Dear Editor,
Joint bleeding is a hallmark of haemophilia A, 1 with recurrent bleeds having the potential to cause irreversible joint damage and crippling arthropathy, often limiting daily activities and requiring surgery. 2 , 3 , 4 Target joints are those joints where three or more bleeds occur in a 6‐month period 5 and are more prone to chronic damage if not properly treated. Prophylaxis with factor VIII (FVIII) concentrates is the current standard of haemophilia care. Its main aim is to prevent the onset and/or progression of joint damage by reducing the frequency and severity of recurrent bleeding episodes, including that into joints, thus preventing haemarthropathy. 2 , 3 Reduced bleeding through long‐term prophylaxis also improves quality of life and allows patients to participate in physical and social activities. 4 , 6 Avoiding surgery to treat or replace damaged joints may also contribute to savings in healthcare costs.
Despite the availability of effective factor replacement therapy, recurrent joint bleeding remains a clinical challenge, particularly in patients with severe haemophilia A. 2 To optimize outcomes, prophylaxis needs to be started early in life and individualized to meet the clinical and lifestyle needs of each patient. 2 , 6 This tailoring should take into account patients’ bleeding phenotype, joint status, lifestyle, degree of physical activity and pharmacokinetic response to clotting factor concentrates. 3 , 6 Patient adherence to prophylaxis is also relevant to achieving good outcomes. 6 Despite acceptance of prophylaxis, adherence rates can be low due to the need for frequent dosing. 7
BAY 94‐9027 (damoctocog alfa pegol; Jivi®; Bayer AG, Germany) is an extended half‐life B‐domain–deleted, site‐specifically PEGylated recombinant FVIII. Due to its prolonged half‐life, BAY 94‐9027 has the potential to maintain FVIII at a higher haemostatic level for longer periods versus standard‐acting agents. 8 This longer half‐life also allows less frequent dosing, which in turn could help to improve adherence when used for prophylaxis. 7
In the phase 2/3 open‐label, partially randomized PROTECT VIII study (NCT01580293), male patients aged 12‐65 years with severe (<1% FVIII) haemophilia A received BAY 94‐9027 for 36 weeks on demand (n = 20) or prophylactically (n = 114). 7 Patients in the prophylaxis group received BAY 94‐9027 25 IU/kg twice weekly for 10 weeks. Those with 0‐1 bleeds in this period were then randomized to BAY 94‐9027 45‐60 IU/kg every 5 days or 60 IU/kg every 7 days for the main study period of 26 weeks. Patients who experienced two or more breakthrough bleeds during the initial 10‐week run‐in period were not eligible for randomization; they increased their dose of BAY 94‐9027 to 30‐40 IU/kg and remained on a twice‐weekly schedule. Patients with good bleed control (up to one breakthrough bleed) who were enrolled after randomization continued with twice‐weekly treatment at 30‐40 IU/kg. All patients who completed 36 weeks of treatment could receive open‐label BAY 94‐9027 in an extension phase. In both the main study and extension, patients could switch their dosing regimen if bleed control was inadequate.
The PROTECT VIII study demonstrated BAY 94‐9027 to be effective at preventing bleeds, at three individually tailored dose regimens at dosing intervals of up to every 7 days. Of the 126 patients who completed the main study, 121 entered the extension that confirmed the safety and efficacy of BAY 94‐9027 prophylaxis with dosing intervals of up to every 7 days for >5 years. 9
Given the importance of preventing joint damage for patients with severe haemophilia A, we performed a post hoc analysis on the PROTECT VIII study data to explore the impact of long‐term BAY 94‐9027 prophylaxis on target joints. The numbers of historic target joints (as judged by the investigators) were recorded at study entry. In addition, the number of new target joints that developed on‐study was evaluated, using the International Society on Thrombosis and Haemostasis (ISTH) definition of a target joint (three or more spontaneous bleeds within 6 months). 5 We also assessed the number of target joints that had resolved by data cut‐off (28 August 2019), using the ISTH definition of joint resolution (a recorded target joint with two or fewer spontaneous bleeds during the last 12 months). 5 These variables were analysed in patients known to have received prophylaxis before study entry and who continued on prophylaxis into the main study and its ongoing extension.
At the data cut‐off for this analysis, there were 82 patients who had been previously treated with FVIII prophylaxis at study entry, and continued prophylaxis with BAY 94‐9027 during the main study and the extension. Patients in the extension were treated either twice weekly (n = 13), every 5 days (Q5D [n = 29]) or every 7 days (Q7D [n = 17]). Patients switching after the first infusion beyond 7 days into the extension were considered together in a ‘variable’ dosage group (n = 23), and comprised patients switching from Q5D (n = 12) or Q7D (n = 11), mostly to a more frequent dosing regimen (Q5D, n = 7 increased to twice weekly; Q7D, n = 6 increased to Q5D and n = 4 increased to twice weekly). Patient characteristics at baseline included the following: median (Q1; Q3) age = 32.5 (24.0; 46.0) years; median (Q1; Q3) body mass index = 24.5 (21.1; 27.7). The median (range) time that these 82 patients had been in the study was 1421 (700‐2071) days – almost 4 years. The median (Q1; Q3) target joint annualized bleeding rate was 0 (0‐1.5) at the end of the main study and 0 (0‐1.4) at the extension cut‐off date.
At baseline, 23 (28%) patients had no historic target joints. In the remaining 59 (72%) patients, baseline target joint burden comprised mostly one or two historic target joints (27 and 18 patients, respectively), although a proportion had three or more target joints (Table 1). The mean (standard deviation) number of historic target joints per patient was 1.4 ± 1.3 for the group as a whole.
Table 1.
Patients with target joint resolution in PROTECT VIII and the extension study, stratified by the number of historic target joints at baseline
| Patients | All patients (N = 82) | |||||
|---|---|---|---|---|---|---|
| 0 Historic target joints (n = 23) | 1 Historic target joint (n = 27) | 2 Historic target joints (n = 18) | 3 Historic target joints (n = 8) | >3 Historic target joints (n = 6) | ||
| Patients with new target joints, n (%) | 3 (13) | 2 (7) | 2 (11) | 1 (13) | 1 (17) | 9 (11) a |
| Patients with target joints (historic or new), n (%) | 3 (13) | 27 (100) | 18 (100) | 8 (100) | 6 (100) | 62 (76) |
| Patients with target joints (historic or new) with at least one of which resolved, n (%) | 1 (33) | 26 (96) | 18 (100) | 8 (100) | 6 (100) | 59 (95) |
| Patients with target joints (historic or new) in which all resolved, n (%) | 1 (33) | 25 (96) | 14 (78) | 7 (88) | 6 (100) | 53 (85) |
Abbreviations: Q5D, every 5 days; Q7D, every 7 days.
Dosing regimens in the main/extension study for patients with 0 historic target joints at baseline: Q5D/Q5D, n = 1; Q7D/variable, n = 2. Dosing regimens in the main/extension study for patients with historic target joints at baseline: twice weekly, n = 1; Q5D/Q5D, n = 1; Q7D/Q7D, n = 1; Q7D/Q5D, n = 1; Q5D/variable, n = 1; Q7D/variable, n = 1.
A total of 113 individual target joints were recorded between the 59 patients reporting historic target joints at baseline (Figure 1). The most common sites for historic target joints were the ankles (47 target joints), elbows (33 target joints) and knees (27 target joints). A total of nine new target joints developed in 9 (11%) patients over the course of the study (~4 years). These also most commonly affected the ankle, elbow or knee joints. Of the 9 patients developing new target joints, 3 had no historic target joints at baseline, while 6 had reported other affected joints at study entry; no patterns were observed between the development of new target joints and the BAY 94‐9027 treatment regimen (Table 1).
Figure 1.
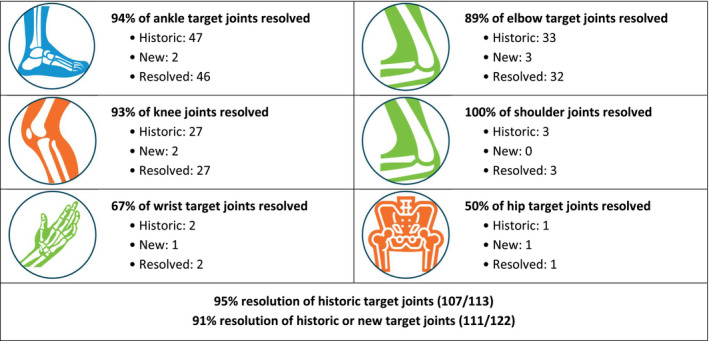
Target joint resolution in PROTECT VIII and the extension study by type of joint
Of a total of 122 historic or new target joints, 111 (91%) had resolved at the data cut‐off. Resolution occurred in all prophylactic regimens. When we examined data for the most common types of target joints at baseline, we found that resolution occurred in 94% of ankle, 93% of knee and 89% of elbow joints. In addition, all shoulder target joints resolved, and 67% and 50% of wrist and hip target joints, respectively, resolved by the data cut‐off point (Figure 1).
Finally, we looked at the data from the perspective of individual patient outcomes among the 62 patients who either had target joints at baseline (n = 59) or had no historic targets at baseline but developed new target joints during the study (n = 3). In this subgroup, 95% (59/62) experienced resolution in at least one target joint and 85% (53/62) achieved resolution in all target joints by data cut‐off, including 1 patient who had six target joints at baseline.
In summary, our post hoc analysis of the PROTECT VIII study found a high rate of resolution of target joints with long‐term prophylaxis with BAY 94‐9027 among patients with severe haemophilia A. At least one target joint resolved in over 90% of patients, and over 80% of patients achieved resolution of all of their target joints. This effect was seen across all prophylactic regimens, including treatment of up to every 7 days.
A potential limitation of our study is that the number of target joints documented at baseline was as reported by the investigator, and may not have reflected the ISTH definition of target joints. In addition, this analysis is not able to comment on joint resolution over time during PROTECT VIII and its extension. However, our findings support BAY 94‐9027 as a valuable prophylactic strategy for patients with severe haemophilia A that improves joint outcomes by preventing repeat joint bleeding and resolving target joints, with the added value of a flexible dosing regimen.
DISCLOSURES
MTR: Grants/research support: Bayer, BioMarin. Consultant/advisory board/speaker: Bayer, Novo Nordisk, Sanofi Genzyme, Takeda. IP: Honoraria or fees for lectures/advisory board: Bayer. SL: Consultant/advisory board: Bayer, Pfizer, Takeda, Teva. Honoraria or fees: Roche, PI Healthcare, Baxter, Alnylam, Grifols, Novo Nordisk, BioMarin. ES: Advisory board/speaker bureau: Shire/Takeda, Bayer, Pfizer, CSL Behring, Novo Nordisk, Grifols, Bioverativ, Sobi, Octapharma, Kedrion, Spark, uniQure, Roche. MEM: Consultant/advisor/speaker: Bayer, CSL Behring, Novo Nordisk, Pfizer, Shire/Takeda, Octapharma, Roche, Sobi, Bioverativ, Kedrion, Grifols.
ACKNOWLEDGEMENTS
Dr Inga Bayh of Bayer AG, Wuppertal, Germany, was responsible for the statistical analysis of this study. This study was supported by Bayer. Medical writing assistance was provided by Fishawack Communications Ltd., which was fully funded by Bayer.
REFERENCES
- 1. Simpson ML, Valentino LA. Management of joint bleeding in hemophilia. Expert Rev Hematol. 2012;5(4):459‐468. [DOI] [PubMed] [Google Scholar]
- 2. Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorb. 2011;1:51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038‐2044. [DOI] [PubMed] [Google Scholar]
- 4. Samuelson Bannow B, Recht M, Négrier C, et al. Factor VIII: long‐established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev. 2019;35:43‐50. [DOI] [PubMed] [Google Scholar]
- 5. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935‐1939. [DOI] [PubMed] [Google Scholar]
- 6. Poon MC, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J. 2016;14(Suppl 1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reding MT, Ng HJ, Poulsen LH, et al. Safety and efficacy of BAY 94–9027, a prolonged‐half‐life factor VIII. J Thromb Haemost. 2017;15(3):411‐419. [DOI] [PubMed] [Google Scholar]
- 8. Shah A, Coyle T, Lalezari S, et al. BAY 94–9027, a PEGylated recombinant factor VIII, exhibits a prolonged half‐life and higher area under the curve in patients with severe haemophilia A: comprehensive pharmacokinetic assessment from clinical studies. Haemophilia. 2018;24(5):733‐740. [DOI] [PubMed] [Google Scholar]
- 9. Lalezari S, Reding MT, Pabinger I, et al. BAY 94–9027 prophylaxis is efficacious and well tolerated for up to >5 years with extended dosing intervals: PROTECT VIII extension interim results. Haemophilia. 2019;25(6):1011‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]


