Abstract
The clinical importance of subclinical, early T cell–mediated rejection (Banff TCMR 1A and borderline lesions) remains unclear, due, in part to the fact that histologic lesions used to characterize early TCMR can be nonspecific. Donor‐derived cell‐free DNA (dd‐cfDNA) is an important molecular marker of active graft injury. Over a study period from June 2017 to May 2019, we assessed clinical outcomes in 79 patients diagnosed with TCMR 1A/borderline rejection across 11 US centers with a simultaneous measurement of dd‐cfDNA. Forty‐two patients had elevated dd‐cfDNA (≥0.5%) and 37 patients had low levels (<0.5%). Elevated levels of dd‐cfDNA predicted adverse clinical outcomes: among patients with elevated cfDNA, estimated glomerular filtration rate declined by 8.5% (interquartile rate [IQR] −16.22% to −1.39%) (−3.50 mL/min/1.73 m2 IQR −8.00 to −1.00) vs 0% (−4.92%, 4.76%) in low dd‐cfDNA patients (P = .004), de novo donor‐specific antibody formation was seen in 40% (17/42) vs 2.7% (P < .0001), and future or persistent rejection occurred in 9 of 42 patients (21.4%) vs 0% (P = .003). The use of dd‐cfDNA may complement the Banff classification and to risk stratify patients with borderline/TCMR 1A identified on biopsy.
Keywords: biomarker, cellular transplantation (non‐islet), clinical research/practice, kidney (allograft) function/dysfunction, kidney failure/injury, monitoring: immune, rejection: T cell mediated (TCMR)
Short abstract
Among patients with borderline and 1A T cell–mediated rejection, a threshold of ≥ 0.5% of donor‐derived cell‐free DNA was associated with increased risk of renal function decline, donor‐specific antibody development, and future episodes of recurrent rejection.
Abbreviations
- ABMR
antibody‐mediated rejection
- C4d
complement 4d
- CXCL9
chemokine (C‐X‐C motif) ligand 9
- dd‐cfDNA
donor‐derived cell‐free DNA
- DNA
deoxyribonucleic acid
- DSA
donor‐specific antibodies
- eGFR
estimated glomerular filtration rate
- FOXP3
forkhead Box p3
- g
glomerulitis
- i
inflammation
- ptc
peritubular capillaritis
- RCV
reference change value
- t
tubulitis
- TCMR
T cell–mediated rejection
- v
vasculitis
1. INTRODUCTION
Banff lesion scores assess the presence and the degree of histopathological changes of renal transplant biopsies, focusing primarily, but not exclusively, on the diagnostic features seen in rejection. These lesion scores may not always be sufficient to reach the Banff diagnostic categories, where additional diagnostic parameters such as histopathological, molecular, serological, and/or clinical markers could help to reach a diagnosis.1
Histologic lesions used to characterize T cell–mediated rejection (TCMR) can also be nonspecific. For example, inflammation‐ (i‐) and tubulitis‐ (t‐)lesions are seen in many other diseases, including acute kidney injury, antibody‐mediated rejection (ABMR), glomerulonephritis, and vasculitis (v), with v‐lesions also occurring in ABMR alone.1, 2
Histological manifestations of acute TCMR are characterized by tubulitis with interstitial inflammatory cell infiltration and arteritis in severe forms. The infiltration of activated T lymphocytes and macrophages occurs into a mildly edematous interstitial lesion and into the tubules, so‐called tubulointerstitial cellular rejection (Banff category 4, type I). In the latter, another major finding is the infiltration of mononuclear cells in the enlarged and activated arterial endothelium, so‐called transplant endarteritis (Banff category 4, type II or III).3
The complexity of pathological interpretation of biopsies especially with varying aspects of TCMR has resulted in some ambiguity around the histologic diagnosis of TCMR.2 Central pathology assessment is often recommended to address inter‐variability of interpretation; however, real‐life clinical practice does not centralize pathology, with many centers using “in house” services to build single‐center experiences and compare outcomes and metrics based on localized pathology reads.
TCMR 1A and borderline diagnoses often overlap; some borderlines behave as rejection and some TCMR do not. Borderline rejection is defined as changes on Banff histology that are not sufficient to diagnose rejection, yet Nankivell et al recently showed that borderline TCMR is associated with inferior graft outcomes despite resolution of inflammatory infiltrates on subsequent biopsies.4 Having a more quantitative method of assessing injury may complement histology and help to identify cases that require further intervention.
Currently, three categories of TCMR histologic diagnosis are described: TCMR, borderline (inflammation less than required for TCMR), and others (neither TCMR nor borderline). Subclasses have been added to the histologic diagnosis of TCMR to provide greater delineation: IA, IB, IIA, IIB, and III (with IIA, IIB, and III based on the assumption that all v‐lesions indicate TCMR).5 As we consider molecular markers of graft injury and rejection, understanding how these newer technologies may complement Banff and support the complex interpretation of histopathology is important.6
The approximate frequencies of the different patterns of acute TCMR include 45%‐70% tubulo‐interstitial, 30%‐55% arteritis, and 2%‐4% glomerular, but these are not used specifically for the categorization of rejection in the Banff 2013 classification.7 Notably, between 20% and 40% of acute TCMR cases also show complement 4d (C4d) positivity along with peritubular capillaritis (ptc), providing evidence of concurrent antibody‐mediated injury.8 The intensity of the interstitial infiltrate or tubulitis has been shown to have no correlation with the severity of the rejection episode,9 with some untreated borderline cases progressing to frank rejection. It has also been suggested that not all interstitial infiltrate is TCMR and so delineating the true pathological features of acute cellular rejection has clear prognostic significance.10, 11
Donor‐derived cell‐free DNA (dd‐cfDNA; AlloSure, CareDx, Brisbane, CA), detected in the blood of transplant recipients, has been proposed as a noninvasive marker of graft injury, which can be caused by TCMR, ABMR, and a number of other pathologies. Importantly, the detection of dd‐cfDNA is not only a simple reflection of tissue injury, but also cellular turnover; in this way, biochemical changes occurring earlier than cell death can also reflect changes in dd‐cfDNA.12 From the moment the allograft is implanted there is a continuous release of allograft donor material into the recipient circulation, which is also cleared continuously. In this way, dd‐cfDNA is a constant and dynamic marker, allowing the assessment of trends, early injury identification and severity.
Early dd‐cfDNA studies identified that not all TCMR diagnoses were associated with an elevation of dd‐cfDNA and were criticized for missing some cases of TCMR 1A.13, 14 However, could dd‐cfDNA be differentiating which TCMR 1As are true active rejection episodes with injury and which may be benign infiltrate without injury? 7
The objective of this study is to test the hypothesis that TCMR 1A and borderline rejection are a heterogeneous cohort which may have variable impact on outcomes and that dd‐cfDNA may help to risk stratify patients and better differentiate which TCMR 1A or borderline cases may progress. Additionally, we aimed to consider the value of injury when assessing histopathology diagnoses described as active rejection, considering that the presence of inflammatory infiltrate is not always concurrent with active and ongoing cellular injury.4 We hypothesize the use of dd‐cfDNA as a complementary tool may support histopathology and potentially improve clinical decision‐making, when assessing whether the changes seen on histopathology are clinically significant.15, 16
2. METHODS
Between June 2017 and May 2019; 79 patients across 11 transplant centers were prospectively surveyed with dd‐cfDNA as part of their standard of care and underwent either surveillance biopsy or biopsies performed for cause. Dd‐cfDNA was measured prior to the event and at time of event. Fifty‐two patients were diagnosed with TCMR 1A based on Banff 2017 criteria (i2,t2, g0, ptc0, C4d negative) and 27 patients were diagnosed with borderline having Banff scores of i1, t1, g0, ptc0, C4d negative, and v0 for 15 patients; and the remaining 12 had scores of i2, t1, and v0 (i = interstitial infiltrate, t = tubulitis, g = glomerulitis, ptc = peritubular capillaritis and v = vasculitis). Patients with other concomitant diagnoses, such as delayed graft function (DGF), calcineurin inhibitor (CNI) toxicity, glomerulopathy or infections such BK or others were excluded to ensure no other cause of injury was causing changes in dd‐cfDNA. Biospies were read locally not centrally, consistent with a real life, unblinded study. All patients were treated with pulsed intravenous steroids, the decision to treat the biopsy was per center clinical protocol, not study mandated, and all patients received the same treatment. Estimated glomerular filtration rate (eGFR) changes, formation of donor‐specific antibodies (DSA), and future rejection events were captured, with dd‐cfDNA levels recorded, before, during and after the event. DSA testing was done prior to the event (all 79 patients were DSA negative), at the time of the event, as well as 1, 3, and 6 months postevent. This was done uniformally and not different between the dd‐cfDNA groups. Routine blood work for creatinine and eGFR was performed more frequently (monthly) and follow‐up biopsy was done for cause in both groups.
2.1. Blood samples and dd‐cfDNA measurements
Venous blood was collected in Streck Cell‐Free DNA blood collection tubes and shipped to the central Clinical Laboratories Improvements Act‐certified laboratory at CareDx, Inc. Details of the standardized specimen processing and analytical methods to determine the percentage of dd‐cfDNA (AlloSure®) have been published.17 The targeted next‐generation sequencing assay employs highly polymorphic single nucleotide polymorphisms to quantify dd‐cfDNA without need for separate genotyping of the recipient or the donor. All measurements were performed by laboratory technicians unaware of the clinical identity of the samples.
2.2. Diagnosis of graft dysfunction and of biopsy‐defined rejection
Information was collected on the number of and clinical indication for renal biopsies for each patient. Biopsies were graded locally according to the Banff 2017 classification scheme for TCMR. Banff lesions were evaluated and no difference was seen between groups. BK viremia and other infections were ruled out with no other concomitant diagnoses given. Biopsy interpretation was performed by the pathologist at the participating transplant center.
2.3. Statistical analyses
Descriptive statistics are used to describe the patient demographics and distribution of dd‐cfDNA measurements obtained from blood samples at the time of rejection diagnosis. A threshold was established to categorize high dd‐cfDNA scores indicating injury vs low dd‐cfDNA scores demonstrating no injury based upon the distribution of data. Comparisons between the high and low dd‐cfDNA groupings were statistically evaluated via Fisher's exact test for categorical variables and Student's t test for continuous variables. Nonparametric comparisons of dd‐cfDNA cumulative distributions between dichotomized groupings were evaluated via Kolmogorov‐Smirnov two‐sample tests. Univariate and multivariate logistic regression methods were used to determine whether univariate analyses were robust when potential confounding factors were included in the models predicting appearance of de novo DSAs, recurrent rejection and greater than a 10% decline in eGFR 6 months post event. Potential confounding factors included patient demographics, diagnosis (TCMR 1A or borderline based on Banff lesions), days posttransplant, biopsy reason (for cause or protocol), and eGFR at the time of AlloSure collection (see Tables S1 and S2 for full model details).
2.4. Patient demographics
Patient demographics stratified by dd‐cfDNA measurement at the time of diagnosis are shown in Table 1. Indications for “for‐cause” biopsy included change in creatinine and change in tacrolimus level, no changes in proteinuria or DSA were found to indicate biopsy. Demographic characteristics were not significantly imbalanced between the groups, other than age, where patients with lower dd‐cfDNA measurements tended to be older than those with high measurements, mean ages were 49.8 and 44.1 years, respectively (P = .0283).
Table 1.
Patient characteristics
| Characteristic | Low donor‐derived cell‐free DNA (dd‐cfDNA) (<0.5) | High dd‐cfDNA (≥0.5) | P value | |
|---|---|---|---|---|
| Age (y) | N | 37 | 42 | .028 |
| Mean (SD) | 49.8 (10.46) | 44.1 (12.14) | ||
| Median (Q1, Q3) | 48 (44, 56) | 43 (36, 55) | ||
| Min, Max | 29, 77 | 23, 69 | ||
| Height (cm) | N | 13 | 17 | .952 |
| Mean (SD) | 165.8 (32.37) | 165.2 (27.78) | ||
| Median (Q1, Q3) | 175 (169, 188) | 172.7 (160, 178) | ||
| Min, Max | 62, 188 | 66, 189 | ||
| Weight (kg) | N | 13 | 17 | .279 |
| Mean (SD) | 96.7 (27.31) | 86.3 (24.06) | ||
| Median (Q1, Q3) | 94.3 (77, 110) | 81 (72, 92) | ||
| Min, Max | 64, 155 | 55, 160 | ||
| Estimated glomerular filtration rate at diagnosis of TCMR1A (mL/min/1.73 m2) | N | 37 | 42 | .340 |
| Mean (SD) | 46.3 (15.18) | 49.7 (14.53) | ||
| Median (Q1, Q3) | 48 (33, 57) | 49 (40, 59) | ||
| Min, Max | 9, 79 | 11, 84 | ||
| Race | White | 29 (78.4%) | 31 (73.8%) | .428 |
| African American | 7 (18.9%) | 11 (26.2%) | ||
| Asian | 1 (2.7%) | 0 | ||
| Gender | Male | 22 (59.5%) | 17 (40.5%) | .117 |
| Female | 15 (40.5%) | 25 (59.5%) | ||
| Kidney disease | Congenital or familial disorders | 1 (7.7%) | 1 (6.7%) | .945 |
| FSGS | 2 (15.4%) | 1 (6.7%) | ||
| Glomerular disease | 1 (7.7%) | 2 (13.3%) | ||
| Hypertensive nephrosclerosis | 4 (30.8%) | 3 (20.0%) | ||
| Neoplasms | 0 | 1 (6.7%) | ||
| Other | 0 | 1 (6.7%) | ||
| PKD | 0 | 2 (13.3%) | ||
| Tubular and interstitial disease | 1 (7.7%) | 0 | ||
| Type II diabetes | 1 (7.7%) | 1 (6.7%) | ||
| Biopsy | For cause | 17 (45.9%) | 21 (50%) | .647 |
| Protocol | 20 (54.1%) | 21 (50%) |
Abbreviations: FSGS, focal segmental glomerulosclerosis; PKD, polycystic kidney disease; TCMR, T cell–mediated rejection.
3. RESULTS
Figure 1 displays a violin plot, where the median dd‐cfDNA level prior to biopsy was 0.19% (interquartile rate [IQR] 0.19‐0.22). At the time of rejection diagnosis dd‐cfDNA measurements are extremely right‐skewed. The plot shows demarcations at 1%, 0.74%, and 0.5% dd‐cfDNA, with the largest demarcation based on this sample observed at the lower threshold of 0.5%.
Figure 1.
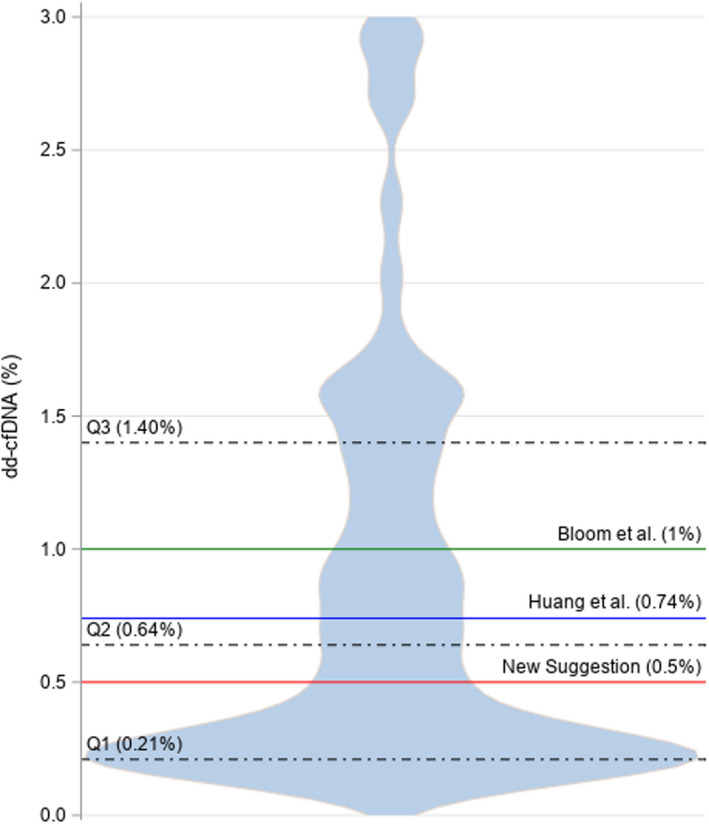
Violin plot shows the probability density at different values smoothed by a kernel density estimator, where the donor‐derived cell‐free DNA (dd‐cfDNA) measurements obtained from the 52 patients diagnosed with T cell–mediated rejection 1A and 27 borderline patients are displayed with IQR and previously published thresholds of clinically significant dd‐cfDNA levels as well as the recommended significant threshold based on the distribution of the data
No significant difference in the distribution of dd‐cfDNA measurements was observed between biopsy type: protocol vs “for‐cause” indication biopsies (P = .7307) or borderline vs TCMR 1A cases (P = .6059; Figure 2). No significant difference was seen across centers with regard to the proportion of high low dd‐cfDNA results P = .56.
Figure 2.

Donor‐derived cell‐free DNA (dd‐cfDNA) distributions. TCMR, T cell–mediated rejection [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3 displays the cumulative distribution functions of the percent change in eGFR from diagnosis of rejection to the next eGFR for the low and high dd‐cfDNA groups. Table 2 provides the descriptive statistics for the percent change in eGFR as well as the absolute eGFR. The low dd‐cfDNA group shows insignificant decline in eGFR 6 months after diagnosis and treatment of rejection (mean = 0.40%, SD = 18.15%, median = 0%), where two subjects demonstrated significant increase (both ≤40%). The high dd‐cfDNA group shows a significantly greater decline in eGFR in comparison (Kolmogorov‐Smirnov two‐sample test P = .0040), having a median decline of 8.5% (mean = 8.54%, SD = 14.98%) 6 months after diagnosis of rejection except in 1 subject who demonstrated a significant eGFR improvement of approximately 33%. Multivariate logistic regression analyses were undertaken to determine whether other factors, beyond dd‐cfDNA grouping, may have contributed to percent change in eGFR >10%. None of the potential confounding factors (Tables S1 and S2) resulted in a relevant difference in the association of significant declines in eGFR and dd‐cfDNA grouping when comparing the univariate logistic regression model to the multivariate model.
Figure 3.
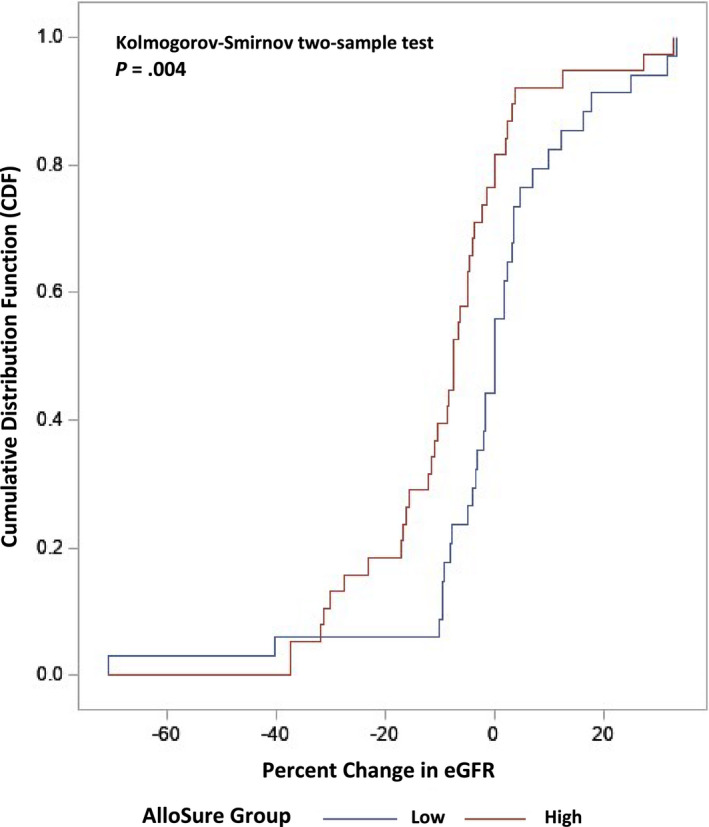
Cumulative distribution functions of the percent change in estimated glomerular filtration rate (eGFR) from diagnosis of T cell–mediated rejection (TCMR) 1A and borderline up to 6 months after event [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Summary statistics for high vs low donor‐derived cell‐free DNA (dd‐cfDNA)
| Measurement | Statistics | All patients | Low (dd‐cfDNA < 0.5%) | High (dd‐cfDNA ≥ 0.5%) | P value |
|---|---|---|---|---|---|
| dd‐cfDNA measurements a (%) | N | 79 | 37 | 42 | ‐ |
| Mean (SD) | 1.05 (1.267) | 0.25 (0.087) | 1.76 (1.40) | ||
| Median (Q1, Q3) | 0.64 (0.21, 1.40) | 0.21 (0.19, 0.29) | 1.40 (0.87, 2.02) | ||
| Min, Max | 0.19, 6.70 | 0.19, 0.49 | 0.52, 6.70 | ||
| Change in estimated glomerular filtration rate (eGFR; 3‐6 mo) | N | 79 | 37 | 42 | .019 b |
| Mean (SD) | −2.85 (7.526) | −0.74 (7.369) | −4.74 (7.247) | ||
| Median (Q1, Q3) | −2.00 (−5.00, 1.00) | 0.00 (−3.00, 2.00) | −3.50 (−8.00, −1.00) | ||
| Min, Max | −29.00, 16.00 | −29.00, 12.00 | −20.00, 16.00 | ||
| % Change in eGFR (3‐6 mo) | N | 79 | 37 | 42 | .004 b |
| Mean (SD) | −4.70 (16.937) | −0.40 (18.149) | −8.54 (14.98) | ||
| Median (Q1, Q3) | −3.89 (−9.89, 2.33) | 0.00 (−4.92, 4.76) | −8.50 (−16.22, −1.39) | ||
| Min, Max | −70.73, 33.33 | −70.73, 33.33 | −37.50, 32.65 | ||
| Presence of donor‐specific antibodies (3‐6 mo) | Percentage | 18/79 (22.9%) | 1/37 (2.7%) | 17/42 (40.5%) | <.001 c |
| Recurrent rejection | Percentage | 9/79 (11.4%) | 0/37 (0.0%) | 9/42 (21.4%) | .003 c |
0.19 is the numerical value used for dd‐cfDNA measurements denoted by <0.19.
Kolmogorov‐Smirnov two‐sample test (high vs low).
Fisher's exact test (high vs low).
One subject (3%) in the low dd‐cfDNA group and 17 subjects (41%) in the high dd‐cfDNA group had de‐novo DSAs develop after their rejection diagnosis (Table 2). A significant difference between the groups regarding the presence of new DSAs and the elevation of dd‐cfDNA was observed (P < .0001). Multivariate logistic regression analyses were used to determine whether additional factors, beyond dd‐cfDNA grouping, may have contributed to the statistical significance observed. None of the potential confounding factors (Tables S1 and S2) resulted in a meaningful difference in the effect of dd‐cfDNA grouping when comparing the univariate logistic regression model to the multivariate model.
There were no subjects in the low dd‐cfDNA group and nine subjects (21%) in the high dd‐cfDNA group that experienced a further episode of rejection (Table 2) (P = .0028). This indicates further rejection is more likely when higher dd‐cfDNA scores are reported. The type of rejection observed included three cases of ABMR and six cases of TCMR ≥1b.
4. DISCUSSION
Banff provides international consensus on renal transplant biopsy reporting and guidance for clinical diagnosis. These criteria allow the renal transplant community to assess current science advances and practice of kidney transplantation. Here we show the ability to support its interpretation with the additional consideration of dd‐cfDNA.
With i‐ and t‐lesions remaining the hallmark of TCMR/borderline diagnoses, conventional renal biopsy assessment alone can sometimes lead to subjective reports and interobserver disagreement.2, 5 Many inflamed biopsies labeled as TCMR 1A or borderline reflect uncertainty, as lesions are not specific and occur in other conditions.9 With the field of transplantation evolving from a “one‐size‐fits‐all” approach toward precision medicine, allowing potential tailoring of therapeutic options, having a patient‐specific strategy is possible only when also considering the molecular characterization of the allograft through biomarkers such dd‐cfDNA to syngergistically complement targeted tissue biopsy.
The results demonstrate that not all TCMR 1A/borderline rejections are equal and that clinically adverse outcomes are associated with elevated dd‐cfDNA levels, despite all patients being managed the same way. The skewness of the violin plot may represent the differentiation seen in the variation of injury, with demarcations seen at 1% and 0.74% being previously published 13, 14 as thresholds for defining rejection. The median dd‐cfDNA level prior to biopsy was 0.19% supporting the baseline previous published using a reference population.18
Considering the strength of this pattern, it is important to note that no statistical differences between the dd‐cfDNA distributions when stratified by protocol vs for‐cause biopsies (P = .7307) or TCMR 1A vs borderline (P = .6059) observed (Figure 2). It may be considered that rejection with low dd‐cfDNA was successfully treated whereas in those with high dd‐cfDNA it was not, as an alternative explanation. However, suggesting half of samples in this series had steroid resistant rejection is unlikely.
Surveillance biopsy data suggest that 20%‐25% of biopsies are positive for subclinical pathology, much of which is infiltration, inflammation, and mild subclinical rejection.19 Yet any outcome benefit for centers performing surveillance biopsies remain equivocal vs centers that perform for‐cause biopsy,20 suggesting that the clinical significance of this pathology remains uncertain and likely not all infiltrates seen on histopathology indicate rejection. We hypothesize that dd‐cfDNA may help to risk stratify borderline and TCMR 1A and could help support biopsies that remain ambiguous based on mild infiltration but have no active tissue injury.2
When considering the heterogeneity of the sample, central review of pathology rather than locally assessed diagnoses may reduce potential intersite variability. However, most centers use “in‐house” pathology building single‐center experiences and so with real‐life clinical practice not routinely centralizing pathology, employing dd‐cfDNA to support standardization and complement risk stratification we believe is a useful addition.
Another limitation is that induction therapy, maintenance medications, and treatment of rejection were not standardized, with centers treating patients according to local practice. However, the majority of centers followed similar protocols, and the distribution of low and high dd‐cfDNA cases was uniform across centers with each having both low and high results. The multivariate analysis performed showed no selection bias or cofounding factors such as immunosuppressive regimens that were affecting results.
The definition of Banff borderline TCMR became ambiguous when the Banff 2005 consensus modified the lower threshold from i1 t1 (10%‐25% interstitial inflammation with mild tubulitis) to i0 t1 (0%‐10% interstitial inflammation with mild tubulitis). With TCMR 1A defined as (t2), (i2), noting tubulitis—t1 as 1‐4 cells whereas t2 is 5‐10 cells—it is also debatable whether 4 vs 6 cells really is different. Inflammation is graded as 0%‐10%, 10%‐25%, and 26%‐50%, which remains arbitrary and so whether a patient with 9% inflammation really differs from one with 11% is where molecular testing may provide insight. Intimal arteritis is currently graded as mild, moderate, and severe, and so the question of how best to quantify these qualitative terms is important. The use of dd‐cfDNA as part of the diagnostic criteria may improve decision‐making in assessing whether the changes seen on histopathology are clinically significant.7, 16
One consideration to further strengthen this observation would be the inclusion of longer term outcomes, as the observation period was limited to 6 months post event. Clayton et al have shown a 30% decline in eGFR between year 1 and 3 was superior to other surrogates; such as acute rejection and doubling of serum creatinine level.21 Large changes in eGFR are not normally considered in TCMR 1A or borderline, with current thinking suggesting that steroids are potentially curative. The results here show that patients with elevated dd‐cfDNA have associated significant eGFR decline (P = .0040). The ability of dd‐cfDNA to identify active injury may allow for better risk stratification of patients more likely to experience a subsequent decline in graft function and inferior long‐term graft outcomes, though this hypothesis would need confirmation with a larger, prospective study.
The impact of acute rejection episodes on increasing the risk of residual, future rejection and late transplant failure has been documented, with graft loss associated with chronic rejection affecting 47% of allografts.22, 23 All recurrent rejections occurred within high dd‐cfDNA levels (Table 2). The rate of further rejection or incomplete treatment of rejection is an important factor in considering the impact to patient and allograft health. Follow‐up biopsies were all for‐cause biopsies, and so there was no ascertainment bias in the diagnosis of further rejection between groups.
Cooper et al have shown poor outcomes in kidney recipients with de novo donor‐specific anti‐HLA antibodies (DSA) detected posttransplant as well as de novo DSA formation that is associated with acute rejection portending worse graft outcomes.24 The results here show increased de novo DSA formation was associated with patients who had high dd‐cfDNA levels, supporting findings by Jordan et al.25
The concept that a rising dd‐cfDNA is a harbinger of inflammation and warrants further evaluation, where the use of DSA testing and biopsy are often used shows the dd‐cfDNA is associated with the formation of dnDSA. Importantly we do not want to confuse the confounding principles of prediction and association, as these data do not sufficiently show dd‐cfDNA anticipates or predicts the formation of DSA (an area of ongoing study).
Loupy et al presented a prospective cohort who underwent surveillance biopsies at 1‐year posttransplant, with concurrent evaluations of graft complement deposition and circulating anti‐HLA antibodies. Of patients with subclinical TCMR at 1 year, only those who further developed de novo DSAs and transplant glomerulopathy showed higher risk of graft loss compared with patients without rejection.26
One hypothesis that could explain the equivocal difference in graft survival between subclinical TCMR (88%) and nonrejection (90%) is that a proportion of those labeled as TCMR did not truly have rejection, and that only those with active injury were those who went on to form DSA. The cause of DSAs remains multifactorial,with the multivariate analysis excluding any confounding factors (inadequate immunosuppressive therapy, PRA, sex, etc); however, currently all we observed is the association of dd‐cfDNA and de‐novo DSA.
Muthukumar et al and others have shown that preselected immune transcripts including FOXP3 (forkhead Box p3) measured by quantitative polymerase chain reaction can noninvasively differentiate acute cellular rejection from non‐acute cellular rejection in kidney transplant recipients. Investigators identified changes in the numbers of FOXP3 positive T cells accompanying acute cellular rejection events and found the same high vs low distribution,27, 28 similar to dd‐cfDNA. Gandolfini et al also showed this high‐low distribution with chemokine (C‐X‐C motif) ligand 9 (CXCL9).29 Measurement of CXCL9 has shown a strong correlation with the high vs low distribution seen in borderline rejection and supports the idea of considering molecular information to provide a more specific assessment of inflammation in kidney allograft biopsies and achieve better prognostic information to allow appropriate treatment to ameliorate allograft failure.
As we learn more about dd‐cfDNA, considering it as a continuous variable is important in its interpretation and may help clinicians understand the value dd‐cfDNA when interpreting results. Moving away from the idea of a threshold and considering reference change value (RCV) seems logical, where our data suggest if a threshold is to be used, a level of 0.5% may be more appropriate with further study recommended.
Not all TCMR 1A/borderline rejections are associated with adverse outcomes and therefore likely represent a composite of more vs less aggressive pathologic states. Further work is needed to support the ideas of this study, but considering the use of companion diagnostic tools to supplement the utility of biopsy and strengthen the interpretation of the Banff system will help to make improved distinctions. When considering dd‐cfDNA provides a more complete characterization of rejection heterogeneity and valuable clinical information for patient treatment when interpreting serial results and assessing the RCV delta.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Matthew Weir, Jonathan Bromberg, Oyedolamu Olaitan, Joseph Melancon, Yasir Qazi, Tarek Alhamad, and Gaurav Gupta are named speakers for CareDx.
Patients were tested with AlloSure dd‐cfDNA as part of standard care. No financial incentives were received for this project and it was done independently. There are no conflicts of interests.
Supporting information
Table S1‐S2
Stites E, Kumar D, Olaitan O, et al. High levels of dd‐cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant. 2020;20:2491–2498. 10.1111/ajt.15822
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Directory of Open Access Journals at https://doaj.org.
REFERENCES
- 1. Roufosse C, Simmonds N, Clahsen‐van Groningen M, et al. A 2018 reference guide to the Banff classification to renal allograft pathology. Transplantation. 2018;102(11):1795‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell mediated rejection despite continued antibody mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2015;26(7):1711‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katsuma AI, Yamakawa T, Nakada Y, Yamamoto I, Yokoo T. Histopathological findings in transplanted kidneys. Ren Replace Ther. 2017;3:6. [Google Scholar]
- 4. Nankivell BJ, Agrawal N, Sharma A, et al. The clinical and pathological significance of borderline T cell mediated rejection. Am J Transplant. 2019;19(5):1452‐1463. [DOI] [PubMed] [Google Scholar]
- 5. Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17(1):28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Troxell ML, Lanciault C. Practical applications in immunohistochemistry: evaluation of rejection and infection in organ transplant. Arch Path Lab Med. 2016;140(9):910‐925. [DOI] [PubMed] [Google Scholar]
- 7. Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell mediated rejection, antibody mediated rejection and prospects for integrative endpoints for next generation clinical trials. Am J Transplant. 2018;18(2):293‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matignon M, Muthukumar T, Seshan SV, Suthanthiran M, Hartono C. Concurrent acute cellular rejection is an independent risk factor for renal allograft failure in patients with C4d‐positive antibody‐mediated rejection. Transplantation. 2012;94(6):603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ivanyi B, Hansen HE, Olsen S. Segmental localization and quantitative characteristics of tubulitis in kidney biopsies from patients undergoing acute rejection. Transplantation. 1993;56(3):581‐585. [DOI] [PubMed] [Google Scholar]
- 10. Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10(9):2066‐2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker JU, Chang A, Nickeleit V, Randhawa P, Roufosse C. Banff borderline changes suspicious for acute T cell‐mediated rejection: where do we stand? Am J Transplant. 2016;16(9):2654‐2660. [DOI] [PubMed] [Google Scholar]
- 12. Volik S, Alcaide M, Morin RD, Collins C. Cell‐free DNA (cfDNA): Clinical significance and utility in cancer shaped by emerging technologies. MCR. 2016;14(10):898‐908. [DOI] [PubMed] [Google Scholar]
- 13. Bloom RD, Bromberg JS, Poggio ED, et al. Cell free DNA and active rejection in kidney allografts. J Am Soc Nephrol. 2017;28(7):2221‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang E, Sethi S, Peng A, et al. Early clinical experience using donor‐derived cell free DNA to detect rejection in kidney transplant recipients. Am J Transplant. 2019;19(6):1663‐1670. [DOI] [PubMed] [Google Scholar]
- 15. Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8(3):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Freitas DG, Sellarés J, Mengel M, et al. The nature of biopsies with "borderline rejection" and prospects for eliminating this category. Am J Transplant. 2012;12(1):191‐201. [DOI] [PubMed] [Google Scholar]
- 17. Grskovic M, Hiller DJ, Eubank LA, et al. Validation of a clinical‐grade assay to measure donor‐derived cell‐free DNA in solid organ transplant recipients. J Mol Diagn. 2016;18(6):890‐902. [DOI] [PubMed] [Google Scholar]
- 18. Bromberg JS, Brennan DC, Poggio E, et al. Biological variation of donor derived cell free DNA in renal transplant recipients: clinical implications. JALM. 2017;2(3):309‐321. [DOI] [PubMed] [Google Scholar]
- 19. Mehta R, Sood P, Hariharan S. Subclinical rejection in renal transplantation: reappraised. Transplantation. 2016;100(8):1610‐1618. [DOI] [PubMed] [Google Scholar]
- 20. Moulin B, Merville P, Renaudin K, et al. Evaluation of protocol biopsy utility 12 months after renal transplantation: a multicenter observational analysis. J Transplant. 2012;2012:781263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clayton PA, Lim WH, Wong G, et al. Relationship between eGFR decline and hard outocmes after kidney transplants. J Am Soc Nephrol. 2016;27(11):3440‐3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pallardo Mateu LM, Sancho Calabuig A, Capdevila Plaza L, et al. Acute rejection and late renal transplant failure: risk factors and prognosis. Nephrol Dial Transplant. 2004;19(Suppl 2):iii38‐iii42. [DOI] [PubMed] [Google Scholar]
- 23. Jevnikar AM, Mannon RB. Late kidney allograft loss: what we know about it, and what we can do about it. Clin J Am Soc Nephrol. 2008;3(Suppl2):S56‐S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cooper JE, Gralla K, Chan K, et al. Clinical significance of post kidney transplant de novo DSA in otherwise stable grafts. Clin Transpl. 2011;35:359‐364. [PubMed] [Google Scholar]
- 25. Jordan S, Sawinski D, Dholakia S. Donor derived cell free DNA initiates de‐novo donor specific antibody (DSA) responses [abstract]. Am J Transplant. 2019;19(Suppl 3):310‐590.32745322 [Google Scholar]
- 26. Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post transplant and outcome of kidney allografts. J AM Soc Nephrol. 2015;26(7):1721‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal allograft recipients. N Engl J Med. 2005;353:2342‐2351. [DOI] [PubMed] [Google Scholar]
- 28. Bunnag S, Allanach K, Jhangri GS, et al. FOXP3 expression in human kidney transplant biopsies is associated with rejection and time post transplant but not with favorable outcomes. Am J Transplant. 2008;8(7):1423‐1433. [DOI] [PubMed] [Google Scholar]
- 29. Gandolfini I, Harris C, Abecassis M, et al. Rapid biolayer interferometry measurements of urinary CXCL9 to detect cellular infiltrates noninvasively after kidney transplant. Kid Int Rep. 2017;2(6):1186‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
The data that support the findings of this study are openly available in the Directory of Open Access Journals at https://doaj.org.


