Abstract
Background
To prescribe early trismus therapy, prognostic factors influencing the restricted mouth opening should be identified first. Our aim is to present an overview of these factors in patients with head and neck cancer.
Methods
PubMed, Cochrane, EMBASE, and CINAHL were searched using terms related to head and neck cancer and mouth opening. Risk of bias was assessed using the “Quality in Prognosis Studies” tool. A best evidence synthesis was performed.
Results
Of the identified 1418 studies, 53 were included. Three studies contained a prognostic multivariate model for a restricted mouth opening.
Conclusions
Patients with head and neck cancer will most likely develop a restricted mouth opening when they have a large tumor near the masticatory muscles that requires extensive cancer treatment. A restricted mouth opening most likely occurs within 6 months after cancer treatment. Further research is necessary on factors related to healing tendency or pain intensity.
Keywords: head and neck neoplasms, mouth neoplasms, mouth opening, oral, prognosis, surgery
1. INTRODUCTION
Trismus, a restricted mouth opening, is considered to be one of the three most burdensome side effects after head and neck cancer treatment. 1 , 2 , 3 Daily activities, such as speaking, eating, and swallowing become more difficult. 4 , 5 , 6 As a consequence, trismus impacts the quality of life. 7 , 8
In order to prevent or to treat trismus, stretching regimens are often prescribed to increase mouth opening. 9 In 2016, a systematic review analyzed the effects of various stretching regimens, but none of them was found to be superior. 10 It has been suggested that early initiation of a therapy for trismus results in a greater improvement in mouth opening. 11 However, when the effectiveness of an early, preventive stretching regimen was analyzed, no significant difference between the exercise group and control group was found. 12 Not all the patients may have been at risk for trismus, which would have hindered the detection of the effectiveness of the therapy. Moreover, the group of patients not at risk of developing trismus was unnecessarily burdened with an intensive stretching regimen.
Thus, factors influencing trismus should be identified so that only the patients at risk for trismus are subjected to therapy. Previous studies examined the factors associated with trismus but the criteria they applied varied (eg, a maximal mouth opening [MMO] of less than 20 mm 13 or less than 35 mm 14 ). They used different assessment methods (eg, objective measurement using a millimeter scale 15 , 16 or perceived difficulties opening the mouth using questionnaires 17 , 18 ), or different study populations (eg, patients receiving radiotherapy 15 , 19 or chemoradiotherapy 19 , 20 ). There is no recent systematic review available on prognostic factors for trismus in patients with head and neck cancer in general.
The aim of this systematic review is to identify the prognostic factors for trismus (measured objectively and subjectively) in patients treated for head and neck cancer.
2. MATERIALS AND METHODS
The protocol for this systematic review is registered in Prospero (Register code: CRD42017071400). The study will be reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.
2.1. Literature search
Four databases were searched for eligible studies: PubMed, Cochrane, Excerpta medica dataBASE (EMBASE), and Cumulative index to nursing and allied health literature (CINAHL). The search strategy was developed in cooperation with an information specialist and included MesH terms and free text regarding head and neck cancer and mouth opening (Supplementary Information S1). All the databases were searched in November 2017. An update was performed in July 2019.
2.2. Eligibility criteria
Prospective longitudinal studies were included if at least two measurement moments, regarding objective measurements of trismus (trismus and MMO) or subjective assessments of trismus (perceived difficulties with opening the mouth), were reported. No distinction was made between active or passive mouth opening measurements. Studies of trismus therapies were excluded, unless they reported data on a restricted mouth opening of a control group that did not receive a form of trismus therapy. (Systematic) reviews, in vitro studies, comments, letters to the editor, and case reports of less than 10 patients were excluded. There were no language or time restrictions. Studies written in languages that could not be understood by the authors were translated. Additionally, a full‐text version had to be available in order to be included for further assessment and data extraction.
2.3. Study selection
After removing any duplicates, the titles and abstracts were assessed for inclusion independently by J.G., P.R., and P.D. The assessors J.G. and P.D. independently assessed the full text versions for inclusion. Any disagreements between them were resolved by discussion. In case no consensus could be reached, a third observer (K.D.) was consulted. Interobserver reliability was measured through Cohen's kappa and percentage of agreement.
Google Scholar, the references of the relevant systematic reviews and the references of the eligible studies were checked by J.G. for studies missed in the database search. When a study was considered eligible, the full text paper was screened and assessed by J.G. and P.D. independently, according to the original protocol.
The studies that only reported descriptive data and did not perform any statistical tests to analyze the influence of factors on trismus, MMO, or perceived difficulties opening the mouth, were excluded.
2.4. Data extraction
One reviewer (J.G.) extracted all required information from the studies, which included sample size, patient characteristics (age, sex), tumor characteristics (tumor localization, T classification, N classification, tumor stage, histology), treatment characteristics (treatment modality), and method of outcome measurements (number of measurement points reported, follow‐up time). Percentage of patients with trismus (based on a cut‐off point), difference in means or medians of MMO between two measurements (one measurement after treatment minus measurement before treatment) were recorded. In case of multivariate prognostic models, the estimated effects and 95% confidence intervals were extracted. Data of the univariate analysis or multivariate analysis were extracted in case trismus, mouth opening, difficulties opening the mouth, were analyzed over time.
A second reviewer (P.D.) extracted data from a random sample of eight studies containing only univariate analyses and the three studies containing multivariate analyses. In case any data were missing or needed clarifying, the corresponding authors were contacted by e‐mail.
2.5. Risk of bias assessment
Included studies were assessed by J.G. and P.D. on risk of bias using the “Quality In Prognosis Studies” tool (QUIPS). 21 This tool is designed to assess the risk of bias in prognostic studies. The tool assesses the following items: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. The risk of bias can be scored low, moderate, or high. We added the option “not applicable” which could be chosen in case the studies did not provide adequate information to be able to assess that specific domain. As attrition is commonly high in studies including patients with head and neck cancer due to early decease, we predefined the following criteria: when the study attrition is more than 20%, but no specifications are given, we assessed the study as high risk of bias on the study attrition domain. When the study attrition is more than 20%, but specifications are given, we assessed the study as moderate risk of bias on the study attrition domain. For the statistical analysis and reporting domain, we scored a high risk of bias if the effect of only one factor on restricted mouth opening was analyzed. J.G. and P.D. were authors of two studies. These studies were assessed by two independent assessors, K.D. and B.G., to reduce the risk of assessor bias.
To assess the overall risk of bias of a study, it was recommended to score the overall risk of bias as “low” if at least all, or the most important domains (determined a priori), were rated as having a low risk of bias. 21 On that basis, we determined the overall risk of bias to be low, if at least five out of six domains were scored with a low risk of bias and the domains “study confounding” and “statistical analysis and reporting” were scored with a low risk of bias. These two domains are of major importance for analyzing the influence of factors on trismus, MMO or perceived difficulties opening the mouth.
In case of disagreement between the reviewers, a consensus meeting was held. If no consensus could be reached, a third reviewer (K.D.) gave a binding verdict.
2.6. Best evidence synthesis
Due to clinical and methodological heterogeneity between the included studies, we did not perform a meta‐analysis. Instead, we performed a best evidence synthesis. Three main domains were taken into account in order to rate the level of evidence: quality, quantity, and consistency. 22 , 23 We determined the evidence to be strong if two or more studies (quantity) with an overall low risk of bias (quality) and relatively consistent findings (consistency) of the analyzed factors across the studies was found. Evidence was determined to be moderate when evidence was provided including one study with an overall low risk of bias and relatively consistent findings of the analyzed factors across the studies. Evidence was determined to be limited when evidence was provided by studies with an overall high risk of bias and relatively consistent findings of the analyzed factors across the studies. Evidence was determined to be limited/moderate when evidence was provided by a study with an overall high risk of bias, but in which a multivariate prognostic model was presented. The evidence was determined to be conflicting in case there were inconsistencies between the findings of the analyzed factors found across the studies.
3. RESULTS
3.1. Study selection
The first search resulted in 1703 hits. After duplicate removal, 1199 papers were included for title and abstract assessment (Cohen's kappa: 0.533, agreement 90%). Although 141 were deemed suitable for full text assessment after a consensus meeting (Cohen's kappa: 0.577, agreement 81%), 40 papers were excluded: 37 were abstracts only (eg, conference abstract or poster abstract), one was a review, one was a comment in a forum, and one full‐text could not be retrieved. The corresponding author was requested to provide the full text article, but no response was received. A further 59 of the available full text papers were excluded because they did not fulfill the inclusion criteria.
The additional check in Google Scholar and the references of the relevant studies and (systematic) reviews, resulted in 16 other papers. 5 , 17 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 After reading the full text, two did not meet the inclusion criteria. 20 , 24 A total of 56 studies were included for risk of bias assessment and data extraction. During the data extraction process, an additional seven studies were excluded, because no statistical analysis was performed to identify any factors influencing trismus (n = 4) 27 , 38 , 39 , 40 or because exercises to increase mouth opening had been undertaken (n = 2), 26 , 41 or because only one measurement moment was reported (n = 1) 42 (Figure 1).
FIGURE 1.
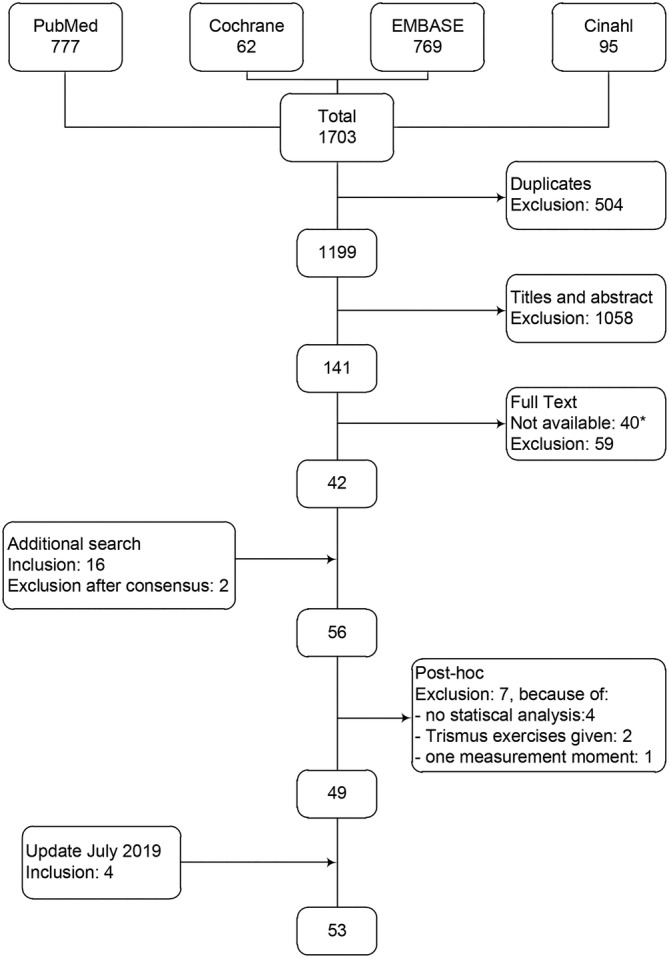
Flowchart. *: 40 studies were not available, because 37 were abstracts only (eg, conference abstract or poster abstract), one was a review, one was a comment in a forum, and one full‐text could not be retrieved
After an update of the search and the removal of duplicates, 203 additional papers were identified. After assessing the titles and abstracts (Cohen's kappa: 0.378, agreement 80%), were included for assessment of the full text (Cohen's kappa: 0.493, agreement 76%). Eventually, four of those studies were included. 43 , 44 , 45 , 46
The above procedures resulted in a final selection of 53 studies. 5 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77
3.2. Study characteristics
Sample sizes ranged from 14 to 641 patients (Table 1). The number of measurement moments ranged from 2 to 20. The longest follow‐up period was 5 years.
TABLE 1.
Data extraction objective and subjective measurements
| Author (year) | Sample size (no. of patients) | Age mean (SD) OR Median (range) | Ratio male:female | Histology | Tumor localization | Stage | Treatment modality a | no. of measures b | Follow‐up c | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Objective measurements—cut‐off point for trismus | ||||||||||
| Yan et al (2003) 13 | 112 | 44.6 [14‐71] | 83:29 | – | Nasopharynx | I‐IV | RT | 7 |
60 |
|
| Scott et al (2011) 14 | 64 | 59 (10) | 40:24 | SCC | Oral cavity, oropharynx | T:1‐4 N:0,+ | S,(C)RT | 3 | 6 | |
| Lee et al (2012) 47 | 152 | – | – | – | HNC | Disease:1‐4 | S,(C)RT | 3 | >6 | |
| Pauli et al (2013) 48 | 75 | 62 [35‐86] |
45:30 |
– |
HNC |
T:0‐4 UICC:I‐IV |
S,(C)RT |
4 |
12 | P: Pauli et al (2016) 49 |
| Pauli et al (2016) 49 | 216 | 60 [29‐87] | 155:62 | – | HNC | T:x‐4 | (C)RT | 4 | 12 | |
| van der Geer et al (2016) 50 | 641 | 62.3 (12.5) |
451:190 |
– |
HNC |
T:x‐4 |
S,(C)RT |
7 |
48 | T: Kamstra et al (2015) 15 |
| Objective measurements—maximal mouth opening measurements | ||||||||||
| Goldstein et al (1999) 51 | 58 | – |
– |
– |
HNC |
– |
RT |
2 |
6‐12 | |
| Wang et al (2005) 52 | 17 | <50 y (n = 8), >50 y (n = 9) |
13:4 |
– |
Nasopharynx |
T:1‐4 N:0‐3 Disease:I‐III |
RT |
20 |
48 | |
| Bragante et al (2012) 54 | 26 | 59.0 (8.8) [45‐74] |
26:0 |
– |
HNC |
INCA:I‐IVB |
(C)RT |
3 |
0 | |
| Mucke et al (2012) 55 | 96 | 62.8 (8.9) [41‐82] |
58:38 |
SCC |
anterior floor of the mouth |
T:1‐4 N:0‐3 |
S,(C)RT |
2 |
2‐24 | |
| Lyons et al (2013) 56 | 62 | <50 y (n = 26), ≥50 y (n = 36) d |
33:29 |
– |
HNC |
T:1‐4 N:0‐3 |
S,(C)RT |
2 |
12‐36 | |
| Lazarus et al (2014) 57 | 29 | 58.5 (9.2) [41‐78] |
23:6 |
– |
HNC |
AJCC:I‐IVA |
(C)RT |
3 |
6 | |
| Safdar et al (2014) 58 | 65 |
Group 1: 59.7 (11.5) Group 2: 60.6 (13.4) |
45:20 |
– |
HNC |
T:1‐4 |
S |
2 |
6 |
Group 1: platysma reconstruction Group 2: submental reconstruction |
| Wetzels et al (2014) 16 | 143 |
Group 1: 68.4 (12.2) Group 2: 66.9 (12.6) Group 3: 62.3 (12.9) |
Group 1: 17:17 Group 2: 28:26 Group 3: 33:22 |
– |
Oral cavity |
T:1‐4 |
S,RT |
4 |
12 |
Group 1: maxilla Group 2: mandible Group 3: tongue/floor of mouth |
| Bragante et al (2015) 53 | 56 | 58.7 (10.8) |
52:4 |
– |
UADT |
Disease:I‐IV |
S,(C)RT |
2 |
0 | |
| Fong et al (2015) 59 | 27 | 58.7 (9.5) |
16:11 |
– |
Nasopharynx |
AJCC:I‐IV |
S,(C)RT |
4 |
12 (after intervention) |
Control Group only |
| Kamstra et al (2015) 15 | 641 | 62.3 (12.5) |
451:190 |
– |
HNC |
T:0‐4, N:0‐3 |
S,(C)RT |
7 |
48 | |
| Manaktala et al (2015) 60 | 24 | – |
– |
– |
HNC |
– |
RT |
5 |
18 Gy | |
| Nayar et al (2016) 61 | 55 | – |
– |
– |
HNC |
– |
S,RT |
2 |
1‐2 | |
| Al‐Saleh et al (2017) 43 | 16 |
Group 1: 54.2 (12.5) Group 2: 50.6 (11.9) |
Group 1: 6:3 Group 2:5:2 |
– | Oral cavity, oropharynx | T:1‐4 N:0‐3 | S | 2 | 1.5‐2 |
Group 1: mandibulotomy surgery Group 2: transoral surgery |
| Lalla et al (2017) 62 | 372 | 59.8 (10.9) |
284:88 |
SCC |
HNC |
– |
S,(C)RT |
2 |
6 | |
| Thor et al (2017) 63 | 196 | 60 (11) |
141:55 |
– |
HNC |
T:0‐4 N:0‐4 |
(C)RT |
4 |
12 | P: Pauli et al (2016) 49 |
| Subjective measurements | ||||||||||
| De Graeff et al (1999) 17 | 75 | 60 [29‐75] |
54:21 |
SCC |
Oral cavity, oropharynx |
AJCC:I‐IV |
S,RT |
3 |
12 | P: De Graeff et al (2000) 29 |
| De Graeff et al (2000) 29 | 107 | 60 [31‐73] |
86:21 |
SCC |
HNC |
AJCC:0‐IV |
S,RT |
5 |
36 | |
| Epstein et al (2000) 5 | 20 | 53.4 [38‐78] |
12:8 |
– |
HNC |
AJCC:I‐IV |
RT |
3 |
6 | |
| Bjordal et al (2001) 28 | 357 | 63 [18‐88] |
256:101 |
– |
HNC |
Disease:I‐IV |
S,(C)RT |
6 |
12 | |
| Hammerlid et al 2001) 18 | 232 | 61 [18‐85] |
162:70 |
– |
HNC |
Disease:I‐IV |
S,(C)RT |
5 |
36 | |
| Ohrn et al (2001) 33 | 18 |
55.4 (9.0) [38‐73] |
10:8 |
SCC ACA |
HNC |
– |
(C)RT |
4 |
1 | |
| Wiltfang et al (2003) 34 | 53 | 54.2 [34‐78] |
48:5 |
SCC |
Oral cavity |
UICC:0‐IV |
S,(C)RT |
4 |
24 | |
| Fang et al (2004) 30 | 77 | 50 [22‐78] |
77:0 |
SCC |
HNC |
AJCC:III,IV |
S, RT |
2 |
24 | P: Fang et al (2005) 31 |
| Abendstein et al (2005) 64 | 167 | 61 [18‐86] |
116:51 |
– |
HNC |
Disease:I‐IV |
S,(C)RT |
3 |
60 | P: Bjordal et al (2001) 28 |
| Fang et al (2005) 31 | 149 | 53 [25‐81] |
138:11 |
SCC |
HNC |
AJCC:III,IV |
(C)RT |
2 |
12 | |
| Nordgren et al (2005) 32 | 89 | 60 |
68:21 |
– |
Pharynx |
Disease:I‐IV |
S,(C)RT |
4 |
60 | P: Bjordal et al (2001) 28 |
| Urdaniz et al (2005) 37 | 60 |
Group 1: 56 Group 2: 57 |
– |
– |
HNC |
T:2‐4 N:0,+ AJCC:III,IV |
(C)RT |
3 |
1 |
Group 1: 72 Gy, 6 wk Group 2: 80.4 Gy, 7 wk |
| Borggreven et al (2007) 65 | 80 | 58 [23‐74] |
47:33 |
SCC |
Oral cavity, oropharynx |
T:2‐4, N:0‐3 |
S,RT |
3 |
12 | |
| Oates et al (2007) 20 | 14 | – |
– |
– |
Nasopharynx |
T:1‐4 N:0‐3 |
(C)RT |
5 |
24 | |
| Bozec et al (2008) 66 | 65 | 61.2 (9.3) [40‐85] |
49:16 |
– |
HNC |
T:2‐4 N:0‐3 |
S,RT |
3 |
12 | |
| Bozec et al (2009) 67 | 41 |
62.3 (9.6) [43‐85] |
33:8 |
SCC |
Oral cavity, Oropharynx |
T:2‐4 N:0‐3 AJCC:II‐IV |
S,(C)RT |
3 |
12 | P: Bozec et al (2008) 66 |
| Rizvi et al (2009) 68 | 37 | 51.8 (9.6) |
18:19 |
– |
HNC |
T:3,4 N:1,2 |
S,RT |
4 |
6 | |
| Vergeer et al (2009) 35 | 241 |
Group 1: ≤65 y (n = 95), >65 y (n = 55) Group 2: ≤65 y (n = 68), >65 y (n = 23) |
Group 1: 104:46 Group 2: 51:40 |
SCC |
HNC |
T:0‐4 N:0‐3, UICC:I‐IV |
S,(C) RT |
5 |
12 |
Group 1: 3D‐RT Group 2: IMRT |
| Yoshimura et al (2009) 69 | 56 | 63 [25‐88] |
46:10 |
SCC |
Oral cavity |
T:1‐3 |
LDR‐BT |
4 |
12 | |
| Chan et al (2012) 36 | 185 | 50.2 (11.4)[24‐81] |
151:34 |
– |
Recurrent nasopharynx |
– |
S,(C)RT |
2 |
6 | |
| Al‐Mamgani et al (2013) 70 | 207 | <65 y (n = 142), ≥65 y (n = 65) |
143:64 |
– |
Oropharynx |
T:1‐4 N:0‐3 AJCC:I‐IV |
(C)RT |
5 |
18 | |
| Kumar et al (2013) 74 | 111 |
Group 1: 55.3 (12.4) Group 2: 53.4 (11.2) |
Group 1: 47:8 Group 2: 49:7 |
SCC |
HNC |
Stage:III‐IVb |
(C)RT |
3 |
6 |
Group 1: palliative RT Group 2: palliative CRT |
| Rathod et al (2013) 71 | 60 |
Group 1: 55 [33‐65] Group 2: 51 [31‐65] |
Group 1: 25:3 Group 2: 29:3 |
SCC |
HNC |
T:1‐3 N:0‐2b AJCC:I‐IV |
RT |
6 |
24 |
Group 1: 3D‐RT Group 2: IMRT |
| Zhao et al (2014) 72 | 83 |
Group 1: 52.0 [22‐81] Group 2: 53.4 [28‐76] |
Group 1: 28:15 Group 2: 27:13 |
– |
Nasopharynx |
T:4 N:3 UICC:2‐4 |
(C)RT |
5 |
24 |
Group 1: CRT + ERF Group 2: CRT |
| Arslan et al (2015) 73 | 40 | 56 [20‐65] |
33:7 |
– |
HNC |
Stage:I‐IVA |
S,(C)RT |
3 |
3 | |
| Landstrom et al (2015) 75 | 19 | 56.6 |
12:7 |
SCC ACA |
HNC |
T:1,2 |
(C)RT |
2 |
12 | |
| Rao et al (2016) 19 | 421 | ≤55 y (n = 191), >55 y (n = 230) |
345:76 |
SCC |
Pharynx, larynx |
T:1‐4 N:0‐3 AJCC:II‐IV |
(C)RT |
12 |
Median 33 | |
| Dzioba et al (2017) 76 | 117 | 58.2 (13.3) |
71:46 |
SCC |
Tongue (oral cavity) |
T:1‐4 AJCC:I‐IVA |
S,(C)RT |
4 |
12 | |
| Gao et al (2018) 77 | 77 | <60 y (n = 48), ≥60 y (n = 29) | 41:36 |
SCC ACC |
Tongue | – | S | 3 | 12 | |
| Tribius et al (2018) 46 | 161 | 60.4 (10.4) | 110:51 | HNC | UICC T:1‐4 N:0‐3 | RT | 3 | 24 | BL = AT | |
| Veluthattil et al (2019) 45 | 25 | ≤60 y (n = 21), >60 y (n = 4) | 11:14 | SCC | Oral cavity | Stage:IVA‐IVC | RT | 2 | 2 | |
Abbreviations: Histology: ACA, adenocarcinoma; ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma. Tumor localization: HNC, head and neck cancer; UADT. upper aero‐digestive tract. Stage: AJCC, stage according to American Joint Committee on Cancer; INCA, stage according to Instituto Nacional de Câncer (National Institute of Cancer Brazil); N, nodes classification; T, tumor classification; UICC, stage according to Union for International Cancer Control. Treatment modality: C, chemotherapy; (C)RT, chemoradiotherapy; LDR‐BT, low‐dose‐rate interstitial brachytherapy; RT, radiotherapy; S, surgery. Remarks: 3D‐RT, three‐dimensional radiotherapy; ERF, extracorporeal radiofrequency; IMRT, intensity modulated radiotherapy; Gy, Groupay of radiation; P, partial overlap of study population; T, total overlap of study population; wk, week.
The text in bold indicates that all the patients in this study received that particular treatment modality.
Number of measurement points reported.
Follow‐up period (in months after treatment).
Calculated from data reported.
3.3. Risk of bias assessment
The overall Cohen's kappa bias assessment score was 0.310 (52% agreement). The source or study population was not described (adequately) in the majority of the studies. These studies were scored with “N/A” on the study participation domain (n = 32; 60%) (Table 2). 5 , 13 , 16 , 18 , 20 , 28 , 30 , 32 , 36 , 37 , 43 , 45 , 46 , 47 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 61 , 64 , 65 , 68 , 69 , 72 , 73 , 75 , 77 Eleven studies (21%) did not report attrition rate. 13 , 19 , 33 , 35 , 51 , 55 , 61 , 70 , 72 , 73 , 77 Some did not report the attrition rate because only the patients with complete data were included. Four studies (8%) were scored with “N/A" on the outcome measurement domain 14 , 16 , 55 , 60 : two studies did not describe the measurement method 55 , 60 and two studies used a measurement method that has not been validated (extra‐oral measurements). 14 , 16 The majority of the studies were scored with a high risk of bias concerning the statistical analysis and reporting domain (n = 42; 79%) because they lacked a multivariate analysis. 5 , 13 , 14 , 17 , 18 , 20 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 43 , 45 , 46 , 47 , 51 , 52 , 54 , 55 , 56 , 57 , 58 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 70 , 72 , 73 , 74 , 75 , 77
TABLE 2.
Quality assessment using the “Quality in Prognosis Studies” tool
| Author (year) | Study participation | Study attrition | Prognostic factor measurement | Outcome measurement | Study confouding | Statistical analysis and reporting | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Objective measurements | |||||||
| Yan et al (2003) 13 | N/A | N/A | L | L | H | H | H |
| Scott et al (2011) 14 | H | H | L | N/A a | L | H | H |
| Lee et al (2012) 47 | N/A | H | L | L | M | H | H |
| Pauli et al (2013) 48 | M | L | L | L | L | M | H |
| Pauli et al (2016) 49 | L | M | M | L | M | M | H |
| van der Geer et al (2016) 50 | M | H | L | L | M | L | H |
| Objective measurements | |||||||
| Goldstein et al (1999) 51 | N/A | N/A | L | L | M | H | H |
| Wang et al (2005) 52 | N/A | M | L | L | H | H | H |
| Bragante et al (2012) 54 | N/A | M | L | L | M | H | H |
| Mucke et al (2012) 55 | N/A | N/A | L | N/A | H | H | H |
| Lyons et al (2013) 56 | N/A | M | L | M | L | H | H |
| Lazarus et al (2014) 57 | N/A | M | L | L | H | H | H |
| Safdar et al (2014) 58 | N/A | L | L | L | H | H | H |
| Wetzels et al (2014) 16 | N/A | M | L | N/A a | L | L | H |
| Bragante et al (2015) 53 | N/A | L | L | L | L | L | L |
| Fong et al (2015) 59 | H | M | L | L | H | M | H |
| Kamstra et al (2015) 15 | L | H | L | M | M | L | H |
| Manaktala et al (2015) 60 | N/A | L | L | N/A | H | H | H |
| Nayar et al (2016) 61 | N/A | N/A | L | L | H | H | H |
| Al‐Saleh et al (2017) 43 | N/A | H | L | M | H | H | H |
| Lalla et al (2017) 62 | H | H | L | M | H | H | H |
| Thor et al (2017) 63 | M | H | L | L | H | H | H |
| Subjective measurements | |||||||
| De Graeff et al (1999) 17 | L | M | L | L | H | H | H |
| De Graeff et al (2000) 29 | L | M | L | L | M | H | H |
| Epstein et al (2000) 5 | N/A | H | L | L | M | H | H |
| Bjordal et al (2001) 28 | N/A | L | L | L | H | H | H |
| Hammerlid et al 2001) 18 | N/A | L | L | M | L | H | H |
| Ohrn et al (2001) 33 | M | N/A | L | L | H | H | H |
| Wiltfang et al (2003) 34 | L | M | L | L | H | H | H |
| Fang et al (2004) 30 | N/A | M | L | L | H | H | H |
| Abendstein et al (2005) 64 | N/A | L | L | L | M | H | H |
| Fang et al (2005) 31 | L | L | L | L | H | H | H |
| Nordgren et al (2005) 32 | N/A | M | L | L | M | H | H |
| Urdaniz et al (2005) 37 | N/A | L | L | L | M | H | H |
| Borggreven et al (2007) 65 | N/A | L | L | L | M | H | H |
| Oates et al (2007) 20 | N/A | L | L | L | H | H | H |
| Bozec et al (2008) 66 | L | H | L | L | L | H | H |
| Bozec et al (2009) 67 | L | M | L | L | L | H | H |
| Rizvi et al (2009) 68 | N/A | L | L | M | H | H | H |
| Vergeer et al (2009) 35 | M | N/A | L | L | H | H | H |
| Yoshimura et al (2009) 69 | N/A | M | M | L | L | M | H |
| Chan et al (2012) 36 | N/A | L | L | L | H | H | H |
| Al‐Mamgani et al (2013) 70 | L | N/A | L | L | M | H | H |
| Rathod et al (2013) 71 | L | H | L | L | L | L | L |
| Zhao et al (2014) 72 | N/A | N/A | L | L | H | H | H |
| Arslan et al (2015) 73 | N/A | N/A | L | L | H | H | H |
| Kumar et al (2013) 74 | L | L | L | L | H | H | H |
| Landstrom et al (2015) 75 | N/A | L | L | L | H | H | H |
| Rao et al (2016) 19 | H | N/A | L | M | L | L | H |
| Dzioba et al (2017) 76 | L | H | L | L | M | M | H |
| Gao et al (2018) 77 | N/A | N/A | L | L | H | H | H |
| Tribius et al (2018) 46 | N/A | L | N/A | L | H | H | H |
| Veluthattil et al (2019) 45 | N/A | M | L | L | H | H | H |
Abbreviations: H, high risk of bias; L, low risk of bias; M, moderate risk of bias; N/A, not applicable.
Extra‐oral measurement.
3.4. Distinguishing the outcome measurements
A distinction was made between objective (eg, using a ruler or calliper) or subjective (eg, using a patient' questionnaire) assessments of restricted mouth opening. An additional distinction was made between the objective studies, namely using a restricted mouth opening as a cut‐off point (n = 6) 13 , 14 , 47 , 48 , 49 , 50 or a decrease in MMO measured in millimeters (n = 16). 15 , 16 , 43 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63
The subjective analyses assessed the perception of a restricted mouth opening either using the European Organization for Research and Treatment for Cancer Quality of Life Questionnaire Head & Neck module‐ 35 (EORTC QLQ H&N35) (n = 29) 17 , 18 , 20 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 45 , 46 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 or an addendum similar to the EORTC QLQ H&N35 (n = 1) 5 or Common Terminology Criteria for Adverse Events (n = 1). 19 The Gothenburg Trismus Questionnaire (n = 3) was used as a secondary endpoint to assess trismus. 48 , 49 , 63
3.5. Univariate analyses
In 16 studies, a single prognostic factor for a decrease in MMO and the patients' perception of difficulties with opening the mouth was analyzed over time (Table 3). 14 , 16 , 35 , 43 , 46 , 53 , 54 , 55 , 56 , 57 , 58 , 62 , 65 , 71 , 72 , 74 Regarding patient related factors, a significant effect was found in relation to sex (in one study in the period between before and after treatment) 14 and the −509 genotype. 56 Patients with a homozygous T allele (TT) in the −509 genotype had a greater reduction in MMO than those with a homozygous C allele (CC) or heterozygous C allele (CT). Tumor related factors included large reductions in MMO when the tumor was located near the oral cavity or oropharynx. 53 , 54 Less reduction was found in other areas, such as the nasopharynx, hypopharynx, larynx, or lymph drainage areas. 53 , 54 No significant effects were found in relation to T classification or N classification. 14 , 16 , 65 Cancer treatment also resulted in a reduction in MMO, with the most occurring after chemoradiotherapy and the least after surgery. 14 The MMO decreased directly after surgery but increased in the 6 months thereafter. When patients received (chemo) radiotherapy, the MMO decreased directly after the treatment, but did not increase in the 6 months thereafter. The MMO decreased even more with an increase in radiation dose. 54
TABLE 3.
Overview of patient, tumor, treatment, and other characteristics as prognostic factors for decrease in maximal mouth opening (objective) and patients' perception of difficulties opening the mouth (subjective)
| Patient characteristics | Time points of analysis | Age | Sex | Dental status | −509 genotype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Objective measures | |||||||||||
| Scott et al (2011) 14 | <55 | 55‐64 | 65+ | Male | Female | Dentate | Edentulous | ||||
| AT‐BT |
−11 [−21;−2] |
−4 [−13;−1] |
−3 [−12;1] |
−8 [−16;−2]a |
−2 [−11;1]a |
−6 [−14;−2] |
−9 [−22;0] |
||||
| 6M‐BT |
−6 [−11;1] |
−4 [−10;3] |
−5 [−] |
−5 [−11;2] |
−1 [−10;3] |
−4 [−10;3] |
−10 [−23;0] |
||||
| Lyons et al (2013) 56 | CC −509 genotype | CT −509 genotype | TT –509 genotype | ||||||||
| AT‐BT |
−8.5 [−4.5;−13.0] b |
−17.0 [−8.0;−26.0] b |
−26.5 [−33.0;−15.0] b |
||||||||
| Wetzels et al (2014) 16 | AT‐BT |
−14.3 (−) c |
−14.9 (−) c |
−13.8 (−) c |
−14.5 (−) c |
||||||
| 6M‐BT |
−9.0 (−) c |
−9.2 (−) c |
−8.1 (−) c |
−9.3 (−) c |
|||||||
| 12M‐BT |
−8.7 (−) c |
−8.5 (−) c |
−8.1 (−) c |
−8.5 (−) c |
|||||||
| Lalla et al (2017) 62 | 6M‐BT |
−3.3 (−) c |
−3.0 (−) c |
||||||||
| Tumor characteristics | Localization | Stage | |||||||||
| Objective measures | |||||||||||
| Scott et al (2011) 14 | Oral | Oropharynx | T‐stage 1,2 | T‐stage 3,4 | N‐stage 0 | N‐stage + | |||||
| AT‐BT |
−7 [−14;−2] |
−5 [−16;−1] |
−5 [−14;−1] |
−9 [−18;−1] |
−5 [−14;−1] |
−8 [−16;−1] |
|||||
| 6M‐BT |
−4 [−10;2] |
−9 [−] |
−3 [−10;3] |
−9 [−16;1] |
−3 [−10;3] |
−8 [−13;1] |
|||||
| Bragante et al (2012) 54 | Mouth | Oropharynx | Hypopharynx | Larynx | Drainage area | Stage I | Stage II | Stage III | Stage IVA | Stage IVB | |
| AT‐BT |
−11.0 (1.7) b |
−11.5 (7.8) b |
−2.0 (0.0) b |
−5.3 (6.3) b |
−2.8 (4.5) b |
−8.0 (−) |
−0.8 (1.5) | −7.8 (5.9) | −4.5 (5.9) | −6.3 (6.9) | |
| Lazarus et al (2014) 57 | Oropharynx | Others | AJCC 1–3 | AJCC 4 | |||||||
| 3M‐BT |
−4.1 (−) c |
−5.0 (−) c |
−3.5 (−) c |
−4.8 (−) c |
|||||||
| 6M‐BT |
−3.8 (−) c |
−6.2 (−) c |
−4.1 (−) c |
−5.0 (−) c |
|||||||
| Wetzels et al (2014) 16 | Maxilla | Mandible | TFM (tongue/floor of mouth) | T‐stage 1 | T‐stage 2 | T‐stage 3 | T‐stage 4 | ||||
| AT‐BT |
−19.1 |
−15.5 |
−10.7 |
−12.0 (−) c |
−16.7 (−) c |
−17.3 (−) c |
−15.0 (−) c |
||||
| 6M‐BT |
−15.1 |
−9.6 |
−5.0 |
−5.7 (−) c |
−10.4 (−) c |
−14.5 (−) c |
−13.8 (−) c |
||||
| 12M‐BT |
−11.8 |
−8.1 |
−7.5 |
−7.1 (−) c |
−9.4 (−) c |
−10.8 (−) c |
−12.0 (−) c |
||||
| Bragante et al (2015) 53 | Oral cavity oropharynx r |
Nasopharynx Hypopharynx Larynx r |
|||||||||
| AT‐BT |
−5.64 (6.42) a |
−1.68 (6.27) a | |||||||||
| Subjective measures | |||||||||||
| Borggreven et al (2007) 65 | Oral cavity | Oropharynx | T‐stage 2 | T‐stage 3,4 | |||||||
| 6M‐BT |
10.6 (−) |
24.2 (−) |
23.5 (−) |
14.3 (−) |
|||||||
| 12M‐6M |
5.6 (−) |
−11.5 (−) |
−11.1 (−) |
3.3 (−) |
|||||||
| Treatment characteristics | Treatment modality | Reconstruction | Radiation dose | ||||||||
| Objective measures | |||||||||||
| Scott et al (2011) 14 | No RT | RT | CRT | No free‐flap | Soft‐free flap | Composite free flap | |||||
| AT‐BT |
−8 [−14;−2] |
−5 [−13;1] |
−9 [−] |
−2 [−9;−1] |
−6 [−16;−2] |
−11 [−12;0] |
|||||
| 6M‐BT |
−1 [−9;4] a |
−7 [−15;0] a |
−7 [−] a |
−1 [−10;4] |
−5 [−11;1] |
−4 [−] |
|||||
| Bragante et al (2012) 54 | RT | CRT | Total dose | ||||||||
| AT‐BT | −5.5 (6.0) | −4.4 (5.5) | R = −0.164 a | ||||||||
| Mucke et al (2012) 55 | S only | S + RT | S+ RT + ORN | ||||||||
| AT‐BT | −22.5% b | −49.2% b | −49.0% b | ||||||||
| Safdar et al (2014) 58 | Platysma flap | Submental flap | |||||||||
| 6M‐BT |
−3.7 |
−4.7 |
|||||||||
| Wetzels et al (2014) 16 | S only | S + RT | RT | No surgery | Local flap | Myocutaneous or free flap | Bone graft/flap | ||||
| AT‐BT |
−13.4 |
−18.2 |
−7.1 |
−11.1 (−) c |
−22.9 (−) c |
−17.9 (−) c |
−17.4 (−) c |
||||
| 6M‐BT |
−4.5 |
−15.0 |
−8.2 |
−5.5 (−) c |
−20.9 (−) c |
−12.9 (−) c |
−12.7 (−) c |
||||
| 12M‐BT |
−4.6 |
−13.9 |
−8.0 |
−5.9 (−) c |
−14.6 (−) c |
−11.9 (−) c |
−9.8 (−) c |
||||
| Al‐Saleh et al (2017) 43 | Mandibu‐lotomy surgery | Transoral surgery | |||||||||
| 1.5‐2AT‐BT |
11.7 |
5.4 |
|||||||||
| Subjective measures | |||||||||||
| Vergeer et al (2009) 35 | 3D‐RT | IMRT | |||||||||
| 6W‐BT |
8.8 |
−7. |
|||||||||
| 6M‐BT |
11.9 |
1.3 |
|||||||||
| Kumar et al (2013) 74 | RT | CRT | |||||||||
| 1M‐BT |
−3.7 |
−12.3 |
|||||||||
| 6M‐BT |
0.0 |
−17.06 |
|||||||||
|
Rathod et al (2013) 71 |
3D‐RT | IMRT | |||||||||
| 3M‐BT |
6 |
−4 |
|||||||||
| 6M‐BT |
16 |
−3 |
|||||||||
| 12M‐BT |
−2 |
−2 |
|||||||||
| 18M‐BT |
2 |
−4 |
|||||||||
| 24M‐BT |
8 |
−9 |
|||||||||
| Zhao et al (2014) 72 | CRT+ ERF | CRT | |||||||||
| 6M‐AT |
−3.5 |
17.1 |
|||||||||
| 12M‐AT |
−2.6 |
18.2 |
|||||||||
| 18M‐AT |
−6.6 |
20.4 |
|||||||||
| 24M‐AT |
−6.4 |
19.0 |
|||||||||
| Other characteristics | Smoking |
Alcohol (>1 daily) |
Mucositis | SES | |||||||
| Objective measures | |||||||||||
| Wetzels et al (2014) 16 | Yes | No | Yes | No | |||||||
| AT‐BT |
−12.9 (−) c |
−15.4 (−) c |
−14.8 (−) c |
−14.3 (−) c |
|||||||
| 6M‐BT |
−8.9 (−) c |
−9.1 (−) c |
−9.1 (−) c |
−9.0 (−) c |
|||||||
| 12M‐BT |
−8.9 (−) c |
−8.2 (−) c |
−10.5 (−) c |
−7.6 (−) c |
|||||||
| Bragante et al (2015) 53 | Yes | No | |||||||||
| AT‐BT |
−5.9 (6.6) a |
−0.6 (5.3) a | |||||||||
| Subjective measures | |||||||||||
| Tribius et al (2018) 46 | Low | Middle | High | ||||||||
| 24M‐AT |
−12.3 |
−30.5 |
−30.6 |
Note: Number of decimals are reported as the authors have reported it. In case two or more decimals are given, one decimal is reported. For the objective measures, a decrease (a negative value), means a worse restricted mouth opening. For the subjective measures, an increase (a positive value), means a worse restricted mouth opening.
Abbreviations: 3D‐RT, three‐dimensional radiotherapy; (n)W, number of weeks after oncological treatment; (n)M, number of months after oncological treatment; AJCC, stage according to American Joint Committee on Cancer; AT, after oncological treatment; BT, before oncological treatment; CRT, chemoradiotherapy; IMRT, intensity modulated radiotherapy; ERF, extracorporeal radiofrequency; RT, radiotherapy; SES, socioeconomic status.
Significant (p<0.05).
Significant in some analyses (p<0.05).
Difference between mean scores calculated.
Conversion centimeters to millimeters.
Value represents median [interquartile range].
Value represents mean score (SD).
Patients who were given conventional three‐dimensional radiotherapy, instead of intensity modulated radiotherapy, perceived more difficulties opening the mouth. 35 , 71 Also, patients who underwent chemotherapy without the addition of extracorporeal radiofrequency perceived more difficulties with opening the mouth compared with those who received additional extracorporeal radiofrequency. 72 Regarding the remaining factors, a greater reduction in MMO was found when mucositis was present compared to when mucositis was not present. 53 MMO was not significantly reduced in relation to alcohol consumption and smoking factors. 16 Patients with a lower social economic status perceived more difficulties with opening the mouth than patients with a middle or high social economic status. 46
3.6. Timing
The highest percentage of patients developed trismus directly after treatment and it continued to increase in the 6 months thereafter (Figure 2A). The percentage of patients with trismus seemed to stabilize 12 months after treatment. MMO decreased directly after treatment and in the 6 months thereafter (Figure 2B) and appeared to stabilize 12 months after treatment. Patients' perception of difficulties with opening the mouth was highly diverse (Figure 2C). The majority of the patients perceived difficulties with opening the mouth directly after treatment, but thereafter the perception varied considerably.
FIGURE 2.
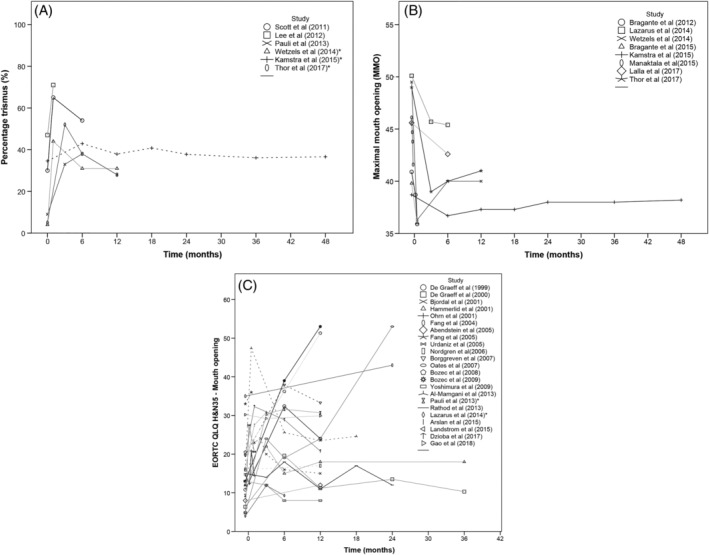
A, Longitudinal evaluation of percentage of patients with trismus. * indicates that study reported trismus as a secondary outcome. Broken lines display studies that had overlapping data with other studies. The studies that contained the largest sample size are displayed as straight lines. B, Longitudinal evaluation of maximal mouth opening. Broken line displays studies that had overlapping data with other studies. The studies that contained the largest sample size are displayed as straight lines. C, Longitudinal evaluation of patient's quality of life score‐domain: difficulties opening the mouth. * indicates that study reported patient's score of perceived difficulties opening the mouth as a secondary outcome. Broken lines display studies that had overlapping data with other studies. The studies that contained the largest sample size are displayed as straight lines
The figures were based on 29 studies. Other studies were not included because: they did not report data on restricted mouth opening at time points before and after oncological treatment (n = 14) 19 , 34 , 35 , 36 , 43 , 46 , 49 , 51 , 55 , 56 , 59 , 61 , 72 , 74 ; the trismus scores were reported as a cumulative incidence 13 ; MMO was reported as a normalized value 52 ; a mean reduction 58 ; or as a median score 14 ; or the scores of the questionnaires were not transformed into symptom scores (n = 3) 5 , 44 , 68 or were reported as a median score. 45 , 75 The data from studies that included the same study population as another study were not displayed either. 50
3.7. Multivariate analyses
Eight studies built multivariate models affecting trismus, mouth opening perceived difficulties opening the mouth. 15 , 16 , 19 , 48 , 49 , 50 , 53 , 76 Three of these studies built and reported prognostic models taking time into account (Table 4). 15 , 53 , 76 Two of these studies analyzed factors affecting MMO, 15 , 53 and one study analyzed the factors affecting perceived difficulties with opening the mouth. 76 Presence of mucositis, deterioration of overall functioning (according to the Karnofsky Performance Status Scale), tumors located near the oral cavity, oropharynx and nasopharynx, nasal cavity and maxillary sinus, shorter time after radiotherapy, female sex, a small baseline mouth opening, large tumor (T stage 4), higher age, and a great target volume (radiotherapy) were significantly associated with a decrease in MMO. A combination of oncological treatment modalities (surgery and (chemo) radiotherapy) and shorter time after oncological treatment) were associated with perceived difficulties with opening the mouth.
TABLE 4.
Prognostic factor models for restricted mouth opening
| Study (year) | Outcome measure | Method for including factor in model | Performed analysis | Factors in the final model | Estimated effect | ||
|---|---|---|---|---|---|---|---|
| Bragante et al (2015) 53 | Reduction in maximal mouth opening | Bivariate analysis (P < .20) | Linear regression analysis | Enter (P < .05) | B | 95% confidence interval | |
| Change in diet consistency after radiotherapy | −0.29 | −4.27;3.69 | |||||
| Radiation field—oral cavity and oropharynx | −2.83 | −6.61;0.96 | |||||
| Mucositis after radiotherapy a | −4.19 | −7.62;−0.80 | |||||
| Difference in Karnofsky Performance Scale a , b | 0.12 | 0.02;0.24 | |||||
| Disease stage: III/IV | −0.90 | −4.26;6.07 | |||||
| Kamstra et al (2015) 15 | Change in maximal mouth opening | Theoretical plausability | Linear mixed model analysis |
Backward stepwise selection (P < .05) (−log likelihood criterion) |
B | 95% confidence interval | |
| Intercept | 12.88 | 10.00;15.77 | |||||
| Location | |||||||
| Oral cavity | 1.57 | −3.50;6.63 | |||||
| Oropharynx and nasopharynx | 1.04 | −4.09;6.18 | |||||
| Salivary glands and ear | 2.56 | −2.57;7.68 | |||||
| Hypoglottic and supraglottic larynx | 3.56 | −1.61;8.73 | |||||
| Glottic and subglottic larynx | 4.40 | −0.76;9.57 | |||||
| Nasal cavity and maxillary sinus | 1.26 | −4.00;6.53 | |||||
| Unknown primary | ‐ | N/A | |||||
| Time after radiotherapy | 4.00 | 3.38;4.63 | |||||
| Male sex | 1.10 | 0.11;2.08 | |||||
| Mouth opening before treatment | 0.69 | 0.65;0.73 | |||||
| Tumor stage: T4 | −1.14 | −2.16;−0.11 | |||||
| Age | −0.05 | −0.08;−0.01 | |||||
| Target volume on primary tumor | −4.76 | −9.36;−0.17 | |||||
| Oral cavity × time | 0.69 | −0.47;1.85 | |||||
| Oropharynx or nasopharynx × time | 0.47 | −0.70;1.64 | |||||
| Salivary glands or ear × time | 0.91 | −0.26;2.08 | |||||
| Hypopharynx or supraglottic larynx × time | 1.27 | 0.09;2.45 | |||||
| Glottic or subglottic larynx × time | 1.48 | 0.30;2.66 | |||||
| Nasal cavity or maxillary sinus × time | 0.62 | −0.57;1.82 | |||||
| Unknown primary × time | ‐ | N/A | |||||
| Mouth opening before treatment × time | −0.10 | −0.11;−0.09 | |||||
| Male sex × time | 0.32 | 0.11;0.54 | |||||
| Baseline age centered at 60 years × time | −0.01 | −0.02;0.00 | |||||
| Tumor stage T4 × time | −0.27 | −0.50;−0.05 | |||||
| Target volume on primary tumor × time | −1.69 | −2.75;−0.64 | |||||
| Dzioba et al (2017) 76 | EORTC QLQ HN35 c | Mixed effect regression analysis |
P > .05 exclusion interaction terms P > .05 exclusion for treatment |
B | 95% confidence interval | ||
| Baseline | 14.65 | 7.4;21.9 | |||||
| Surgery and radiotherapy | 2.24 | −7.6;12.0 | |||||
| Surgery and chemoradiotherapy | 14.59 | 5.5;23.7 | |||||
| 1 month after treatment | 12.42 | 5.2;19.6 | |||||
| 6 months after treatment | 11.30 | 3.7;18.9 | |||||
| 1 year after treatment | 2.86 | −5.3;11.0 |
Significantly contributing to the model.
Karnofsky Performance Scale: an index used to classify functional impairment, using a scale of 0‐100.
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—Head and Neck cancer Module 35: a validated quality of life questionnaire, specifically for head and neck cancer related symptoms.
3.8. Best evidence synthesis
There is moderate evidence that the presence of mucositis and a deterioration of overall functioning (according to the Karnofsky Performance Status Scale) results in a reduction of MMO (Table 5). There is limited to moderate evidence that target volume, time after treatment, and baseline mouth opening results in a reduction of MMO, and that time after treatment results in higher scores of perceived difficulties opening the mouth. There is conflicting evidence that the factors age localization, age, T classification, reconstruction after surgery, different types of treatment modalities, and sex affect MMO, and that the different types of treatment modalities affect perceived difficulties opening the mouth as well.
TABLE 5.
Best evidence synthesis of prognostic factors on MMO and on scores for perceived difficulties opening the mouth
| Prognostic factor | Studies | Number of patients per study | Total number of patients | Associations (+, −, or ±) | Level of evidence |
|---|---|---|---|---|---|
| Maximal mouth opening reduction | |||||
| Disease stage | [54;57;53] | 26;29;56 | 111 | − | Moderate |
| Presence of mucositis | [53;53] | 56 | 56 | + | Moderate |
| Deterioration of overall functioning (Karnofsky Performance Status Scale a ) | [53] | 56 | 56 | + | Moderate |
| Diet consistency | [53] | 56 | 56 | − | Moderate |
| Larger target volume | [15] | 641 | 641 | + | Limited/Moderate |
| Shorter time after treatment | [15] | 641 | 641 | + | Limited/Moderate |
| Smaller baseline mouth opening | [15] | 641 | 641 | + | Limited/Moderate |
|
Localization |
[16;53;15] | 143;56;641 | 840 | + |
Conflicting Oral cavity (predominantly maxilla) and oropharynx vs other localizations b |
| 54 | 26 | 26 | ± | ||
| [14;57;53 c ] | 64;29;56 | 149 | − | ||
| Age | [15] | 641 | 641 | + | Conflicting b |
| [14] | 64 | 64 | − | ||
|
T classification |
[15] | 641 | 641 | + |
Conflicting T classification stage 4 vs other stages. b |
| [14;16] | 64;143 | 207 | − | ||
|
Reconstruction |
[58] | 65 | 65 | + |
Conflicting Platysma flap vs submental flap b |
| [14;16] | 64;143 | 207 | − | ||
|
Treatment modalities |
[16;43] | 143;16 | 159 | + |
Conflicting b Multiple treatment modalities vs single treatment modality;(Chemo) radiotherapy vs surgery > 6months |
| [14;55] | 64;96 | 160 | ± | ||
| [54] | 26 | 26 | − | ||
| Sex | [15] | 641 | 641 | + | Conflicting |
| [14] | 64 | 64 | ± | ||
| [16;62] | 143;372 | 515 | − | ||
| Dental status | [14;16] | 64;143 | 207 | − | Limited |
| Alcohol | [16] | 143 | 143 | − | Limited |
| Smoking | [16] | 143 | 143 | − | Limited |
| N classification | [14] | 64 | 64 | − | Limited |
| −509 genotype | [56] | 62 | 62 | ± | Limited |
| Higher radiation dose | [54] | 26 | 26 | + | Limited |
| Increased score on perceived difficulties opening the mouth | |||||
| Shorter time after treatment | [76] | 117 | 117 | + | Limited/moderate |
|
Treatment modalities |
[74;76] | 111;117 | 228 | − |
Conflicting Multiple treatment modalities vs single treatment modality;Chemo radiotherapy vs radiotherapy; Three dimensional radiotherapy vs intensity modulated radiotherapy >6 months b |
| [35;71] | 241;60 | 301 | ± | ||
| Higher social economic status | [46] | 161 | 161 | + | Limited |
| No addition of electrofrequency | [72] | 83 | 83 | + | Limited |
| Localization | [65] | 80 | 80 | − | Limited |
| T stage | [65] | 80 | 80 | − | Limited |
Note: [number], reference of study, univariate analysis; [number], reference of study, multivariate analysis; +, significant association found between factor and outcome measure; −, no significant association found between factor and outcome measure; ±, partial association found between elements within a factor and outcome measure.
Karnofsky Performance Scale: an index used to classify functional impairment, using a scale of 0‐100.
Significant associations found between factor and outcome measure on the basis of a particular categorization. This particular categorization is written in italics.
This study analyzed the effects of “radiation field in the area of the oral cavity and oropharynx” on maximal mouth opening, and is therefore included as part of the potential prognostic factor: “localization.”
Conflicting evidence was mainly the result of a different categorization of a particular factor across the studies. For instance, a significant association between factor tumor localization and MMO was found, if tumor localization was categorized in the two categories: “oral cavity and oropharynx” vs “nasopharynx, hypopharynx and larynx.” 53 However, no significant association was found between factor tumor localization and MMO, if tumor localization was categorized in the two categories “oral cavity” vs “oropharynx.” 14
Significant associations were found between a reduction in MMO and the factors: T classification: if stage 4 was compared to other stages; treatment modalities, if multiple treatment modalities were compared to a single treatment modality or (chemo)radiotherapy was compared to surgery more than 6 months after treatment; reconstruction, if platysma flap was compared with a submental flap. A significant association between higher scores on perceived difficulties opening the mouth and the factor treatment modality was found, if multiple treatment modalities were compared to one single treatment modality or chemoradiotherapy was compared to radiotherapy alone. The largest reductions on MMO were found for a greater target volume (limited to moderate evidence) and the presence of mucositis after radiotherapy (moderate evidence) (Table 4, estimated effects). The greatest increases for perceived difficulties opening the mouth were found for a combination of treatment modalities given (conflicting evidence) and time after treatment (limited to moderate evidence) (Table 4, estimated effects).
4. DISCUSSION
4.1. Key results
A restricted mouth opening is most likely in patients with head and neck cancer who have a large tumor near the masticatory muscles that requires extensive cancer treatment. A restricted mouth opening is most likely to occur in the first 6 months after cancer treatment.
4.2. Quality of studies
Overall, the quality of the studies was poor. Most studies had a high risk of bias. Two studies had a low risk of bias, but these studies did not build a multivariate prognostic model. Factors that were most likely to affect trismus, MMO, or perceiving difficulties opening the mouth, were identified and described. These studies had moderate, limited, or conflicting levels of evidence. Levels of strong evidence were not reached. Nonetheless, this systematic review gives insight into the factors that should be taken into account in future research on a restricted mouth opening in patients with head and neck cancer.
4.3. Prognostic factors
Moderate evidence was found for the influence of mucositis after radiotherapy on a reduction in MMO. 53 The effect of mucositis on mouth opening is probably related to the associated healing tendency and the associated pain, since it was noted that MMO decreased in the presence of mucositis and increased when the mucositis resolved. 78 The effects of pain on MMO, analyzed in the form of pain medication or alcohol (which may act as a pain killer as well) have also been reported. 16 , 47 , 48 The effects of factors related to the healing tendency or pain intensity on a restricted mouth opening should be explored further in future studies.
The healing process might also influence the impact of other factors (such as time after treatment and different types of treatment modalities) on a restricted mouth opening. If time passes, it is likely that the affected tissues will heal. The MMO might become less restricted or even increase over time. 15 The healing process might also differ per treatment modality. For instance, in one study, the differences in MMO reduction between surgery and (chemo) radiotherapy over time were displayed: patients who had surgery had a decrease in MMO directly after treatment, but the MMO increased in the 6 months thereafter, whereas the patients who received (chemo) radiotherapy had a decrease in MMO directly after treatment, but the MMO did not increase in the 6 months thereafter. The healing process after (chemo) radiotherapy takes more time than after surgery. 14
Besides the healing process, tumor localization might influence MMO as well, although the evidence is conflicting. The greatest reduction in MMO is most likely when the tumor is located near risk structures. Risk structures involve the temporomandibular joint and the masticatory muscles. A decrease in MMO and an increase in perceived difficulties with opening the mouth were found when the tumor was located in proximity of these risk structures, such as the oral cavity, oropharynx and nasopharynx, nasal cavity, and maxillary sinus. A former systematic review on risk factors for trismus included only one study (the Goldstein et al 51 ) that found that the MMO was reduced by 18% (SD 17%) when the temporomandibular joint and/or the pterygoid muscles were affected. 9 A later review concluded that the masticatory related structures generally affect MMO, but the masseter muscle had the strongest influence. 79 More recently, the ipsilateral medial pterygoid muscle 19 , 80 and the masseter muscle 49 , 80 were identified as the structures most likely to result in a decrease in MMO.
A larger target volume, and also a stage IV tumor, resulted in a large reduction of MMO. 15 Both findings are in contrast with other studies. 14 , 16 , 54 , 57 , 65 Presumably, a significant effect was found for such a large tumor, because more risk structures were involved and more extensive cancer treatment was necessary.
There is limited to moderate evidence that baseline mouth opening affect MMO. 15 A smaller baseline mouth opening results in a larger decrease in MMO. This large decrease in MMO means that the risk of trismus will be greater. As an elaboration of this found effect, a baseline mouth opening of 46 mm or less was determined, as a cut‐off point for developing trismus. 80
The described effects of sex and age on MMO are conflicting. One study found that males tend to have a larger decrease over time than females. 14 Another study found that the decrease was the same in males and females over time. 16 Yet another study found that females had a higher risk of a decrease in mouth opening than males. 15 Regarding age, one study found that the mouth opening of younger patients decreased more over time than of older patients. 14 However, another study found that older patients had a higher risk of a decrease in mouth opening than younger patients. 15 The effects of sex and age may have been confounded by other factors not reported or analyzed in those studies. For instance, the genotype of the patients might have influenced the effect. 56 Patients with the homozygous TT −509 genotype experienced a greater reduction in MMO than patients with the homozygous CC or heterozygous CT −509 genotype. However, the evidence for the influence of the −509 genotype is limited.
4.4. Objective and subjective measures over time
Diverse patterns were seen over time regarding perceived difficulties with opening the mouth. Patients' perceptions of difficulties with opening the mouth might be influenced by different factors over time, such as pain, dry mouth, overall emotional functioning, or treatment modalities. 81 , 82
4.5. Strength and limitations
The strength of this study is that we had no restriction concerning publication year or publication language. Four databases were searched in order to include as many studies as possible. Due to the different aims of the studies and subsequently the different designs of the studies, it was challenging to structure and interpret the data. Due to clinical and methodological heterogeneity, no meta‐analysis was conducted. Instead, we performed a best evidence synthesis. Due to this synthesis, we were still able to gain insight into which prognostic factors should be taken into account from the 53 included studies. The results of this systematic review should be viewed cautiously because of high risk of bias in the source studies.
We used the QUIPS tool to assess bias but it was not really suitable for those studies whose primary aim was not to analyze trismus prognostic factors, making it difficult to assess the studies. Hence, the overall kappa score was low.
4.6. Future research
Large sample size studies are recommended with multiple structured measurement moments to analyze prognostic factors. The effects of factors related to healing tendency and pain intensity on trismus, decrease in MMO, and perceived difficulties with opening the mouth should be studied further.
5. CONCLUSION
A restricted mouth opening is most likely when the patient with head and neck cancer has a large tumor located in close proximity to the mastication muscles or temporomandibular joint that requires extensive cancer treatment. A restricted mouth opening will most likely occur in the first 6 months after cancer treatment. More research is needed on the effect of factors related to healing tendency and pain intensity on a restricted mouth opening.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Data S1: Supporting information.
ACKNOWLEDGMENTS
We would like to thank S. van der Werf for her assistance with building the best possible search strategy for this review; K. Delli and B. Gareb for their input and assessment of risk of bias; K.C. Bragante and J.W. Wetzels for sharing data of their studies, X. Lu for translation of an article.
van der Geer SJ, van Rijn PV, Roodenburg JLN, Dijkstra PU. Prognostic factors associated with a restricted mouth opening (trismus) in patients with head and neck cancer: Systematic review. Head & Neck. 2020;42:2696–2721. 10.1002/hed.26327
Registration: Prospero CRD42017071400.
REFERENCES
- 1. Kamstra JI, Jager‐Wittenaar H, Dijkstra PU, et al. Oral symptoms and functional outcome related to oral and oropharyngeal cancer. Support Care Cancer. 2011;19:1327‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199‐212. [DOI] [PubMed] [Google Scholar]
- 3. Bensadoun RJ, Riesenbeck D, Lockhart PB, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18:1033‐1038. [DOI] [PubMed] [Google Scholar]
- 4. Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health‐related quality of life in patients attending a Maxillofacial Oncology clinic. Oral Oncol. 2008;44:430‐438. [DOI] [PubMed] [Google Scholar]
- 5. Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson‐Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389‐398. [DOI] [PubMed] [Google Scholar]
- 6. Jansma J, Vissink A, Bouma J, Vermey A, Panders AK, Gravenmade EJ. A survey of prevention and treatment regimens for oral sequelae resulting from head and neck radiotherapy used in Dutch radiotherapy institutes. Int J Radiat Oncol Biol Phys. 1992;24:359‐367. [DOI] [PubMed] [Google Scholar]
- 7. Johnson J, Johansson M, Ryden A, Houltz E, Finizia C. Impact of trismus on health‐related quality of life and mental health. Head Neck. 2015;37:1672‐1679. [DOI] [PubMed] [Google Scholar]
- 8. Louise Kent M, Brennan MT, Noll JL, et al. Radiation‐induced trismus in head and neck cancer patients. Support Care Cancer. 2008;16:305‐309. [DOI] [PubMed] [Google Scholar]
- 9. Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40:879‐889. [DOI] [PubMed] [Google Scholar]
- 10. Kamstra JI, van Leeuwen M, Roodenburg JL, Dijkstra PU. Exercise therapy for trismus secondary to head and neck cancer: a systematic review. Head Neck. 2017;39:160‐169. [DOI] [PubMed] [Google Scholar]
- 11. Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer. 2013;21:951‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loorents V, Rosell J, Karlsson C, Lidback M, Hultman K, Borjeson S. Prophylactic training for the prevention of radiotherapy‐induced trismus—a randomised study. Acta Oncol. 2014;53:530‐538. [DOI] [PubMed] [Google Scholar]
- 13. Yan WP, Chen LH, Xu ZX, Deng XG. Etiological analysis of the sequelae of radiotherapy for nasopharyngeal carcinoma: a follow‐up study of 112 cases. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:1002‐1005. [PubMed] [Google Scholar]
- 14. Scott B, D'Souza J, Perinparajah N, Lowe D, Rogers SN. Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Oral Maxillofac Surg. 2011;49:106‐111. [DOI] [PubMed] [Google Scholar]
- 15. Kamstra JI, Dijkstra PU, van Leeuwen M, Roodenburg JL, Langendijk JA. Mouth opening in patients irradiated for head and neck cancer: a prospective repeated measures study. Oral Oncol. 2015;51:548‐555. [DOI] [PubMed] [Google Scholar]
- 16. Wetzels JW, Merkx MA, de Haan AF, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1‐year prospective study. Head Neck. 2014;36:1754‐1762. [DOI] [PubMed] [Google Scholar]
- 17. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. A prospective study on quality of life of patients with cancer of the oral cavity or oropharynx treated with surgery with or without radiotherapy. Oral Oncol. 1999;35:27‐32. [DOI] [PubMed] [Google Scholar]
- 18. Hammerlid E, Silander E, Hornestam L, Sullivan M. Health‐related quality of life three years after diagnosis of head and neck cancer—a longitudinal study. Head Neck. 2001;23:113‐125. [DOI] [PubMed] [Google Scholar]
- 19. Rao SD, Saleh ZH, Setton J, et al. Dose‐volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2016;55:99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oates JE, Clark JR, Read J, et al. Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:533‐540. [DOI] [PubMed] [Google Scholar]
- 21. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280‐286. [DOI] [PubMed] [Google Scholar]
- 22. West S, King V, Carey TS, Lohr KN, Sutton SF, Lux L. Systems to Rate the Strength of Scientific Evidence: Summary. 2002;Technology Assessment No. 47. AHRQ Publication No. 02‐E016. [PMC free article] [PubMed]
- 23. Lohr KN. Rating the strength of scientific evidence: relevance for quality improvement programs. International J Qual Health Care. 2004;16:9‐18. [DOI] [PubMed] [Google Scholar]
- 24. Infante‐Cossio P, Torres‐Carranza E, Cayuela A, Hens‐Aumente E, Pastor‐Gaitan P, Gutierrez‐Perez JL. Impact of treatment on quality of life for oral and oropharyngeal carcinoma. Int J Oral Maxillofac Surg. 2009;38:1052‐1058. [DOI] [PubMed] [Google Scholar]
- 25. Krasin MJ, Wiese KM, Spunt SL, et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head‐and‐neck sarcomas. Int J Radiat Oncol Biol Phys. 2012;82:355‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity‐modulated radiotherapy vs. conventional radiotherapy for early‐stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981‐991. [DOI] [PubMed] [Google Scholar]
- 27. Verdonck‐de Leeuw IM, Buffart LM, Heymans MW, et al. The course of health‐related quality of life in head and neck cancer patients treated with chemoradiation: a prospective cohort study. Radiother Oncol. 2014;110:422‐428. [DOI] [PubMed] [Google Scholar]
- 28. Bjordal K, Ahlner‐Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope. 2001;111:1440‐1452. [DOI] [PubMed] [Google Scholar]
- 29. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long‐term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98‐106. [DOI] [PubMed] [Google Scholar]
- 30. Fang FM, Chien CY, Kuo SC, Chiu HC, Wang CJ. Changes in quality of life of head‐and‐neck cancer patients following postoperative radiotherapy. Acta Oncol. 2004;43:571‐578. [DOI] [PubMed] [Google Scholar]
- 31. Fang FM, Tsai WL, Chien CY, et al. Changing quality of life in patients with advanced head and neck cancer after primary radiotherapy or chemoradiation. Oncology. 2005;68:405‐413. [DOI] [PubMed] [Google Scholar]
- 32. Nordgren M, Jannert M, Boysen M, et al. Health‐related quality of life in patients with pharyngeal carcinoma: a five‐year follow‐up. Head Neck. 2006;28:339‐349. [DOI] [PubMed] [Google Scholar]
- 33. Ohrn KE, Sjoden PO, Wahlin YB, Elf M. Oral health and quality of life among patients with head and neck cancer or haematological malignancies. Support Care Cancer. 2001;9:528‐538. [DOI] [PubMed] [Google Scholar]
- 34. Wiltfang J, Grabenbauer G, Block‐Birkholz A, Leher A, Neukam FW, Kessler P. Beurteilung der Lebensqualität von Patienten mit Plattenepithelkarzinomen der Mundhöhle. Strahlenther Onkol. 2003;179:682‐689. [DOI] [PubMed] [Google Scholar]
- 35. Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity‐modulated radiotherapy reduces radiation‐induced morbidity and improves health‐related quality of life: results of a nonrandomized prospective study using a standardized follow‐up program. Int J Radiat Oncol Biol Phys. 2009;74:1‐8. [DOI] [PubMed] [Google Scholar]
- 36. Chan YW, Chow VL, Wei WI. Quality of life of patients after salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Cancer. 2012;118:3710‐3718. [DOI] [PubMed] [Google Scholar]
- 37. Urdaniz JIA, de la Vega FA, Burgaleta AM, et al. Quality of life in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Comparison of two protocols using the EORTC questionnaires (QLQ‐C30, H&N35). Clin Trans Oncol. 2005;7:398‐403. [DOI] [PubMed] [Google Scholar]
- 38. Carnaby‐Mann G, Crary MA, Schmalfuss I, Amdur R. "Pharyngocise": randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head‐and‐neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:210‐219. [DOI] [PubMed] [Google Scholar]
- 39. Kekatpure VD, Manjula BV, Mathias S, Trivedi NP, Selvam S, Kuriakose MA. Reconstruction of large composite buccal defects using single soft tissue flap—analysis of functional outcome. Microsurgery. 2013;33:184‐190. [DOI] [PubMed] [Google Scholar]
- 40. Zheng Y, Han F, Xiao W, et al. Analysis of late toxicity in nasopharyngeal carcinoma patients treated with intensity modulated radiation therapy. Radiat Oncol. 2015;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pauli N, Fagerberg‐Mohlin B, Andrell P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53:502‐509. [DOI] [PubMed] [Google Scholar]
- 42. Lindblom U, Garskog O, Kjellen E, et al. Radiation‐induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53:620‐627. [DOI] [PubMed] [Google Scholar]
- 43. Al‐Saleh MA, Punithakumar K, Lagravere M, et al. Three‐dimensional morphological changes of the temporomandibular joint and functional effects after mandibulotomy. J Otolaryngol Head Neck Surg. 2017;46:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mu JW, Zhang MJ, Luan BQ, Wu J, Sun P. Quality of life in Chinese patients with laryngeal cancer after radiotherapy. Medicine. 2018;97:e11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veluthattil A, Sudha S, Kandasamy S, Chakkalakkoombil S. Effect of hypofractionated, palliative radiotherapy on quality of life in late‐stage oral cavity cancer: a prospective clinical trial. Indian J Palliat Care. 2019;25:383‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tribius S, Meyer MS, Pflug C, et al. Socioeconomic status and quality of life in patients with locally advanced head and neck cancer. Strahlenther Onkol. 2018;194:737‐749. [DOI] [PubMed] [Google Scholar]
- 47. Lee R, Slevin N, Musgrove B, Swindell R, Molassiotis A. Prediction of post‐treatment trismus in head and neck cancer patients. Br J Oral Maxillofac Surg. 2012;50:328‐332. [DOI] [PubMed] [Google Scholar]
- 48. Pauli N, Johnson J, Finizia C, Andrell P. The incidence of trismus and long‐term impact on health‐related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52:1137‐1145. [DOI] [PubMed] [Google Scholar]
- 49. Pauli N, Olsson C, Pettersson N, et al. Risk structures for radiation‐induced trismus in head and neck cancer. Acta Oncol. 2016;55:788‐792. [DOI] [PubMed] [Google Scholar]
- 50. van der Geer SJ, Kamstra JI, Roodenburg JL, et al. Predictors for trismus in patients receiving radiotherapy. Acta Oncol. 2016;55:1318‐1323. [DOI] [PubMed] [Google Scholar]
- 51. Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:365‐373. [DOI] [PubMed] [Google Scholar]
- 52. Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, Hsiung CY. The degree and time‐course assessment of radiation‐induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope. 2005;115:1458‐1460. [DOI] [PubMed] [Google Scholar]
- 53. Bragante K, Wienandts P, Mozzini C, Pinto R, da Motta N, Jotz G. Jaw mobility changes in patients with upper aerodigestive tract cancer undergoing radiation therapy. Med Oral Patol Oral Cir Bucal. 2015;20:e693‐e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bragante KC, Nascimento DM, Motta NW. Evaluation of acute radiation effects on mandibular movements of patients with head and neck cancer. Rev Bras Fisioter. 2012;16:141‐147. [DOI] [PubMed] [Google Scholar]
- 55. Mucke T, Koschinski J, Wagenpfeil S, et al. Functional outcome after different oncological interventions in head and neck cancer patients. J Cancer Res Clin Oncol. 2012;138:371‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lyons AJ, Crichton S, Pezier T. Trismus following radiotherapy to the head and neck is likely to have distinct genotype dependent cause. Oral Oncol. 2013;49:932‐936. [DOI] [PubMed] [Google Scholar]
- 57. Lazarus CL, Husaini H, Hu K, et al. Functional outcomes and quality of life after chemoradiotherapy: baseline and 3 and 6 months post‐treatment. Dysphagia. 2014;29:365‐375. [DOI] [PubMed] [Google Scholar]
- 58. Safdar J, Liu FY, Moosa Y, Xu ZF, Li ZN, Sun CF. Submental versus platysma flap for the reconstruction of complex facial defects following resection of head and neck tumors. Pak J Med Sci. 2014;30:739‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fong SS, Ng SS, Lee HW, et al. The effects of a 6‐month Tai Chi Qigong training program on temporomandibular, cervical, and shoulder joint mobility and sleep problems in nasopharyngeal cancer survivors. Integr Cancer Ther. 2015;14:16‐25. [DOI] [PubMed] [Google Scholar]
- 60. Manaktala N, Boaz K, Natarajan S, et al. Anticipating oral mucositis in oral cancer patients undergoing fractionated radiotherapy: a cytological correlation. Res J Pharm Biol Chem Sci. 2015;6:294‐301. [Google Scholar]
- 61. Nayar S, Brett R, Clayton N, Marsden J. The Effect of a Radiation Positioning Stent (RPS) in the Reduction of Radiation Dosage to the Opposing Jaw and Maintenance of Mouth opening after Radiation Therapy. Eur J Prosthodont Restor Dent. 2016;24:71‐77. [PubMed] [Google Scholar]
- 62. Lalla RV, Treister N, Sollecito T, et al. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis. 2017;23:1134‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thor M, Olsson CE, Oh JH, et al. Temporal patterns of patient‐reported trismus and associated mouth‐opening distances in radiotherapy for head and neck cancer: a prospective cohort study. Clin Otolaryngol. 2017;43(1):22‐30. 10.1111/coa.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abendstein H, Nordgren M, Boysen M, et al. Quality of life and head and neck cancer: a 5 year prospective study. Laryngoscope. 2005;115:2183‐2192. [DOI] [PubMed] [Google Scholar]
- 65. Borggreven PA, Aaronson NK, Verdonck‐de Leeuw IM, et al. Quality of life after surgical treatment for oral and oropharyngeal cancer: a prospective longitudinal assessment of patients reconstructed by a microvascular flap. Oral Oncol. 2007;43:1034‐1042. [DOI] [PubMed] [Google Scholar]
- 66. Bozec A, Poissonnet G, Chamorey E, et al. Free‐flap head and neck reconstruction and quality of life: a 2‐year prospective study. Laryngoscope. 2008;118:874‐880. [DOI] [PubMed] [Google Scholar]
- 67. Bozec A, Poissonnet G, Chamorey E, et al. Quality of life after oral and oropharyngeal reconstruction with a radial forearm free flap: prospective study. J Otolaryngol Head Neck Surg. 2009;38:401‐408. [PubMed] [Google Scholar]
- 68. Rizvi TA, Rashid M, Ahmed B, et al. Quality of life assessment in patients with locally advanced head and neck malignancy after ablative surgery and reconstruction with microvascular free flaps. J Coll Physicians Surg Pak. 2009;19:108‐112. [PubMed] [Google Scholar]
- 69. Yoshimura R, Shibuya H, Miura M, et al. Quality of life of oral cancer patients after low‐dose‐rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2009;73:772‐778. [DOI] [PubMed] [Google Scholar]
- 70. Al‐Mamgani A, van Rooij P, Tans L, Verduijn GM, Sewnaik A, Baatenburg de Jong RJ. A prospective evaluation of patient‐reported quality‐of‐life after (chemo)radiation for oropharyngeal cancer: which patients are at risk of significant quality‐of‐life deterioration? Radiother Oncol. 2013;106:359‐363. [DOI] [PubMed] [Google Scholar]
- 71. Rathod S, Gupta T, Ghosh‐Laskar S, Murthy V, Budrukkar A, Agarwal J. Quality‐of‐life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity‐modulated radiation therapy (IMRT) compared to three‐dimensional conformal radiotherapy (3D‐CRT): evidence from a prospective randomized study. Oral Oncol. 2013;49:634‐642. [DOI] [PubMed] [Google Scholar]
- 72. Zhao C, Chen J, Yu B, Chen X. Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non‐invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol. 2014;90:853‐858. [DOI] [PubMed] [Google Scholar]
- 73. Arslan SS, Demir N, Cengiz M, Karaduman AA. Swallowing and quality of life outcomes early after radiation therapy in head and neck cancer patients. Fiz Rehab. 2015;26:128‐134. [Google Scholar]
- 74. Kumar A, Sharma A, Mohanti BK, et al. A phase 2 randomized study to compare short course palliative radiotherapy with short course concurrent palliative chemotherapy plus radiotherapy in advanced and unresectable head and neck cancer. Radiother Oncol. 2015;117:145‐151. [DOI] [PubMed] [Google Scholar]
- 75. Landstrom FJ, Reizenstein J, Adamsson GB, Beckerath M, Moller C. Long‐term follow‐up in patients treated with curative electrochemotherapy for cancer in the oral cavity and oropharynx. Acta Otolaryngol. 2015;135:1070‐1078. [DOI] [PubMed] [Google Scholar]
- 76. Dzioba A, Aalto D, Papadopoulos‐Nydam G, et al. Functional and quality of life outcomes after partial glossectomy: a multi‐institutional longitudinal study of the head and neck research network. J Otolaryngol Head Neck Surg. 2017;46;(1):56. 10.1186/s40463-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gao N, Fu K, He W. Assessment of the quality of life of tongue base cancer patients after reconstruction with anterolateral thigh perforator flap. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:214‐218. [DOI] [PubMed] [Google Scholar]
- 78. Sirapracha J, Sessirisombat S. Comparative study on the maximum mouth opening between dynamic and static jaw exercise in irradiated head and neck cancer patients: a randomized control trial. J Oral Maxillofac Surg Med Pathol. 2018;30:307‐312. [Google Scholar]
- 79. Buglione M, Cavagnini R, Di Rosario F, et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: xerostomia and trismus (Part 2). Literature review and consensus statement. Crit Rev Oncol Hematol. 2016;102:47‐54. [DOI] [PubMed] [Google Scholar]
- 80. Kraaijenga SA, Hamming‐Vrieze O, Verheijen S, et al. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck. 2019;41:1387‐1394. [DOI] [PubMed] [Google Scholar]
- 81. van der Geer SJ, van Rijn PV, Kamstra JI, Roodenburg JLN, Dijkstra PU. Criterion for trismus in head and neck cancer patients: a verification study. Support Care Cancer. 2019;27:1129‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loh SY, Mcleod RWJ, Elhassan HA. Trismus following different treatment modalities for head and neck cancer: a systematic review of subjective measures. Eur Arch Otorhinolaryngol. 2017;274:2695‐2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information.


