Abstract
Aim
Comparing people with Type 2 diabetes mellitus with and without heart failure in terms of metabolic control, therapeutic regimen and comorbidities.
Methods
The Prospective Diabetes Registry (DPV) is a longitudinal documentation system for demographics, medical care and outcome in people with diabetes mellitus. It consists of follow‐up data from people with diabetes mellitus who have agreed to be recorded in the registry. Clinical data are submitted by general practitioners, specialists and clinics throughout Germany and Austria. Some 289 954 people with Type 2 diabetes mellitus (years 2000 to 2015) were analysed using demographic statistics and adjustment for confounders based on linear and logistic regression analysis.
Results
People with Type 2 diabetes mellitus (ICD code: E11) and heart failure (ICD code: I50) (N = 14 723) were older, more often women and presented with longer diabetes duration compared with those without heart failure. After adjustment for age, gender and diabetes duration, people with heart failure showed lower HbA1c, higher BMI and more intense insulin therapy. Analysis revealed that people with heart failure were more often treated with insulin, and more frequently received anti‐hypertensives and lipid‐lowering medication. They presented with lower systolic and diastolic BP. People with heart failure more frequently showed a history of comorbidities.
Conclusion
Heart failure is common in diabetes mellitus, but the prevalence in the DPV is lower frequent than expected. The reason for improved metabolic control in heart failure may be intensified therapy with insulin, lipid‐lowering medication and anti‐hypertensives in this cohort.
What's new?
Heart failure is a common comorbidity of diabetes mellitus. Prevalence data from intervention studies do not represent real‐world data because the people in studies are often pre‐specified with relation to several underlying diseases. For Germany, data on heart failure prevalence among people with Type 2 diabetes mellitus from large representative cohorts are currently unavailable. Data on diabetes treatment and therapeutic regimens regarding other comorbidities in people with Type 2 diabetes and heart failure are rare.
The Prospective Diabetes Registry (DPV) delivers data from nearly 290 000 people with Type 2 diabetes in specialized diabetes care and thus represents real‐world data.
The DPV provides data on clinical situation, diabetes treatment and therapeutic regimens regarding other comorbidities in people with Type 2 diabetes and heart failure from all over Germany.
The DPV is commonly used to document the real‐world treatment of people with diabetes and either heart failure or other comorbidities. The DPV clearly shows insufficient treatment of dyslipidaemia in people with diabetes and heart failure.
What's new?
Heart failure is a common comorbidity of diabetes mellitus. Prevalence data from intervention studies do not represent real‐world data because the people in studies are often pre‐specified with relation to several underlying diseases. For Germany, data on heart failure prevalence among people with Type 2 diabetes mellitus from large representative cohorts are currently unavailable. Data on diabetes treatment and therapeutic regimens regarding other comorbidities in people with Type 2 diabetes and heart failure are rare.
The Prospective Diabetes Registry (DPV) delivers data from nearly 290 000 people with Type 2 diabetes in specialized diabetes care and thus represents real‐world data.
The DPV provides data on clinical situation, diabetes treatment and therapeutic regimens regarding other comorbidities in people with Type 2 diabetes and heart failure from all over Germany.
The DPV is commonly used to document the real‐world treatment of people with diabetes and either heart failure or other comorbidities. The DPV clearly shows insufficient treatment of dyslipidaemia in people with diabetes and heart failure.
Introduction
Type 2 diabetes mellitus and heart failure are of growing interest in public health due to their high prevalence, high rate of hospitalization and mortality, late complications and comorbidity. Epidemiological data suggest an association between diabetes mellitus and heart failure. The Framingham Heart Study established diabetes mellitus as a risk factor for the development of heart failure 1. Pathogenic mechanisms are multimodal, and cardiovascular comorbidities play a central role, with people with diabetes presenting with elevated BP (1.5–2‐fold), a 2–4‐fold higher risk of coronary artery disease and a 34% higher risk of atrial fibrillation 2, 3. People with diabetes show an increased risk of myocardial infarction (+77%) and ischaemic stroke (+68%) 4. Disturbed calcium‐handling, activation of the renin–angiotensin–aldosterone system, exaggerated oxidative stress caused by hyperglycaemia and insulin resistance account for diabetes‐specific structural cardiac remodelling resulting in morphological and functional changes, and contributing to a failing heart 5, 6, 7. In addition, diabetic nephropathy and obesity are independent risk factors for heart failure 8, 9. Hyperglycaemic excursions have been proven to elevate the risk of heart failure, as shown in the United Kingdom Prospective Diabetes Study; a 1% elevation in HbA1c correlates with an 8% increase in heart failure risk 10. Taken together, these factors contribute to the 5‐fold increased risk of heart failure seen in people with Type 2 diabetes, compared with people without diabetes 11. In the Framingham Heart Study, women showed a 5‐fold risk elevation and men a 2.4‐fold risk elevation for heart failure if diabetes was present. Comparable with people without diabetes, heart failure in diabetes shows a correlation with older age; older people (72–76 years) present with a prevalence of 22%, whereas in younger people (64–68 years) the prevalence is ~ 11% 12.
People with heart failure are prone to higher diabetes risk. The prevalence of Type 2 diabetes ranges from 24% to 38% in heart failure studies like Olmsted County or Medicare beneficiaries, respectively. In decompensated heart failure (Acute Decompensated Heart Failure National Registry; ADHERE), the incidence of Type 2 diabetes mellitus rises to 44% 13, 14. The results of the Prospective Comparison of ARNI (Angiotensin Receptor Neprilysin Inhibitor) With ACEI (Angiotensin‐Converting Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) study unmask the real situation: 73% of study participants presented with impaired glucose metabolism (35% Type 2 diabetes, 13% with newly diagnosed Type 2 diabetes, 25% with prediabetes) 15. The combination of diabetes and heart failure is associated with a worse prognosis; people with Type 2 diabetes and heart failure present with a 10‐fold elevated mortality risk compared with those with heart failure, but without diabetes 16.
The aim of this study was to evaluate the prevalence of heart failure in a large cohort of people with Type 2 diabetes and to compare the characteristics, medical treatment and comorbidities of those with and without heart failure.
Participants and methods
The Prospective Diabetes Registry (Diabetes‐Patienten‐Verlaufsdokumentation; DPV), a longitudinal standardized documentation system for demographics, medical care and outcome in people with all diabetes types, was used for data evaluation. It consists of follow‐up data from more than 400 centres [general practitioners (GPs), specialists and clinics] throughout Germany and Austria, with one each in Luxembourg and Switzerland (http://buster.zibmt.uni-ulm.de/dpv/dateien/DPV-Flyer.pdf). The DPV is one of the largest registries in Europe for people with diabetes. Every 6 months, locally documented data are anonymized and analysed at the University of Ulm. The DPV Initiative was approved by the ethics committee of the University of Ulm (process number 202/09) and data collection was approved by local review boards. The DPV is thus representative of current German and German‐speaking diabetes care, and it is part of large cohort analyses worldwide like the Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT‐2 Inhibitors (CVD‐REAL) study 17 or analyses describing regional differences in diabetes treatment and outcomes in Germany 18. For the presented analysis, data from 289 954 adults with Type 2 diabetes treated between 2000 and 2015 were included. Classification of diabetes type and comorbidity was performed using ICD coding (E11 for Type 2 diabetes mellitus and I50 for heart failure status) by the treating physician. Diagnosis of heart failure followed evaluation of clinical signs and symptoms, or laboratory values such as brain natriuretic peptide. Variables included in the analysis were: demographic data (age, gender, diabetes duration, age at diabetes diagnosis), clinical data (HbA1c, lipid variables, liver enzymes, BMI, BP), medical treatment (lipid‐lowering medication, anti‐hypertensive and anti‐diabetic medication) as well as cardiovascular status and diabetic late complications. eGFR was calculated following the Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease 2012 (CKD‐EPI). For each individual, the last documented examination was taken into consideration.
Statistical analysis
People with diabetes were categorized by age (< 40, 40–59, 60–79, ≥ 80 years). Median values with lower (Q1) and upper quartiles (Q3) were calculated for continuous variables, categorical data like comorbidities and medical treatment were assessed as percentages. To compare the two groups of people, those with vs. those without heart failure, the χ2 test was used for categorical variables and the Wilcoxon rank sum test for continuous variables. Adjustment of P‐values for multiple comparisons was done using the false discovery rate 19. Following adjustment for age, gender and duration of diabetes between the groups, separate linear regression models were fitted for HbA1c, BMI, systolic and diastolic BP, insulin dose, age (< 40, 40–59, 60–79, ≥ 80 years) and diabetes duration (< 10, 10–19, 20–29, ≥ 30 years). To prevent over‐adjustment, the analysis includes these three variables as confounders only. Separate logistic regression models were applied to analyse the proportion of people treated with insulin, the frequency of anti‐hypertensive and lipid‐lowering medication, and comorbidities. Results of linear and logistic regression models were presented as adjusted estimates (LSmeans) with lower and upper confidence limits (95% CI).
For statistical analysis, SAS v. 9.4 (SAS Institute Inc., Cary, NC, USA) was used. All two‐sided P‐values < 0.01 were considered statistically significant.
Results
Demographic data and basic characteristics are summarized in Table 1. In total, 289 954 people (52% men) were available for analysis, median age was 70 years (Q1;Q3: 61;78 years), median age at diabetes diagnosis was 59 (49;69) years and median diabetes duration was 8 (3;15) years. The prevalence of heart failure based on ICD coding was 5.1%, and was closely correlated with age (Fig. 1).
Table 1.
Demographic data and clinical characteristics of the participants
| All | People without heart failure | People with heart failure | P‐valuea | ||||
|---|---|---|---|---|---|---|---|
| N | Median (Q1;Q3) | N | Median (Q1;Q3) | N | Median (Q1;Q3) | ||
| Men, % | 289 954 | 52 | 275 231 | 53 | 14 723 | 47 | < 0.0001 |
| Age (years) | 289 954 | 70 (61;78) | 275 231 | 70 (60;77) | 14 723 | 77 (70;83) | < 0.0001 |
| Age at diagnosis (years) | 289 954 | 59 (49;69) | 275 231 | 59 (49;68) | 14 723 | 65 (55;73) | < 0.0001 |
| Diabetes duration (years) | 289 954 | 8 (3;15) | 275 231 | 8 (3;14) | 14 723 | 11 (5;16) | < 0.0001 |
| BMI (kg/m2) | 255 983 | 30 (26;34) | 243 628 | 30 (26;34) | 12 355 | 30 (26;35) | > 0.05 |
| HbA1c (mmol/mol) | 258 478 | 54 (45;68) | 245 516 | 54 (45;69) | 12 962 | 54 (45;65) | < 0.0001 |
| HbA1c (%) | 258 478 | 7.1 (6.3;8.4) | 245 516 | 7.1 (6.3;8.4) | 12 962 | 7.1 (6.3;8.1) | < 0.0001 |
| Systolic BP (mmHg) | 265 184 | 132 (120;145) | 251 490 | 132 (120;145) | 13 694 | 130 (120;140) | < 0.0001 |
| Diastolic BP (mmHg) | 265 095 | 80 (70;80) | 251 406 | 80 (70;80) | 13 689 | 75 (70;80) | < 0.0001 |
| LDL‐cholesterol (mmol/l) | 173 652 | 2.8 (2.1;3.6) | 165 346 | 2.8 (2.2;3.6) | 8 306 | 2.6 (2.0;3.4) | < 0.0001 |
| HDL‐cholesterol (mmol/l) | 177 241 | 1.1 (1.0;1.4) | 168 915 | 1.1 (0.9;1.4) | 8 326 | 1.1 (0.9;1.4) | < 0.0001 |
| Triglycerides (mmol/l) | 192 287 | 1.8 (1.2;2.5) | 182 826 | 1.8 (1.2;2.6) | 9 461 | 1.6 (1.1;2.3) | < 0.0001 |
| Alanine aminotransferase (U/l) | 59 972 | 24 (17;37) | 54 402 | 24 (17;37) | 5 570 | 22 (15;32) | < 0.0001 |
| Gamma glutamyl‐transferase (U/l) | 61 355 | 38 (23;74) | 55 922 | 38 (23;72) | 5 433 | 46 (26;93) | < 0.0001 |
| eGFR (ml min−1 1.73 m−2)b | 247 535 | 68 (47;87) | 234 038 | 69 (49;88) | 13 497 | 48 (32;66) | < 0.0001 |
| Medication and comorbitity | |||||||
| All (N = 289 954) |
People without heart failure (N = 275 231) |
People with heart failure (N = 14 723) |
P‐valuea | ||||
| Prevalence (%) | Prevalence (%) | Prevalence (%) | < 0.0001 | ||||
| Lifestyle only | 24 | 24 | 19 | < 0.0001 | |||
| Oral antidiabetic drugs only | 26 | 26 | 20 | < 0.0001 | |||
| Insulin only | 30 | 29 | 42 | < 0.0001 | |||
| Oral antidiabetic drugs + Insulin | 20 | 31 | 18 | < 0.0001 | |||
| Oral antidiabetic drugs/GLP‐1 receptor agonist | 46 | 47 | 38 | < 0.0001 | |||
| Insulin | 51 | 50 | 61 | < 0.0001 | |||
| Anti‐hypertensives | 51 | 50 | 72 | < 0.0001 | |||
| Lipid‐lowering drugs | 26 | 25 | 33 | < 0.0001 | |||
| Coronary heart disease | 123 | 11 | 36 | < 0.0001 | |||
| Myocardial infarction | 9 | 9 | 15 | < 0.0001 | |||
Data are medians (Q1;Q3) unless otherwise indicated
Wilcoxon test for continuous variables, and chi‐square test for binary variables; P‐values adjusted for multiple comparisons by false discovery rate.
eGFR was calculated following the Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease 2012 (CKD EPI).
Figure 1.
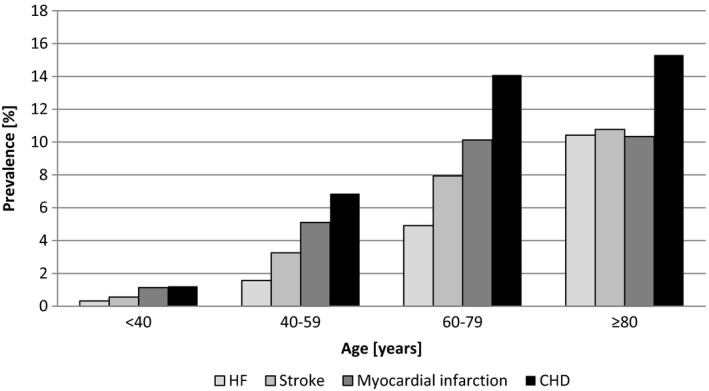
Prevalence of heart failure (HF) and other cardiovascular diseases (CVD) in the DPV.
Demographic, clinical and therapeutic data
People with heart failure were significantly older [median 76 (Q1;Q3: 70;83) vs. 70 (60;77) years), P < 0.0001], more often women (47% vs. 53%, P<0.0001), with longer diabetes duration [11 (5;16) vs. 8 (3;14) years, P < 0.0001] and higher age at diabetes diagnosis [65 (55;73) vs. 59 (49;68) years, P < 0.0001] (Table 1). For the age categories < 40, 40–59, 60–79 and ≥ 80 years, the proportion of women with heart failure was 48%, 33%, 46% and 67%, respectively, showing that the gender distribution of heart failure correlates strongly with increasing age and is reversed in people aged 80 or older. This accounts for the high number of women with heart failure in the registry.
Lower levels for LDL‐cholesterol [2.6 (2.0;3.4) vs. 2.8 (2.2;3.6) mmol/l] and triglycerides [1.6 (1.1;2.3) vs. 1.8 (1.2;2.6) mmol/l, P < 0.0001] were detected among people with heart failure. Regarding liver enzymes, people with heart failure presented with elevated values for gamma‐glutamyl transferase [46 (26;93) vs. 38 (23;72) U/l, P < 0.0001], but lower values for alanine aminotransferase [22 (15;32) vs. 24 (17;37) U/l, P < 0.0001] (Table 1).
Following adjustment for age, gender and diabetes duration, people with heart failure showed improved blood glucose control [HbA1c, mean 59 (95% CI 58–61) vs. 61 (60–62) mmol/mol; 7.6 (7.5–7.7)% vs. 7.7 (7.6–7.8)%, P < 0.0001]. Analysis revealed lower levels for systolic BP [134 (133–135) vs. 137 (136–137) mmHg, P < 0.0001] and diastolic BP [76 (76–77) vs. 78 (77–78) mmHg, P < 0.0001]. BMI was higher in people with heart failure [31.8 (31.7–32.0) vs. 30.4 (30.3–30.6) kg/m2, P < 0.0001] (Table 2). People with heart failure present with worse renal function [eGFR, CKD EPI: 58 (58–59) vs. 68 (67–69) ml min−1 1.73 m−2, P < 0.0001] (Table 2).
Table 2.
Data analyses adjusted for age, sex and diabetes duration
| People without heart failure (N = 275 231) Mean (lower mean–upper mean) | People with heart failure (N = 14 723) Mean (lower mean–upper mean) | P‐valuea | |
|---|---|---|---|
| HbA1c (mmol/mol) | 61 (60–62) | 59 (58–61) | < 0.0001 |
| HbA1c (%) | 7.7 (7.6–7.8) | 7.6 (7.5–7.7) | < 0.0001 |
| BMI (kg/m2) | 30.4 (30.3–30.6) | 31.8 (31.7–32.0) | < 0.0001 |
| Insulin dose (IU/kg body weight) | 0.59 (0.57–0.61) | 0.63 (0.60–0.67) | 0.0141 |
| Systolic BP (mmHg) | 137 (136–137) | 134 (133–135) | < 0.0001 |
| Diastolic BP (mmHg) | 78 (77–78) | 76 (76–77) | < 0.0001 |
| eGFR (ml min−1 1.73 m−2)b | 68 (67–69) | 58 (58–59) | < 0.0001 |
| Coronary heart disease (%) | 10 (10–11) | 32 (31–32) | < 0.0001 |
| Myocardial infarction (%) | 8 (8–8) | 13 (12–13) | < 0.0001 |
| Angina pectoris (%) | 0.5 (0.4–0.5) | 2 (2–2) | < 0.0001 |
| Stroke (%) | 6 (6–6) | 10 (10–11) | < 0.0001 |
| Diabetic foot (%) | 6 (5–6) | 11 (10–11) | < 0.0001 |
| Diabetic neuropathy (%) | 44 (44–44) | 46 (45–47) | < 0.0001 |
| Microalbuminuria (%) | 31 (31–31) | 29 (29–30) | < 0.0005 |
| Antihypertensives (%) | 50 (50–50) | 70 (69–70) | < 0.0001 |
| Lipid‐lowering drugs (%) | 25 (25–25) | 32 (31–33) | < 0.0001 |
| Insulin therapy (%) | 50 (50–51) | 58 (57–59) | < 0.0001 |
Linear and logistic regression models were applied to analyse the data, adjusted means with lower and upper confidence interval (95% CI) are given.
P‐values for continuous variables are from F‐tests from multiple regressions
eGFR was calculated following the Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease 2012 (CKD EPI).
Medical treatment
People with heart failure were treated more often with insulin (61% vs. 50%, P < 0.0001) and less frequently with oral anti‐diabetic drugs (OAD) alone or in combination with glucagon‐like peptide 1 (GLP‐1) receptor agonists (38% vs. 47%, P < 0.0001) or lifestyle intervention (19% vs. 24%, P < 0.0001) (Table 1). Younger people with heart failure (< 40 years) were treated with OAD (43%) more often than people without heart failure in the same group, for whom lifestyle interventions were more frequent (32%). Insulin therapy was used more often in people without heart failure than in people with heart failure in this age group (29% vs. 40%, P < 0.0001). All other people with heart failure were more often treated with insulin only, whereas in the non‐heart failure group, OAD and lifestyle intervention were common in all age groups (Fig. 2).
Figure 2.
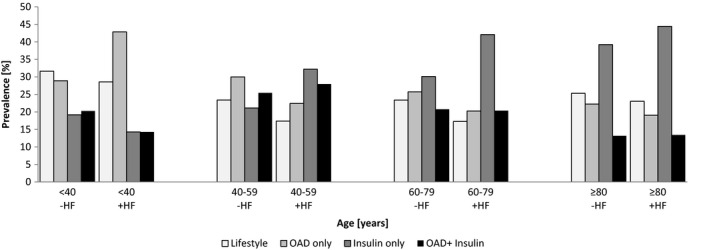
Anti‐diabetic treatment according to age and heart failure (HF) status. OAD, oral anti‐diabetic drug.
Adjusted analysis revealed that people with diabetes and heart failure received higher insulin doses than their counterparts without heart failure [mean 0.63 (95% CI 0.60–0.67) vs. 0.59 (0.57–0.61) IU/kg body weight, P = 0.0141]. Regarding treatment for cardiovascular comorbidities, people with heart failure more often received anti‐hypertensive treatment (70% vs. 50%), and lipid‐lowering medication (32% vs. 25%) (Table 2).
The most pronounced effect was seen in the youngest age group: 67% of these received anti‐hypertensive medication and only 33% lipid‐lowering treatment, compared with 21% and 10% in people without heart failure, respectively. The differences in the proportions who received anti‐hypertensive medication and lipid‐lowering treatment between people with heart failure or without heart failure were less profoundly different with increasing age categories (Fig. 3).
Figure 3.

Lipid‐lowering and anti‐hypertensive therapy in people with Type 2 diabetes according to age and heart failure (HF) status.
Comorbidities
Following adjustment for age, gender and diabetes duration, coronary artery disease (32% vs. 10%), myocardial infarction (13% vs. 8%), angina (2% vs. 0.5%) and stroke (10% vs. 6%) were present more often in people with heart failure compared with those without (P < 0.0001 for all variables; Table 2). The same applied to diabetic late complications like diabetic neuropathy (46% vs. 44%) and diabetic foot (11% vs. 6%, P < 0.0001 for all variables), but not for microalbuminuria, which was reported more often for people without heart failure (29% vs. 31%, P < 0.0005) (Table 2).
Discussion
The aim of our data analysis was to evaluate the prevalence and treatment of heart failure as well as comorbidities among a large Type 2 diabetes cohort. The DPV consists mainly of German and Austrian data. The prevalence of heart failure in the DPV was 5.1% and showed a significant association with age. Comparative data are available from the Framingham Study (prevalence of heart failure 2.5%, determined by clinical signs and symptoms, comparable with documentation in the DPV) and the National Health and Nutrition Examination Survey (prevalence of heart failure 1.9%, determined by individual's self‐reporting). In population‐based national registries such as the Kaiser Permanente Northwest Registry, 14.2% of the population with diabetes presented with heart failure, identified by heart failure‐related therapeutic regimen changes. An US‐based cohort of people with diabetes and age > 65 years pointed to a 22.3% prevalence of heart failure as assessed by the New York Heart Association (NYHA) state evaluation. Compared with these data, the prevalence of heart failure might be underestimated in the DPV. Reasons for this might be diagnosis criteria used, which in the DPV result from ICD coding of the disease only, without unselected screening, whereas in other cohorts, heart failure screening was done by discrete clinical evaluation.
Within the DPV, women are more often affected by heart failure, although the distribution between genders differed by age group. Up to age 79 years, the diagnosis was more frequent in men, from age 80 years, more women were diagnosed. This is in line with observations from other studies 20, 21.
Within the DPV, people with diabetes and heart failure are older and have longer diabetes duration. These facts were taken into account by adjusting for age and diabetes duration. People with heart failure presented with slightly better metabolic control in terms of glucose and cholesterol values. Intensified diabetes treatment of people with heart failure is obvious; insulin alone is the most frequent treatment option (42%), followed by OAD alone (20%) or OAD plus insulin (18%). People with heart failure receive significantly higher doses of insulin per kg body weight (1.1‐fold higher compared with those without heart failure when adjusted for age, sex and diabetes duration). People without heart failure are treated less often with insulin (29%), and more often with OAD (26%) or lifestyle intervention (24%).
Comorbidities may influence the therapeutic decision in heart failure. Diabetic nephropathy with reduced eGFR was frequent in people with heart failure, limiting the use of OAD. Also, in cases of heart failure, more stringent metabolic control is aimed for, resulting in more frequent use of insulin. Therefore, the better metabolic control in people with heart failure could be a result of more aggressive insulin use. In addition, heart failure is known to be closely correlated with underlying insulin resistance, and insulin resistance is a contributor to the development of heart failure, leading to increased insulin requirements 22. The causal relationship between heart failure and insulin requirement cannot be answered by this data evaluation. In a meta‐analysis of 37 229 people, Castagno et al. showed that intensive glycaemic control had no impact on the risk of heart failure in people with Type 2 diabetes 23. Udell and co‐workers found that glucose‐lowering drugs or strategies increased the risk of heart failure compared with standard care, and the magnitude of this effect varied with the method of glucose‐lowering used, with the highest risk being associated with the use of peroxisome proliferator‐activated receptor agonists followed by an intermediate risk with dipeptidyl peptidase‐4 inhibitors and no risk with insulin glargine 24. The positive association between glucose‐lowering therapy and increased risk of heart failure may be caused by therapy‐induced weight gain. Target‐based intensive glycaemic control strategies and intensive weight loss were also not associated with the development of heart failure.
In addition, people with Type 2 diabetes and heart failure in the DPV present with significantly lower levels of LDL‐cholesterol and lower levels of triglycerides. This is closely correlated with more intense lipid‐lowering therapy among people with heart failure. However, people with Type 2 diabetes do not reach the guideline recommendation of LDL < 2.6 mmol/l, or < 1.8 mmol/l if cardiovascular disease is present, and this shows clear and clinically relevant undertreatment with lipid‐lowering drugs.
Hypertension and BP control are critical in diabetes treatment. Up to 66% of the population with diabetes present with hypertension as a part of the metabolic syndrome 25. For risk reduction, the common ESC/EASD guideline suggests a BP target of < 140/85 mmHg. In the DPV, both groups on average fulfil the recommendation; people with heart failure, however, presented with significantly lower BP and greater use of anti‐hypertensive agents.
Regarding further components of the metabolic syndrome, obesity is of central influence in disease progression, both for diabetes and heart failure 3. Following the adjustment for age, gender and diabetes duration, people with heart failure presented with a significantly higher BMI, especially younger people. Obviously, BMI has a higher impact in these people.
The observed differences in both groups reach statistical significance due to the large numbers of people. In clinical routines, the differences (0.1% in HbA1c or 0.2 mmol/l in LDL cholesterol) are not considered major. But even if this is recognized, the analysis clearly shows that the comorbidity heart failure is not properly taken into account in diabetes treatment.
In several prospective studies, additional cardiovascular diseases turned out to be predictive of heart failure development and progression 26. In the DPV, after adjustment for age, gender and diabetes duration, nearly one third of people with heart failure showed coronary heart disease (3‐fold increase compared with those without heart failure), with 13% having had a myocardial infarction (1.6‐fold increase), 70% of people with heart failure present with medication for hypertension (1.4‐fold increase), and 10% were post stroke (1.6‐fold increase). The prevalence of comorbidities increased with age.
By contrast, other cardiovascular diseases occur more often at a younger age in people with heart failure. In total, diabetes seems have a greater impact in people with heart failure, with substantial harm to metabolism and the micro‐ and macrovascular system, as both micro‐ and macrovascular defects are significantly more often detected in people with heart failure.
Current data suggest that anti‐diabetic therapy needs to be individualized and comorbidities have to be treated earlier and more aggressively. Anti‐hyperglycaemic medications should be considered in terms of not only their blood sugar‐lowering properties, but also their cardiovascular safety 27, 28, 29, 30.
Limitations of the study
As with every real‐world data collection, the quality of the data relies strongly on data provision. In the DPV, GPs, diabetologists and clinics enter data upon evaluation. The data are therefore heterogeneous and disease diagnosis may correspond to signs and symptoms as well as specialized analyses like echocardiography or laboratory results. Most of the people in the DPV are followed regularly and the last documented data available for the described time span was taken into account in the analysis. The data are not influenced by any study‐related inclusion and exclusion criteria which results in a real‐world scenario.
Given the large number of people, even small – clinical irrelevant – differences reach statistical significance. The comorbidity panel is highly different in the heart failure cohort, as is the occurrence of treatment for hypertension and dyslipidaemia, but treatment results in terms of vital and laboratory parameters are similar and do not reach guideline recommendations. Therefore, the obvious weakness of registry data turns out to be a strong signal towards the need for control of therapeutic success.
The authors are aware that the statistical analysis adjusts only for age, gender and diabetes duration, and that other age‐dependent confounders like renal function may influence the analysis. To not over‐adjust with other age‐dependent confounders, we decided to use only the very basic variables into the statistic model.
Conclusion
The prevalence of diagnosed and documented heart failure by ICD coding in the DPV is 5.1% and might be underestimated if compared with other national registries or studies. Heart failure prevalence potentiates with increasing age. As expected, people with diabetes mellitus and heart failure are older, multimorbid and present with more diabetes‐associated late complications. With regard to this, multifaceted and more aggressive treatments are required and actually used among the critical ill people, resulting in better metabolic control in terms of glucose and lipid control as well as BP.
Funding sources
The study was financially supported by the Federal Ministry of Education and Research within the German Competence Network for Diabetes mellitus (grant number: 01GI1106) which is integrated in the German Centre for Diabetes Research (DZD, FKZ82DZD14A02) as of January 2015. The DPV registry receives additional support by the European Foundation for the Study of Diabetes (EFSD) www.europeandiabetesfoundation.org. Further financial support was provided by the German Diabetes Society (DDG) www.deutsche-diabetes-gesellschaft.de. A geographical listing of sites contributing data for the DPV is available at http://www.d-p-v.eu.
Competing interests
None declared.
Acknowledgments
We thank all participating centres of the DPV initiatives, especially those who collaborated in this investigation. Special thanks to A. Hungele and R. Ranz for support and the development of the DPV documentation software and K. Fink and E. Bollow for the DPV data management (clinical data managers and software developers, Ulm University, Germany).
Author contributions
D.S. and B.S. wrote the manuscript and researched data. D.T. contributed to the discussion and reviewed/edited the manuscript. R.W.H. performed statistical analysis. A.S., R.W.H., N.H., S.M, H.J.Z. researched data and contributed to discussion and reviewed/edited the manuscript.
Diabet. Med. 37: 1291–1298(2020)
References
- 1. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 2. Stratmann B, Tschoepe D. Heart in diabetes: not only a macrovascular disease. Diabetes Care 2011; 34(Suppl 2): S138–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus ‐ mechanisms, management, and clinical considerations. Circulation 2016; 133: 2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Van Belle E et al. Impact of diabetes mellitus on long‐term survival in patients with congestive heart failure. Eur Heart J 2004; 25: 656–662. [DOI] [PubMed] [Google Scholar]
- 5. Cesario DA, Brar R, Shivkumar K. Alterations in ion channel physiology in diabetic cardiomyopathy. Endocrinol Metab Clin North Am 2006; 35: 601–610. [DOI] [PubMed] [Google Scholar]
- 6. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004; 25: 543–567. [DOI] [PubMed] [Google Scholar]
- 7. Stuhlmann GI. Cellular mechanism of insulin resistance. J Clin Invest 2000; 106: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL et al. CHARM Investigators and Committees . Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374: 543–550. [DOI] [PubMed] [Google Scholar]
- 9. Kenchiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 2009; 119: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001; 103: 2668–2673. [DOI] [PubMed] [Google Scholar]
- 11. Halle M, Gitt AK, Hanefeld M, Kellerer M, Marx N, Meier JJ et al. Diabetes and heart failure: a practically oriented critical appraisal. Dtsch Med Wochenschr 2012; 137: 437–441. [DOI] [PubMed] [Google Scholar]
- 12. Zhou L, Deng W, Zhou L, Fang P, He D, Zhang W et al. Prevalence, incidence and risk factors of chronic heart failure in the type 2 diabetic population: systematic review. Curr Diabetes Rev 2009; 5: 171–184. [DOI] [PubMed] [Google Scholar]
- 13. From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ et al. Diabetes in heart failure: Prevalence and impact on outcome in the population. Am J Med 2006; 119: 591–599. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 15. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B et al. Risk related to pre‐diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 2016; 9e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 17. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL Study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation 2017; 136: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartmann B, Bramlage P, Lanzinger S, Danne T, Hummel M, Kaltheuner M et al. Regional differences in type 2 diabetes treatment and outcomes in Germany – an analysis of the German DPV und DIVE registries. Diabetes Metab Res Rev 2018; e3049. [DOI] [PubMed] [Google Scholar]
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57: 289–300. [Google Scholar]
- 20. Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A et al. Prevalence of heart failure and left ventricular dysfunction in the general population: the Rotterdam Study. Eur Heart J 1999; 20: 447–455. [PubMed] [Google Scholar]
- 21. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 22. Knops M, Doehner W. Insulinresistenz bei Herzinsuffizienz: Pathophysiologie, Messmethoden, Therapie. Diabetes Stoffw Herz 2012; 21: 295–306. [Google Scholar]
- 23. Castagno D, Baird‐Gunning J, Jhund PS, Biondi‐Zoccai G, MacDonald MR, Petrie MC et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta‐analysis. Am Heart J 2011; 162: 938–948. [DOI] [PubMed] [Google Scholar]
- 24. Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose‐lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta‐analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015; 3: 356–366. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson PM, Cederholm J, Zethelius BR, Eliasson BR, Eeg‐Olofsson K, Gudbj Rnsdottir S. Trends in blood pressure control in patients with type 2 diabetes: data from the Swedish National Diabetes Register (NDR). Blood Press 2011; 20: 348–354. [DOI] [PubMed] [Google Scholar]
- 26. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999; 159: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 27. Schneider CA, Pfister R, Erdmann E. Diabetes and heart failure. Herz 2010; 35: 140–146. [DOI] [PubMed] [Google Scholar]
- 28. Scheen AJ. Cardiovascular effects of new oral glucose‐lowering agents: DPP‐4 and SGLT‐2 inhibitors. Circ Res 2018; 122: 1439–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol 2018; 6: 105–113. [DOI] [PubMed] [Google Scholar]
- 30. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019;393: 31–39. [DOI] [PubMed] [Google Scholar]


