Abstract
Aims
To examine the manifestation of cardiovascular or renal disease (CVRD) in patients with type 2 diabetes (T2D) initially free from CVRD as well as the mortality risks associated with these diseases.
Methods
Patients free from CVRD were identified from healthcare records in England, Germany, Japan, the Netherlands, Norway and Sweden at a fixed date. CVRD manifestation was defined by first diagnosis of cardiorenal disease, or a stroke, myocardial infarction (MI) or peripheral artery disease (PAD) event. The mortality risk associated with single CVRD history of heart failure (HF), chronic kidney disease (CKD), MI, stroke or PAD was compared with that associated with CVRD‐free status.
Results
Of 1 177 896 patients with T2D, 772 336 (66%) were CVRD‐free and followed for a mean of 4.5 years. A total of 137 081 patients (18%) developed a first CVRD manifestation, represented by CKD (36%), HF (24%), stroke (16%), MI (14%) and PAD (10%). HF or CKD was associated with increased cardiovascular and all‐cause mortality risk: hazard ratio (HR) 2.02 (95% confidence interval [CI] 1.75–2.33) and HR 2.05 (95% CI 1.82–2.32), respectively. HF and CKD were separately associated with significantly increased mortality risks, and the combination was associated with the highest cardiovascular and all‐cause mortality risk: HRs 3.91 (95% CI 3.02–5.07) and 3.14 (95% CI 2.90–3.40), respectively.
Conclusion
In a large multinational study of >750 000 CVRD‐free patients with T2D, HF and CKD were consistently the most frequent first cardiovascular disease manifestations and were also associated with increased mortality risks. These novel findings show these cardiorenal diseases to be important and serious complications requiring improved preventive strategies.
Keywords: diabetic nephropathy, heart failure, macrovascular disease, observational study, type 2 diabetes, SGLT2 inhibitor
1. INTRODUCTION
Type 2 diabetes (T2D) affects more than 425 million patients worldwide,1 with a high prevalence of cardiorenal disease, heart failure (HF) 6%–27% 2 , 3 and chronic kidney disease (CKD) 4%–20%. 2 , 4 Hence, HF and CKD are becoming pandemic complications in T2D. 2 , 5 , 6 , 7 HF and CKD are severe conditions that, separately and in combination, are associated with high symptom burden and cardiovascular risk, mortality risk and healthcare costs, particularly in patients with T2D. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The seriousness of these diseases is further accentuated because a failing heart could lead to kidney failure, and vice versa, through multifaceted inter‐organ crosstalk 16 driving a vicious cycle resulting in cardiorenal syndrome (CRS). 17
Residual risks of HF and CKD in T2D have been reported despite established preventive and cardiovascular disease (CVD) treatment strategies. One recent study reported that optimal management of CVD risk factors in T2D might neutralize the excess risk of myocardial infarction (MI) and stroke; but not the risk of HF, which remained high when compared to patients without T2D. 18 Other reports showed that the prevalence of and mortality risk associated with CKD in clinical practice remains high despite use of renin‐angiotensin inhibition, this being the most commonly used treatment to slow renal function decline in T2D. 19 , 20 Consequently, optimization of classic CVD risk factor treatment may ameliorate the risk of atherosclerotic diseases, 21 but less effectively control risk of HF and CKD in T2D. 22
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors belong to a novel glucose‐lowering drug class, for which cardiovascular outcome trials have shown consistent risk‐reducing effects on hospitalization for and worsening of HF 23 , 24 , 25 , 26 and CKD, 27 , 28 , 29 even in patients with T2D who have no history of established CVD. 28 , 30 , 31 The high HF and CKD prevalence and associated risks 18 , 19 , 20 show an important unmet clinical need that might be considered when choosing preventive strategies in early stages of T2D, taking recent paradigm‐shifting results into account. 22 , 28 , 30 However, current knowledge of the temporal development of HF and CKD, hereafter referred to as cardiorenal disease, in patients with T2D is scarce, but critically important to understand when deciding future cardiovascular preventive strategies.
In this multinational and contemporary cohort study, using well‐established data sources, the objectives were, first to investigate the temporal development of cardiorenal disease among cardiovascular or renal disease (CVRD)‐free patients. Second, to evaluate the risks associated with the type of first CVRD event.
2. MATERIALS AND METHODS
In this study, we used the unique features of available healthcare registries and corresponding healthcare systems' secondary data across six countries: England, Germany, Japan, the Netherlands, Norway and Sweden.
In England, records from the Clinical Practice Research Datalink (CPRD) Aurum data were used. 32 CPRD Aurum is a database containing routinely collected data on more than 20 million patients from 873 primary care practices in England (10% of English practices), of whom seven million (13% of the population of England) were alive and currently contributing to the database as of September 2018 (Supporting Information, pp. 4–5).
Data from Germany were obtained from the Betriebskrankenkassen, a sickness‐fund database consisting of up to 5.1 million insured individuals who are covered by statutory health insurance (Supporting Information, pp. 5–6). The characteristics of patients with T2D are similar to those of other European populations. 33
In Japan, we used the Medical Data Vision Co., Ltd, a hospital‐based database containing administrative claims and laboratory data, linked to the Diagnostic Procedure Combination (flat‐fee payment system) inpatient hospital payment system, covering more than 20 million patients from more than 300 hospitals across the country, corresponding to approximately 20% of the total population in Japan (Supporting Infomation, pp. 6). The demographic characteristics, including the age and sex distributions of these patients, are very similar to those of national statistics in Japan. 34 , 35 , 36
In the Netherlands, data were obtained from the PHARMO Database Network (Supporting Information, pp. 6–7). This population‐based network of electronic healthcare databases combines and links, through validated algorithms, patient‐level data from different primary and secondary healthcare settings, including data from general practices, in‐ and outpatient pharmacies, clinical laboratories, hospitals and the cancer, pathology and perinatal registries. Detailed information on the methodology and the validation of the record linkage method has been described previously. 37 , 38
Both Norway and Sweden have comprehensive, nationwide public healthcare systems (Supporting Information, pp. 7–8). 33 , 39 , 40 All citizens have a unique personal identification number, which is mandatory for all administrative purposes, including any contact with the healthcare system, as well as drug purchases, thus providing a comprehensive medical history of the population. Individual patient‐level data from the Prescribed Drug Registers, the Cause of Death Registers and the National Patient Registers covering all hospitalizations with discharge diagnoses and all outpatient hospital visits, were linked using the personal identification number.
2.1. CVRD‐free patients
All T2D patients (see definition in Supporting Information, pp. 9) 33 , 39 with no recorded history of cardiovascular or renal disease, defined as stroke, MI, angina pectoris (including the use of nitrates), unstable angina pectoris, atrial fibrillation, HF, coronary revascularization, peripheral artery disease (PAD), peripheral artery revascularization, and CKD; hereinafter referred to as CVRD‐free patients with T2D (Table S1). Diseases were searched in prescribed drug and hospital records in all countries except in England and the Netherlands, where additional general practice records were searched. All CVRD‐free patients with T2D were indexed at a fixed date, selected in each individual participating country separately to secure a balance between sufficient follow‐back (patient history) versus follow‐up time: England 2010, Germany 2014; Japan 2016; the Netherlands 2012; Norway 2010 and Sweden 2007 (Table S2). The manifestation of CVRD was examined by observing the CVRD‐free patients with T2D from index to the first recorded CVRD event, thus with different follow‐up times in each country.
2.2. Single‐CVRD manifestation groups
Patients with a single CVRD manifestation, but who were otherwise CVRD‐free, were additionally identified at index. Consecutively, seven additional groups were defined with a single CVRD manifestation, that is, only stroke, MI, PAD, cardiorenal disease (HF or CKD), and its separate components HF, CKD and CRS. These first CVRD manifestation groups were defined in each country and on the same index dates as the CVRD‐free cohorts, and risk of outcomes in these cohorts were compared with the CVRD‐free cohort.
2.3. Outcomes
A first CVRD event was defined in all countries by the first recorded outside‐ or in‐hospital diagnosis of HF (including hypertensive HF), CKD (including diabetic nephropathy, acute kidney failure, CKD, unspecified kidney disease, hypertensive kidney failure and dialysis), cardiorenal disease (diagnosis of HF or CKD), stroke (including ischaemic and haemorrhagic stroke), MI and PAD (Table S3). The following outcomes were used for the estimation of risk associations: all‐cause death (death from any cause), CVD death (death caused by CVD) and CVRD outcomes as described above.
2.4. Statistical analysis
All statistical analyses were performed in each country separately according to a prespecified statistical analysis plan. Baseline characteristics were described using standard statistical measures, such as mean and SD values for numerical variables and frequencies and percentages for categorical variables. The CVD‐free populations are described separately by country and overall where the overall summary is weighted according to the number of patients from each country. The description of the different risk groups is presented as a weighted summary of all countries in the same manner.
The cumulative incidence of the first CVRD disease manifestation among CVRD‐free patients with T2D was analysed using a cumulative incidence function, where the competing risk of the other events, as well as death were taken into account. Patients without an event were censored at end of follow‐up, or when leaving the database. Diagnoses were searched in all available data within each country and, if more than one event occurred at the same date, the main (primary) diagnosis was primarily defined as the event. For sensitivity analyses, diagnoses in first position as well as first and second position were used to define the manifestation event. The results are presented separately by country in cumulative incidence plots (ie, the proportion of patients with an event over time), as well as a description of the relative proportion of event types among patients who experienced an event during follow‐up. All analyses of the cumulative incidence are descriptive, and no formal comparative analyses between countries have been carried out. In order to explore how the risk of all‐cause death, CVD death, MI, stroke and PAD is associated with a baseline manifestation of different CVRD types, we compared patients with only one (single presence) of cardiorenal disease, HF, CKD, CRS, MI, stroke or PAD) with the CVRD‐free population. For example, in patients with single history of HF, those with a concurrent diagnosis of MI were excluded. The bidirectional analyses estimating risk of CKD when there was a single presence of HF and vice versa, used the same analytical approach as described above. The analyses were performed for each endpoint separately using a Cox regression model, with risk group, age and gender as independent variables and with years since index date as timescale. Analyses were performed within each country, and then the hazard ratios (HRs) were pooled for each risk estimate using a random effects meta‐analysis approach. The estimated HRs are presented with 95% confidence intervals (CIs), both per country and for the pooled estimate. No adjustment for multiplicity was performed, but as no inference was based on the results, this was not needed.
In several countries (Japan, Norway and Sweden), where estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) measurements were available for a random subset of patients, the validity of the CKD definition was tested using a simplistic method whereby all patients were classified as CKD 'yes/no' based on all available data (one diagnosis was enough), and the latest available eGFR measurement was used. Using this approach, the predictive probability of eGFR on CKD diagnosis was tested using receiver‐operating characteristic (ROC) curve analysis, including area under the ROC curve. The optimal threshold was estimated using the Youden index. We also tested the validity of the CKD diagnoses set only in primary care and outpatient hospital visits separately.
3. RESULTS
From a total of 1 177 896 general patients with T2D identified across the six included countries, 772 336 (66%) were CVRD‐free at index (Table 2). The distribution was similar in all countries (Table S4). Patients were followed for a mean of 4.5 years, resulting in a total of 3.5 million patient‐years. Patients in Germany, Japan and the Netherlands were in general somewhat older than in the other countries (Table 1). There were minor differences in use of antidiabetic and cardiovascular risk‐lowering therapies between the populations studied, as known from previous reports. Less abundant registration of low‐dose aspirin in Germany, is probably explained by its prescription‐free availability compared to the other countries.
TABLE 2.
Pooled baseline characteristics of patients with type 2 diabetes in England, Germany, Japan, the Netherlands, Norway and Sweden
| All patients with T2D | CVRD‐free (reference group) | Cardiorenal disease | HF | CKD | Cardiorenal syndrome | Stroke | MI | PAD | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 1 177 896 | 772 336 | 60 466 | 17 626 | 42 840 | 8464 | 34 449 | 9376 | 11 633 |
| Age, years (SD) | 67.7 (12.7) | 65.2 (12.6) | 70.9 (12.2) | 73.2 (12.2) | 70.0 (11.9) | 74.7 (11.6) | 71.3 (10.8) | 70.0 (11.0) | 70.2 (10.5) |
| Women, n (%) | 507 305 (43.1) | 345 928 (44.8) | 28 417 (47.0) | 8907 (50.5) | 19 510 (45.5) | 4221 (49.9) | 14 084 (40.9) | 2681 (28.6) | 4290 (36.9) |
| CVD, n (%) | 318 578 (27.0) | 0 (0.0) | 60 466 (100.0) | 17 626 (100.0) | 42 840 (100.0) | 8464 (100.0) | 34 449 (100.0) | 9376 (100.0) | 11 633 (100.0) |
| Stroke, n (%) | 84 467 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 34 449 (100.0) | 0 (0.0) | 0 (0.0) |
| Myocardial infarction, n (%) | 90 839 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9376 (100.0) | 0 (0.0) |
| UAP, n (%) | 45 699 (3.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AP, n (%) | 148 903 (12.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PAD, n (%) | 47 630 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 633 (100.0) |
| Atrial fibrillation, n (%) | 85 828 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiorenal disease, n (%) | 1956 69 (16.6) | 0 (0.0) | 60 466 (100.0) | 17 626 (100.0) | 42 840 (100.0) | 8464 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HF, n | 115 224 (9.8) | 0 (0.0) | 17 626 (29.2) | 17 626 (100.0) | 0 (0.0) | 8464 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CKD, n | 120 597 (10.2) | 0 (0.0) | 42 840 (70.8) | 0 (0.0) | 42 840 (100.0) | 8464 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiorenal syndrome, n (%) | 40 152 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8464 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Microvascular disease, n (%) | 216 342 (18.4) | 790 03 (10.2) | 24 642 (40.8) | 3996 (22.7) | 20 646 (48.2) | 4876 (57.6) | 8251 (24.0) | 2024 (21.6) | 4674 (40.2) |
| CVD prevention, n (%) | 918 896 (78.0) | 558 306 (72.3) | 49 703 (82.2) | 14 525 (82.4) | 35 178 (82.1) | 7394 (87.4) | 28 312 (82.2) | 8364 (89.2) | 10 096 (86.8) |
| Low‐dose aspirin, n (%) | 336 312 (28.6) | 141 815 (18.4) | 15 126 (25.0) | 4100 (23.3) | 11 026 (25.7) | 2134 (25.2) | 14 665 (42.6) | 5685 (60.6) | 5116 (44.0) |
| Statins, n (%) | 562 183 (47.7) | 322 219 (41.7) | 27 872 (46.1) | 6658 (37.8) | 21 214 (49.5) | 3563 (42.1) | 17 437 (50.6) | 6384 (68.1) | 6770 (58.2) |
| Anti‐hypertensives, n (%) | 772 986 (65.6) | 449 539 (58.2) | 44 807 (74.1) | 13 268 (75.3) | 31 539 (73.6) | 6780 (80.1) | 23 449 (68.1) | 7469 (79.7) | 8346 (71.7) |
| ACE, n (%) | 312 719 (26.5) | 160 777 (20.8) | 20 517 (33.9) | 6371 (36.1) | 14 146 (33.0) | 3027 (35.8) | 10 035 (29.1) | 3947 (42.1) | 4277 (36.8) |
| ARBs, n (%) | 329 812 (28.0) | 208 624 (27.0) | 20 214 (33.4) | 5448 (30.9) | 14 766 (34.5) | 3112 (36.8) | 9692 (28.1) | 2522 (26.9) | 3227 (27.7) |
| Beta blockers, n (%) | 379 213 (32.2) | 165 474 (21.4) | 19 373 (32.0) | 8103 (46.0) | 11 270 (26.3) | 4035 (47.7) | 9067 (26.3) | 5881 (62.7) | 3412 (29.3) |
| High ceiling diuretics, n (%) | 220 509 (18.7) | 73 498 (9.5) | 21 459 (35.5) | 9787 (55.5) | 11 672 (27.2) | 5815 (68.7) | 4895 (14.2) | 1892 (20.2) | 2080 (17.9) |
| Aldosterone antagonists, n (%) | 46 933 (4.0) | 13 639 (1.8) | 4197 (6.9) | 2891 (16.4) | 1306 (3.0) | 1191 (14.1) | 826 (2.4) | 412 (4.4) | 354 (3.0) |
| Metformin, n (%) | 647 512 (55.0) | 444 534 (57.6) | 25 634 (42.4) | 7647 (43.4) | 17 987 (42.0) | 2186 (25.8) | 17 480 (50.7) | 5550 (59.2) | 7063 (60.7) |
| Sulphonylureas, n (%) | 339 157 (28.8) | 218 948 (28.3) | 16 024 (26.5) | 4424 (25.1) | 11 600 (27.1) | 1677 (19.8) | 10 207 (29.6) | 2628 (28.0) | 3715 (31.9) |
| DPP‐4 inhibitors, n (%) | 265 896 (22.6) | 189 488 (24.5) | 14 206 (23.5) | 4631 (26.3) | 9575 (22.4) | 2346 (27.7) | 7341 (21.3) | 1836 (19.6) | 1807 (15.5) |
| SGLT2 inhibitors, n (%) | 13 732 (1.2) | 9908 (1.3) | 445 (0.7) | 157 (0.9) | 288 (0.7) | 53 (0.6) | 116 (0.3) | 77 (0.8) | 52 (0.4) |
| GLP‐1RAs, n (%) | 14 869 (1.3) | 10 859 (1.4) | 1122 (1.9) | 270 (1.5) | 852 (2.0) | 153 (1.8) | 246 (0.7) | 139 (1.5) | 125 (1.1) |
| Meglitinides, n (%) | 63 381 (5.4) | 30 574 (4.0) | 2924 (4.8) | 738 (4.2) | 2186 (5.1) | 469 (5.5) | 1262 (3.7) | 312 (3.3) | 394 (3.4) |
| Thiazolidinediones, n (%) | 50 547 (4.3) | 48 183 (6.2) | 3645 (6.0) | 553 (3.1) | 3092 (7.2) | 197 (2.3) | 1739 (5.0) | 379 (4.0) | 659 (5.7) |
| Acarbose, n (%) | 101 896 (8.7) | 58 521 (7.6) | 4210 (7.0) | 1145 (6.5) | 3065 (7.2) | 670 (7.9) | 1891 (5.5) | 486 (5.2) | 429 (3.7) |
| Insulin, n (%) | 366 462 (31.1) | 183 429 (23.7) | 22 057 (36.5) | 6052 (34.3) | 16 005 (37.4) | 3817 (45.1) | 10 266 (29.8) | 2853 (30.4) | 4068 (35.0) |
Abbreviations: ACE, angiotensin‐converting enzyme; UAP, unstable angina pectoris; AP, angina pectoris; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; CVRD, cardiovascular or renal disease; DPP‐4, dipeptidyl‐peptidase‐4; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; SGLT2, sodium‐glucose co‐transporter‐2; UAP, xxx.
TABLE 1.
Baseline characteristics of patients with type 2 diabetes without history of cardiovascular or renal disease in six countries
| All CVRD‐free | England | Germany | Japan | Netherlands | Norway | Sweden | |
|---|---|---|---|---|---|---|---|
| Index year | n/a | 2010 | 2014 | 2016 | 2012 | 2010 | 2007 |
| Number of patients | 772 336 | 66 412 | 136 635 | 299 965 | 36 903 | 94 683 | 137 738 |
| Age, years (SD) | 65.2 (12.6) | 59.8 (13.12) | 66.2 (11.9) | 67.5 (12.3) | 67.1 (12.1) | 61.2 (14.6) | 63.9 (12.5) |
| Females, n (%) | 345 928 (44.8) | 30 950 (46.6) | 61 203 (44.8) | 124 306 (41.4) | 18 340 (49.7) | 46 623 (49.2) | 64 506 (46.8) |
| Follow‐up time, years | 3 502 197 | 511 372 | 514 213 | 768 430 | 169 754 | 477 442 | 1 060 986 |
| CVD prevention, n (%) | 558 306 (72.3) | 56 133 (84.5) | 111 496 (81.6) | 182 275 (60.8) | 33 003 (89.4) | 69 007 (72.9) | 106 392 (77.2) |
| Low‐dose aspirin, n (%) | 141 815 (18.4) | 23 514 (35.4) | 7778 (5.7) | 32 169 (10.7) | 7595 (20.6) | 30 050 (31.7) | 40 709 (29.6) |
| Statins, n (%) | 322 219 (41.7) | 47 176 (71.0) | 44 447 (32.5) | 101 924 (34.0) | 247 60 (67.1) | 451 23 (47.7) | 58 789 (42.7) |
| Anti‐hypertensives, n (%) | 449 539 (58.2) | 39 192 (59.0) | 94 501 (69.2) | 149 173 (49.7) | 24 107 (65.3) | 56 539 (59.7) | 86 027 (62.5) |
| ACE inhibitors, n (%) | 173 160 (22.4) | 27 196 (41.0) | 59 644 (43.7) | 14 736 (4.9) | 12 383 (33.6) | 17 377 (18.4) | 41 824 (30.4) |
| ARBs, n (%) | 219 303 (28.4) | 9167 (13.8) | 33 264 (24.3) | 104 459 (34.8) | 10 679 (28.9) | 32 220 (34.0) | 29 514 (21.4) |
| Beta blockers, n (%) | 165 474 (21.4) | 8161 (12.3) | 54 315 (39.8) | 27 536 (9.2) | 13 231 (35.9) | 23 284 (24.6) | 38 947 (28.3) |
| High ceiling diuretics, n (%) | 73 498 (9.5) | 3514 (5.3) | 14 587 (10.7) | 25 020 (8.3) | 3854 (10.4) | 9002 (9.5) | 17 521 (12.7) |
| Aldosterone antagonists, n (%) | 13 639 (1.8) | 418 (0.6) | 1997 (1.5) | 4128 (1.4) | 1109 (3.0) | 1375 (1.5) | 4612 (3.3) |
| Metformin, n (%) | 444 534 (57.6) | 54 180 (81.6) | 98 046 (71.8) | 88 841 (29.6) | 29 000 (78.6) | 70 813 (74.8) | 103 654 (75.3) |
| Sulphonylureas, n (%) | 218 948 (28.3) | 23 530 (35.4) | 24 648 (18.0) | 75 970 (25.3) | 13 950 (37.8) | 33 535 (35.4) | 47 315 (34.4) |
| DPP‐4 inhibitors, n (%) | 189 488 (24.5) | 2229 (3.4) | 29 130 (21.3) | 154 933 (51.7) | 1917 (5.2) | 1279 (1.4) | 0 (0.0) |
| SGLT2 inhibitors, n (%) | 9908 (1.3) | 0 (0.0) | 1122 (0.8) | 8786 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| GLP‐1RAs, n (%) | 10 859 (1.4) | 977 (1.5) | 3037 (2.2) | 6000 (2.0) | 485 (1.3) | 360 (0.4) | 0 (0.0) |
| Meglitinides, n (%) | 30 574 (4.0) | 467 (0.7) | 3353 (2.5) | 17 808 (5.9) | 39 (0.1) | 249 (0.3) | 8658 (6.3) |
| Thiazolidinediones, n (%) | 48 183 (6.2) | 9102 (13.7) | 215 (0.2) | 25 566 (8.5) | 1032 (2.8) | 4074 (4.3) | 8194 (5.9) |
| Acarbose, n (%) | 58 521 (7.6) | 202 (0.3) | 1288 (0.9) | 54 354 (18.1) | 45 (0.1) | 642 (0.7) | 1990 (1.4) |
| Insulin, n (%) | 183 429 (23.7) | 8399 (12.6) | 29 648 (21.7) | 80 267 (26.8) | 10 220 (27.7) | 14 986 (15.8) | 39 909 (29.0) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; CVRD, cardiovascular or renal disease; DPP‐4, dipeptidyl‐peptidase‐4; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; SGLT2, sodium‐glucose co‐transporter‐2.
3.1. First CVRD manifestation
Of 772 336 CVRD‐free patients with T2D, 137 081 (18%) developed a first CVRD manifestation during follow‐up. Cardiorenal disease was consistently the most frequent first manifestation and was increased early across all countries (Figure 1 and Figure S1A). The proportion of cardiorenal disease manifestations in initially CVRD‐free patients with T2D was 60%, consisting of 24% HF and 36% CKD, and this was four‐, four‐, and sixfold more common than stroke (16%), MI (14%) and PAD (10%), respectively. Cardiorenal disease was the most frequent manifestation in all countries: England 67%, Germany 67%, Japan 70%, the Netherlands 57%, Norway 52% and Sweden 48% (Figure S1B).
FIGURE 1.
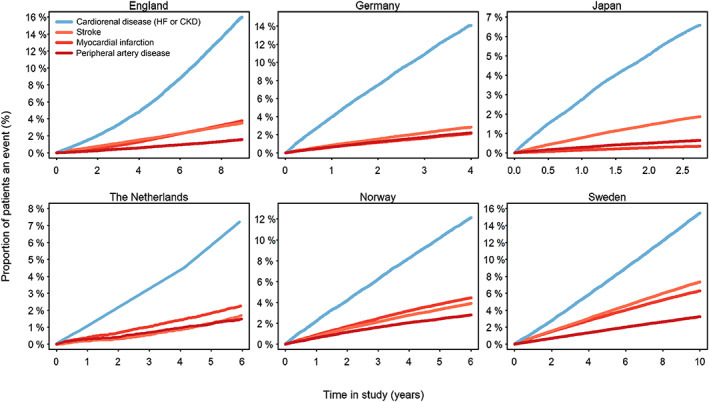
Cardiovascular manifestation during follow‐up in initially cardiovascular or renal disease‐free patients with type 2 diabetes. CKD, chronic kidney disease; HF, heart failure
3.2. Single presence of CVRD and risks
The groups with single presence of different CVRDs were generally older (70.0 to 74.7 years) than the general T2D population (67.7 years; Table 2), with similar trends in all countries (Table S5). CVRD‐free patients were, in general, younger and had received less CVD preventive treatment. Event rates of HF, CKD, stroke, MI and PAD in the CVRD‐free cohort were 13.4, 18.5, 7.5, 6.5 and 5.0 events per 1000 patient‐years, respectively (Table S6). When comparing the groups with single presence of CVRD with the CVRD‐free group, single presence of cardiorenal disease was associated with an increased all‐cause and CVD mortality risk compared to CVRD‐free with T2D : HRs 2.02 (95% CI 1.75–2.33) and 2.05 (1.82–2.32), respectively (Figure 2). The separate components of cardiorenal disease (HF, CKD and the combination of the two in CRS) were all associated with increased risks of all‐cause, CVD mortality and CVD events (Figures 2 and 3). Single presence of CRS was associated with the highest risks of all‐cause and CVD mortality compared to CVRD‐free status: HR 3.14 (95% CI 2.90–3.40) and HR 3.91 (95% CI 3.02–5.07), respectively. Similar patterns were seen in all countries (Figure S2). Single presence of cardiorenal disease and presence of its separate components were also associated with significantly increased risk of MI, stroke and PAD compared to CVRD‐free status, demonstrating similar risk patterns as seen for mortality (Figure 3) consistently in all countries (Figure S3).
FIGURE 2.
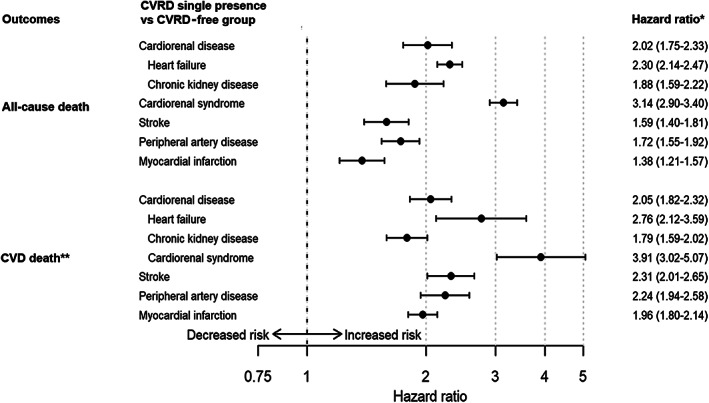
Pooled death risks associated with the single presence groups of cardiovascular or renal disease (CVRD) compared to a CVRD‐free type 2 diabetes group. Cardiorenal disease defined as heart failure (HF) or chronic kidney disease (CKD). Cardiorenal syndrome defines as the presence of both HF and CKD). *Adjusted for age and sex. **Cardiovascular disease (CVD) death was not obtainable in Germany, Japan and the Netherlands
FIGURE 3.
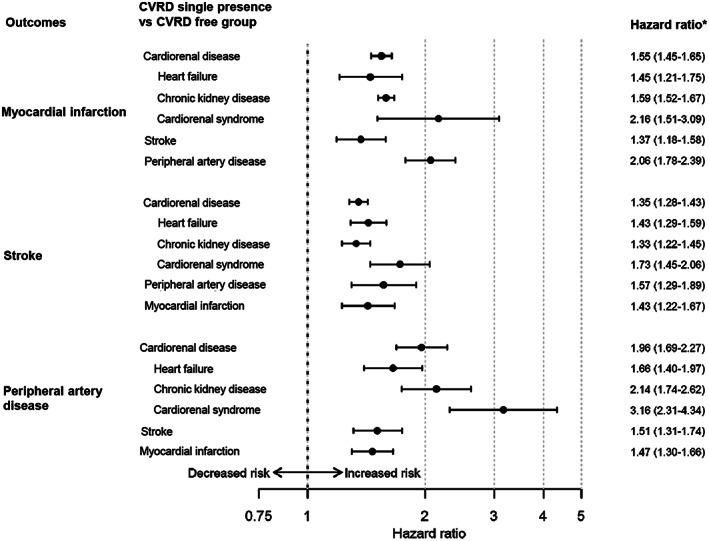
Pooled cardiovascular risks associated with the single presence groups of cardiovascular or renal disease (CVRD) compared to a CVRD‐free type 2 diabetes group. Cardiorenal disease defined as heart failure (HF) or chronic kidney disease (CKD). Cardiorenal syndrome defines as the presence of both HF and CKD). *Adjusted for age and sex
3.3. Bi‐directional risk associations between HF and CKD
Compared to CVRD‐free status, single presence of HF was associated with increased risk of incident CKD (HR 2.30 [95% CI 2.00–2.65]) and CKD was associated with increased risk of HF (HR 1.99 [95% CI 1.75–2.26]), consistent across all countries (Figure S4).
3.4. Sensitivity analyses
Validation of the CKD definition using available eGFR data showed robust sensitivity and specificity results across multiple countries (Figure S5A). Detailed validation of the CKD diagnosis definition set during outpatient clinic and primary care visits showed similar results (Figures S5B and S5C). Use of only first diagnosis or first and second diagnosis when identifying CVRD manifestation showed similar findings (Figure S6).
4. DISCUSSION
In this large population‐based study including more than one million general patients with T2D across six countries and populations of different ethnicities in Europe and Asia, we have shown that the majority (66%) were CVRD‐free, that is, had no recorded history of cardiovascular or renal disease. In approximately 780 000 CVRD‐free patients with T2D, cardiorenal disease was consistently the most frequent first CVRD disease manifestation (60%) and was four, four and six times more common than stroke, MI and PAD, respectively. In addition, single presence of HF or CKD was associated with a high risk of death and CVD complications compared with CVRD‐free with T2D status, more so than single presence of MI, stroke or PAD. We also showed a bi‐directional risk association, where single presence of HF was associated with an approximately twofold increased risk of incident CKD and vice versa, confirming existing knowledge regarding interlinked pathophysiology leading to CRS. 17 These novel findings show that cardiorenal disease is an important and potentially fatal complication in T2D, representing an unmet clinical need which should be considered when choosing future optimal preventive strategies in the management of these patients, adding a cardiorenal preventive approach to an already existing and quietly successful atherosclerotic preventive approach. 18 , 21
Shah et al 41 assessed cardiovascular manifestation in 34 198 patients with T2D using data from England. However, comparisons with the present study are challenging because of large differences in objectives and methods. For example, the T2D population free from CVD in the study by Shah et al 41 was differently defined and was likely to have higher baseline CVD risks, data were less contemporary, different outcome definitions were used compared to the present study, and CKD was not considered. For more detailed discussions, see Supporting Information, pp. 10–12.
We demonstrated that incident HF is one of the most frequent first manifestations of CVRD in T2D across several countries. This is supported by recent reports confirming that T2D is the most powerful risk factor for incident HF 42 and that risk of incident HF is developing earlier in patients with T2D when compared to individuals without diabetes. 43
The absence of hospitalization for coronary artery disease prior to the incident HF events suggests that diabetic cardiomyopathy, 44 caused by left ventricular hypertrophy 45 and increased myocardial fibrosis, 46 , 47 , 48 , 49 or myocardial metabolic derangement, could form part of the explanation of the HF manifestation in the present study. Consequently, the increased likelihood of incident diabetic cardiomyopathy might be less impacted by the extensive use of preventive treatment with statins, low‐dose aspirin and anti‐hypertensives compared to MI and stroke risk. 22 Moreover, another large observational study of more than 270 000 patients with T2D has shown that well managed classic risk factors might neutralize MI and stroke risks, but not the risk of HF. 18 These findings suggest that HF preventive treatment is particularly challenging in T2D. 22
We have also shown that single presence of HF in otherwise CVRD‐free patients with T2D is associated with increased risks of both death and further CVD risk. Many studies have, conversely, shown that T2D increases the risk of death in patients with HF, 50 , 51 , 52 , 53 and the present study adds knowledge of how HF increases risk of death in CVRD‐free patients with T2D. This finding, in combination with the high incidence rates, is particularly important to understand when assessing the potential of new primary preventive strategies to lower risks of incident HF.
Chronic kidney disease was, next to HF, the other most frequent first CVRD manifestation in initially CVRD‐free patients with T2D across all countries. To our knowledge little is known about the development of incident CKD in a real‐world CVRD‐free T2D population, also in comparison to development of other CVD events. The association between poor kidney function, for example, low eGFR or high albuminuria, and CVD or mortality risk has been firmly established in previous reports. 10 , 15 We have shown that presence of CKD is associated with increased CVD and mortality risks in a T2D population across several countries. These findings support that CKD is strongly associated with CVD 10 and that the combination with T2D is associated with severe consequences, which require improved treatment strategies. 54 , 55
Finally, we have shown that HF in T2D is associated with a high (twofold) risk of CKD and vice versa, confirming that multifaceted crosstalk forms a vicious circle between the failing cardiorenal organs. 16 , 17 , 56 To our knowledge, few epidemiological data on CRS and its associations with CVD and death risk have been reported. We have shown that single presence of CRS is associated with very high (three‐ to fourfold) CVD and mortality risks compared to CVRD‐free with T2D status. This shows that all components of cardiorenal disease, HF, CKD and especially CRS, are associated with serious risks. While the increased complication risks of HF and CKD are known, little has been reported about the associated risks in patients with T2D who are otherwise free from cardiovascular and renal disease. The gravity of cardiorenal diseases has been underlined by Ronco et al, 57 who suggest a novel outcome, major adverse renal and cardiac events (MARCE), to include cardiorenal disease for future trials and observational studies. This was also reinforced in the cardiorenal scientific statement from the American Heart Association in 2019. 17
In the present study, we have shown that cardiorenal disease might be a frequent and fatal unmet clinical need in the large proportion of patients with T2D without cardiovascular or renal disease, demanding new priorities when choosing preventive treatment strategies. The novel SGLT2 inhibitor drug class has been reported to have strong risk‐reducing effects on both HF and CKD in patients without established cardiovascular and renal disease, 28 , 30 and may contribute significantly to an improved primary preventive strategy in addition to the important CVD risk factor management in T2D. 54 , 55 , 58 , 59
To our knowledge, this study is the first to address the development of cardiorenal disease across multiple countries. Despite differences in ethnicity, healthcare systems and treatment guidelines across six countries, we found robustly similar findings of disease manifestations and associated risks in approximately 780 000 patients. The definition of CKD used in the present study was validated across multiple countries (Supporting Information, pp. 34).
Our findings should be interpreted within the context of several potential limitations. Data sources have different properties regarding coverage of treatment level (primary or hospital care), and population proportion covered might in turn have led to insufficient information to ensure that patients were truly CVD‐free at index. The robustness of CVRD manifestation and associated risks is, however, supported by observations in a diversity of registry properties and healthcare systems across the countries, for example, access to primary and hospital care (England, Germany, the Netherlands) versus hospital care only (Japan, Norway and Sweden), full population (Norway, Sweden), representative population data (England, Germany, Japan and the Netherlands), and finally different ethnic populations (European and Asian). Only outcomes requiring hospital care were used which might have led to underestimation of less severe conditions, such as those managed in primary care. Validation of HF diagnoses from hospital care has been evaluated but not in a CVRD‐free T2D population such as that in the present study. 60 , 61 However, high validity of HF diagnoses are probable because the patients with T2D in this cohort are healthy and relatively young and more careful diagnosing is expected compared with older and more comorbid patients. Residual confounding attributable to variables not covered by the registries, such as diabetes duration, laboratory records, low number of proteinuria measurements, measurements of cardiac function, smoking, alcohol intake, diet, physical activity, stress and environmental factors, might have influenced the results.
In conclusion, in patients with T2D without a history of CVRD, across six countries in Europe and Asia, cardiorenal disease (HF or CKD) was consistently the most frequent first CVRD manifestation and was associated with significantly increased risk of all‐cause and CVD death. These novel findings show that cardiorenal disease is an important T2D complication that needs improved preventive strategies.
CONFLICTS OF INTEREST
K.I.B. has received grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. J.B. holds a full‐time position at AstraZeneca as an epidemiologist. J.W.E. has received honoraria or research grants from AstraZeneca, NovoNordisk, Bayer, Sanofi and MSD. A.N. has received honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. H.H has received lecture fees and travel expenses from Alexion, Baxter, NovoNordisk, Noxxon, Janssen and AstraZeneca. G.C.M.L. has no competing interests. M.T. is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic‐Baltic is a client. S.O. is a full‐time employee of AstraZeneca. E.G.P. is an employee of Team Gesundheit GmbH and conducted work on behalf of Kantar Health. J.O. is an employee of the PHARMO Institute for Drug Outcomes Research, an independent research institute that performs financially supported studies for government and related healthcare authorities and for several pharmaceutical companies. R.Z. and T.Y. are full‐time employees of AstraZeneca. I.K. declares grants from Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, Mitsubishi Tanabe Pharma Corp., Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd, Toa Eiyo Ltd, honoraria from Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, Mitsubishi Tanabe Pharma Corp., Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd and Toa Eiyo Ltd, and lecture/other fees from AstraZeneca. T.K. declares grants from Asahi Mutual Life Insurance Co., Boehringer Ingelheim Japan, Daiichi Sankyo Co. Ltd, Kowa Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Corp., MSD K.K., Novo Nordisk Pharma Ltd, Sanofi K.K. and Takeda Pharmaceutical Co. Ltd and lecture/other fees from AstraZeneca K.K., Astellas Pharma Inc., Boehringer Ingelheim Japan, Daiichi Sankyo Co. Ltd, Eli Lilly Japan K.K., Kowa Pharmaceutical Co. Ltd, Kyowa Hakko Kirin Co., Ltd, Mitsubishi Tanabe Pharma Corp., MSD K.K., Ono Pharmaceutical Co. Ltd, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd, Sanwa Kagaku Kenkyusho Co. Ltd, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Co, Ltd.
AUTHOR CONTRIBUTIONS
All authors participated in the research design. M.T. performed the data management and statistical analyses for all countries after discussion with all authors. Statistical analyses were performed in Japan, Norway and Sweden by M.T., Germany by E.G., the Netherland by J.O., and England by R.Z. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility in the decision to submit for publication.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We are grateful to Susanna Jerström and Helena Goike at AstraZeneca for logistical support and valuable comments on the manuscript. Urban Olsson, Statisticon AB, is acknowledged for database management. All authors are guarantors of the manuscript. Data from the Norwegian Patient Register have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian patient register is intended nor should be inferred.
Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab. 2020;22:1607–1618. 10.1111/dom.14074
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14074.
Funding information AstraZeneca
REFERENCES
- 1. Federation ID . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. http://www.idf.org/diabetesatlas. [Google Scholar]
- 2. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2018;24:968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28(3):612‐616. [DOI] [PubMed] [Google Scholar]
- 4. Collaboration GBDCKD . Global, regional, and national burden of chronic kidney disease, 1990‐2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298‐302. [DOI] [PubMed] [Google Scholar]
- 6. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853‐872. [DOI] [PubMed] [Google Scholar]
- 8. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699‐703. [DOI] [PubMed] [Google Scholar]
- 9. Bruck K, Stel VS, Gambaro G, et al. CKD prevalence varies across the european general population. J Am Soc Nephrol. 2016;27(7):2135‐2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gansevoort RT, Correa‐Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339‐352. [DOI] [PubMed] [Google Scholar]
- 11. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296‐1305. [DOI] [PubMed] [Google Scholar]
- 12. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577‐1589. [DOI] [PubMed] [Google Scholar]
- 13. Kerr M, Bray B, Medcalf J, O'Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(Suppl 3):iii73‐iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015;33(8):811‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. A collaborative meta‐analysis of high‐risk population cohorts. Kidney Int. 2011;79(12):1341‐1352. [DOI] [PubMed] [Google Scholar]
- 16. Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio‐renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32(1):18‐25. [DOI] [PubMed] [Google Scholar]
- 17. Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement From the American Heart Association. Circulation. 2019;139(16):e840‐e878. [DOI] [PubMed] [Google Scholar]
- 18. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633‐644. [DOI] [PubMed] [Google Scholar]
- 19. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532‐2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregg EW, Li Y, Wang J, et al. Changes in diabetes‐related complications in the United States, 1990‐2010. N Engl J Med. 2014;370(16):1514‐1523. [DOI] [PubMed] [Google Scholar]
- 22. Greene SJ, Butler J. Primary prevention of heart failure in patients with type 2 diabetes mellitus. Circulation. 2019;139(2):152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 24. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 25. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 26. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 27. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 28. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
- 29. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 30. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 31. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes and chronic kidney disease in primary and secondary cardiovascular prevention groups: results from the randomized CREDENCE trial. Circulation. 2019;140:739‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) Aurum. Int J Epidemiol. 2019;48:1740‐1740g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2018;21(4):968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular Events Associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628‐2639. [DOI] [PubMed] [Google Scholar]
- 35. Tanabe M, Motonaga R, Terawaki Y, Nomiyama T, Yanase T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. J Diabetes Investig. 2017;8(2):227‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanabe M, Nomiyama T, Motonaga R, Murase K, Yanase T. Reduced vascular events in type 2 diabetes by biguanide relative to sulfonylurea: study in a Japanese hospital database. BMC Endocr Disord. 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herings RM, Pedersen L. Pharmacy‐based medical record linkage systems In: Kimmel S, Hennessey S, eds. Pharmacoepidemiology. 5th ed. Oxford, United Kingdom: John Wiley & Sons, Ltd; 2012:270‐286. [Google Scholar]
- 38. van Herk‐Sukel MP, van de Poll‐Franse LV, Lemmens VE, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46(2):395‐404. [DOI] [PubMed] [Google Scholar]
- 39. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709‐717. [DOI] [PubMed] [Google Scholar]
- 40. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20(2):344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Avery CL, Loehr LR, Baggett C, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60(17):1640‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879‐1884. [DOI] [PubMed] [Google Scholar]
- 44. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294‐e324. [DOI] [PubMed] [Google Scholar]
- 45. Levelt E, Mahmod M, Piechnik SK, et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. 2016;65(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582‐592. [DOI] [PubMed] [Google Scholar]
- 47. Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41(4):611‐617. [DOI] [PubMed] [Google Scholar]
- 48. Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5(11):715‐724. [DOI] [PubMed] [Google Scholar]
- 49. Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes. 2015;6(7):943‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dauriz M, Targher G, Laroche C, et al. Association between diabetes and 1‐year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: results from the ESC‐HFA heart failure long‐term registry. Diabetes Care. 2017;40(5):671‐678. [DOI] [PubMed] [Google Scholar]
- 51. From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119(7):591‐599. [DOI] [PubMed] [Google Scholar]
- 52. Johansson I, Dahlstrom U, Edner M, Nasman P, Ryden L, Norhammar A. Prognostic implications of type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol. 2016;68(13):1404‐1416. [DOI] [PubMed] [Google Scholar]
- 53. Targher G, Dauriz M, Laroche C, et al. In‐hospital and 1‐year mortality associated with diabetes in patients with acute heart failure: results from the ESC‐HFA heart failure long‐term registry. Eur J Heart Fail. 2017;19(1):54‐65. [DOI] [PubMed] [Google Scholar]
- 54. Cherney DZI, Repetto E, Wheeler DC, et al. Impact of cardio‐renal‐metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 55. Garcia‐Carro C, Vergara A, Agraz I, et al. The new era for reno‐cardiovascular treatment in type 2 diabetes. J Clin Med. 2019;8(6).1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62:298‐302. [DOI] [PubMed] [Google Scholar]
- 57. Ronco C, Ronco F, McCullough PA. A call to action to develop integrated curricula in cardiorenal medicine. Blood Purif. 2017;44(4):251‐259. [DOI] [PubMed] [Google Scholar]
- 58. Verma S, Juni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet. 2019;393(10166):3‐5. [DOI] [PubMed] [Google Scholar]
- 59. Connelly KA, Bhatt DL, Verma S. Can we declare a victory against cardio‐renal disease in diabetes? Cell Metab. 2018;28(6):813‐815. [DOI] [PubMed] [Google Scholar]
- 60. Brynildsen J, Hoiseth AD, Nygard S, et al. [Diagnostic accuracy for heart failure ‐ data from the Akershus Cardiac Examination 2 Study]. Tidsskr Nor Laegeforen. 2015;135(19):1738‐1744. [DOI] [PubMed] [Google Scholar]
- 61. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787‐791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


