Abstract
Objective
To review the literature on the mechanism of action of onabotulinumtoxinA in chronic migraine.
Background
OnabotulinumtoxinA is a chronic migraine preventive treatment that significantly reduces headache frequency. The traditional mechanism described for onabotulinumtoxinA – reducing muscle contractions – is insufficient to explain its efficacy in migraine, which is primarily a sensory neurological disease.
Methods
A narrative literature review on the mechanism of action of onabotulinumtoxinA in chronic migraine.
Results
Following injection into tissues, onabotulinumtoxinA inhibits soluble N‐ethylmaleimide‐sensitive fusion attachment protein receptor (SNARE)‐mediated vesicle trafficking by cleaving one of its essential proteins, soluble N‐ethylmaleimide‐sensitive fusion attachment protein (SNAP‐25), which occurs in both motor and sensory nerves. OnabotulinumtoxinA inhibits regulated exocytosis of motor and sensory neurochemicals and proteins, as well as membrane insertion of peripheral receptors that convey pain from the periphery to the brain, because both processes are SNARE dependent. OnabotulinumtoxinA can decrease exocytosis of pro‐inflammatory and excitatory neurotransmitters and neuropeptides such as substance P, calcitonin gene‐related peptide, and glutamate from primary afferent fibers that transmit nociceptive pain and participate in the development of peripheral and central sensitization. OnabotulinumtoxinA also decreases the insertion of pain‐sensitive ion channels such as transient receptor potential cation channel subfamily V member 1 (TRPV1) into the membranes of nociceptive neurons; this is likely enhanced in the sensitized neuron. For chronic migraine prevention, onabotulinumtoxinA is injected into 31‐39 sites in 7 muscles of the head and neck. Sensory nerve endings of neurons whose cell bodies are located in trigeminal and cervical ganglia are distributed throughout the injected muscles, and are overactive in people with migraine. Through inhibition of these sensory nerve endings, onabotulinumtoxinA reduces the number of pain signals that reach the brain and consequently prevents activation and sensitization of central neurons postulated to be involved in migraine chronification.
Conclusion
OnabotulinumtoxinA likely acts via sensory mechanisms to treat chronic migraine.
Keywords: migraine, headache, botulinum, trigeminal system
Abbreviations
- BoNTA
botulinum toxin type A
- CGRP
calcitonin gene‐related peptide
- CNS
central nervous system
- FGFR3
fibroblast growth factor receptor 3
- NAPs
neurotoxin‐associated proteins
- NO
nitric oxide
- P2X3
purinergic receptor P2X ligand‐gated ion channel 3
- PACAP 38
pituitary adenylate cyclase‐activating peptide‐38
- PSG
polysialoganglioside
- SNAP‐25
soluble N‐ethylmaleimide‐sensitive fusion attachment protein
- SNARE
soluble N‐ethylmaleimide‐sensitive fusion attachment protein receptor
- SV2
synaptic vesicle protein 2
- TRPA1
transient receptor potential cation channel subfamily A member 1
- TRPV1
transient receptor potential cation channel subfamily V member 1
Introduction
Botulinum neurotoxin type A (BoNTA) is a potent inhibitor of muscle contraction that acts by preventing the release of acetylcholine at the neuromuscular junction. This property led to the development of an injectable formulation, commonly referred to as BOTOX (onabotulinumtoxinA), for the treatment of ocular conditions characterized by focal muscle overactivity, particularly blepharospasm and strabismus. 1 , 2 Subsequently, the clinical use expanded and onabotulinumtoxinA became a first‐line treatment for cervical dystonia and a treatment for upper and lower limb spasticity in adults 3 and pediatrics. In clinical trials, treatment of cervical dystonia 4 , 5 , 6 and spasticity 7 , 8 , 9 with onabotulinumtoxinA reduced both muscle contractions and pain. The clinical use of onabotulinumtoxinA expanded to other conditions that involve abnormal muscle contractions. 10 In the early 1990s, some patients described improvement in their migraine following treatment of facial lines with onabotulinumtoxinA. 11 Since migraine is primarily a sensory disease, these reports raised the possibility that onabotulinumtoxinA had an ability to block activation of nociceptive pathways. The literature on onabotulinumtoxinA has largely focused on its mechanism of action at the neuromuscular junction, and there is a gap in understanding how it may affect the sensory system as well. 12 Thus, this narrative literature review aims to summarize our current understanding of the mechanism of action for onabotulinumtoxinA for the treatment of chronic migraine.
In 2010, 2 double‐blind placebo‐controlled trials confirmed onabotulinumtoxinA’s effectiveness for the prevention of headaches in chronic migraine patients. In these trials, a migraine‐specific injection paradigm (155‐195 U, 31‐39 injection sites in head and neck muscles, which correspond to areas innervated by sensory nerves) resulted in significant reduction of headache and migraine days per month compared to placebo (Table 1). 13 These results led to the regulatory approval for chronic migraine in 2010, and lent credence to the idea that onabotulinumtoxinA treatment could modulate sensory neurons, 14 which is the focus of this review.
Table 1.
Efficacy of OnabotulinumtoxinA in the Treatment of Chronic Migraine†
| Variable‡ | OnabotulinumtoxinA (n = 688) | Placebo (n = 696) | Mean Intergroup Difference (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Frequency of headache days§ | ||||
| Baseline | 19.9 | 19.8 | 0.1 | .498 |
| Change from baseline | −8.4 | −6.6 | −1.8 (−2.52, −1.13) | <.001¶ |
| Frequency of migraine/probable migraine days | ||||
| Baseline | 19.1 | 18.9 | 0.2 | .328 |
| Change from baseline | −8.2 | −6.2 | −2.0 (−2.67, −1.27) | <.001¶ |
| Total headache impact test‐6 score | ||||
| Baseline | 65.5 | 65.4 | 0.1 | .638 |
| Change from baseline | −4.8 | −2.4 | −2.4 (−3.11, −1.72) | <.001†† |
OnabotulinumtoxinA Mechanism Overview
OnabotulinumtoxinA contains 900‐kDa BoNTA protein complex consisting of the 150‐kDa botulinum neurotoxin and several nontoxic, neurotoxin‐associated proteins (NAPs). The NAPs are thought to play a role in the pharmacologic actions of the neurotoxin, including structural stability of the neurotoxin, 15 protection from proteolysis, 16 and/or binding kinetics. 17 OnabotulinumtoxinA acts on peripheral nerve terminals to interfere with specific events in the synaptic vesicle cycle. Briefly, at nerve terminals, synaptic vesicles undergo fusion to the cell membrane and are recycled. Vesicles containing neurotransmitters and neuropeptides destined for synaptic release undergo docking, priming, and fusion with the neuronal membrane. 18 These steps require the crucial formation of the protein assembly SNARE complex (soluble N‐ethylmaleimide‐sensitive fusion‐attachment protein receptor) (Fig. 1, top panel). 19 Vesicular contents include small molecules in small synaptic vesicles (eg, acetylcholine and glutamate), or neuropeptides in large dense core vesicles (eg, calcitonin gene‐related peptide [CGRP], pituitary adenylate cyclase activating peptide 38 [PACAP 38], and Substance P). Large dense core vesicle cargo include proteins and receptors (eg, transient receptor potential cation channel subfamily V member 1 [TRPV1], transient receptor potential cation channel subfamily A member 1 [TRPA1], purinergic receptor P2X ligand‐gated ion channel 3 [P2X3], etc.) whose insertion into the lipid bilayer of the synaptic membrane is critical for proper pain signaling. 20 , 21 In some cases, vesicles fuse with the nerve terminal membrane through constitutive exocytosis, 22 which is a housekeeping function. In other cases, fusion of synaptic vesicles with nerve terminal membrane is SNARE mediated. SNARE ability to regulate exocytosis is most commonly associated with electrical activity in the nerve. Synaptic vesicles that have fully fused with the membrane then undergo recycling and the process begins again.
Fig. 1.

Mechanism of onabotulinumtoxinA at the synapse. The top panel shows fusion of large dense core synaptic vesicles with the nerve terminal membrane in the absence of onabotulinumtoxinA. By step 4, neurotransmitters contained in the synaptic vesicles are released into the synapse and receptors/ion channels are inserted into the nerve terminal membrane. The bottom panel shows the steps of onabotulinumtoxinA action at nerve terminals. The end result is that synaptic vesicles cannot fuse with the nerve terminal membrane, preventing release of neurotransmitters at the synapse, and inhibiting insertion of receptors/ion channels into the nerve terminal membrane.
The intraneuronal target for onabotulinumtoxinA is SNAP‐25 (synaptosomal‐associated protein‐25 kDa), one of the SNARE proteins critical for vesicular fusion. Following injection, onabotulinumtoxinA is distributed to the extracellular space. When the neurotoxin encounters nerve terminals 23 the heavy chain of the botulinum neurotoxin binds with relatively low affinity to a polysialoganglioside (PSG), including GT1b (KD ~200 nM) 24 , 25 , 26 (Fig. 1, bottom panel). A second receptor with greater affinity, synaptic vesicle protein 2 (SV2) (KD ~100 nM), 27 , 28 , 29 , 30 , 31 is a vesicle protein that is exposed to the extracellular space during vesicular fusion. 32 The heavy chain potentially binds to a higher‐affinity receptor, fibroblast growth factor receptor 3 (FGFR3) (KD ~15 nM 33 ).
OnabotulinumtoxinA, bound to the receptors, is endocytosed, and once it enters the endosome, the light chain dissociates from the heavy chain and translocates into the intracellular cytosol where it specifically cleaves SNAP‐25. 32 The light chain’s proteolytic cleavage of this essential component of the SNARE protein complex prevents the fusion of the synaptic vesicle to the inner surface of the cellular membrane. Impacted synaptic vesicles can neither release their contents into the synaptic cleft, nor deliver receptors or ion channels carried as cargo (eg, TRPV1, P2X3) into neuronal membranes. This latter effect is illustrated by onabotulinumtoxinA interfering with trafficking of thermoTRP channels. 22 The downstream inhibitory effects depend on the target organ, whether it be nociceptors, motor, or autonomic nerves innervating skeletal or smooth muscle, or glands.
In the nerve terminal, the light chain endopeptidase escapes immediate degradation via specific interactions on the presynaptic terminal including the presence of a dileucine motif, 34 interactions with membrane‐bound septins, 35 and specific deubiquinating enzymes, 36 and consequently, maintains persistent proteolytic cleavage of SNAP‐25. OnabotulinumtoxinA‐cleaved SNAP‐25 can still form stable, although nonfunctional SNARE complexes within neurons. These faulty complexes can have a relatively prolonged life within the synaptic terminal 37 , 38 After exposure to BoNTA, cleaved SNAP‐25 persisted beyond the latest timepoint, 80 days, in cultured spinal cord cells. 39 Together, these mechanisms (sustained proteolytic activity and prolonged faulty SNARE complexes) likely contribute to the long‐acting, nerve/tissue‐target–dependent effects of onabotulinumtoxinA. However, because the neuronal types studied in these preclinical experiments are not necessarily representative of mature motor nerves, and the experimental conditions diverge from the clinical situation, the translatability of the results to the clinical situation is unclear. Nevertheless, clinically, the effects of onabotulinumtoxinA last approximately 3 to 4 months in motor nerves 40 , 41 and 6 to 9 months in autonomic nerves. 42 , 43 The onabotulinumtoxinA light chain is ultimately ubiquinated 36 and neurotransmission is restored. 44 During recovery, the presence of sprouts in motor neurons 45 and their paucity in autonomic nerves 46 may also contribute to duration in specific nerve/tissue targets.
Rationale for OnabotulinumtoxinA for Chronic Migraine Treatment
Sensory effects of onabotulinumtoxinA in migraine are supported by findings from preclinical studies, which established that BoNTA inhibits the release of neuropeptides such as substance P 48 , 49 and CGRP 50 from primary sensory (first order) neurons. Sensory effects of onabotulinumtoxinA are also demonstrated in the formalin pain model, in which subcutaneous injection of onabotulinumtoxinA dose dependently inhibits the delayed pain response to formalin without affecting the acute pain response and without inducing muscle weakness. 51 In clinical studies, there were early suggestions of a dissociation between pain and muscle relaxation in cervical dystonia, with some studies reporting more prevalent improvements in pain than muscle contractions. 5 , 6 Additionally, a spasticity study that specifically examined the relationship between pain and muscle tone found only a weak correlation between them, consistent with the notion that onabotulinumtoxinA‐associated improvements in muscle tone and pain are separate dimensions. 47 OnabotulinumtoxinA has also shown benefits in the treatment of other pain disorders, including painful diabetic neuropathy, a primary sensory disorder. 52 The combination of these findings provides a clinical basis for understanding the sensory effects of onabotulinumtoxinA in chronic migraine and are further confirmed in the laboratory studies described in the subsequent section.
Migraine Pathophysiology and OnabotulinumtoxinA Mechanisms of Action in Chronic Migraine Prevention
OnabotulinumtoxinA Inhibits Neurotransmitter and Neuropeptide Release
The initiation of migraine pain occurs at the periphery when nociceptive neurons that innervate the dura and potentially the pia mater become active and release vasoactive and pro‐inflammatory neuropeptides and neurotransmitters that further irritate them and mediate their prolonged activation. 58 The vasodilatory neuropeptides CGRP and PACAP‐38, as well as the neurotransmitter nitric oxide (NO), are potent vasodilators involved in migraine pathophysiology. 59 , 60 , 61 , 62 , 63 , 64 , 65
At the synaptic cleft, onabotulinumtoxinA attenuates the release of neuropeptides and neurotransmitters that activate and modulate receptors that have been implicated in migraine pathophysiology. 48 , 50 , 66 , 67 , 68 , 69 This is supported by preclinical findings that show that onabotulinumtoxinA inhibits the release of glutamate, 51 substance P, 49 and CGRP 50 from primary sensory (ie, first‐order neurons in dorsal root and trigeminal ganglia) nerve terminals. 51 Regarding CGRP, recent reviews of its role in the headache phase of migraine 70 , 71 , 72 and the rationale behind the successful prevention of migraine with drugs that reduce its presence in the periphery 73 support the possibility that a part of onabotulinumtoxinA’s mechanism of action in migraine prevention may involve the reduction of CGRP release from peripheral nerve terminals of meningeal and trigeminal nociceptors. Two lines of evidence support this possibility. The first is in vitro animal experiments showing that onabotulinumtoxinA inhibits the release of CGRP from sensory neurons. 50 , 74 The second is a clinical study showing that onabotulinumtoxinA reduces interictal CGRP plasma levels in chronic migraine patients who are deemed treatment responders but not those deemed treatment nonresponders. 75
In cases in which chronic migraine is associated with chronic muscle tenderness, occipital allodynia, 56 , 76 , 77 and altered expression of genes related to inflammation in the calvarial periosteum, 56 , 76 , 77 extracranial injection of onabotulinumtoxinA may exert its effects through direct action on extracranial nerves (Fig. 2). However, extracranial injection of onabotulinumtoxinA also has a clear path to intracranial nerves, 55 , 78 which may account for its effects on migraine headaches that originate intracranially. Functional evidence for these intracranial‐to‐extracranial and extracranial‐to‐intracranial pathways comes from the finding that extracranial administration of onabotulinumtoxinA inhibits responses of C‐ but not Aδ‐fibers to stimulation of their intracranial meningeal receptive fields with ligands of TRPV1 and TRPA1 channels. 57
Fig. 2.
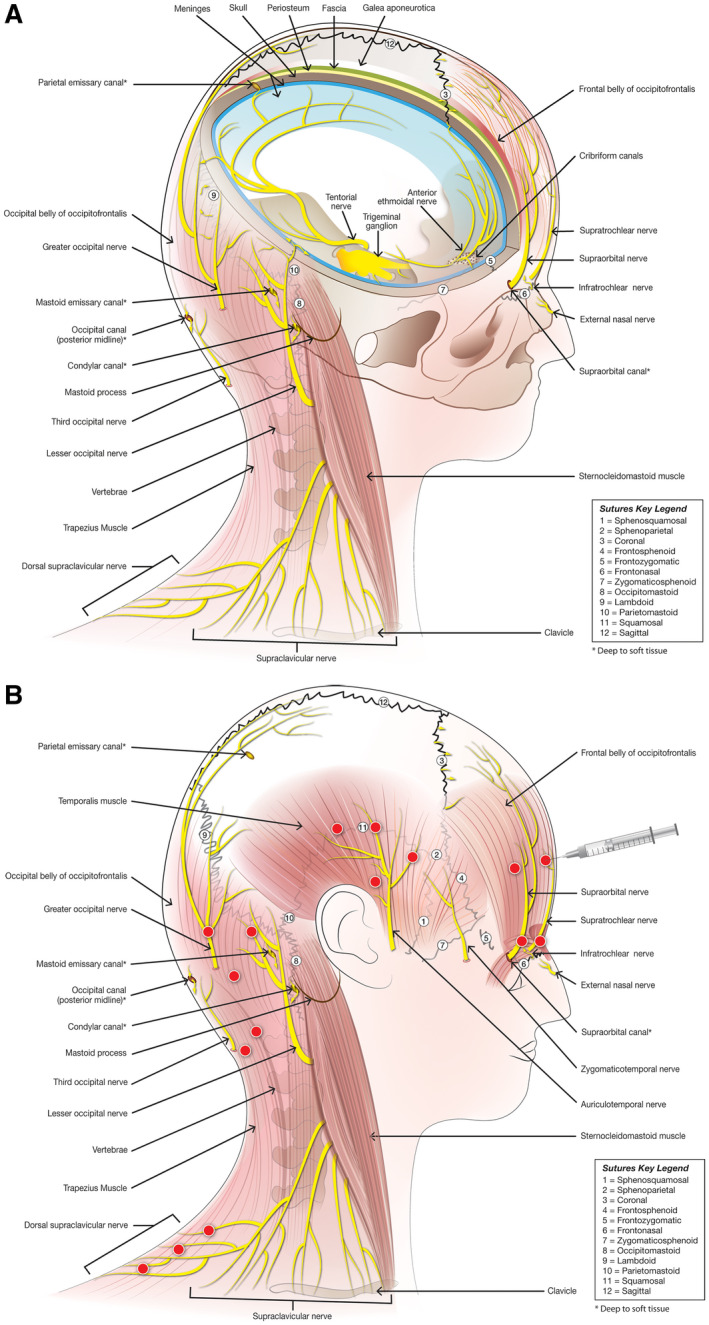
Neuroanatomy relevant to onabotulinumtoxinA injections sites. (A) Nerves originating from the trigeminal ganglion innervate intracranial structures and extend extracranially through cranial sutures. Spinal nerves originating from cervical dorsal root ganglia 2 and 3 innervate pericranial muscles and extend intracranially through cranial sutures, emissary canals, and fissures. (B) Extracranial injection sites correspond to anatomical region of extracranial nerves, many of which are adjacent to cranial sutures, emissary canals, and fissures.
The mechanism by which injection of onabotulinumtoxinA into extracranial muscles can affect intracranial neurons is still under investigation, and several possibilities exist, including effects of onabotulinumtoxinA on collateral branches of trigeminal axons that cross from the inside to the outside 78 , 79 or cervical axons that cross from the outside to inside 55 of the skull via suture lines, emissary canals, and fissures, as demonstrated in rats 55 , 78 , 79 and humans 79 , 80 (Fig. 2). The mechanisms by which injections of onabotulinumtoxinA reduce activation along peripheral and central pathways, such as those that mediate migraine headache, include (1) increased threshold for nociceptive activation by reducing circulating levels of neuropeptides (eg, CGRP) and neurotransmitters; 75 , 81 (2) decreased TRPV1‐immunoreactive neurons in the trigeminal ganglion following peripheral injections into the face; 82 (3) reduced activation of dorsal horn neurons 83 , 84 and expression of NO synthase in the central nervous system (CNS) after injections of onabotulinumtoxinA into the intraplantar fascia and relevant muscles, respectively; 85 and (4) reduced numbers of dendrites and synapses in central sensory processes, as exemplified in the hypoglossal nucleus after injection of onabotulinumtoxinA into the tongue, 86 which were also associated with changes in nucleolar and cell body shape. Given recent evidence against transsynaptic transfer of onabotulinumtoxinA, 87 , 88 it is now believed that central sensitization, synaptic plasticity, and other CNS effects attributed to onabotulinumtoxinA are secondary to the decreased peripheral input. 89
OnabotulinumtoxinA Inhibits Ion Channel Insertion into Synaptic Membranes
Migraine headache is commonly associated with throbbing and increased headache intensity caused by mild elevation in intracranial pressure due to coughing, sneezing, or bending over. 90 , 91 , 92 , 93 These symptoms are believed to result from sensitization of nerve endings of first‐order sensory neurons – a functional switch involving upregulation of pain‐related ion channels on nociceptive nerve terminals and cell bodies, including TRPA1, TRPV1, and sodium channels. Repeated stimulation of trigeminal nerve endings and their eventual sensitization can lead to the development of central sensitization, ongoing pain, allodynia (pain caused by stimuli that do not normally evoke pain), and hyperalgesia (increased sensitivity to pain). 94 , 95
The effects of onabotulinumtoxinA on ionic channels expression in nociceptors have not been directly tested in people with migraine because the involved nerves are located in head and neck areas that are not readily accessible to biopsies. However, in patients with overactive bladder, onabotulinumtoxinA is injected directly into bladder muscle and submucosal area, which, given patients’ consent, may be sampled for study. Epithelial cells in the urinary bladder express TRPV1 and P2X3 receptors, which are believed to be involved in conveying sensory information such as the urge to urinate. 96 , 97 In suburothelial tissue obtained from patients with detrusor overactivity (a condition of overactive bladder), onabotulinumtoxinA significantly decreased and normalized the pretreatment elevated TRPV1 and P2X3 levels, and improved both clinical and urodynamic measures. 97 The decrease in P2X3 receptors and, to a lesser extent, TRPV1 receptors after onabotulinumtoxinA treatment were significantly correlated with improvements in sensations of urgency, but not with changes in maximum detrusor pressure or the volume at which patients felt they could no longer delay urination. Along this line, in a capsaicin human pain model, subcutaneous administration of onabotulinumtoxinA to the forehead reduced capsaicin‐induced pain intensity and duration, most likely through downregulation of TRPV1 receptors on unmyelinated c‐fiber nociceptors, 98 and in a population of female patients with chronic migraine, a polymorphism in the TRPV1 gene was associated with a greater likelihood of response to onabotulinumtoxinA. 99
Selectivity of OnabotulinumtoxinA Effects
The inhibitory effects of onabotulinumtoxinA on SNARE‐mediated processes are not observed in all neurons. Outside the context of migraine, onabotulinumtoxinA does not give rise to local anesthesia, 100 suggesting that it does not interact with large‐diameter myelinated axons carrying tactile information from the skin to the spinal cord. In the context of migraine, where the headache phase depends on activity in unmyelinated C‐ and thinly myelinated Aδ‐fibers in the dura, onabotulinumtoxinA appears to selectively inhibit activation and sensitization of the unmyelinated C‐ but not thinly myelinated Aδ‐fibers, 101 as well as their activation by mustard oil and capsaicin 57 or cortical spreading depolarization/depression. 102 The latter result is supported by the effectiveness of onabotulinumtoxinA in treating chronic migraine patients both with and without aura 103 (the former presumably related to cortical spreading depolarization/depression). As far as selectivity is concerned, a recent preclinical study found that another migraine medication, humanized CGRP monoclonal antibodies, inhibits Aδ‐ but not C‐type neurons in the trigeminal ganglion. 104 These preclinical findings as well as emerging clinical experience 105 , 106 , 107 suggest the interesting possibility that a combination treatment that blocks both the C‐ (onabotulinumtoxinA) and the Aδ‐ (CGRP monoclonal antibodies and CGRP receptor antagonists) meningeal nociceptors may be more effective than a monotherapy that blocks only one of these pathways. While these animal‐based selectivities await additional confirmation in humans, the enigma of how onabotulinumtoxinA exerts its selective effects on different classes of sensory neurons remains unanswered.
Rationale for OnabotulinumtoxinA Injection Paradigm for Chronic Migraine
For chronic migraine prevention, onabotulinumtoxinA is injected into 31‐39 sites in 7 muscles of the head and neck. 53 The injection sites correlate closely with the sensory innervation of the face, scalp, and cervical region. These include the supratrochlear and supraorbital nerves, which travel through the corrugator, procerus, and frontalis muscles; the auriculotemporal and zygomaticotemporal nerves; the greater and lesser occipital nerves traveling along the occipitofrontalis complex to innervate the adjacent scalp; the third occipital nerve traveling through the cervical paraspinal muscles; and the supraclavicular nerves traveling through the trapezius (Fig. 2). Following intramuscular injection, onabotulinumtoxinA diffuses within the tissue to affect nerves within a circumscribed region. 108
The primary role of trigeminal and cervical neurons in migraine 54 , 55 , 56 and the inhibition of SNARE‐mediated processes by onabotulinumtoxinA are consistent with an inhibitory action of onabotulinumtoxinA on these nerves. SNARE‐mediated processes in these nerve terminals include the vesicular release of inflammatory and nociceptive neuropeptides and neurotransmitters and the insertion of pain‐encoding receptors into the membrane of unmyelinated c‐fibers. 57
The aforementioned sensory effects of onabotulinumtoxinA suggest that it may also be useful for episodic migraine. Although several of the early randomized, controlled studies in episodic migraine showed an efficacy signal, 109 , 110 they did not use the PREEMPT paradigm (ie, 31‐39 injection sites in head and neck muscles) that was demonstrated to be effective in chronic migraine phase 3 studies. Thus, the efficacy and safety of onabotulinumtoxinA in episodic migraine has not been fully explored. Real‐world evidence using the PREEMPT paradigm has demonstrated clinical benefit in patients with episodic migraine. 111
Conclusions
Mechanism of Action in the Synapse
Although onabotulinumtoxinA is primarily known for its inhibition of muscle contraction, it is an effective treatment for the prevention of chronic migraine – a sensory neurological disease. The common basis for these clinical outcomes is onabotulinumtoxinA inhibition of SNARE‐mediated vesicle trafficking, which occurs in both motor and sensory nerves. OnabotulinumtoxinA inhibits regulated exocytosis of motor and sensory neurochemicals and proteins, as well as membrane insertion of peripheral receptors that convey pain from the periphery to the brain in pathological conditions such as chronic migraine because both processes are SNARE dependent (Fig. 1). OnabotulinumtoxinA can decrease exocytosis of pro‐inflammatory and excitatory neurotransmitters and neuropeptides such as substance P, CGRP, and glutamate from primary afferent fibers that transmit nociceptive pain and participate in the development of peripheral and central sensitization. OnabotulinumtoxinA also decreases the insertion of pain‐sensitive ion channels such as TRPV1 into the membranes of nociceptive neurons. Prolonged activation of sensory neurons is likely to increase insertion of TRPV1 channels into the membrane. In vivo studies have demonstrated that treatment reduced sensory neuron excitability and sensitization, consistent with increasing the pain threshold for migraine.
Mechanism of Action in Migraine
The main sensory input to the face and head comes from the trigeminal nerve, which innervates muscles, meninges, and other tissues, with contributions from cervical and occipital nerves. Numerous pericranial injections of onabotulinumtoxinA are likely needed to target the vast projection regions of trigeminal and cervical nerves, in order to attenuate their overall input to central neurons, which appear to become sensitized and perpetuate chronic migraine when activated repeatedly or continuously by pain signals they receive from the periphery (Fig. 2). Through this antidromic influence, onabotulinumtoxinA injected into the periphery can reduce the number of pain signals that travel along sensory nerves from the dura to the spinal trigeminal nucleus, which indirectly prevents the development of hyperexcitability of spinal, brainstem, thalamic, and cortical neurons involved in migraine pathophysiology.
Beyond Headache
Investigations into the mechanism of onabotulinumtoxinA action in chronic migraine and other conditions with prominent sensory components (eg, overactive bladder) have broadened our understanding of its therapeutic benefit. In contemplating which, if any, other diseases may benefit from onabotulinumtoxinA treatment, it will be important to consider and be guided by the extent to which SNARE‐mediated processes play a role in pathology.
Statement of Authorship
Category 1
(a) Conception and Design
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
(b) Acquisition of Data
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
(c) Analysis and Interpretation of Data
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
Category 2
(a) Drafting the Manuscript
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
(b) Revising It for Intellectual Content
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
Category 3
(a) Final Approval of the Completed Manuscript
Rami Burstein, Andrew M. Blumenfeld, Stephen D. Silberstein, Aubrey Manack Adams, Mitchell F. Brin
Acknowledgments
Writing and editorial support for development of this manuscript was provided by Mary Ann Chapman, of Visage Communications, Inc., Spokane, WA, USA, with administrative support provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA; and was funded by NIH grants R37‐NS0797681, RO1‐NS094198 (RB) and grants from Allergan plc (Dublin, Ireland).
Conflict of Interest: MFB and AMA are employees of Allergan. RB is a consultant to Allergan. AMB has served on advisory boards for Allergan, Amgen, Alder, Teva, Supernus, Promius, Eaglet, and Lilly; and has received funding for speaking from Allergan, Amgen, Pernix, Supernus, Depomed, Avanir, Promius, Teva, and Eli Lilly and Company. SDS is a consultant and/or advisory panel member for and has received honoraria from Alder Biopharmaceuticals, Allergan, Amgen, Avanir, eNeura, ElectroCore Medical, Labrys Biologics, Medscape, Medtronic, Neuralieve, NINDS, Pfizer, and Teva. His employer receives research support from Allergan, Amgen, Cumberland Pharmaceuticals, ElectroCore Medical, Labrys Biologics, Eli Lilly, Mars, and Troy Healthcare. RB received grant support from Allergan, Ely Lilly, Teva, Dr. Reddy., and the NIH. He is also a consultant to Allergan, Alder, Amgen, Biohaven, Electrocore, Johnson & Johnson, Neurolief, Percept, Teav, Theranica, and Trigemina.
Funding: NIH grants R37‐NS0797681, RO1‐NS094198 (RB) and grants from Allergan.
References
- 1. Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044‐1049. [DOI] [PubMed] [Google Scholar]
- 2. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985;103:347‐350. [DOI] [PubMed] [Google Scholar]
- 3. Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsui JK, Eisen A, Mak E, Carruthers J, Scott A, Calne DB. A pilot study on the use of botulinum toxin in spasmodic torticollis. Can J Neurol Sci. 1985;12:314‐316. [DOI] [PubMed] [Google Scholar]
- 5. Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double‐blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;2:245‐247. [DOI] [PubMed] [Google Scholar]
- 6. Brin MF, Fahn S, Moskowitz C, et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov Disord. 1987;2:237‐254. [DOI] [PubMed] [Google Scholar]
- 7. Dengler R, Neyer U, Wohlfarth K, Bettig U, Janzik HH. Local botulinum toxin in the treatment of spastic drop foot. J Neurol. 1992;239:375‐378. [DOI] [PubMed] [Google Scholar]
- 8. Memin B, Pollak P, Hommel M, Perret J. Treatment of spasticity with botulinum toxin. Rev Neurol (Paris). 1992;148:212‐214. [PubMed] [Google Scholar]
- 9. Benecke R. Botulinum toxin for spasms and spasticity in the lower extremities In: Jankovic J, Hallett M, eds. Therapy With Botulinum Toxin. New York: Marcel Dekker, Inc.; 1994:557‐566. [Google Scholar]
- 10. Brin MF, Blitzer A. History of onabotulinumtoxinA therapeutic In: Carruthers A, Carruthers J, eds. Botulinum Toxin E‐Book, 4th edn. New York, NY: Elsevier; 2018:1‐12. [Google Scholar]
- 11. Binder WJ, Brin MF, Blitzer A, Schoenrock L. Botulinum toxin A (Botox) for treatment in migraine headache: An open label assessment. Headache. 1999;39:344.29517174 [Google Scholar]
- 12. Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open‐label study. Otolaryngol Head Neck Surg. 2000;123:669‐676. [DOI] [PubMed] [Google Scholar]
- 13. Dodick DW, Turkel CC, DeGryse RE, et al. Assessing clinically meaningful treatment effects in controlled trials: Chronic migraine as an example. J Pain. 2015;16:164‐175. [DOI] [PubMed] [Google Scholar]
- 14. Jakubowski M, McAllister PJ, Bajwa ZH, Ward TN, Smith P, Burstein R. Exploding vs. imploding headache in migraine prophylaxis with botulinum toxin A. Pain. 2006;125:286‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kukreja RV, Singh BR. Comparative role of neurotoxin‐associated proteins in the structural stability and endopeptidase activity of botulinum neurotoxin complex types A and E. Biochemistry. 2007;46:14316‐14324. [DOI] [PubMed] [Google Scholar]
- 16. Sharma SK, Singh BR. Hemagglutinin binding mediated protection of botulinum neurotoxin from proteolysis. J Nat Toxins. 1998;7:239‐253. [PubMed] [Google Scholar]
- 17. Ghosal KJ, Patel K, Singh BR, Hale ML. Role of critical elements in botulinum neurotoxin complex in toxin routing across intestinal and bronchial barriers. PLoS One. 2018;13:e0199524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imig C, Min SW, Krinner S, et al. The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron. 2014;84:416‐431. [DOI] [PubMed] [Google Scholar]
- 19. Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3:a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devesa I, Ferrandiz‐Huertas C, Mathivanan S, et al. alphaCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc Natl Acad Sci U S A. 2014;111:18345‐18350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng J, Wang J, Steinhoff M, Dolly JO. TNFalpha induces co‐trafficking of TRPV1/TRPA1 in VAMP1‐containing vesicles to the plasmalemma via Munc18‐1/syntaxin1/SNAP‐25 mediated fusion. Sci Rep. 2016;6:21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrandiz‐Huertas C, Mathivanan S, Wolf CJ, Devesa I, Ferrer‐Montiel A. Trafficking of thermoTRP channels. Membranes (Basel). 2014;4:525‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rummel A. Two feet on the membrane: Uptake of clostridial neurotoxins. Curr Top Microbiol Immunol. 2017;406:1‐37. [DOI] [PubMed] [Google Scholar]
- 24. Yowler BC, Schengrund CL. Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry. 2004;43:9725‐9731. [DOI] [PubMed] [Google Scholar]
- 25. Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co‐receptor GT1b‐insight into the toxin‐neuron interaction. PLoS Pathog. 2008;4:e1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamark C, Berntsson RP, Masuyer G, et al. Glycans confer specificity to the recognition of ganglioside receptors by botulinum neurotoxin A. J Am Chem Soc. 2017;139:218‐230. [DOI] [PubMed] [Google Scholar]
- 27. Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011‐2014. [DOI] [PubMed] [Google Scholar]
- 28. Strotmeier J, Mahrhold S, Krez N, et al. Identification of the synaptic vesicle glycoprotein 2 receptor binding site in botulinum neurotoxin A. FEBS Lett. 2014;588:1087‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benoit RM, Frey D, Hilbert M, et al. Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature. 2014;505:108‐111. [DOI] [PubMed] [Google Scholar]
- 30. Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592‐596. [DOI] [PubMed] [Google Scholar]
- 31. Yao G, Zhang S, Mahrhold S, et al. N‐linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol. 2016;23:656‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12:535‐549. [DOI] [PubMed] [Google Scholar]
- 33. Jacky BP, Garay PE, Dupuy J, et al. Identification of fibroblast growth factor receptor 3 (FGFR3) as a protein receptor for botulinum neurotoxin serotype A (BoNT/A). PLoS Pathog. 2013;9:e1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez‐Salas E, Steward LE, Ho H, et al. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc Natl Acad Sci U S A. 2004;101:3208‐3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vagin O, Tokhtaeva E, Garay PE, et al. Recruitment of septin cytoskeletal proteins by botulinum toxin A protease determines its remarkable stability. J Cell Sci. 2014;127(Pt 15):3294‐3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai YC, Kotiya A, Kiris E, et al. Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type A intoxication. Proc Natl Acad Sci U S A. 2017;114:E5158‐E5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bajohrs M, Rickman C, Binz T, Davletov B. A molecular basis underlying differences in the toxicity of botulinum serotypes A and E. EMBO Rep. 2004;5:1090‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montecucco C, Molgo J. Botulinal neurotoxins: Revival of an old killer. Curr Opin Pharmacol. 2005;5:274‐279. [DOI] [PubMed] [Google Scholar]
- 39. Keller JE, Neale EA, Oyler G, Adler M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456:137‐142. [DOI] [PubMed] [Google Scholar]
- 40. Glogau R, Kane M, Beddingfield F, et al. OnabotulinumtoxinA: A meta‐analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg. 2012;38:1794‐1803. [DOI] [PubMed] [Google Scholar]
- 41. Wein T, Esquenazi A, Jost WH, Ward AB, Pan G, Dimitrova R. OnabotulinumtoxinA for the treatment of poststroke distal lower limb spasticity: A randomized trial. PM&R. 2018;10:693‐703. [DOI] [PubMed] [Google Scholar]
- 42. Naumann M, Lowe NJ, Kumar CR, Hamm H, Hyperhidrosis Clinical Investigators Group . Botulinum toxin type a is a safe and effective treatment for axillary hyperhidrosis over 16 months: A prospective study. Arch Dermatol. 2003;139:731‐736. [DOI] [PubMed] [Google Scholar]
- 43. Nitti VW, Ginsberg D, Sievert KD, et al. Durable efficacy and safety of long‐term onabotulinumtoxinA treatment in patients with overactive bladder syndrome: Final results of a 3.5‐year study. J Urol. 2016;196:791‐800. [DOI] [PubMed] [Google Scholar]
- 44. Dolly JO, Lawrence GW. Chapter 3: Molecular basis for the therapeutic effectiveness of botulinum neurotoxin type A. Neurourol Urodyn. 2014;33(Suppl. 3):S14‐S20. [DOI] [PubMed] [Google Scholar]
- 45. de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haferkamp A, Schurch B, Reitz A, et al. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type a in overactive neurogenic bladder. Eur Urol. 2004;46:784‐791. [DOI] [PubMed] [Google Scholar]
- 47. Wissel J, Ganapathy V, Ward AB, et al. OnabotulinumtoxinA improves pain in patients with post‐stroke spasticity: Findings from a randomized, double‐blind, placebo‐controlled trial. J Pain Symptom Manage. 2016;52:17‐26. [DOI] [PubMed] [Google Scholar]
- 48. Purkiss J, Welch M, Doward S, Foster K. Capsaicin‐stimulated release of substance P from cultured dorsal root ganglion neurons: Involvement of two distinct mechanisms. Biochem Pharmacol. 2000;59:1403‐1406. [DOI] [PubMed] [Google Scholar]
- 49. Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000;38:245‐258. [DOI] [PubMed] [Google Scholar]
- 50. Durham PL, Cady R, Cady R. Regulation of calcitonin gene‐related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache. 2004;44:35‐43. [DOI] [PubMed] [Google Scholar]
- 51. Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin‐induced pain. Pain. 2004;107:125‐133. [DOI] [PubMed] [Google Scholar]
- 52. Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: A randomized double‐blind crossover trial. Neurology. 2009;72:1473‐1478. [DOI] [PubMed] [Google Scholar]
- 53. Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well‐tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406‐1418. [DOI] [PubMed] [Google Scholar]
- 54. Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560‐564. [DOI] [PubMed] [Google Scholar]
- 55. Noseda R, Melo‐Carrillo A, Nir RR, Strassman AM, Burstein R. Non‐trigeminal nociceptive innervation of the posterior dura: Implications to occipital headache. J Neurosci. 2019;39:1867‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perry CJ, Blake P, Buettner C, et al. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: Implications for extracranial origin of headache. Ann Neurol. 2016;79:1000‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Strassman AM, Novack V, Brin MF, Burstein R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors' responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia. 2016;36:875‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dodick DW. A phase‐by‐phase review of migraine pathophysiology. Headache. 2018;58(Suppl. 1):4‐16. [DOI] [PubMed] [Google Scholar]
- 59. Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene‐related peptide is a potent vasodilator. Nature. 1985;313:54‐56. [DOI] [PubMed] [Google Scholar]
- 60. Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109‐142. [PubMed] [Google Scholar]
- 61. Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: Mechanisms and role in headache generation. Headache. 2012;52:1411‐1427. [DOI] [PubMed] [Google Scholar]
- 62. Kaiser EA, Russo AF. CGRP and migraine: Could PACAP play a role too? Neuropeptides. 2013;47:451‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Russo AF. Overview of neuropeptides: Awakening the senses? Headache. 2017;57(Suppl. 2):37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Russo AF. CGRP as a neuropeptide in migraine: Lessons from mice. Br J Clin Pharmacol. 2015;80:403‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jansen‐Olesen I, Hougaard Pedersen S. PACAP and its receptors in cranial arteries and mast cells. J Headache Pain. 2018;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McMahon HT, Foran P, Dolly JO, Verhage M, Wiegant VM, Nicholls DG. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma‐aminobutyric acid, aspartate, and met‐enkephalin release from synaptosomes. Clues to the locus of action. J Biol Chem. 1992;267:21338‐21343. [PubMed] [Google Scholar]
- 67. Hanna‐Mitchell AT, Wolf‐Johnston AS, Barrick SR, et al. Effect of botulinum toxin A on urothelial‐release of ATP and expression of SNARE targets within the urothelium. Neurourol Urodyn. 2015;34:79‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Collins VM, Daly DM, Liaskos M, et al. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int. 2013;112:1018‐1026. [DOI] [PubMed] [Google Scholar]
- 69. Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987‐993. [DOI] [PubMed] [Google Scholar]
- 70. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies – Successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338‐350. [DOI] [PubMed] [Google Scholar]
- 71. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lukacs M, Tajti J, Fulop F, Toldi J, Edvinsson L, Vecsei L. Migraine, neurogenic inflammation, drug development – Pharmacochemical aspects. Curr Med Chem. 2017;24:3649‐3665. [DOI] [PubMed] [Google Scholar]
- 73. Ong JJY, De Felice M. Migraine treatment: Current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15:274‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Joussain C, Le Coz O, Pichugin A, et al. Botulinum neurotoxin light chains expressed by defective herpes simplex virus type‐1 vectors cleave SNARE proteins and inhibit CGRP release in rat sensory neurons. Toxins (Basel). 2019;11:123‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cernuda‐Morollon E, Ramon C, Martinez‐Camblor P, Serrano‐Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156:820‐824. [DOI] [PubMed] [Google Scholar]
- 76. Blake P, Nir RR, Perry CJ, Burstein R. Tracking patients with chronic occipital headache after occipital nerve decompression surgery: A case series. Cephalalgia. 2019;39:556‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. 2019;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622‐1631. [DOI] [PubMed] [Google Scholar]
- 80. Schueler M, Neuhuber WL, De Col R, Messlinger K. Innervation of rat and human dura mater and pericranial tissues in the parieto‐temporal region by meningeal afferents. Headache. 2014;54:996‐1009. [DOI] [PubMed] [Google Scholar]
- 81. Cady R, Turner I, Dexter K, Beach ME, Cady R, Durham P. An exploratory study of salivary calcitonin gene‐related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache. 2014;54:269‐277. [DOI] [PubMed] [Google Scholar]
- 82. Shimizu T, Shibata M, Toriumi H, et al. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type‐A. Neurobiol Dis. 2012;48:367‐378. [DOI] [PubMed] [Google Scholar]
- 83. Cui M, Aoki KR. Mechanisms of the antinociceptive effect of subcutaneous BOTOX(R): Inhibition of peripheral and central nociceptive processing In: Olesen J, ed. Preventive Pharmacotherapy of Headache Disorders. Oxford: Oxford University Press; 2004:158‐162. [Google Scholar]
- 84. Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785‐793. [DOI] [PubMed] [Google Scholar]
- 85. Mariotti R, Bentivoglio M. Botulinum toxin induces nitric oxide synthase activity in motoneurons. Neurosci Lett. 1996;219:25‐28. [DOI] [PubMed] [Google Scholar]
- 86. Sumner BE. Ultrastructural responses of the hypoglossal nucleus to the presence in the tongue of botulinum toxin, a quantitative study. Exp Brain Res. 1977;30:313‐321. [DOI] [PubMed] [Google Scholar]
- 87. Cai BB, Francis J, Brin MF, Broide RS. Botulinum neurotoxin type A‐cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience. 2017;352:155‐169. [DOI] [PubMed] [Google Scholar]
- 88. Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO. Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission. Biochem J. 2012;441:443‐452. [DOI] [PubMed] [Google Scholar]
- 89. Weise D, Weise CM, Naumann M. Central effects of botulinum neurotoxin‐evidence from human studies. Toxins (Basel). 2019;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981;1:143‐147. [DOI] [PubMed] [Google Scholar]
- 91. Rasmussen BK, Jensen R, Olesen J. A population‐based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia. 1991;11:129‐134. [DOI] [PubMed] [Google Scholar]
- 92. Liveing E. On Megrim, Sick Headache. Nijmegen: Arts & Boeve Publishers; 1873. [Google Scholar]
- 93. Daley ML, Pasupathy H, Griffith M, Robertson JT, Leffler CW. Detection of loss of cerebral vascular tone by correlation of arterial and intracranial pressure signals. IEEE Trans Biomed Eng. 1995;42:420‐424. [DOI] [PubMed] [Google Scholar]
- 94. Woolf CJ. Evidence for a central component of post‐injury pain hypersensitivity. Nature. 1983;306:686‐688. [DOI] [PubMed] [Google Scholar]
- 95. Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986;6:1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yiangou Y, Facer P, Ford A, et al. Capsaicin receptor VR1 and ATP‐gated ion channel P2X3 in human urinary bladder. BJU Int. 2001;87:774‐779. [DOI] [PubMed] [Google Scholar]
- 97. Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977‐982. [DOI] [PubMed] [Google Scholar]
- 98. Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt‐Nielsen L. Subcutaneous Botulinum toxin type A reduces capsaicin‐induced trigeminal pain and vasomotor reactions in human skin. Pain. 2009;141:60‐69. [DOI] [PubMed] [Google Scholar]
- 99. Moreno‐Mayordomo R, Ruiz M, Pascual J, et al. CALCA and TRPV1 genes polymorphisms are related to a good outcome in female chronic migraine patients treated with OnabotulinumtoxinA. J Headache Pain. 2019;20:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155‐188. [PubMed] [Google Scholar]
- 101. Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia. 2014;34:853‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Melo‐Carillo A, Strassman AM, Schain AJ, Adams A, Brin MF, Burstein R. Extracranial injections of onabotulinumtoxinA in combination with iv injection of atogepant attenuates activation of HT and WDR neurons by CSD [abstract]. Presented at the International Headache Conference, Dublin, Ireland, 5‐8 September 2019, IHC2019L‐878. [Google Scholar]
- 103. Cernuda‐Morollon E, Martinez‐Camblor P, Ramon C, Larrosa D, Serrano‐Pertierra E, Pascual J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache. 2014;54:987‐995. [DOI] [PubMed] [Google Scholar]
- 104. Melo‐Carrillo A, Strassman AM, Nir RR, et al. Fremanezumab‐A humanized monoclonal anti‐CGRP antibody‐inhibits thinly myelinated (Adelta) but not unmyelinated (C) meningeal nociceptors. J Neurosci. 2017;37:10587‐10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Armanious M, Khalil N, Lu Y, Jimenez‐Sanders R. A retrospective analysis to evaluate the response of the addition of erenumab to onabotulinumtoxinA for the prevention of intractable chronic migraine wihtout aura. Presented at the International Headache Congress, 5‐8 Septebmer 2019, Dublin, Ireland. [Google Scholar]
- 106. Yuan H, Baggaley S, Digre K, Ozudogru S. CGRP antibodies as adjunctive prophylactic therapy for chronic migraine patients receiving onabotulinumtoxinA (BOTOX) injections. Presented at the International Headache Congress, 5‐8 September, 2019, Dublin, Ireland. [Google Scholar]
- 107. Boudreau GP, Demers C. Treatment of chronic migraine with erenumab alone or as an add on therapy: A real world prospective observational study Presented at the International Headache Congress, Dublin, Ireland, 5‐8 September 2019. [Google Scholar]
- 108. Trindade de Almeida AR, Marques E, de Almeida J, Cunha T, Boraso R. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatol Surg. 2007;33(Suppl. 1):S37‐S43. [DOI] [PubMed] [Google Scholar]
- 109. Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache. 2000;40:445‐450. [DOI] [PubMed] [Google Scholar]
- 110. Barrientos N, Chana P. Botulinum toxin type A in prophylactic treatment of migraine headaches: A preliminary study. J Headache Pain. 2003;4:146‐151. [Google Scholar]
- 111. Alpuente A, Gallardo VJ, Torres‐Ferrus M, Alvarez‐Sabin J, Pozo‐Rosich P. Early efficacy and late gain in chronic and high‐frequency episodic migraine with onabotulinumtoxinA. Eur J Neurol. 2019;26:1464‐1470. [DOI] [PubMed] [Google Scholar]
- 112. Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921‐936. [DOI] [PubMed] [Google Scholar]


