Abstract
New Findings
-
What is the central question of this study?
What are the effects of a 2 week period of severe food restriction on vascular reactivity of resistance arteries and on cardiac structure and function?
-
What is the main finding and its importance?
This study showed, for the first time, that a 2 week period of severe food restriction in adult male Fischer rats caused endothelial dysfunction in mesenteric arteries and increased the susceptibility to ischaemia–reperfusion‐induced arrhythmias and cardiac pathology. Our findings might have ramifications for cardiovascular risk in people who experience periods of inadequate caloric intake.
Abstract
Severe food restriction (sFR) is a common dieting strategy for rapid weight loss. Male Fischer rats were maintained on a control (CT) or sFR (40% of CT food intake) diet for 14 days to mimic low‐calorie crash diets. The sFR diet reduced body weight by 16%. Haematocrits were elevated by 10% in the sFR rats, which was consistent with the reduced plasma volume. Mesenteric arteries from sFR rats had increased sensitivity to vasoconstrictors, including angiotensin II [maximum (%): CT, 1.30 ± 0.46 versus sFR, 11.5 ± 1.6; P < 0.0001; n = 7] and phenylephrine [maximum (%): CT, 78.5 ± 2.8 versus sFR, 94.5 ± 1.7; P < 0.001; n = 7] and reduced sensitivity to the vasodilator acetylcholine [EC50 (nm): CT, 49.2 ± 5.2 versus sFR, 71.6 ± 6.8; P < 0.05; n = 7]. Isolated hearts from sFR rats had a 1.7‐fold increase in the rate of cardiac arrhythmias in response to ischaemia–reperfusion and more cardiac pathology, including myofibrillar disarray with contractions and cardiomyocyte lysis, than hearts from CT rats. The sFR dietary regimen is similar to very low‐calorie commercial and self‐help weight‐loss programmes, which provide ∼800–1000 kcal day−1. Therefore, these findings in rats warrant the study of cardiovascular function in individuals who engage in extreme dieting or are subjected to bouts of very low caloric intake for other reasons, such as socioeconomic factors and natural disasters.
Keywords: inadequate food intake, langendorff, vascular reactivity

1. INTRODUCTION
Studies of caloric restriction regimens vary tremendously, from starvation (e.g. total caloric restriction) to those that are mild (e.g. restricting food intake to 80–85% of food eaten by a control group fed on an ad libitum basis) or intermittent (e.g. limiting the window of food availability, although caloric intake is similar to non‐food restricted animals). The consequences to cardiovascular health vary considerably based upon these food restriction models, because cardiac function is altered in different ways depending upon the degree and timing of caloric restriction.
Starvation and cachexia cause a severe state of malnutrition that results in myocardial autophagy, atrophy and, ultimately, heart failure (Hariharan et al., 2010; Lee et al., 2015; Rahman et al., 2016). In contrast, caloric restriction without malnutrition increases life expectancy in organisms from yeast to humans (Fontana & Partridge, 2015; Testa, Biasi, Poli, & Chiarpotto, 2014). This prolongation of life expectancy is associated with marked reductions in the incidence of cardiovascular disease (Colman et al., 2009; Kagawa, 1978; Mattison et al., 2012). Animal studies of intermittent fasting that do not involve weight loss have reported cardiovascular benefit (Ahmet, Wan, Mattson, Lakatta, & Talan, 2005). In fact, intermittent fasting has been suggested as a treatment option for prevention of cardiovascular events and attenuation of ischaemia–reperfusion (I/R) injury in individuals with established heart disease, such as cardiomyopathy and heart failure (Harvie et al., 2011; Ma et al., 2019).
Less is known about the effects of very low‐calorie dieting on cardiovascular function. Therefore, we investigated the effects of a severe food‐restricted (sFR) diet on cardiovascular function in an animal model that mimics low‐calorie crash diets. In this model, daily caloric and nutrient intake is reduced by 60% compared with the control (CT) group on an ad libitum diet (de Souza et al., 2015, 2018), and body weight (BW) decreases by 15–20% over a 2 week period. The rate and duration of weight loss in this model is similar to what occurs in many individuals who engage in limited crash dieting (<1000 calories day−1 for ≥2 weeks) (Lissner, Andres, Muller, & Shimokata, 1990).
In this study, we investigated the effects of sFR on vascular reactivity in response to vasoconstrictors and vasodilators in mesenteric and femoral arteries of male Fischer rats. In addition to studying the effect of sFR on endothelial function, we studied the effect of sFR on the function and morphology of the isolated heart in response to I/R. We hypothesized that this sFR regimen that mimics low‐calorie crash diets will have adverse effects on endothelial function and cardiac structure and function that would become apparent after the stress of I/R.
We focused these studies on the acute effects of sFR, which we define as those effects exhibited during a relatively short period (14 days), in contrast to chronic effects, which are typically based on much longer periods of dietary restriction (e.g. months) (Okoshi et al., 2001; Sugizaki et al., 2005). In contrast to our previous studies in female rats (de Souza et al., 2018), we conducted this study on male rats because men are also exposed to periods of sFR either voluntarily (e.g. crash dieting for personal or professional reasons) or not (e.g. war, natural disasters, poverty).
2. METHODS
2.1. Ethical approval
All procedures were approved by the Georgetown University Animal Care and Use Committee (#2017‐0068) and conducted in accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of rats used in this study. At the end of the experiment, rats were anaesthetized with 2.5% isoflurane in O2 at 3 l min−1. After the absence of the pedal reflex, animals were killed by excision of the heart.
2.2. Animals
All experiments were conducted on male Fischer rats initially weighing 250–300 g at 4 months of age. The rats were purchased from Envigo Corp. (Frederick, MD, USA). All rats were housed in individual cages on a 12 h–12 h light–dark cycle at room temperature (24°C).
2.3. Diet
All rats were single housed and received a standard rat diet composed of 62.3% carbohydrate, 24.6% protein and 13.1% lipid (Rodent diet 20, #5053; LabDiet, Marlborough, MA, USA) with ad libitum access to water for the duration of the experiment. The sFR rats received 40% of the control diet (i.e. 60% reduction in normal caloric intake), as we described previously (de Souza et al., 2015). At the end of the 2 week dietary regimen, all rats were killed between 08.00 and 09.00 h before harvesting of plasma and tissues for further study.
2.4. Food consumption and water intake
Food consumption, water intake and BW were determined daily at 17.00 h during the 2 week sFR period. Food and water intake were measured gravimetrically, as described by West et al. (2013).
2.5. Haematocrit and urine volumes
Blood was collected from the caudal artery in 25 μl capillary tubes and centrifuged for 1 min at 14,000g to precipitate red blood cells. Haematocrits were determined using a capillary micro‐haematocrit reader (JM Scientific, Boston, MA, USA). The urine volume was determined by weighing the volume of liquid collected from individual rats housed for 24 h in Nalgene metabolic cages designed for rats (Ancare Corporation, Bellmore, NY, USA), as described by de Souza et al. (2015).
2.6. Sodium and potassium
Blood was collected from the caudal artery and by abdominal aortic puncture. Samples were centrifuged at 13,000g for 10 min to isolate the plasma, which was stored in sterilized tubes at −80°C until analysis. Sodium and potassium plasma concentrations were determined by flame photometry (model #2655‐10; Cole‐Parmer, Vernon Hills, IL, USA).
2.7. Tissue size and mass
Immediately after the sFR period ended, rats were killed and wet weights determined for epididymal, interscapular brown adipose tissue, inguinal and retroperitoneal fat beds, the heart, left and right kidneys and adrenal glands. In addition, the length of the tibia was determined using callipers.
2.8. Myography
Mesenteric and femoral arteries were mounted onto wires in a myography apparatus (Multi Myograph System 620M; DMT‐USA, Inc., Ann Arbor, MI, USA) and then preconstricted with 10−5 mol l−1 phenylephrine (PE) to determine the maximal response and ensure vessel function. If a vessel did not show five consecutive vasoconstrictor responses to PE that were similar in magnitude, it was not used for further experiments. Concentration–response curves to angiotensin II (Ang II) and PE followed by ACh and sodium nitroprusside (SNP) were then conducted as we described previously (Wang et al., 2014).
2.9. Isolated heart function
Between 10 and 15 min after an i.p. injection of 200 IU heparin, rats were anaesthetized with isoflurane (2.5% isoflurane in 3 l min−1 O2). The thorax was opened and the heart dissected and prepared for Langendorff perfusion (Krebs's Ringer solution composition: 118.4 mmol/l NaCl, 4.7 mmol/l KCl, 1.2 mmol/l KH2PO4, 1.2 mmol/l MgSO4·7H2O, 2.0 mmol/l CaCl2·2H2O, 11.7 mmol/l glucose and 26.5 mmol/l NaHCO3) through the aortic stump as previously described (Li et al., 2018; von Lueder et al., 2015). The perfusion flow was maintained at a constant rate (8–10 ml min−1) at 37°C with constant oxygenation (5% CO2 and 95% O2). A balloon was inserted into the left ventricle through the left atrium for isovolumetric recordings of left ventricular pressures. Coronary perfusion was measured with a transducer connected to an aortic cannula and coupled to a data‐acquisition system (PowerLab; ADInstruments, Colorado Springs, CO, USA) (Nunes et al., 2017). Global ischaemia was achieved by complete occlusion of inflow lines for 30 min. Reperfusion effects were evaluated after flow reinstatement for 60 min. Isolated hearts placed in the Langendorff apparatus but not subjected to I/R served as basal controls for the I/R experiments. The incidence of left ventricular arrhythmias during the reperfusion period was counted manually and expressed as a percentage of the total reperfusion period by J.F.Q.A., who was blinded to sample identification.
2.10. Cardiac histology and morphology
Heart tissues were immersed in buffered 10% formalin at room temperature and then embedded in paraffin, sectioned (5 micrometers) and mounted on glass slides. Tissue sections were subjected to Haematoxylin and Eosin (H & E) or Masson's Trichrome staining. Morphometric analysis was performed by N.S., who was blinded to sample identification by systematic uniform random sampling with the Fiji software (Schindelin et al., 2012) version ImageJ2 (Rueden et al., 2017) using 25 randomly selected images. The diameter of single cardiac myocytes was measured on H & E‐stained images of myocardium. For quantification of collagen deposition in the left ventricular myocardium, the percentage of fibrosis was calculated using morphometric grid images from Masson's Trichrome‐stained slides (Mendez & Keys, 1960).
2.11. Statistical analysis
Statistical comparisons were assessed using GraphPad Prism software (v.8; GraphPad Inc., La Jolla, CA, USA). The effect of sFR was analysed by two‐way ANOVA (repeated measures when appropriate) followed by Tukey's post hoc test. Data are expressed as means ± SD. Significance was defined as P < 0.05. The Gaussian kernel density estimates for univariate observations were used to analyse cardiomyocyte diameter from H & E‐stained hearts in CT and sFR groups. Estimates were computed by the density function in the R statistical program (v.3.6.1 by R‐core, R‐core@R‐project.org). The algorithm disperses the mass of the empirical distribution function over a regular grid of ≥512 points, after which it uses fast Fourier transformation to convolve this approximation with a discretized version of the kernel and then uses linear approximation to evaluate the density at the specified points. The statistical properties of a kernel were determined by , which is always one, and . Mean squared error‐equivalent bandwidths (for different kernels) are proportional to , which is scale invariant and, for our kernels, equal to . Unequal variance based on two‐sample t tests was used to test the significance between groups.
3. RESULTS
3.1. Acute effects of sFR on BW, food consumed and water intake
Before beginning the sFR protocol, there were no differences in daily food consumed (Figure 1a), water intake (Figure 1b) or initial BW (Figure 1c) between the CT and sFR groups. Daily food intake in the sFR group was 40% of that in the CT group for the duration of the 2 week sFR period (Figure 1a). The sFR rats drank ∼20–30% less water throughout the sFR period (Figure 1b). Over 2 weeks, the CT group slowly gained BW, whereas BW in the sFR group continued to fall; by day 14, the sFR rats had BWs that were 84% of their initial weight, i.e. a 16% reduction in BW (Figure 1c).
FIGURE 1.
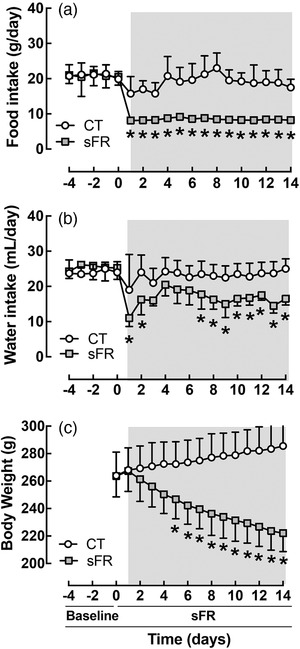
Effect of severe food restriction (sFR) on food and water intake and body weight (BW) in male rats. Shown is daily food consumed (a), water intake (b) and BW (c) before and during maintenance on a CT (open circles) or sFR (filled squares) diet for 2 weeks (grey area). * P < 0.05 versus CT, by two‐way ANOVA with repeated measures (diet and time) and Tukey's post hoc test. Values are expressed as the mean ± SD; n = 8 per group
3.2. Acute effects of sFR on tissue size and mass
Compared with the CT rats, the epididymal, inguinal and retroperitoneal fat beds in the sFR group were markedly reduced by 41, 47 and 58%, respectively, but no effect of sFR was observed on interscapular brown adipose tissue (Table 1). Furthermore, heart mass and the heart mass index were 13 and 14% lower, respectively, and the masses of the left and right kidneys were 16 and 20% lower, respectively, in the sFR group compared with CT rats. In contrast, the adrenal gland mass was 1.3‐fold higher. Severe food restriction did not decrease tibial length (the bones increased in length in both groups to the same extent over the duration of the experiment), indicating that losses in body and tissue mass were not a result of impaired growth.
TABLE 1.
Effect of a 2 week sFR diet on BW and individual tissue size and weight
| Tissue | CT | sFR | sFR/CT | P‐value |
|---|---|---|---|---|
| BW (g) | 286 ± 6.1, n = 8 | 222 ± 4.8, n = 8 | 0.77 | <0.001* |
| Epididymal fat (g) | 4.97 ± 0.29, n = 8 | 2.95 ± 0.39, n = 8 | 0.59 | <0.001* |
| Inguinal fat (g) | 3.37 ± 0.22, n = 6 | 1.77 ± 0.29, n = 5 | 0.53 | <0.01* |
| Retroperitoneal fat (g) | 3.26 ± 0.24, n = 7 | 1.36 ± 0.30, n = 7 | 0.42 | <0.001* |
| IBAT (mg) | 198 ± 3.4, n = 5 | 197 ± 4.3, n = 4 | 0.99 | 1.0 |
| Heart (g) | 1.17 ± 0.031, n = 8 | 1.01 ± 0.032, n = 8 | 0.86 | <0.01* |
| HMI (g cm−1) | 0.31 ± 0.007, n = 7 | 0.27 ± 0.008, n = 8 | 0.87 | <0.01* |
| Left kidney (g) | 0.930 ± 0.093, n = 8 | 0.779 ± 0.041, n = 8 | 0.84 | <0.01* |
| Right kidney (g) | 0.932 ± 0.020, n = 8 | 0.745 ± 0.035, n = 6 | 0.80 | <0.001* |
| Adrenal gland (mg) | 41.8 ± 3.0, n = 8 | 53.1 ± 4.2, n = 8 | 1.27 | <0.05* |
| Tibia (cm) | 3.80 ± 0.040, n = 7 | 3.73 ± 0.037, n = 8 | 0.98 | 0.15 |
Immediately after the 2 week sFR period ended, the tissues were isolated, and the size and or mass was measured. Values are reported as the mean ± SD, n = 6 per group; *P < 0.05 versus CT, by Student's unpaired t test. Abbreviations: BW, body weight; CT, control; HMI, heart mass index; IBAT, interscapular brown adipose tissue; and sFR, severe food restriction.
3.3. Acute effects of sFR on haematocrit and plasma sodium and potassium
The haematocrit was slightly elevated in the sFR compared with the CT group [(%): CT, 45.2 ± 1.1 (n = 6) versus sFR, 51.1 ± 1.4 (n = 8); P < 0.01]. Plasma Na+ [(mmol l−1): CT, 168 ± 5.5 (n = 6) versus sFR, 171 ± 5.6 (n = 7)] and plasma K+ [(mmol l−1): CT, 5.30 ± 0.11 (n = 6) versus sFR, 4.96 ± 0.20 (n = 7)] were not significantly affected by the sFR diet.
3.4. Acute effects of sFR on vasomotricity in mesenteric arteries
Angiotensin II caused mesenteric arteries from sFR rats to vasoconstrict until tachyphylaxis occurred at increasing doses of Ang II (Figure 2a; Table 2). In contrast, mesenteric arteries from CT rats responded minimally to Ang II. Mesenteric arteries from both CT and sFR rats vasoconstricted in response to PE; however, the maximal response to PE was 1.2‐fold higher (Figure 2b; Table 2). The EC50 was 50% lower in the sFR compared with the CT rats, indicating that sFR caused an increase in potency for PE‐induced vasoconstriction. No differences were observed in the maximal response to ACh‐ or SNP‐induced vasorelaxation or in the SNP EC50 between CT and sFR rats (Figure 2c; Table 2). However, the EC50 for ACh was 1.5‐fold higher in the sFR rats, indicating sFR caused a reduction in potency for ACh‐induced relaxation.
FIGURE 2.
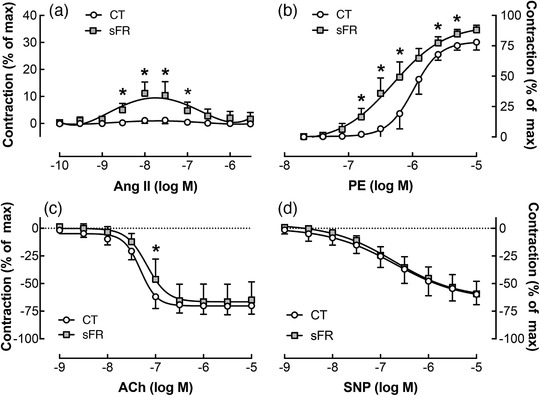
Effects of severe food restriction (sFR) on vasomotricity in mesenteric arteries from male rats. Shown are concentration–response curves in mesenteric arteries from control (CT; open circles) and sFR (filled squares) rats after the addition of angiotensin II (Ang II; a), phenylephrine (PE; b), ACh (c) and sodium nitroprusside (SNP; d). Values are expressed as the mean ± SD; * P < 0.05 versus CT, by two‐way ANOVA with repeated measures (diet and response) and Tukey's post hoc test; n = 7 per group
TABLE 2.
Effect of a 2 week sFR diet on vascular reactivity parameters in mesenteric and femoral vessels
| Mesenteric | Femoral | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Parameter | CT | sFR | P‐value | CT | sFR | P‐value |
| Angiotensin II | Max, % | 1.30 ± 0.46 | 11.5 ± 1.6 | <0.0001 | 13.2 ± 4.3 | 24.7 ± 9.3 | n.s. |
| AUC | 1.83 ± 0.66 | 19.7 ± 3.5 | <0.001 | 22.6 ± 7.6 | 20.1 ± 5.2 | n.s. | |
| Phenylephrine | Max, % | 78.5 ± 2.8 | 94.5 ± 1.7 | <0.001 | 91.2 ± 4.9 | 83.6 ± 1.8 | n.s. |
| EC50 (nm) | 1060 ± 11 | 552 ± 110 | <0.01 | 270 ± 51 | 527 ± 84 | <0.02 | |
| ACh | Max, % | 65.8 ± 2.5 | 66.5 ± 5.2 | n.s. | 80.9 ± 2.4 | 74.9 ± 3.6 | n.s. |
| EC50 (nm) | 49.2 ± 5.2 | 71.6 ± 6.8 | <0.05 | 99.8 ± 7.30 | 139 ± 27 | n.s. | |
| SNP | Max, % | 69.3 ± 1.7 | 67.2 ± 4.1 | n.s. | 78.7 ± 2.8 | 79.8 ± 3.3 | n.s. |
| EC50 (nm) | 318 ± 120 | 243 ± 70 | n.s. | 32.6 ± 2.9 | 53.6 ± 19 | n.s. | |
Immediately after the 2 week sFR period ended, values were determined from vascular reactivity experiments using wire myography on mesenteric and femoral vessels. Values are reported as the mean ± SD, n = 6 per group; *P < 0.05 versus CT, by Student's unpaired t test. Abbreviations: AUC, area under the curve; CT, control; EC50, concentration of drug that gives half‐maximal response to either the vasoconstrictor or the vasodilator; Max %, percentage of maximal response to either the vasoconstrictor or the vasodilator; sFR, severe food restriction; and SNP, sodium nitroprusside.
3.5. Acute effects of sFR on vasomotricity in femoral arteries
Angiotensin II caused greater vasoconstriction in femoral compared with mesenteric vessels in both CT and sFR rats (P < 0.05, two‐way ANOVA with repeated measures, factors: vessel and response; Figures 2a and 3a). However, no significant differences in the maximal response to Ang II were observed in the femoral artery between CT and sFR rats (Figure 3a; Table 2). There was a marked twofold increase in the EC50 to PE‐induced vasoconstriction in the femoral artery of sFR rats, indicating a reduction in PE potency; however, there was no difference in the maximal PE response between CT and sFR femoral arteries (Figure 3b; Table 2). Furthermore, no significant differences were observed between CT and sFR rats in the maximal response or EC50 values for ACh (Figure 3c; Table 2) or SNP (Figure 3d; Table 2).
FIGURE 3.
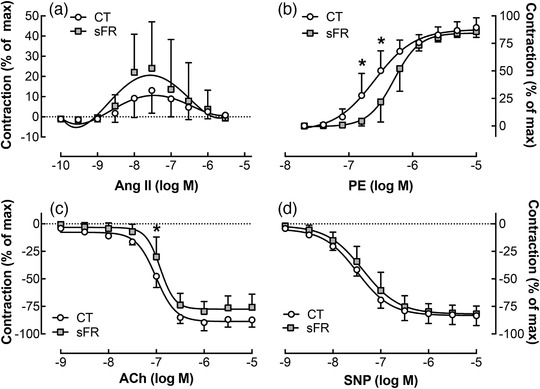
Effects of severe food restriction (sFR) on vasomotricity in femoral arteries from male rats. Shown are concentration–response curves in femoral vessels from control (CT; open circles) and sFR (filled squares) rats after the addition of angiotensin II (Ang II; a), phenylephrine (PE; b), ACh (c) and sodium nitroprusside (SNP; d). Values are expressed as the mean ± SD; * P < 0.05 versus CT, same time point, by two‐way ANOVA with repeated measures (diet and response) and Tukey's post hoc test; CT, n = 7; sFR, n = 6
3.6. Acute effects of sFR on isolated perfusion pressure and heart rate in response to I/R
There were no differences in basal levels or in the effect of I/R on perfusion pressure (PP; Figure 4a), diastolic left ventricular perfusion pressure (dLVP; Figure 4b), the maximal (Max) rate of change of left ventricular pressure (dP/dt; Figure 4c), the minimal (Min) dP/dt (Figure 4d) and heart rate (HR; Figure 4e) between the CT and sFR animal groups. After 30 min of ischaemia, reperfusion caused a marked increase in PP (Figure 4a) and Min dP/dt (Figure 4d) and a marked decrease in dLVP (Figure 4b) and Max dP/dt (Figure 4c); however, there was no effect of diet on any of these parameters nor were there any differences in HR between CT and sFR rats during reperfusion (Figure 4e).
FIGURE 4.
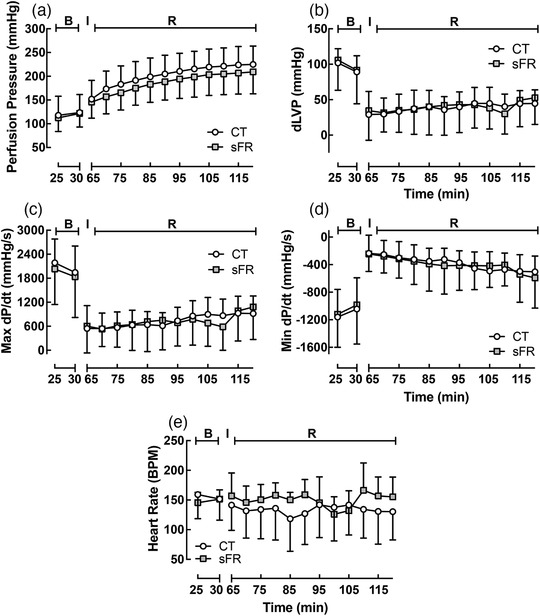
Effect of severe food restriction (sFR) on isolated heart from male rats before and after ischaemia–reperfusion (I/R). Shown is perfusion pressure (PP; a), diastolic left ventricular pressure (dLVP; b), maximal rate of change of left ventricular perfusion pressure (Max dP/dt; c), minimal rate of change of left ventricular perfusion pressure (Min dP/dt; d) and heart rate (HR; e) in isolated hearts from control (CT; open circles) and sFR (filled squares) rats before [basal (B)] the 30 min ischaemic (I) period and after reperfusion (R). CT, n = 6; sFR, n = 7
3.7. Acute effects of sFR on arrhythmias in response to I/R
Ischaemia caused arrhythmias in isolated hearts (Figure 5). The percentage of arrhythmias was 1.7‐fold higher in hearts from the sFR rats compared with the CT rats.
FIGURE 5.
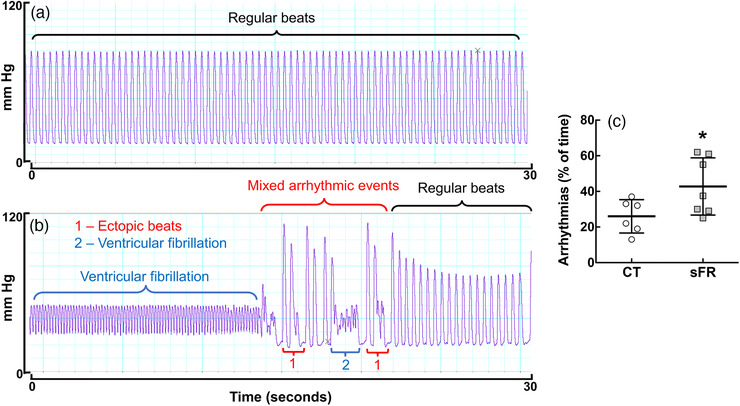
Effect of severe food restriction (sFR) on incidence of arrhythmias in isolated heart from male rats during ischaemia. Shown is a representative trace of pressure pulses over 30 s during the 30 min reperfusion period from control (CT; a) and sFR (b) rats. In this 30 s period shown, there were no arrhythmic events in the CT hearts, whereas the sFR rats exhibited ventricular fibrillation and ectopic beats before returning to regular beats. (c) Quantification of the percentage of arrhythmias during the 30 min ischaemic period in isolated hearts from CT and sFR rats. * P < 0.05 versus CT, by Student's unpaired t test; CT, n = 6; sFR, n = 7
3.8. Acute effects of sFR on cardiac morphology
The H & E staining of Langendorff‐perfused hearts from sFR rats revealed disorganized myofibrils of the left myocardium. The typical pattern of myofibrils orientated in parallel and cross‐striation of cardiac myocytes observed in CT rats (Figure 6a,c) was missing in sFR rats, and multiple contractile lesions were present (Figure 6b,d). The hearts from sFR rats also exhibited focal lysis of cardiomyocytes (Figure 6d). A significant difference in the Gaussian distribution of cardiomyocyte diameter was observed between the CT and sFR groups (Figure 6g). The mean thickness of cardiac myocytes in the CT group was 11.5 μm (SD = 0.76 μm; out of an average of 261 cells counted per animal; n = 5 per group). In contrast, only 16.6% of cardiomyocytes from the sFR group had cell diameters within one standard deviation of the mean of the CT cardiomyocytes; 51.8 ± 0.67% of sFR cardiomyocytes were atrophied (diameter <10.7 μm) and 31.6 ± 1.2% were hypertrophied (diameter >12.3 μm) (Figure 6h). Morphometric analysis of Masson's Trichrome staining (Figure 6e,f) showed that there were 21% fewer collagen fibers in the myocardium of sFR rats compared with the CT group (Figure 6i).
FIGURE 6.
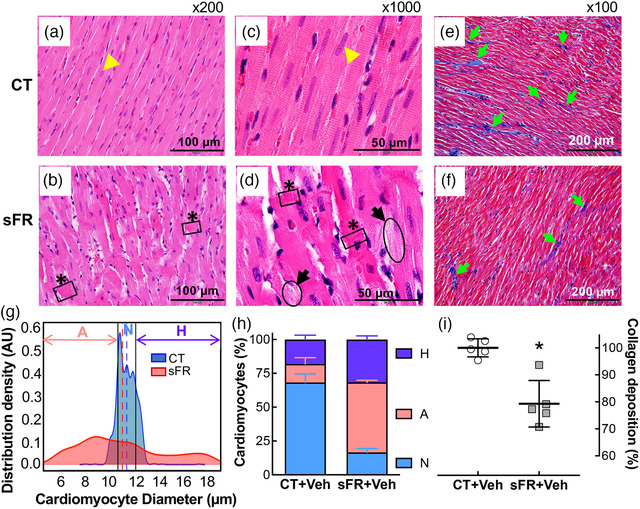
Effect of severe food restriction (sFR) on cardiac pathology. Shown are representative heart sections stained with Haematoxylin and Eosin (H & E; a–d) or Masson's Trichrome (e,f) from control (CT; a,c,e) and sFR (b,d,f) rats. (a–d) The H & E staining showed myofibrillar disarray with contractions (black boxes highlighted asterisks) and cardiomyocyte lysis (black ovals highlighted by black arrows) in sFR rats at ×200 (a,b) and ×1000 (c,d) magnification. (e,f) Masson's Trichrome staining of collagen, indicated by blue staining (green arrows) at ×100 magnification, revealed that there was less collagen present in the sFR group compared with the CT rats. The H & E and Masson's Trichrome images are representative of five rats per group; a yellow arrowhead indicates the nucleus. (g) Gaussian kernel density estimates of cardiomyocyte diameter from H & E‐stained hearts in CT (aqua curve) and sFR (pink curve) groups. The vertical dashed lines represent the mean in each group. (h) Quantification of the percentage of cardiomyocytes below (pink bar) and above (purple bar) the normal (aqua bar) range of cardiomyocyte diameter revealed greater numbers of atrophied and hypertrophied cardiomyocytes in sFR hearts; * P < 0.001 versus CT by Gaussian kernel density analysis. (i) Percentage of collagen staining in sFR rat hearts normalized to the staining in the CT group; * P < 0.05 versus CT, by Student's unpaired t test. Abbreviations: A, atrophied cardiomyocytes; H, hypertrophied cardiomyocytes; and N, normal cardiomyocytes
3.9. Acute effects of sFR on cardiac morphology after I/R
Langendorff‐perfused hearts from sFR rats exhibited more severe necrotic damage compared with the CT group in response to I/R (Figure 7a–d). Sections stained with H & E revealed that despite the presence of similar degrees of oedema in the pericellular space in the myocardium of sFR and CT rats, cardiomyocytes from sFR rats exhibited many multifocal areas of damage (Figure 7b) along with eosinophilic areas undergoing contractile lesions (Figure 7d). Higher‐magnification analyses showed necrotic lesions that were consistent with coagulative necrosis of cardiac myocytes, with the presence of multiple contraction bands. Ischaemia–reperfusion had significant effects on the Gaussian distribution of cardiomyocyte diameter on CT and sFR groups (P < 0.0001; Figures 6g and 7g). A significant difference in the Gaussian distribution was also observed between the CT+I/R and sFR+I/R groups (Figure 7g). After I/R, the majority (92.9 ± 1.7%) of cardiomyocytes in the CT group had atrophied (i.e. reduced wall thickness), whereas only 6.9 ± 1.7% had diameters in the normal range (Figure 7h). The majority (62.6 ± 1.6%) of cardiomyocytes in the sFR group were also atrophied, whereas 25.3 ± 1.2% were hypertrophied (i.e. increased wall thickness). Morphometric analysis of Masson's Trichrome staining (Figure 7e,f) showed there were 21% fewer collagen fibers content in the myocardium of sFR rats compared with the CT group (Figure 7i). There was no detectable effect of I/R on collagen fibres between the CT or sFR rat hearts by two‐way ANOVA with repeated measures (P = 0.18; no interaction).
FIGURE 7.
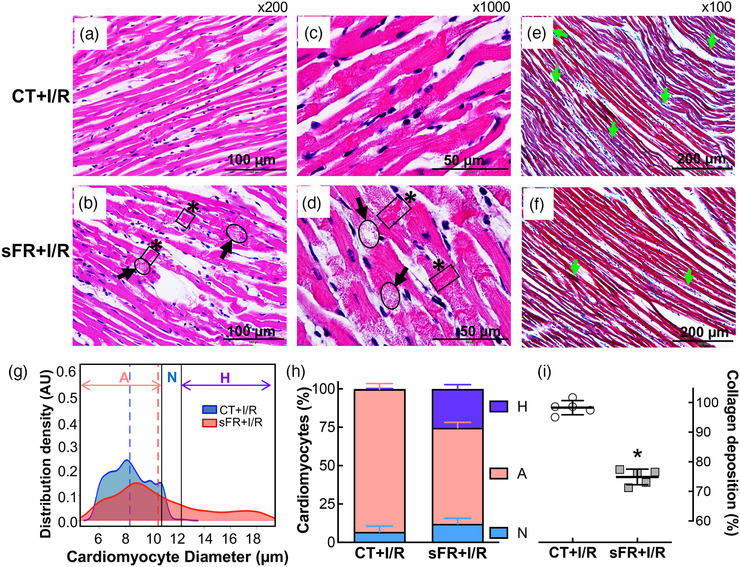
Effect of severe food restriction (sFR) on cardiac pathology after ischaemia–reperfusion (I/R). Shown are representative heart sections stained with Haematoxylin and Eosin (H & E; a–d) or Masson's Trichrome (e,f) from control (CT; a,c,e) and sFR (b,d,f) rats after I/R. (a–d) The H & E staining showed myofibrillar disarray with contractions (black boxes highlighted by asterisks) and cardiomyocyte lysis (black ovals highlighted by black arrows) in sFR rats at ×200 (a,b) and ×1000 (c,d) magnification. (e,f) Masson's Trichrome staining of collagen, indicated by blue staining (green arrow) at ×100 magnification, revealed that there was less collagen present in the sFR group compared with the CT rats. The H & E and Masson's Trichrome images are representative of five rats per group; a yellow arrowhead indicates the nucleus. (g) Gaussian kernel density estimates of cardiomyocyte diameter from H & E‐stained hearts in CT (aqua curve) and sFR (pink curve) groups after I/R. The vertical dashed lines represent the mean in each group. (h) Quantification of the percentage of cardiomyocytes below (pink bar) and above (purple bar) the normal (aqua bar) range of cardiomyocyte diameter revealed greater numbers of atrophied and hypertrophied cardiomyocytes in sFR hearts after I/R; * P < 0.0001 versus CT by Gaussian kernel density analysis. (i) Percentage of collagen staining in sFR rat hearts after I/R normalized to the staining in the CT group; * P < 0.05 versus CT, by Student's Unpaired t test. Abbreviations: A, atrophied cardiomyocytes; H, hypertrophied cardiomyocytes; and N, normal cardiomyocytes
4. DISCUSSION
A major finding of this study was that a caloric‐ and nutrient‐restricted diet that resulted in a 16% reduction in BW over a 2 week period caused endothelial dysfunction in adult male Fischer rats. Vasoreactivity experiments in mesenteric resistance arteries showed that sFR increased responsiveness to the vasoconstrictors Ang II and PE but attenuated sensitivity to the vasodilator ACh. In addition, sFR vessels were also less compliant in response to the vasodilator ACh, which acts upon vascular endothelial cells to cause nitric oxide release.
The increased vasoconstrictor response to Ang II could be attributable to increased expression of angiotensin type 1 receptor (AT1R). Angiotensin II promotes vasoconstriction by binding to the AT1R, and we showed that sFR increased AT1R mRNA levels in mesenteric arteries (de Souza et al., 2018).
Increased sensitivity to PE could be attibutable to increased activity of the α1‐adrenoceceptor, which we demonstrated to occur after sFR (de Souza et al., 2015). Another cause of increased sensitivity to PE could be increased circulating Ang II, because we previously showed that sFR increases plasma Ang II (de Souza et al., 2018). Although the vasoconstrictor response to PE in mesenteric vessels from Fischer rats is similar to what is observed in other rodents, the vasoconstrictor response to Ang II appears blunted (Javeshghani, Sairam, Neves, Schiffrin, & Touyz, 2006). This suggests that the sensitivity of mesenteric arteries to Ang II is dependent upon the animal model.
The maximal response to ACh in mesenteric vessels was not affected, but the EC50 increased 1.5‐fold. This reduction in ACh potency observed in sFR mesenteric vessels could be attributable to reduced nitric oxide, because we previously found that sFR lowered plasma nitric oxide concentrations (de Souza et al., 2015). This impairment in vasorelaxation is likely to be endothelium dependent, because the response to SNP, the nitric oxide mimetic, was not altered by sFR.
Endothelial dysfunction occurs in hypertension and other cardiovascular diseases (Rodrigo et al., 1997; Strawn, Gallagher, Dean, Ganten, & Ferrario, 1997). There are also many examples of endothelial dysfunction in animal models of diet‐induced obesity and diabetes (Bhatta et al., 2017; Dunn, Hilgers, & Das, 2017; Zuloaga et al., 2016). There is much less known regarding endothelial dysfunction during caloric restriction. Razzak, Abu‐Hozaifa, Bamosa, and Ali (2011) showed improved endothelium‐dependent vasodilatory responses of rat aortic rings to ACh after intermittent fasting. Dolinsky et al. (2010) reported that flow‐mediated vasodilatation in the femoral artery was significantly improved in calorie‐restricted spontaneously hypertensive rats compared with non‐food‐restricted control spontaneously hypertensive rats. In contrast to our study, the dietary regimens in these published studies were associated with attenuated BW gain, not BW loss. One study investigated vascular reactivity in offspring of pregnant rats that were calorie restricted during the second half of their pregnancy (50% calorie restriction from day 11 of gestation to term, ∼10 days; Durrant, Khorram, Buchholz, & Pearce, 2014). The 8‐month‐old offspring of these rats had increased arterial stiffness and wall thickness compared with the offspring from control mothers; however, vascular reactivity of resistance vessels was not assessed in that study. Thus, our study extends these findings of others by demonstrating that small mesenteric resistance vessels exhibit endothelial dysfunction after only 2 weeks of sFR.
The second major finding of our study is that the 2 week sFR diet increased the number of arrhythmias induced by I/R. The incidence of arrhythmias nearly doubled in sFR rats. This observation contrasts with a recent study of I/R on male Sprague–Dawley rats, which showed that intermittent fasting (every other day feeding) protected the heart from I/R‐induced damage (Ahmet et al., 2005). Of note, the rats on the intermittent fasting protocol did not lose BW. Thus, the difference between protection and susceptibility to I/R is likely to be related to the degree and rate of BW loss.
Endothelial dysfunction is a risk factor for cardiovascular disease (Roustit, Loader, Deusenbery, Baltzis, & Veves, 2016). Atrial fibrillation (AF), the most prevalent type of cardiac arrhythmia in adults, is associated with endothelial dysfunction (Polovina, Lip, & Potpara, 2015). Individuals with endothelial dysfunction (measured by peripheral arterial tonometry) had a hazard ratio of 4.18 for increased risk of AF recurrence and a hazard ratio of 3.62 for increased risk of any atrial arrhythmia after pulmonary vein isolation (Matsuzawa et al., 2016). The Framingham Heart Study examined relationships between incident AF and endothelial function by flow‐mediated dilatation in offspring and third‐generation cohorts and found that lower flow‐mediated dilatation was associated with a 20% increased risk of incident AF (Shaikh et al., 2016). Thus, our observation of endothelial dysfunction in the mesenteric artery warrants investigation of potential dysfunction in other vessels that could contribute to increased susceptibility to arrhythmias after I/R in sFR rats.
In humans, hypokalaemia was shown to promote ectopic ventricular beats and prolonged QT intervals and to precipitate arrhythmias (Fisler, 1992). We did not measure potassium directly in cardiac tissue. Therefore, although the lack of difference in plasma potassium between CT and sFR rats suggests that potassium is not a key player in the induction of cardiac arrhythmias, we cannot rule out the possibility that plasma concentrations do not reflect tissue levels and that potassium levels are reduced in cardiac tissue from sFR rats as a result of the significant reduction in food intake and thus potassium intake. In this regard, potassium can be regulated in a tissue‐specific manner (Palmer & Clegg, 2016). Electrolyte imbalance might also contribute to the increased susceptibility to arrhythmias as a result of dehydration owing to reduced water intake.
Sympathetic nervous system (SNS) activation could further increase susceptibility towards arrhythmias in the whole animal. Two weeks of sFR has been shown to increase plasma and cardiac levels of noradrenaline and adrenaline (de Souza et al., 2015; McKnight, Rupp, Beamish, & Dhalla, 1996). This increased SNS activity compensates for the reduced cardiac output as a result of the loss of myocardial mass (Webb, Kiess, & Chan‐Yan, 1986). Thus, increased SNS activity can increase the occurrence of ventricular arrhythmias (Zipes, 2008). Although the increased incidence of arrhythmias observed in our study was in an isolated heart model, with no influence of SNS activity, the SNS might further increase the susceptibility of the heart towards arrhythmias in the sFR animal.
The third major finding of this study is that hearts from sFR rats had evidence of cardiac pathology even before injury was induced by I/R and that even worse cardiac pathology was observed after I/R. In contrast, there were no differences detected in PP, dLVP, dP/dt or HR between these two groups either before or after I/R. These observations raise concerns that standard tests of cardiac function, which measure PP, left ventricular function and HR, could miss underlying cardiac pathology attributable to inadequate caloric intake. Consequently, an individual who is at greater risk for ischaemia‐induced arrhythmias as a result of maintaining a severe calorie‐restricted diet could be overlooked by standard cardiological diagnostics, and this increased risk might become apparent only after an ischaemic event.
The heart is composed of four major cell types: cardiomyocytes, fibroblasts, endothelial and perivascular cells (Anversa, Olivetti, Melissari, & Loud, 1980). Fibroblasts are the primary cells responsible for the production of collagen, which plays a key role in maintaining tissue architecture and distributing the force generated by myocytes on the ventricular chamber (Dostal, 2001). Histological staining of sFR hearts in the present study showed less collagen deposition than in the CT hearts. This observation can be explained by the lower heart mass and heart mass index in sFR rats. Changes in rates of collagen synthesis and deposition in response to injury were shown to occur over several days and weeks in rats (Sajanti et al., 1999). Therefore, it is not surprising that there was no effect of the 30 min I/R period on collagen deposition in either the CT or the sFR rats.
Previous studies of individuals on very low‐calorie diets have warned of dangerous cardiovascular consequences. The US Food and Drug Administration and the US Centers for Disease Control and Prevention discovered a pattern of sudden death attributable to intractable ventricular arrhythmias in individuals who had been dieting for prolonged periods of time and who had lost large amounts of weight (Isner, Sours, Paris, Ferrans, & Roberts, 1979). A study by the National Heart, Lung and Blood Institute of 17 of these dieters who died unexpectedly from intractable ventricular arrhythmias suggested that rapid weight loss owing to severe caloric restriction (300–400 kcal day−1; 2.5 kg BW loss week−1; 35% BW loss over 5 months) damaged the heart (Isner et al., 1979). This conclusion is supported by case reports and small studies of patients on fasting diets that have reported sudden cardiac death and myofibrillar damage (Ahmed, Flynn, & Alpert, 2001). The chief finding was myocardial atrophy, which is consistent with protein calorie malnutrition in humans (Ramalingaswami, 1968; Schnitker, Mattman, & Bliss, 1951) and monkeys (Chauhan, Nayak, & Ramalingaswami, 1965).
What is particularly worrisome is that myocardial atrophy of myocardial cells occurred after merely 2 weeks on a diet that provided 40% of normal caloric intake, which is at least twice as much kilocalories per day as in the above related studies (Chauhan et al., 1965; Ramalingaswami, 1968; Schnitker et al., 1951). This short period of time is not uncommon among dieters on very low caloric diets. These findings support a recent study by Tith et al. (2019) that followed women with bulimia nervosa for up to 12 years and found a striking increase in the risk of cardiovascular disease, including ischaemic heart disease, atherosclerosis and cardiac conduction defects. Thus, our studies in rats warrant investigation of cardiovascular function in individuals subjected to short periods of sFR either because of crash dieting or other causes (e.g. natural disasters, socioeconomic factors).
We did not measure physical activity in these rats. Therefore, we cannot rule out the possibility that some of the weight loss was attributable to increased physical activity in the sFR animals; however, our studies in female rats suggest that sFR animals are less active than CT animals (Aline de Souza, personal communication). Physical activity also has implications for endothelial and cardiovascular function, because sedentary behaviour is associated with increased dysfunction (Lavie, Ozemek, Carbone, Katzmarzyk, & Blair, 2019).
In addition to the reduction in BW, the sFR diet increased the haematocrit, which is consistent with reduced plasma volume and our findings in adult female Fischer rats (de Souza et al., 2018). These findings are also similar to changes in haematocrit observed in men and women on very low‐calorie diets. Obese subjects on prolonged very low‐calorie diets had elevated haematocrits (Poggi et al., 1994). What remains unknown is what would happen if obese animals or those with other types of metabolic disease were subjected to sFR for 2 weeks. Would endothelial and cardiovascular dysfunction associated with these conditions be worsened or blunted? Lastly, it is important to note that our studies in males cannot be extrapolated to females. It will be important in future studies to determine whether a similar dietary regimen increases the susceptibility of the female rat heart to cardiac arrhythmias, particularly given that women are more at risk for AF then men (Ko et al., 2016).
In conclusion, we found that a 16% reduction in BW, induced by sFR over a 2 week period in adult male Fischer rats, increased endothelial dysfunction and susceptibility to I/R‐induced arrhythmias and cardiac pathology. Our findings in Fischer rats have ramifications for cardiovascular risk in humans who experience periods of inadequate caloric intake. Very low‐calorie commercial and self‐help weight‐loss programmes provide ∼800–1000 kcal day−1 (1993; Gudzune et al., 2015). Caloric intake can also be inadequate for other voluntary reasons (e.g. bulimia nervosa; Tith et al., 2019) or involuntary reasons (e.g. poverty, natural disasters, war). Our study suggests that these individuals are at increased risk of adverse cardiovascular events and thus would benefit from more frequent screening for cardiovascular disease. Clearly, more research is needed to assess the impact of short‐term sFR on cardiovascular health of both men and women and to develop interventions that could reduce the risk of cardiac dysfunction and disease in this population.
COMPETING INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
Conception and design of the experiments: J.F.Q.A., A.M.A.d.S., N.S. and K.S. Collection, analysis and interpretation of data: J.F.Q.A., A.M.A.d.S., N.S., X.W., H.J., J.W. and K.S. Drafting and revising the article critically for important intellectual content: J.F.Q.A., N.S., A.M.A.d.S., H.J., J.W. and K.S. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
ACKNOWLEDGEMENTS
The authors are thankful to Drs Crystal A. West, Carolyn M. Ecelbarger and Robert C. Speth for critical feedback.
This study was supported by grants from the US National Institutes of Health (NIH), including R01‐HL121456 (K.S.) and R21‐AG060730 (K.S. and H.J.); a Georgetown University Partners‐in‐Research Award (K.S. and N.S.); an American Heart Association (AHA) Innovation Award (K.S.) and an AHA Postdoctoral Fellowship Award (A.M.A.d.S.); and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) in partnership with Federal University of Minas Gerais, Brazil (J.F.Q.A.).
Almeida JFQ, Shults N, de Souza AMA, Ji H, Wu X, Woods J, Sandberg K. Short‐term very low caloric intake causes endothelial dysfunction and increased susceptibility to cardiac arrhythmias and pathology in male rats. Experimental Physiology. 2020;105:1172–1184. 10.1113/EP088434
Edited by: Philip Atherton
REFERENCES
- (1993). Very low‐calorie diets. National Task Force on the Prevention and Treatment of Obesity, National Institutes of Health. JAMA, 270, 967–974. [PubMed] [Google Scholar]
- Ahmed, W. , Flynn, M. A. , & Alpert, M. A. (2001). Cardiovascular complications of weight reduction diets. American Journal of the Medical Sciences, 321, 280–284. [DOI] [PubMed] [Google Scholar]
- Ahmet, I. , Wan, R. , Mattson, M. P. , Lakatta, E. G. , & Talan, M. (2005). Cardioprotection by intermittent fasting in rats. Circulation, 112, 3115–3121. [DOI] [PubMed] [Google Scholar]
- Anversa, P. , Olivetti, G. , Melissari, M. , & Loud, A. V. (1980). Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. Journal of Molecular and Cellular Cardiology, 12, 781–795. [DOI] [PubMed] [Google Scholar]
- Bhatta, A. , Yao, L. , Xu, Z. , Toque, H. A. , Chen, J. , Atawia, R. T. , … Caldwell, R. W. (2017). Obesity‐induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovascular Research, 113, 1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan, S. , Nayak, N. C. , & Ramalingaswami, V. (1965). The heart and skeletal muscle in experimental protein malnutrition in rhesus monkeys. Journal of Pathology and Bacteriology, 90, 301–309. [DOI] [PubMed] [Google Scholar]
- Colman, R. J. , Anderson, R. M. , Johnson, S. C. , Kastman, E. K. , Kosmatka, K. J. , Beasley, T. M. , … Weindruch, R. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science, 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, A. A. , de Menezes, R. C. , Abreu, A. R. , Araujo, G. R. , Costa, D. C. , & Chianca, D. A., Jr . (2015). Increased α1‐adrenoreceptor activity is required to sustain blood pressure in female rats under food restriction. Life Sciences, 128, 55–63. [DOI] [PubMed] [Google Scholar]
- de Souza, A. M. A. , West, C. A. , de Abreu, A. R. R. , Pai, A. V. , Mesquita, L. B. T. , Ji, H. , … Sandberg, K. (2018). Role of the renin angiotensin system in blood pressure allostasis‐induced by severe food restriction in female Fischer rats. Scientific Reports, 8, 10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky, V. W. , Morton, J. S. , Oka, T. , Robillard‐Frayne, I. , Bagdan, M. , Lopaschuk, G. D. , … Dyck, J. R. (2010). Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension, 56, 412–421. [DOI] [PubMed] [Google Scholar]
- Dostal, D. E. (2001). Regulation of cardiac collagen: Angiotensin and cross‐talk with local growth factors. Hypertension, 37, 841–844. [DOI] [PubMed] [Google Scholar]
- Dunn, S. M. , Hilgers, R. , & Das, K. C. (2017). Decreased EDHF‐mediated relaxation is a major mechanism in endothelial dysfunction in resistance arteries in aged mice on prolonged high‐fat sucrose diet. Physiological Reports, 5, e13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant, L. M. , Khorram, O. , Buchholz, J. N. , & Pearce, W. J. (2014). Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: Effects of metyrapone. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 306, R401–R410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisler, J. S. (1992). Cardiac effects of starvation and semistarvation diets: Safety and mechanisms of action. American Journal of Clinical Nutrition, 56, 230S–234S. [DOI] [PubMed] [Google Scholar]
- Fontana, L. , & Partridge, L. (2015). Promoting health and longevity through diet: From model organisms to humans. Cell, 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudzune, K. A. , Doshi, R. S. , Mehta, A. K. , Chaudhry, Z. W. , Jacobs, D. K. , Vakil, R. M. , … Clark, J. M. (2015). Efficacy of commercial weight‐loss programs: An updated systematic review. Annals of Internal Medicine, 162, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan, N. , Maejima, Y. , Nakae, J. , Paik, J. , Depinho, R. A. , & Sadoshima, J. (2010). Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation‐induced autophagy in cardiac myocytes. Circulation Research, 107, 1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie, M. N. , Pegington, M. , Mattson, M. P. , Frystyk, J. , Dillon, B. , Evans, G. , … Howell, A. (2011). The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. International Journal of Obesity (2005), 35, 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner, J. M. , Sours, H. E. , Paris, A. L. , Ferrans, V. J. , & Roberts, W. C. (1979). Sudden, unexpected death in avid dieters using the liquid‐protein‐modified‐fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation, 60, 1401–1412. [DOI] [PubMed] [Google Scholar]
- Javeshghani, D. , Sairam, M. R. , Neves, M. F. , Schiffrin, E. L. , & Touyz, R. M. (2006). Angiotensin II induces vascular dysfunction without exacerbating blood pressure elevation in a mouse model of menopause‐associated hypertension. Journal of Hypertension, 24, 1365–1373. [DOI] [PubMed] [Google Scholar]
- Kagawa, Y. (1978). Impact of Westernization on the nutrition of Japanese: Changes in physique, cancer, longevity and centenarians. Preventive Medicine, 7, 205–217. [DOI] [PubMed] [Google Scholar]
- Ko, D. , Rahman, F. , Schnabel, R. B. , Yin, X. , Benjamin, E. J. , & Christophersen, I. E. (2016). Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nature Reviews. Cardiology, 13, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie, C. J. , Ozemek, C. , Carbone, S. , Katzmarzyk, P. T. , & Blair, S. N. (2019). Sedentary behavior, exercise, and cardiovascular health. Circulation Research, 124, 799–815. [DOI] [PubMed] [Google Scholar]
- Lee, S. R. , Ko, T. H. , Kim, H. K. , Marquez, J. , Ko, K. S. , Rhee, B. D. , & Han, J. (2015). Influence of starvation on heart contractility and corticosterone level in rats. Pflugers Archiv. European Journal of Physiology, 467, 2351–2360. [DOI] [PubMed] [Google Scholar]
- Li, X. , Sun, M. , Men, S. , Shi, Y. , Ma, L. , An, Y. , … Du, Z. (2018). The inflammatory transcription factor C/EBPβ plays a critical role in cardiac fibroblast differentiation and a rat model of cardiac fibrosis induced by autoimmune myocarditis. International Heart Journal, 59, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Lissner, L. , Andres, R. , Muller, D. C. , & Shimokata, H. (1990). Body weight variability in men: Metabolic rate, health and longevity. International Journal of Obesity, 14, 373–383. [PubMed] [Google Scholar]
- Ma, X. , Mani, K. , Liu, H. , Kovacs, A. , Murphy, J. T. , Foroughi, L. , … Diwan, A. (2019). Transcription factor EB activation rescues advanced αB‐crystallin mutation‐induced cardiomyopathy by normalizing desmin localization. Journal of the American Heart Association, 8, e010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa, Y. , Suleiman, M. , Guddeti, R. R. , Kwon, T. G. , Monahan, K. H. , Lerman, L. O. , … Lerman, A. (2016). Age‐dependent predictive value of endothelial dysfunction for arrhythmia recurrence following pulmonary vein isolation. Journal of the American Heart Association, 5, e003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison, J. A. , Roth, G. S. , Beasley, T. M. , Tilmont, E. M. , Handy, A. M. , Herbert, R. L. , … de Cabo, R. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature, 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight, K. A. , Rupp, H. , Beamish, R. E. , & Dhalla, N. S. (1996). Modification of catecholamine‐induced changes in heart function by food restriction in rats. Cardiovascular Drugs and Therapy, 10(Suppl 1), 239–246. [DOI] [PubMed] [Google Scholar]
- Mendez, J. , & Keys, A. (1960). Density and composition of mammalian muscle. Metabolism, 9, 184–188. [Google Scholar]
- Nunes, A. D. , Souza, A. P. , Macedo, L. M. , Alves, P. H. , Pedrino, G. R. , Colugnati, D. B. , … Castro, C. H. (2017). Influence of antihypertensive drugs on aortic and coronary effects of Ang‐(1‐7) in pressure‐overloaded rats. Brazilian Journal of Medical and Biological Research, 50, e5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoshi, M. P. , Okoshi, K. , Pai, V. D. , Pai‐Silva, M. D. , Matsubara, L. S. , & Cicogna, A. C. (2001). Mechanical, biochemical, and morphological changes in the heart from chronic food‐restricted rats. Canadian Journal of Physiology and Pharmacology, 79, 754–760. [PubMed] [Google Scholar]
- Palmer, B. F. , & Clegg, D. J. (2016). Physiology and pathophysiology of potassium homeostasis. Advances in Physiology Education, 40, 480–490. [DOI] [PubMed] [Google Scholar]
- Poggi, M. , Palareti, G. , Biagi, R. , Legnani, C. , Parenti, M. , Babini, A. C. , … Coccheri, S. (1994). Prolonged very low calorie diet in highly obese subjects reduces plasma viscosity and red cell aggregation but not fibrinogen. International Journal of Obesity and Related Metabolic Disorders, 18, 490–496. [PubMed] [Google Scholar]
- Polovina, M. M. , Lip, G. Y. , & Potpara, T. S. (2015). Endothelial (dys)function in lone atrial fibrillation. Current Pharmaceutical Design, 21, 622–645. [DOI] [PubMed] [Google Scholar]
- Qiu, H. Y. , Henrion, D. , & Levy, B. I. (1994). Endogenous angiotensin II enhances phenylephrine‐induced tone in hypertensive rats. Hypertension, 24, 317–321. [DOI] [PubMed] [Google Scholar]
- Rahman, A. , Jafry, S. , Jeejeebhoy, K. , Nagpal, A. D. , Pisani, B. , & Agarwala, R. (2016). Malnutrition and cachexia in heart failure. JPEN. Journal of Parenteral and Enteral Nutrition, 40, 475–486. [DOI] [PubMed] [Google Scholar]
- Ramalingaswami, V. (1968). Nutrition and the heart. Cardiologia, 52, 57–68. [DOI] [PubMed] [Google Scholar]
- Razzak, R. L. , Abu‐Hozaifa, B. M. , Bamosa, A. O. , & Ali, N. M. (2011). Assessment of enhanced endothelium‐dependent vasodilation by intermittent fasting in Wistar albino rats. Indian Journal of Physiology and Pharmacology, 55, 336–342. [PubMed] [Google Scholar]
- Rodrigo, E. , Maeso, R. , Muñoz‐García, R. , Navarro‐Cid, J. , Ruilope, L. M. , Cachofeiro, V. , & Lahera, V. (1997). Endothelial dysfunction in spontaneously hypertensive rats: Consequences of chronic treatment with losartan or captopril. Journal of Hypertension, 15, 613–618. [DOI] [PubMed] [Google Scholar]
- Roustit, M. , Loader, J. , Deusenbery, C. , Baltzis, D. , & Veves, A. (2016). Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. Journal of Clinical Endocrinology and Metabolism, 101, 3401–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden, C. T. , Schindelin, J. , Hiner, M. C. , DeZonia, B. E. , Walter, A. E. , Arena, E. T. , & Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajanti, J. , Björkstrand, A. S. , Finnilä, S. , Heikkinen, E. , Peltonen, J. , & Majamaa, K. (1999). Increase of collagen synthesis and deposition in the arachnoid and the dura following subarachnoid hemorrhage in the rat. Biochimica et Biophysica Acta, 1454, 209–216. [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. , … Cardona, A. (2012). Fiji: An open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitker, M. A. , Mattman, P. E. , & Bliss, T. L. (1951). A clinical study of malnutrition in Japanese prisoners of war. Annals of Internal Medicine, 35, 69–96. [DOI] [PubMed] [Google Scholar]
- Shaikh, A. Y. , Wang, N. , Yin, X. , Larson, M. G. , Vasan, R. S. , Hamburg, N. M. , … McManus, D. D. (2016). Relations of arterial stiffness and brachial flow‐mediated dilation with new‐onset atrial fibrillation: The Framingham Heart Study. Hypertension, 68, 590–596. [DOI] [PubMed] [Google Scholar]
- Strawn, W. B. , Gallagher, P. , Dean, R. H. , Ganten, D. , & Ferrario, C. M. (1997). Endothelial injury in transgenic (mRen‐2)27 hypertensive rats. American Journal of Hypertension, 10, 51–57. [DOI] [PubMed] [Google Scholar]
- Sugizaki, M. M. , Carvalho, R. F. , Aragon, F. F. , Padovani, C. R. , Okoshi, K. , Okoshi, M. P. , … Cicogna, A. C. (2005). Myocardial dysfunction induced by food restriction is related to morphological damage in normotensive middle‐aged rats. Journal of Biomedical Science, 12, 641–649. [DOI] [PubMed] [Google Scholar]
- Testa, G. , Biasi, F. , Poli, G. , & Chiarpotto, E. (2014). Calorie restriction and dietary restriction mimetics: A strategy for improving healthy aging and longevity. Current Pharmaceutical Design, 20, 2950–2977. [DOI] [PubMed] [Google Scholar]
- Tith, R. M. , Paradis, G. , Potter, B. J. , Low, N. , Healy‐Profitos, J. , He, S. , & Auger, N. (2019). Association of bulimia nervosa with long‐term risk of cardiovascular disease and mortality among women. JAMA Psychiatry, 77, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lueder, T. G. , Wang, B. H. , Kompa, A. R. , Huang, L. , Webb, R. , Jordaan, P. , … Krum, H. (2015). Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circulation. Heart failure, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- Wang, D. , Wang, C. , Wu, X. , Zheng, W. , Sandberg, K. , Ji, H. , … Wilcox, C. S. (2014). Endothelial dysfunction and enhanced contractility in microvessels from ovariectomized rats: Roles of oxidative stress and perivascular adipose tissue. Hypertension, 63, 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, J. G. , Kiess, M. C. , & Chan‐Yan, C. C. (1986). Malnutrition and the heart. CMAJ: Canadian Medical Association Journal, 135, 753–758. [PMC free article] [PubMed] [Google Scholar]
- West, C. A. , Shaw, S. , Sasser, J. M. , Fekete, A. , Alexander, T. , Cunningham, M. W., Jr. , … Baylis, C. (2013). Chronic vasodilation increases renal medullary PDE5A and α‐ENaC through independent renin‐angiotensin‐aldosterone system pathways. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 305, R1133–R1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipes, D. P. (2008). Heart–brain interactions in cardiac arrhythmias: Role of the autonomic nervous system. Cleveland Clinic Journal of Medicine, 75(Suppl 2), S94–S96. [DOI] [PubMed] [Google Scholar]
- Zuloaga, K. L. , Johnson, L. A. , Roese, N. E. , Marzulla, T. , Zhang, W. , Nie, X. , … Alkayed, N. J. (2016). High fat diet‐induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. Journal of Cerebral Blood Flow and Metabolism, 36, 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]


