Abstract
Next‐generation tobacco products and nicotine delivery systems such as heat‐not‐burn tobacco products and electronic cigarettes, the usage of which is expected to have a beneficial impact on public health, have gained popularity over the past decade. However, the risks associated with the long‐term use of such products are still incompletely understood. Although the risks of these products should be clarified through epidemiological studies, such studies are normally performed based on each product category, not product‐by‐product. Therefore, investigation of the risk on a product‐by‐product basis is important to provide specific scientific evidence. In the current study, we performed the 40‐day repeated exposure of in vitro human bronchial epithelial tissues to cigarette smoke (CS) or vapor from our proprietary novel tobacco vapor product (NTV). In addition, tissue samples exposed to CS were switched to NTV or CS exposure was stopped at 20 days to reflect a situation where smokers switched to NTV or ceased to smoke. All tissue samples were assessed in terms of toxicity, inflammation and transcriptomic alterations. Tissue samples switched to NTV and the cessation of exposure samples showed recovery from CS‐induced damage although there was a time‐course difference. Moreover, repeated exposure to NTV produced negligible effects on the tissue samples while CS produced cumulative effects. Our results suggest that the use of NTV, including switching to NTV from cigarette smoking, has fewer effects on bronchial epithelial tissues than continuing smoking.
Keywords: exposure cessation, exposure switching, organotypic three‐dimensional cultures, repeated exposure, vapor product
Short abstract
We carried out the 40‐day repeated exposure of in vitro bronchial epithelial tissues to cigarette smoke (CS) or vapor from novel tobacco vapor product (NTV) and intermediate switching from CS exposure to NTV exposure. Long‐term exposure to NTV resulted in negligible effect on the tissues. Moreover, the tissues that intermediately switched to NTV exposure showed recovery from CS‐induced damage similar to exposure cessation. These results implied that exposure to NTV showed few effects on bronchial epithelial tissues.
1. INTRODUCTION
Novel vapor products, including e‐cigarettes and heat‐not‐burn (HnB) products, have gained substantial popularity. These vapor products produce low yields of potentially harmful constituents in their vapor because of the absence of combustion (Forster et al., 2018; Jaccard et al., 2017; Margham et al., 2016; Takahashi et al., 2018). For example, our proprietary novel tobacco vapor product (NTV) generates the vapor by electrical heating of nicotine‐free liquid, and it evaporates tobacco‐derived flavors and nicotine when passing through the tobacco capsule containing granulated tobacco leaves. Therefore, they are expected to reduce the health risks associated with cigarette smoking. Cigarette smoke (CS) is a known risk factor for chronic inflammatory diseases in respiratory and cardiovascular organs (Forey, Thornton, & Lee, 2011; Go et al., 2014). CS acts as a trigger of oxidative stress and inflammatory responses in the pathogenesis of such diseases (Barreiro et al., 2010; King, 2015; Oh & Sin, 2012; Rahman, Swarska, Henry, Stolk, & MacNee, 2000; Tavilani, Nadi, Karimi, & Goodarzi, 2012). In this context, various in vitro studies were carried out to evaluate the reduced‐risk potential of novel tobacco products in comparison with conventional cigarettes. Iskandar et al. previously reported that exposure to the vapor from a tobacco heating system clearly reduced inflammatory responses and perturbation of transcriptomic profiles compared with CS (Iskandar et al., 2017). Taylor et al. also reported that cells exposed to e‐cigarette vapor had a lower level of reactive oxygen species and related oxidative stress responses than those exposed to CS (Taylor et al., 2016). In addition, we previously conducted a comparative study using a commercially available HnB product, an e‐cigarette, our proprietary NTV and conventional cigarettes, and showed that emissions from all the novel tobacco products were less toxic and less reactive in a human bronchial epithelial cell line than those from conventional cigarettes were (Munakata et al., 2018). Moreover, Philips et al. previously showed that switching from CS exposure to tobacco heating system vapor halted the progression of chronic obstructive pulmonary disease and atherosclerosis in apolipoprotein E knockout mice (Phillips et al., 2016). Several clinical studies reported that smokers who switched to novel tobacco products showed a decrease in levels of biomarkers of exposure, and improvement in biomarkers of potential harm (BoPH) (Haziza et al., 2016; Haziza et al., 2019; Ludicke et al., 2018; Yuki, Takeshige, Nakaya, & Futamura, 2018). However, clinical studies and animal tests have limitations. In clinical studies of tobacco products, it is difficult to detect an improvement in BoPH levels following smoking cessation or switching to novel tobacco products because BoPH levels are easily affected by the lifestyle of individuals. Furthermore, species differences between animals and humans can affect the translation of results from animals to humans (Martignoni, Groothuis, & de Kanter, 2006; Vatner, 2016).
Advanced in vitro tests are considered useful in addressing these issues, because current advances of in vitro techniques have enabled the differentiation of cells from respiratory organs into tissue‐like structures, which incorporate pseudostratified columnar epithelial cells in an air‐liquid interface culture. These three‐dimensional (3D) cultures maintain a tissue‐like structure for extended periods if not affected by external stimuli (Baxter et al., 2015), therefore allowing for long‐term and repeated exposure studies. Our previous studies showed that repeated exposure of 3D cultured bronchial tissues to whole CS exacerbated inflammatory responses (Ishikawa & Ito, 2017), and this phenomenon was recapitulated by repeated exposure to the total particulate matter (TPM) of CS (Ito, Ishimori, & Ishikawa, 2018). We also reported that repeated exposure to whole CS perturbed the metabolomic and transcriptomic profiles in 3D bronchial epithelial tissues, and omics analyses indicated the tissues were under severe oxidative stress with enhanced inflammatory responses using epidermal growth factor receptor as an upstream regulator. The tissues also showed the hypersecretion of mucin proteins on the apical surface (Ishikawa, Matsumura, Kitamura, Takanami, & Ito, 2019). These phenomena are consistent with the adverse outcome pathway involved in decreased lung function as previously reported (Luettich et al., 2017). Therefore, the combination of 3D cultured bronchial epithelial tissues and repeated exposure to CS might help investigate important processes in the pathogenesis of inflammatory respiratory diseases.
In the current study, we repeatedly exposed 3D bronchial epithelial tissues to CS TPM and the aerosol collected mass (ACM) of NTV, and performed intermediate switching from CS exposure to NTV exposure or ceased CS exposure to mimic the situation of smokers who switched to NTV or ceased smoking (Figure 1). As noted above, NTV contains fewer harmful chemical constituents, because even direct heating of tobacco leaves are not associated with the vapor generation, thus we expected that repeated exposure and switching of NTV shows a similar effect with no exposure conditions and exposure cessation respectively. In addition, in vitro switching studies are useful to gain mechanistic insights into the risk reduction associated with NTV use.
FIGURE 1.
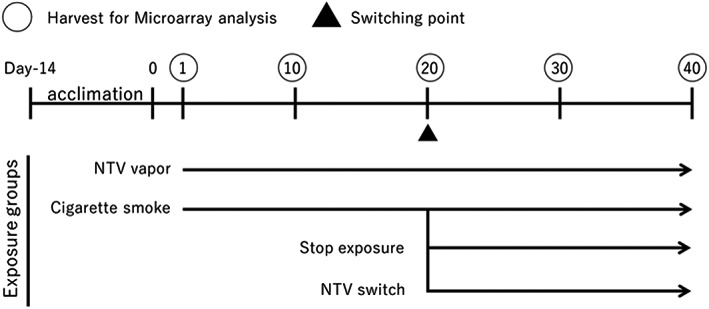
Experimental design. Tissue samples were acclimated for 14 days before exposure, to recover from any damage that occurred during shipping. RNA was extracted at 1, 10, 20, 30 and 40 days (circled) for microarray analysis. Basolateral medium was collected at each medium change, which was performed every 2 or 3 days, and subjected to analyses. NTV, novel tobacco vapor product
2. MATERIALS AND METHODS
2.1. Cell culture
3D cultured bronchial epithelial cells (MucilAir™) and MucilAir culture medium were purchased from Epithelix Sàrl. Cell culture was performed in accordance with the manufacturer's instructions. The donor was a healthy nonsmoker, 53‐year‐old caucasian. MucilAir tissues were acclimated for 14 days before starting exposure to allow spontaneous inflammatory states to diminish (Ito et al., 2018).
2.2. Preparation of cigarette smoke total particulate matter and novel tobacco vapor aerosol collected mass
CS‐TPM and NTV‐ACM were prepared in accordance with our previous report (Ito et al., 2018; Takahashi et al., 2018). CS was generated from 3R4F reference cigarettes purchased from the University of Kentucky, and NTV (PLOOM TECH, manufactured by Japan Tobacco Inc.) was conditioned at 22 ± 2°C and 60% ± 5% relative humidity for at least 48 hours before use. The ventilation hole of the 3R4F was blocked and then applied to a Borgwaldt RM20H smoking machine, and smoke was generated under the International Organization for Standardization Intense smoking regimen (a 55 mL puff taken over 2 seconds, repeated every 30 seconds) (ISO20778, 2018). The TPM was collected on a 45‐mm diameter Cambridge filter pad and extracted using dimethyl sulfoxide (DMSO; Sigma‐Aldrich). The NTV was generated in the same manner as for the 3R4F cigarettes but without the vent‐block (ISO20768, 2018), because the NTV does not have any ventilation holes (the structure of NTV can be found in the previous report; Takahashi et al., 2018). The initial concentration of CS‐TPM and NTV‐ACM were adjusted to 40 mg/mL with DMSO. The CS‐TPM and NTV‐ACM in DMSO solution were then immediately aliquoted for single use and stored at 80°C until use. Each aliquot was thawed just before each medium changing and diluted with the culture medium to create the exposure medium at 20 μg/mL for the CS‐TPM and approximately 75 μg/mL for the NTV‐ACM respectively to align the nicotine concentration, because the nicotine concentration in the ACM of NTV is approximately 3.75‐fold lower than that in CS‐TPM (Takahashi et al., 2018).
2.3. Study design for repeated exposure
After a 14‐day acclimation period, tissue samples were continuously exposed to CS‐TPM or NTV‐ACM for 40 days. Other tissue samples were exposed to CS‐TPM for 20 days and were then switched to NTV‐ACM exposure or to clean medium for a further 20 days to mimic switching to NTV or smoking cessation. Control cultures were maintained without exposure. The medium was changed every 2 or 3 days, by transferring the culture inserts into the new 24‐well culture plate and each well was filled with 700 μL of clean medium or the exposure medium. The concentration of CS and the day of intermediate switching or cessation of exposure was decided based on the results of our previous repeated CS‐TPM exposure study, where we observed a sudden increase in interleukin (IL)‐8 secretion from day 20 (Ito et al., 2018). The study design is shown in Figure 1.
2.4. Adenylate kinase assay
Cytotoxicity induced by repeated exposure was determined using a Toxilight bioassay kit (Lonza) in accordance with the manufacturer's instructions. Basolateral medium sampled at each exposure day was mixed with an adenylate kinase (AK) detection reagent supplied with the kit, and the luminescence of each sample was measured using Tecan infinite® Pro200 (Tecan) after 10 minutes incubation at room temperature. The activity of AK at each exposure day was determined using six replicate samples.
2.5. Measurement of interleukin‐8 secretion
Basolateral medium obtained at each medium change was assessed to determine IL‐8 secretion levels. Concentrations of IL‐8 were measured using a Human Cytokine Magnetic Kit (Merck Millipore) using the Bio‐plex® 200 (Bio‐Rad). Only IL‐8 levels were determined because augmentation of IL‐8 secretion was observed in our previous study (Ito et al., 2018). IL‐8 secretion levels were determined using a minimum of three samples for each exposure day.
2.6. Determination of matrix metalloproteinase 9 secretion
Matrix metalloproteinase (MMP)‐9 levels in the basolateral medium from each exposure group were determined by gelatin zymography. The samples were mixed with nonreducing sodium dodecyl sulfate (SDS) sample buffer and were subjected to polyacrylamide gel electrophoresis. SDS gel electrophoresis was performed using 7.5% acrylamide gels containing 0.9 mg/mL of gelatin. The gels were then washed twice using 2.5 mm Tris‐HCl buffer at pH 7.5 containing 0.5% Triton X‐100 and 150 mm NaCl followed by incubation in 2.5 mm Tris‐HCl buffer at pH 7.5 containing 20 mm NaCl and 10 mm CaCl2 for 20 hours. The gels were stained using 0.1% Coomassie blue and photographed using a LAS‐4000 (GE Healthcare). The intensity values of the untreated control at each exposure day were used for normalization.
Chemicals used for gelatin zymography were purchased from FUJIFILM Wako Pure Chemical Corporation. MMP‐9 secretion levels were determined using triplicate samples for each exposure day.
2.7. Statistical analysis of biological assays
All data are shown as the mean and standard error of the replicates. Parametric one‐ and two‐way analysis of variance (ANOVA) followed by Welch's t‐test with Bonferroni corrections were performed to identify statistically significant differences compared with the control samples for each exposure day. The threshold for statistical significance was set as P < .05. Statistical analyses were performed with JMP® version 14 (SAS Institute).
2.8. RNA preparation
Total RNA was isolated from the tissues at exposure days 1, 20, 30 and 40. RNA purification was performed using the Qiagen RNeasy® Mini Kit (Qiagen) in accordance with the manufacturer's instructions. RNA quality was checked using the ratio of A260 to A280 with Tecan® infinite Pro200, and the ratio of 18S to 28S RNA using a 2100 Bioanalyzer (Agilent Technologies).
2.9. Microarray analysis
Transcriptome data were obtained by microarray analysis conducted by Takara Bio, Inc. cDNA was synthesized from 250 ng of total RNA and then biotinylated cRNA was generated using a GeneChip® 3′ IVT PLUS Reagent Kit (Affymetrix). Fragmentation of cRNA was performed using the GeneChip® Hybridization, Wash and Stain Kit, and 15 μg of biotinylated fragmented cRNA was hybridized to a GeneChip® HG‐U133 Plus 2.0 array for 16 hours at 60 rpm in a 45°C GeneChip® Hybridization Oven 645 (Affymetrix). The arrays were then inserted into a GeneChip® Fluidics Station 450 (Affymetrix) for washing and staining, followed by scanning using a GeneChip® 3000 7G Scanner (Affymetrix). After scanning, expression data were obtained using Affymetrix GeneChip® Command Console software and Affymetrix Expression Console Software 1.4. Transcriptomic data are available in ArrayExpress at accession number E‐MTAB‐8155. All these procedures were in accordance with the Affymetrix GeneChip® Expression Analysis Technical Manual.
2.10. Microarray data processing
Raw data were normalized to the 75th percentile and baseline‐transformed to the mean of the untreated control for each exposure day, and then summarized using GC‐Robust Multiarray Average in GeneSpring Version 14.0 (Agilent Technologies). The summarized data were filtered using a coefficient of variation of < 50%. The filtered gene list was statistically evaluated using a moderated t‐test with correction using the Benjamini‐Hochberg False Discovery ratio (FDR). Statistical significance was identified as FDR‐corrected P < .05 between the exposed samples and the controls at each exposure day. The filtered genes were regarded as differentially expressed probes. Volcano plots were described using R statistics software, with an absolute log2 fold‐change >1.0 and an FDR‐corrected P < .05 as the threshold. Heatmap images were described using R with the heatmap.2 function in the “gplots” packages (Warnes et al., 2020) (Figure S1; see Supporting Information). Transcriptomics datasets used in current study are available in ArrayExpress at accession number E‐MTAB‐8155.
2.11. Ingenuity® pathway analysis
Differentially expressed probes identified after microarray data processing were analyzed using Ingenuity® pathway analysis (IPA) software and each probe was annotated to identify differentially expressed genes (DEGs). Canonical pathway analysis was performed to investigate significantly perturbed biological pathways. An absolute z‐score >2.0 and corrected P < .0005 was used as the strict threshold for continuing CS exposure, and an absolute z‐score >2.0 and corrected P < .05 was used for 10 days after ceasing exposure.
3. RESULTS
3.1. Adenylate kinase activity analysis
We analyzed the activity of AK in basolateral medium as a cytotoxicity indicator. Marked augmentation of AK activity was observed after day 27 for CS exposure repetition, while intermediate switching from CS exposure to NTV or cessation of exposure at day 20 showed less AK activity than continuous CS exposure after day 30. Exposure repetition of NTV throughout the experimental period showed no statistically significant increase in AK activity (Figure 2). Two‐way ANOVA test on the exposure group, exposure duration and their interaction resulted in the F score 46.9210, 31.9361 and 8.6898 respectively, with statistical significance at P < .0001 for both the factors and the interaction.
FIGURE 2.
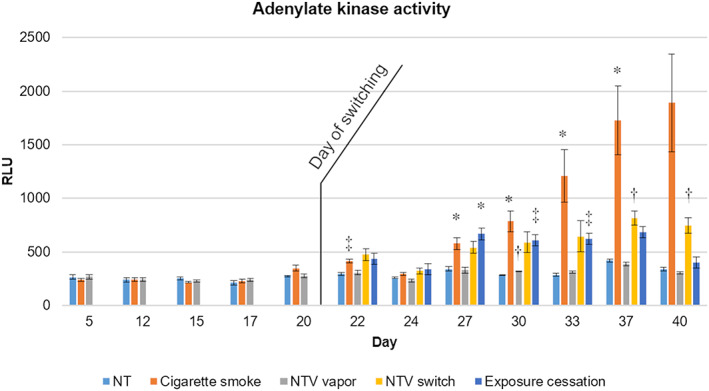
Adenylate kinase activity. Adenylate kinase activity was measured at each time point throughout the experimental period. Data shown are the means of a minimum of six replicates and the error bars indicate standard errors. Statistical significance against untreated tissue controls at each day was calculated using one‐way ANOVA followed by Welch's t‐test with Bonferroni corrections, and is shown as *P < .05, †P < .01, ‡P < .005, §P < .001, respectively. NT, untreated control, NTV, novel tobacco vapor product
3.2. Secretion of interleukin‐8
The time course of altered IL‐8 secretion in basolateral medium was analyzed to investigate the inflammatory state of tissues (Figure 3A). CS exposure elicited a statistically significant increase in IL‐8 at day 12 and further augmentation was found from days 20 to 30. A decline in IL‐8 secretion in CS‐exposed tissue samples was observed after day 33, possibly because of excess cumulative cell damage. Intermediate switching from CS exposure to NTV exposure or cessation of exposure at day 20 resulted in a decline of IL‐8 secretion thereafter. Exposure repetition of NTV throughout the experimental period showed no statistical changes in IL‐8 secretion. Two‐way ANOVA test on the exposure group, exposure duration and their interaction resulted in the F score 42.1132, 15.7628 and 3.4749 respectively, with statistical significance of P < .0001 for both the factors and the interaction.
FIGURE 3.
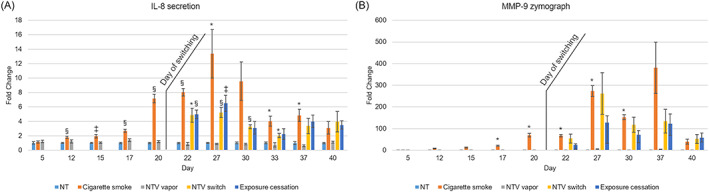
Secretion of inflammatory mediators. IL‐8 and MMP‐9 secreted into the basolateral medium was measured at each time point throughout the experimental period. A, IL‐8. B, MMP‐9. Data shown are means of a minimum of three replicates and the error bars indicate standard errors. Statistical significance against untreated tissue controls on each day was calculated using one‐way ANOVA followed by Welch's t‐test with Bonferroni corrections, and is shown as *P < .05, †P < .01, ‡P < .005, §P < .001, respectively. IL, interleukin; MMP‐9, matrix metalloproteinase‐9; NT, untreated control, NTV, novel tobacco vapor product
3.3. Secretion of matrix metalloproteinase‐9
The time course of altered MMP‐9 secretion in basolateral medium was also measured as a different inflammatory mediator (Figure 3B). Similar to changes in IL‐8 secretion, CS exposure repetition augmented MMP‐9 secretion in an oscillating pattern from day 20, and a statistically significant difference was observed through day 17 to day 30. Intermediate switching from CS exposure to NTV or cessation of exposure at day 20 showed no further significant increase of MMP‐9 secretion. Two‐way ANOVA test on the exposure group, exposure duration and their interaction resulted in the F score 75.4224, 15.1321 and 4.2076 respectively, with a statistical significance of P < .0001 for both the factors and the interaction.
3.4. Microarray analysis of each exposure group
We performed transcriptomic analyses using RNA extracted from tissue samples at days 1, 20, 30 and 40. Volcano plots of each exposure group on each sampled day are shown in Figure 4. Only 22 DEGs were identified in the single exposure of the CS group (day 1), while a marked increase in DEGs was observed after day 20 in the CS exposure group. Switching to NTV exposure decreased DEGs at day 30, which was still observed at day 40. Ten days after the cessation of exposure (day 30) there was no obvious change in DEG number, but this had returned to baseline at day 40. The 40‐day exposure repetition of NTV did not significantly alter the gene expressions throughout the experimental period. All DEGs are summarized in Table S1 (see Supporting Information).
FIGURE 4.
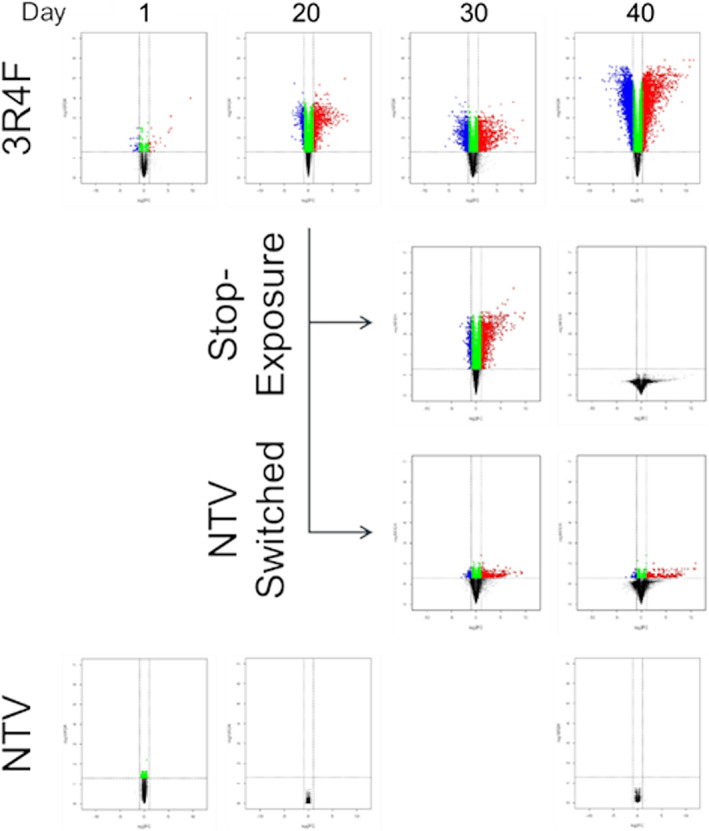
Volcano plot with DEGs identified in each exposure group on each day. DEGs are indicated in volcano plots (P < .05, absolute log2 fold‐change >1.0). Red and blue dots indicate upregulated and downregulated DEGs, respectively. Repeated exposure to vapor from the NTV for 30 days was not performed. DEGs, differentially expressed genes; NTV, novel tobacco vapor product
3.5. Time‐course analysis of transcriptomic alteration in the cigarette smoke‐exposed group
We analyzed the transcriptomic alterations in CS‐exposed tissues by comparing the DEGs in a Venn diagram (Figure 5A). Most DEGs identified in tissues after 20‐ and 30‐day repeated exposures of CS were common: 90% and 97% of DEGs at days 20 and 30, respectively, overlapped with DEGs identified at day 40 (Figure 5A), and 2475 of 4058 DEGs had new transcriptomic alterations at day 40. However, canonical pathway analysis with each identified DEG revealed that pathways perturbed by CS exposure repetition were similar regardless of the duration of CS exposure (Figure 5B). For example, inflammation‐related pathways (such as “IL‐6 Signaling,” “IL‐8 Signaling” and “NF‐κB‐Signaling” in IPA software) were predicted to be activated throughout the CS exposure period.
FIGURE 5.
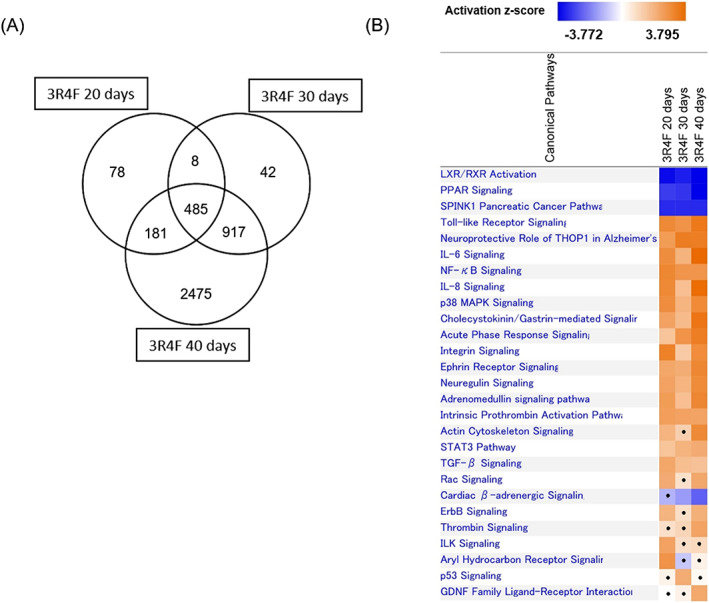
Time‐course analysis of transcriptomic alterations resulting from repeated exposure to cigarette smoke. A, Venn diagram of differentially expressed genes. B, Canonical pathway analysis of differentially expressed genes at days 20, 30 and 40. Significantly perturbed pathways are shown in the heatmap with z‐score. Positive and negative value of z‐score represent predicted activation and inactivation of the biological pathways respectively. Pathways did not reach statistical significance (P ≥ .05) are shown with dot. IL, interleukin; LXR/RXR, liver/retinoid X receptor; MAPK, mitogen‐activated protein kinase; NF‐κB, nuclear factor kappaB; PPAR, peroxisome proliferator‐activated receptor; TGF, transforming growth factor
3.6. Effects of intermediate switching to novel tobacco vapor and cessation of exposure on the transcriptomic profile
The DEGs of each exposure group at days 30 and 40 were subjected to Venn diagram analysis (Figure 6A and 6B) to compare transcriptomic alterations. Most DEGs were common to the exposure groups; however, 219 DEGs specific to the cessation of the exposure group were identified at day 30. Canonical pathway analysis revealed these DEGs were related to cell cycle regulation. Because only three specific DEGs in the NTV switching group and no DEGs in the cessation of the exposure group were found at day 40 respectively, we did not perform canonical pathway analysis with DEGs at day 40.
FIGURE 6.
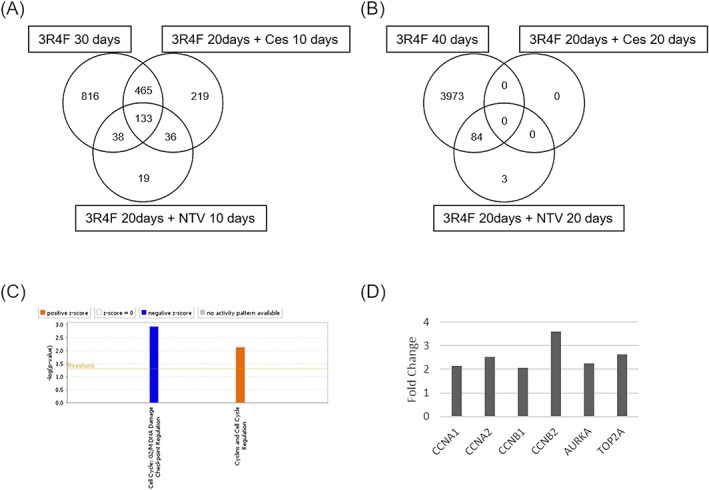
Transcriptomics analysis of tissues after the switching point. A, Venn diagram of the DEGs identified in the tissue samples exposed to cigarette smoke for 30 days, 10 days after switching to NTV and 10 days after cessation of exposure. B, Venn diagram of the DEGs identified in the tissue samples exposed to cigarette smoke for 40 days, 20 days after switching to NTV and 20 days after cessation of exposure. C, Canonical pathway analysis with 219 DEGs identified in samples 10 days after cessation of exposure. D, Expression level of the genes involved in the pathways shown in (C). AURKA, aurora kinase A; CCNA, cyclin A; CCNB, cyclin B; Ces; cessation of exposure; DEGs, differentially expressed genes; NTV, novel tobacco vapor product; TOP2A, topoisomerase II‐alpha
4. DISCUSSION
In the current study, we performed a 40‐day repeated tissue exposure to CS to reproduce the tissues of habitual smokers, and these results were compared with intermediate switching from 20‐day CS exposure to NTV or fresh medium to mimic smokers who switched to NTV or ceased smoking, respectively. We also performed a 40‐day repeated tissue exposure to NTV to mimic the long‐term use of NTV (Figure 1).
AK release from the CS‐exposed group during the first half of the 40‐day exposure revealed that the concentration of CS (20 μg/mL) was low in terms of cytotoxicity (Figure 2), because AK release was comparable with the control tissues. However, a significant increase in AK release was observed after day 22, and an increasing trend continued until day 40. A slight increase in AK release was also observed in the NTV switching group and cessation of the exposure group, but the amplitude was relatively low. This suggested that a further accumulation of cell damage did not occur after switching. Similarly, the secretion of IL‐8 was relatively low until day 16, which then markedly increased thereafter, suggesting inflammatory exacerbation by CS exposure repetition (Figure 3A), even at a low‐dose level as observed in our previous study (Ito et al., 2018). A reduction in IL‐8 level was observed after day 33. Taken together with the increase in AK release during the latter half of the experimental period, this suggested that the decrease in IL‐8 secretion by CS exposure repetition was caused by an excess accumulation of cell damage. Intermediate switching from CS exposure to NTV exposure or the cessation of smoking attenuated the secretion of IL‐8. A previous study reported that levels of IL‐8 and other inflammatory mediators in sputum from asymptomatic smokers with normal lung function were decreased 1 year after smoking cessation (Willemse et al., 2005); this suggests that the tissues observed in the current study are reflective of a similar situation in terms of progressing from active smoking to cessation. Therefore, switching to NTV might be similar to smoking cessation in terms of inflammation.
Interestingly, although the augmentation and decline of MMP‐9 secretion were observed by CS exposure repetition and switching at day 20, respectively (Figure 3B), there was a slight difference with IL‐8 secretion. Increased MMP‐9 secretion oscillated after day 27, and even after switching, increased MMP‐9 secretion was observed at day 27 in the NTV switch group and cessation of the exposure group although it did not reach statistical significance. This suggests that a bias of tissue destruction caused by continuous exposure to CS is not easily recovered, and that a certain period without CS exposure is required. Although there may be difference of lifespan between in vitro cultured epithelium and in vivo epithelium, this result is consistent with the previous study in which Louhelainen et al. reported that the MMP‐9 levels in sputum of smokers were higher than that of nonsmokers, and even 6 months after cessation, the levels remained elevated (Louhelainen et al., 2010). Decline of MMP‐9 secretion was observed in the CS exposure repetition group; however, it might be caused by cumulative cell damage as similar to the secretion of IL‐8. In contrast, the decline of MMP‐9 secretion observed in the NTV switch group and exposure cessation group could mean the attenuation of inflammatory responses because only a slight increase in AK release was observed in these groups. Importantly, MMP‐9 is also known to be associated with acute as well as chronic respiratory diseases, thus considered as the potential biomarker for monitoring airway remodeling (reviewed by Grzela, Litwiniuk, Zagorska, & Grzela, 2016). Therefore, it is suggested that switching to NTV and exposure cessation can contribute to attenuate the progression of CS‐induced airway remodeling.
Transcriptomic analysis suggested cumulative damage in tissues exposed to CS for 40 days, because consecutive increases in DEG counts were observed throughout the experimental period (Figure 4). DEGs identified at days 20 and 30 mostly overlapped, and 2475 DEGs showed newly changed expression levels at day 40 (Figure 5A). However, canonical pathway analysis with IPA software revealed that perturbed biological pathways were similar throughout the CS exposure repetition (Figure 5B); inflammatory response‐related and xenobiotic metabolism‐related pathways (e.g., “IL‐6 Signaling,” “IL‐8 Signaling” and “NF‐κB‐Signaling” and “Aryl hydrocarbon receptor signaling” pathway in IPA software) were perturbed. This result is consistent with the transcriptomic perturbations of human airway epithelium, which Spira et al. previously reported that the pathways related to inflammation, xenobiotic responses and redox signaling were perturbed in the large airway of smokers (Spira et al., 2004). Further analysis of common DEGs in the CS exposure group suggested that the amplitude of these pathway perturbations might show an increasing trend; expression changes in DEGs appeared to amplify as CS exposure repetition increased (Figure S1; see Supporting Information).
Similar to the attenuation of the inflammatory mediator secretions, DEG counts were also decreased after switching to NTV or cessation of exposure, suggesting pathways perturbed by CS exposure until day 20 returned to normal conditions after switching by day 40 (Figure 4) similar to the secretion of inflammatory mediators (Figure 3A and 3B). These results are consistent with the previous clinical study, in which Spira et al. found that gene expressions altered by cigarette smoking could be reverted to normal within 2 years after smoking cessation (Spira et al., 2004). A marked difference between these groups was observed at day 30; NTV switching showed a rapid decrease in DEG counts, whereas gene expression changes were maintained in the cessation of the exposure group at day 30 (Figure 4). Therefore, we considered that process in recovery from CS‐induced biological perturbation were different. We further analyzed differences in the gene expression profiles of each group, and found that DEGs mostly overlapped between the continuous exposure group, NTV switching group and cessation of the exposure group (Figure 6A). However, there were 219 DEGs with newly altered expression levels in the cessation of the exposure group at day 30, and canonical pathway analysis revealed that these genes were related to cell cycle regulation (Figure 6C); gene expression levels of cyclin A, cyclin B, Aurora kinase A and DNA topoisomerase II alpha in the exposure cessation tissue samples were upregulated (Figure 6D). This implied that the cells were in transition from late G2 phase to M phase (Gong & Ferrell, 2010; Sakaguchi & Kikuchi, 2004; Willems et al., 2018). Although further investigation is needed, the perturbation of these pathways might reflect ongoing tissue repair because progression of the cell cycle is essential for the proliferation of cells. In addition, repeated exposure to NTV for 40 days at a comparable nicotine concentration with CS produced no effects on IL‐8 and MMP‐9 secretions or gene expression profiles, because the levels were similar to those of the untreated controls (Figure 3A and 3B) and no DEGs were detected (Figure 4). These results support the hypothesis that exposure to NTV is similar to nonsmoking.
To the best of our knowledge, this is the first report investigating the long‐term effects of switching to NTV in an in vitro bronchial epithelial 3D culture model. Overall, our results suggest that exposure to NTV is similar to nonsmoking and that switching to NTV is similar to smoking cessation. However, the in vitro 3D culture model used in the current study lacks various cells that are present in human tissues in vivo, such as immune cells and smooth muscle cells, and cell‐to‐cell interactions between these cells and epithelial cells are important not only to maintain tissue homeostasis but also for the alteration of morphological and functional changes as well as the other biological perturbations (e.g., inflammation, oxidative stress responses) (Aufderheide, Ito, Ishikawa, & Emura, 2017; Chandorkar et al., 2017; Ishikawa, Ishimori, & Ito, 2017; Iskandar et al., 2015; Marescotti et al., 2019). The results of the current study are limited to the alteration of inflammatory state and global transcriptome, but the morphological and functional changes, and their repairs should be investigated further in future studies.
In addition, the tissues we used in the current study were derived from a single donor, but primary cells could show donor‐specific characteristics depending on the original characteristics; therefore, further study with several different donor tissues would be needed. Taken together, there is still scope for improvement in terms of more accurately reflecting the respiratory epithelium. Moreover, we constitutively exposed tissue samples to NTV at a concentration that was aligned to CS in terms of nicotine levels; however, product use and vaping behavior, the method and frequency of vaping of individual users should be taken into account in the experimental design because these conditions will be different depending on the product specifications and the smoking/vaping experiences of users (Evans & Hoffman, 2014; St Helen et al., 2016). Although further scientific evidence, including clinical and nonclinical studies, is necessary to elucidate the reduced risk potential of NTV, we think the results revealed in the current study suggest that NTV has the potential for reduced risk compared with combustible cigarettes.
FUNDING
This study was supported by Japan Tobacco Inc. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
This work involved an NTV product (PLOOM TECH, manufactured by Japan Tobacco). All authors are employees of Japan Tobacco Inc.
Supporting information
Figure S1 Transcriptomics analysis of common differentially expressed genes (DEGs) throughout the exposure period in cigarette smoke‐exposed tissues. (A) Heatmap image of common DEGs. Lines in the heatmap represent the fold change value of each identified DEG. (B) Canonical pathway analysis of common DEGs.
Table S1 Differentially expressed genes in each exposure group at each sampling day. The table shows the gene symbol, Entrez gene name, P‐value,and log2 fold change value for each differentially expressed gene (P <.05, log2FC ≥2.0) in alphabetical order.
ACKNOWLEDGEMENTS
We are grateful to Dr. Tomoki Nishino and Dr. Hitoshi Fujimoto for helpful discussions and suggestions on this project.
Ito S, Matsumura K, Ishimori K, Ishikawa S. In vitro long‐term repeated exposure and exposure switching of a novel tobacco vapor product in a human organotypic culture of bronchial epithelial cells. J Appl Toxicol. 2020;40:1248–1258. 10.1002/jat.3982
REFERENCES
- Aufderheide, M. , Ito, S. , Ishikawa, S. , & Emura, M. (2017). Metaplastic phenotype in human primary bronchiolar epithelial cells after repeated exposure to native mainstream smoke at the air‐liquid interface. Experimental and Toxicologic Pathology, 69(5), 307–315. 10.1016/j.etp.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Barreiro, E. , Peinado, V. I. , Galdiz, J. B. , Ferrer, E. , Marin‐Corral, J. , Sanchez, F. , … ENIGMA in COPD Project . (2010). Cigarette smoke‐induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. American Journal of Respiratory and Critical Care Medicine, 182(4), 477–488. 10.1164/rccm.200908-1220OC [DOI] [PubMed] [Google Scholar]
- Baxter, A. , Thain, S. , Banerjee, A. , Haswell, L. , Parmar, A. , Phillips, G. , & Minet, E. (2015). Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia. Toxicology in Vitro, 29(5), 864–875. 10.1016/j.tiv.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Chandorkar, P. , Posch, W. , Zaderer, V. , Blatzer, M. , Steger, M. , Ammann, C. G. , … Wilflingseder, D. (2017). Fast‐track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Scientific Reports, 7(1), 1–13. 10.1038/s41598-017-11271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, S. E. , & Hoffman, A. C. (2014). Electronic cigarettes: Abuse liability, topography and subjective effects. Tobacco Control, 23(Suppl 2), ii23–ii29. 10.1136/tobaccocontrol-2013-051489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forey, B. A. , Thornton, A. J. , & Lee, P. N. (2011). Systematic review with meta‐analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulmonary Medicine, 11, 36 10.1186/1471-2466-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, M. , Fiebelkorn, S. , Yurteri, C. , Mariner, D. , Liu, C. , Wright, C. , … Proctor, C. (2018). Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regulatory Toxicology and Pharmacology, 93, 14–33. 10.1016/j.yrtph.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Go, A. S. , Mozaffarian, D. , Roger, V. L. , Benjamin, E. J. , Berry, J. D. , Blaha, M. J. , … Stroke Statistics Subcommittee . (2014). Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation, 129(3), e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, D. , & Ferrell, J. E. Jr. (2010). The roles of cyclin A2, B1, and B2 in early and late mitotic events. Molecular Biology of the Cell, 21(18), 3149–3161. 10.1091/mbc.E10-05-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzela, K. , Litwiniuk, M. , Zagorska, W. , & Grzela, T. (2016). Airway remodeling in chronic obstructive pulmonary disease and asthma: The role of matrix metalloproteinase‐9. Archivum Immunologiae et Therapiae Experimentalis (Warsz), 64(1), 47–55. 10.1007/s00005-015-0345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haziza, C. , de La Bourdonnaye, G. , Donelli, A. , Skiada, D. , Poux, V. , Weitkunat, R. , … Ludicke, F. (2019). Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the menthol tobacco heating system 2.2 for three months (Part 2). Nicotine & Tobacco Research, 1–11. ntz084 10.1093/ntr/ntz084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haziza, C. , de La Bourdonnaye, G. , Skiada, D. , Ancerewicz, J. , Baker, G. , Picavet, P. , & Ludicke, F. (2016). Evaluation of the Tobacco Heating System 2.2. Part 8: 5‐Day randomized reduced exposure clinical study in Poland. Regulatory Toxicology and Pharmacology, 81(Suppl 2), S139–S150. 10.1016/j.yrtph.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Ishikawa, S. , Ishimori, K. , & Ito, S. (2017). A 3D epithelial‐mesenchymal co‐culture model of human bronchial tissue recapitulates multiple features of airway tissue remodeling by TGF‐beta1 treatment. Respiratory Research, 18(1), 195 10.1186/s12931-017-0680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, S. , & Ito, S. (2017). Repeated whole cigarette smoke exposure alters cell differentiation and augments secretion of inflammatory mediators in air‐liquid interface three‐dimensional co‐culture model of human bronchial tissue. Toxicology in Vitro, 38, 170–178. 10.1016/j.tiv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Ishikawa, S. , Matsumura, K. , Kitamura, N. , Takanami, Y. , & Ito, S. (2019). Multi‐omics analysis: Repeated exposure of a 3D bronchial tissue culture to whole‐cigarette smoke. Toxicology in Vitro, 54, 251–262. 10.1016/j.tiv.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Iskandar, A. R. , Martinez, Y. , Martin, F. , Schlage, W. K. , Leroy, P. , Sewer, A. , … Hoeng, J. (2017). Comparative effects of a candidate modified‐risk tobacco product aerosol and cigarette smoke on human organotypic small airway cultures: A systems toxicology approach. Toxicological Research, 6(6), 930–946. 10.1039/c7tx00152e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar, A. R. , Xiang, Y. , Frentzel, S. , Talikka, M. , Leroy, P. , Kuehn, D. , … Hoeng, J. (2015). Impact assessment of cigarette smoke exposure on organotypic bronchial epithelial tissue cultures: A comparison of mono‐culture and coculture model containing fibroblasts. Toxicological Sciences, 147(1), 207–221. 10.1093/toxsci/kfv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO20768 . (2018). Vapour products—Routine analytical vaping machine—Definitions and standard conditions.
- ISO20778 . (2018). Cigarettes—Routine analytical cigarette smoking machine—Definitions and standard conditions with an intense smoking regime.
- Ito, S. , Ishimori, K. , & Ishikawa, S. (2018). Effects of repeated cigarette smoke extract exposure over one month on human bronchial epithelial organotypic culture. Toxicology Reports, 5, 864–870. 10.1016/j.toxrep.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard, G. , Tafin Djoko, D. , Moennikes, O. , Jeannet, C. , Kondylis, A. , & Belushkin, M. (2017). Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regulatory Toxicology and Pharmacology, 90, 1–8. 10.1016/j.yrtph.2017.08.006 [DOI] [PubMed] [Google Scholar]
- King, P. T. (2015). Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clinical and Translational Medicine, 4(1), 68 10.1186/s40169-015-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhelainen, N. , Stark, H. , Mazur, W. , Rytila, P. , Djukanovic, R. , & Kinnula, V. L. (2010). Elevation of sputum matrix metalloproteinase‐9 persists up to 6 months after smoking cessation: a research study. BMC Pulmonary Medicine, 10, 13 10.1186/1471-2466-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludicke, F. , Picavet, P. , Baker, G. , Haziza, C. , Poux, V. , Lama, N. , & Weitkunat, R. (2018). Effects of Switching to the Tobacco Heating System 2.2 menthol, smoking abstinence, or continued cigarette smoking on biomarkers of exposure: A randomized, controlled, open‐label, multicenter study in sequential confinement and ambulatory settings (Part 1). Nicotine & Tobacco Research, 20(2), 161–172. 10.1093/ntr/ntw287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luettich, K. , Talikka, M. , Lowe, F. J. , Haswell, L. E. , Park, J. , Gaca, M. D. , & Hoeng, J. (2017). The adverse outcome pathway for oxidative stress‐mediated EGFR activation leading to decreased lung function. Applied in vitro Toxicology, 3(1), 99–109. 10.1089/aivt.2016.0032 [DOI] [Google Scholar]
- Marescotti, D. , Serchi, T. , Luettich, K. , Xiang, Y. , Moschini, E. , Talikka, M. , … Hoeng, J. (2019). How complex should an in vitro model be? Evaluation of complex 3D alveolar model with transcriptomic data and computational biological network models. ALTEX, 36(3), 388–402. 10.14573/altex.1811221 [DOI] [PubMed] [Google Scholar]
- Margham, J. , McAdam, K. , Forster, M. , Liu, C. , Wright, C. , Mariner, D. , & Proctor, C. (2016). Chemical composition of aerosol from an E‐cigarette: A quantitative comparison with cigarette smoke. Chemical Research in Toxicology, 29(10), 1662–1678. 10.1021/acs.chemrestox.6b00188 [DOI] [PubMed] [Google Scholar]
- Martignoni, M. , Groothuis, G. M. , & de Kanter, R. (2006). Species differences between mouse, rat, dog, monkey and human CYP‐mediated drug metabolism, inhibition and induction. Expert Opinion on Drug Metabolism & Toxicology, 2(6), 875–894. 10.1517/17425255.2.6.875 [DOI] [PubMed] [Google Scholar]
- Munakata, S. , Ishimori, K. , Kitamura, N. , Ishikawa, S. , Takanami, Y. , & Ito, S. (2018). Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco‐ and nicotine‐containing products. Regulatory Toxicology and Pharmacology, 99, 122–128. 10.1016/j.yrtph.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Oh, J. Y. , & Sin, D. D. (2012). Lung inflammation in COPD: Why does it matter? F1000 Medicine Reports, 4, 23 10.3410/M4-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, B. , Veljkovic, E. , Boue, S. , Schlage, W. K. , Vuillaume, G. , Martin, F. , … Hoeng, J. (2016). An 8‐month systems toxicology inhalation/cessation study in Apoe−/− mice to investigate cardiovascular and respiratory exposure effects of a candidate modified risk tobacco product, THS 2.2, compared with conventional cigarettes. Toxicological Sciences, 149(2), 411–432. 10.1093/toxsci/kfv243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, I. , Swarska, E. , Henry, M. , Stolk, J. , & MacNee, W. (2000). Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax, 55(3), 189–193. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10679536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, A. , & Kikuchi, A. (2004). Functional compatibility between isoform α and β of type II DNA topoisomerase. Journal of Cell Science, 117(Pt 7), 1047–1054. 10.1242/jcs.00977 [DOI] [PubMed] [Google Scholar]
- Spira, A. , Beane, J. , Shah, V. , Liu, G. , Schembri, F. , Yang, X. , … Brody, J. S. (2004). Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proceedings of the National Academy of Sciences of the United States of America, 101(27), 10143–10148. 10.1073/pnas.0401422101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen, G. , Ross, K. C. , Dempsey, D. A. , Havel, C. M. , Jacob, P. 3rd , & Benowitz, N. L. (2016). Nicotine delivery and vaping behavior during ad libitum E‐cigarette access. Tobacco Regulatory Science, 2(4), 363–376. 10.18001/TRS.2.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y. , Kanemaru, Y. , Fukushima, T. , Eguchi, K. , Yoshida, S. , Miller‐Holt, J. , & Jones, I. (2018). Chemical analysis and in vitro toxicological evaluation of aerosol from a novel tobacco vapor product: A comparison with cigarette smoke. Regulatory Toxicology and Pharmacology, 92, 94–103. 10.1016/j.yrtph.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Tavilani, H. , Nadi, E. , Karimi, J. , & Goodarzi, M. T. (2012). Oxidative stress in COPD patients, smokers, and non‐smokers. Respiratory Care, 57(12), 2090–2094. 10.4187/respcare.01809 [DOI] [PubMed] [Google Scholar]
- Taylor, M. , Carr, T. , Oke, O. , Jaunky, T. , Breheny, D. , Lowe, F. , & Gaca, M. (2016). E‐cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicology Mechanisms and Methods, 26(6), 465–476. 10.1080/15376516.2016.1222473 [DOI] [PubMed] [Google Scholar]
- Vatner, S. F. (2016). Why so few new cardiovascular drugs translate to the clinics. Circulation Research, 119(6), 714–717. 10.1161/CIRCRESAHA.116.309512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes, G. R. , Bolker, B. , Bonebakker, L. , Gentleman, R. , Liaw, W. H. A. , Lumley, T. , … Warnes, G. (2020). gplots: Various R Programming Tools for Plotting Data. Retrieved from https://CRAN.R-project.org/package=gplots
- Willems, E. , Dedobbeleer, M. , Digregorio, M. , Lombard, A. , Lumapat, P. N. , & Rogister, B. (2018). The functional diversity of Aurora kinases: A comprehensive review. Cell Division, 13, 7 10.1186/s13008-018-0040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemse, B. W. , ten Hacken, N. H. , Rutgers, B. , Lesman‐Leegte, I. G. , Postma, D. S. , & Timens, W. (2005). Effect of 1‐year smoking cessation on airway inflammation in COPD and asymptomatic smokers. European Respiratory Journal, 26(5), 835–845. 10.1183/09031936.05.00108904 [DOI] [PubMed] [Google Scholar]
- Yuki, D. , Takeshige, Y. , Nakaya, K. , & Futamura, Y. (2018). Assessment of the exposure to harmful and potentially harmful constituents in healthy Japanese smokers using a novel tobacco vapor product compared with conventional cigarettes and smoking abstinence. Regulatory Toxicology and Pharmacology, 96, 127–134. 10.1016/j.yrtph.2018.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Transcriptomics analysis of common differentially expressed genes (DEGs) throughout the exposure period in cigarette smoke‐exposed tissues. (A) Heatmap image of common DEGs. Lines in the heatmap represent the fold change value of each identified DEG. (B) Canonical pathway analysis of common DEGs.
Table S1 Differentially expressed genes in each exposure group at each sampling day. The table shows the gene symbol, Entrez gene name, P‐value,and log2 fold change value for each differentially expressed gene (P <.05, log2FC ≥2.0) in alphabetical order.


