculostomy‐associated cerebrospinal fluid infections. BMC Infect Dis 2015;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong B, Winkel A, Stumpp N, Abdallata M, Saryyevaa A et al. Detection of bacterial DNA on neurostimulation systems in patients without overt infection. Clin Neurol Neurosurg 2019;184:105399. [DOI] [PubMed] [Google Scholar]
- 28. Lalkhen AG, Mahindra C, Patel J. Microbiological evaluation of the extension wire and percutaneous epidural lead anchor site following a “2‐stage cut‐down” spinal cord stimulator procedure. Pain Pract 2017;17:886–891. [DOI] [PubMed] [Google Scholar]
- 29. Simopoulos T, Sharma S, Aner M, Gill JS. A temporary vs permanent anchored percutaneous lead trial of spinal cord stimulation: a comparison of patient outcomes and adverse events. Neuromodulation 2018;21:508–512. [DOI] [PubMed] [Google Scholar]
- 30. Yusuf E, Bamps S, Thüer B et al. A multidisciplinary infection control bundle to reduce the number of spinal cord stimulator infections. Neuromodulation 2017;20:563–566. [DOI] [PubMed] [Google Scholar]


